Abstract
Conjugating cells of the ciliate Tetrahymena thermophila were electroporated in the presence of plasmid DNA containing a paromomycin-resistant ribosomal RNA gene (rDNA). Cells were selected with paromomycin following 12-24 hr of growth on nonselective medium. Resistant cells appeared after 2-3 days. Processing vectors containing the micronuclear rDNA and somatic vectors containing the macronuclear gene transformed the cells, with the former yielding frequencies up to 900 transformants per microgram of plasmid DNA. A ribosomal protein gene (rpL29) conferring cycloheximide resistance also transformed conjugating cells. The transformation efficiency of the plasmid containing only the rpL29 gene was increased by insertion of an rDNA replication origin and by cotransformation and preselection with an rDNA vector. These results indicate that electroporation can be used for the production of large numbers of transformed Tetrahymena.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackburn E. H., Karrer K. M. Genomic reorganization in ciliated protozoans. Annu Rev Genet. 1986;20:501–521. doi: 10.1146/annurev.ge.20.120186.002441. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H. Structure and function of telomeres. Nature. 1991 Apr 18;350(6319):569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- Brunk C. F., Navas P. Transformation of Tetrahymena thermophila by electroporation and parameters effecting cell survival. Exp Cell Res. 1988 Feb;174(2):525–532. doi: 10.1016/0014-4827(88)90322-9. [DOI] [PubMed] [Google Scholar]
- Cech T. R., Brehm S. L. Replication of the extrachromosomal ribosomal RNA genes of Tetrahymena thermophilia. Nucleic Acids Res. 1981 Jul 24;9(14):3531–3543. doi: 10.1093/nar/9.14.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleffmann G. The transient division block in the D9ts mutant of Tetrahymena is inducible at every cell cycle stage. J Cell Sci. 1989 Mar;92(Pt 3):349–352. doi: 10.1242/jcs.92.3.349. [DOI] [PubMed] [Google Scholar]
- Del Sal G., Manfioletti G., Schneider C. A one-tube plasmid DNA mini-preparation suitable for sequencing. Nucleic Acids Res. 1988 Oct 25;16(20):9878–9878. doi: 10.1093/nar/16.20.9878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerder F. P., Debault L. E. Cytofluorimetric analysis of nuclear DNA during meiosis, fertilization and macronuclear development in the ciliate Tetrahymena pyriformis, syngen 1. J Cell Sci. 1975 May;17(3):471–493. doi: 10.1242/jcs.17.3.471. [DOI] [PubMed] [Google Scholar]
- Doerder F. P., Frankel J., Jenkins L. M., DeBault L. E. Form and pattern in ciliated protozoa: analysis of a genic mutant with altered cell shape in Tetrahymena pyriformis, Syngen 1. J Exp Zool. 1975 May;192(2):237–258. doi: 10.1002/jez.1401920214. [DOI] [PubMed] [Google Scholar]
- Engberg J., Nielsen H. Complete sequence of the extrachromosomal rDNA molecule from the ciliate Tetrahymena thermophila strain B1868VII. Nucleic Acids Res. 1990 Dec 11;18(23):6915–6919. doi: 10.1093/nar/18.23.6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel J., Jenkins L. M., Doerder F. P., Nelsen E. M. Mutations affecting cell division in Tetrahymena pyriformis. I. Selection and genetic analysis. Genetics. 1976 Jul;83(3 PT2):489–506. [PMC free article] [PubMed] [Google Scholar]
- Gorovsky M. A., Yao M. C., Keevert J. B., Pleger G. L. Isolation of micro- and macronuclei of Tetrahymena pyriformis. Methods Cell Biol. 1975;9(0):311–327. doi: 10.1016/s0091-679x(08)60080-1. [DOI] [PubMed] [Google Scholar]
- Greider C. W. Telomeres. Curr Opin Cell Biol. 1991 Jun;3(3):444–451. doi: 10.1016/0955-0674(91)90072-7. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Horowitz S., Gorovsky M. A. An unusual genetic code in nuclear genes of Tetrahymena. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2452–2455. doi: 10.1073/pnas.82.8.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerka-Dziadosz M., Frankel J. A mutant of Tetrahymena thermophila with a partial mirror-image duplication of cell surface pattern. I. Analysis of the phenotype. J Embryol Exp Morphol. 1979 Jan;49:167–202. [PubMed] [Google Scholar]
- Larson D. D., Blackburn E. H., Yaeger P. C., Orias E. Control of rDNA replication in Tetrahymena involves a cis-acting upstream repeat of a promoter element. Cell. 1986 Oct 24;47(2):229–240. doi: 10.1016/0092-8674(86)90445-9. [DOI] [PubMed] [Google Scholar]
- Love H. D., Jr, Allen-Nash A., Zhao Q. A., Bannon G. A. mRNA stability plays a major role in regulating the temperature-specific expression of a Tetrahymena thermophila surface protein. Mol Cell Biol. 1988 Jan;8(1):427–432. doi: 10.1128/mcb.8.1.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martindale D. W., Allis C. D., Bruns P. J. Conjugation in Tetrahymena thermophila. A temporal analysis of cytological stages. Exp Cell Res. 1982 Jul;140(1):227–236. doi: 10.1016/0014-4827(82)90172-0. [DOI] [PubMed] [Google Scholar]
- Orias E., Flacks M., Satir B. H. Isolation and ultrastructural characterization of secretory mutants of Tetrahymena thermophila. J Cell Sci. 1983 Nov;64:49–67. doi: 10.1242/jcs.64.1.49. [DOI] [PubMed] [Google Scholar]
- Orias E., Hamilton E. P., Flacks M. Osmotic shock prevents nuclear exchange and produces whole-genome homozygotes in conjugating Tetrahymena. Science. 1979 Feb 16;203(4381):660–663. doi: 10.1126/science.760210. [DOI] [PubMed] [Google Scholar]
- Orias E., Larson D., Hu Y. F., Yu G. L., Karttunen J., Løvlie A., Haller B., Blackburn E. H. Replacement of the macronuclear ribosomal RNA genes of a mutant Tetrahymena using electroporation. Gene. 1988 Oct 30;70(2):295–301. doi: 10.1016/0378-1119(88)90201-6. [DOI] [PubMed] [Google Scholar]
- Pennock D. G., Thatcher T., Bowen J., Bruns P. J., Gorovsky M. A. A conditional mutant having paralyzed cilia and a block in cytokinesis is rescued by cytoplasmic exchange in Tetrahymena thermophila. Genetics. 1988 Nov;120(3):697–705. doi: 10.1093/genetics/120.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennock D. G., Thatcher T., Gorovsky M. A. A temperature-sensitive mutation affecting cilia regeneration, nuclear development, and the cell cycle of Tetrahymena thermophila is rescued by cytoplasmic exchange. Mol Cell Biol. 1988 Jul;8(7):2681–2689. doi: 10.1128/mcb.8.7.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfert H. M., Hipke H., Schmidt W. Isolation and phenotypic characterization of Tetrahymena thermophila size mutants: the relationship between cell size and regulation of DNA content. J Cell Sci. 1984 Apr;67:203–215. doi: 10.1242/jcs.67.1.203. [DOI] [PubMed] [Google Scholar]
- Smith D. L., Doerder F. P. Multiple effects of mutation on expression of alternative cell surface protein genes in Tetrahymena thermophila. Genetics. 1992 Jan;130(1):97–104. doi: 10.1093/genetics/130.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Long range periodicities in mouse satellite DNA. J Mol Biol. 1975 May 5;94(1):51–69. doi: 10.1016/0022-2836(75)90404-0. [DOI] [PubMed] [Google Scholar]
- Spangler E. A., Blackburn E. H. The nucleotide sequence of the 17S ribosomal RNA gene of Tetrahymena thermophila and the identification of point mutations resulting in resistance to the antibiotics paromomycin and hygromycin. J Biol Chem. 1985 May 25;260(10):6334–6340. [PubMed] [Google Scholar]
- Struhl K. The new yeast genetics. 1983 Sep 29-Oct 5Nature. 305(5933):391–397. doi: 10.1038/305391a0. [DOI] [PubMed] [Google Scholar]
- Suhr-Jessen P. B., Orias E. Mutants of TETRAHYMENA THERMOPHILA with Temperature-Sensitive Food Vacuole Formation. I. Isolation and Genetic Characterization. Genetics. 1979 Aug;92(4):1061–1077. doi: 10.1093/genetics/92.4.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney R., Yao M. C. Identifying functional regions of rRNA by insertion mutagenesis and complete gene replacement in Tetrahymena thermophila. EMBO J. 1989 Mar;8(3):933–938. doi: 10.1002/j.1460-2075.1989.tb03454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tondravi M. M., Yao M. C. Transformation of Tetrahymena thermophila by microinjection of ribosomal RNA genes. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4369–4373. doi: 10.1073/pnas.83.12.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkewitz A. P., Madeddu L., Kelly R. B. Maturation of dense core granules in wild type and mutant Tetrahymena thermophila. EMBO J. 1991 Aug;10(8):1979–1987. doi: 10.1002/j.1460-2075.1991.tb07727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M. C., Blackburn E., Gall J. G. Amplification of the rRNA genes in Tetrahymena. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1293–1296. doi: 10.1101/sqb.1979.043.01.147. [DOI] [PubMed] [Google Scholar]
- Yao M. C., Yao C. H. Accurate processing and amplification of cloned germ line copies of ribosomal DNA injected into developing nuclei of Tetrahymena thermophila. Mol Cell Biol. 1989 Mar;9(3):1092–1099. doi: 10.1128/mcb.9.3.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
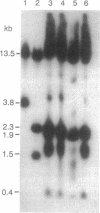
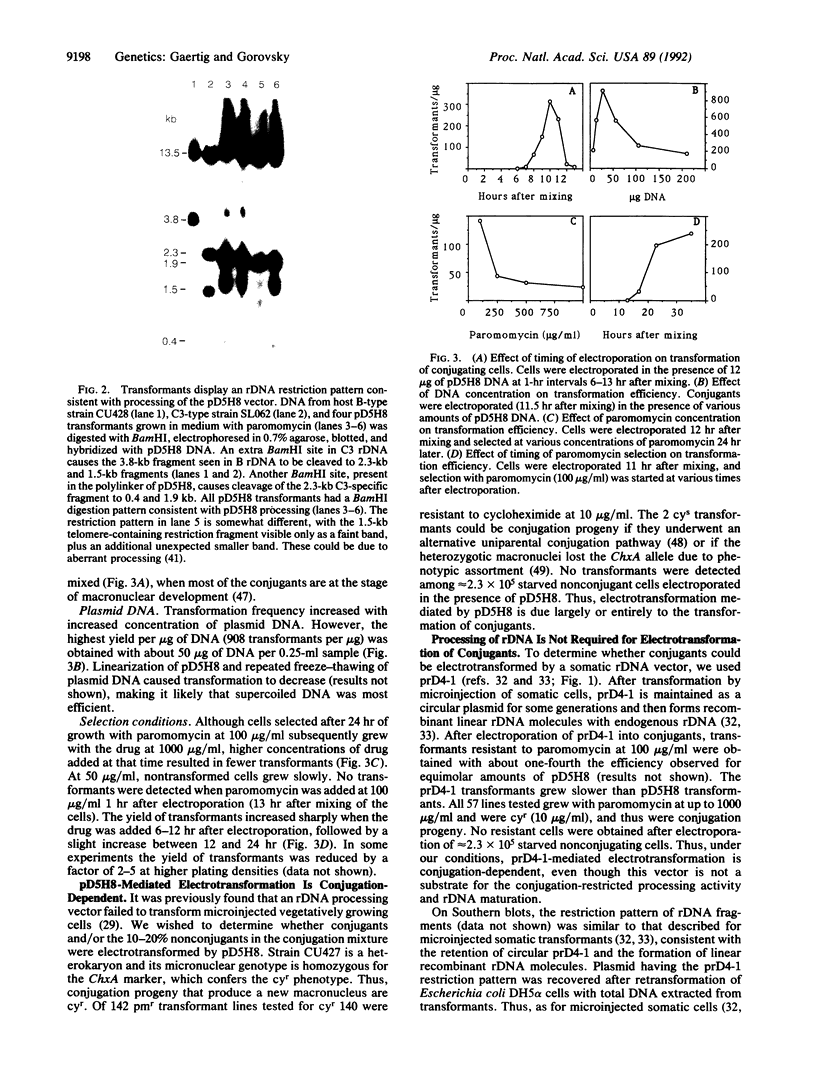
- Yao M. C., Yao C. H. Transformation of Tetrahymena to cycloheximide resistance with a ribosomal protein gene through sequence replacement. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9493–9497. doi: 10.1073/pnas.88.21.9493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G. L., Blackburn E. H. Amplification of tandemly repeated origin control sequences confers a replication advantage on rDNA replicons in Tetrahymena thermophila. Mol Cell Biol. 1990 May;10(5):2070–2080. doi: 10.1128/mcb.10.5.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G. L., Blackburn E. H. Transformation of Tetrahymena thermophila with a mutated circular ribosomal DNA plasmid vector. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8487–8491. doi: 10.1073/pnas.86.21.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G. L., Hasson M., Blackburn E. H. Circular ribosomal DNA plasmids transform Tetrahymena thermophila by homologous recombination with endogenous macronuclear ribosomal DNA. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5151–5155. doi: 10.1073/pnas.85.14.5151. [DOI] [PMC free article] [PubMed] [Google Scholar]