In this study melanopsin was transfected into pyramidal cells of the cerebral cortex to study G protein signaling in these cells with high temporal resolution.
Keywords: melanopsin, Gαq-11 signaling, pyramidal neuron, 5-HT2A receptor
Abstract
Our understanding of G protein-coupled receptors (GPCRs) in the central nervous system (CNS) has been hampered by the limited availability of tools allowing for the study of their signaling with precise temporal control. To overcome this, we tested the utility of the bistable mammalian opsin melanopsin to examine G protein signaling in CNS neurons. Specifically, we used biolistic (gene gun) approaches to transfect melanopsin into cortical pyramidal cells maintained in organotypic slice culture. Whole cell recordings from transfected neurons indicated that application of blue light effectively activated the transfected melanopsin to elicit the canonical biphasic modulation of membrane excitability previously associated with the activation of GPCRs coupling to Gαq-11. Remarkably, full mimicry of exogenous agonist concentration could be obtained with pulses as short as a few milliseconds, suggesting that their triggering required a single melanopsin activation-deactivation cycle. The resulting temporal control over melanopsin activation allowed us to compare the activation kinetics of different components of the electrophysiological response. We also replaced the intracellular loops of melanopsin with those of the 5-HT2A receptor to create a light-activated GPCR capable of interacting with the 5-HT2A receptor interacting proteins. The resulting chimera expressed weak activity but validated the potential usefulness of melanopsin as a tool for the study of G protein signaling in CNS neurons.
NEW & NOTEWORTHY
In this study melanopsin was transfected into pyramidal cells of the cerebral cortex to study G protein signaling in these cells with high temporal resolution.
g protein-coupled receptors (GPCRs) play a fundamental role in regulating neuronal excitability in the central nervous system (CNS). Many studies have used brain slices to address mechanistic aspects of GPCR signaling, and these studies have contributed to our current understanding of the effects of GPCR activation and the various signaling mechanisms utilized by these receptors to regulate downstream ion channels. These advances notwithstanding, access limitations intrinsic to brain slices have made it difficult to address the temporal aspects of GPCR signaling. Given the transient nature of synaptic transmission, this represents a significant omission. Additionally, it is now widely recognized that GPCR signaling is regulated by a variety of intracellular receptor interacting proteins (Bockaert et al. 2010), many of which likely sculpt signaling in the temporal domain. However, gaining a clear understanding of how these proteins regulate signaling in a physiological context has been hampered by methodological limitations. In the present work we report on the use of melanopsin to examine the temporal aspects of G protein signaling in cortical neurons.
Opsins are light-activated GPCRs of the rhodopsin family and thus are closely related to other GPCRs sensing neurotransmitters such as serotonin (5-HT), norepinephrine, and acetylcholine (ACh) (Fredriksson et al. 2003). They comprise a diverse group that can couple to heterotrimeric G proteins of the Gαi/o, Gαq-11, and Gαs subtypes (Feuda et al. 2012; Koyanagi and Terakita 2014; Porter et al. 2012). However, in contrast to other GPCRs in this family, opsins are not activated by a diffusible molecule (agonist) but rather by the light-induced isomerization of retinal, which is covalently bound to the opsin. In the dark state opsins bind 11-cis-retinal, which suppresses the intrinsic activity of the opsin (Melia et al. 1997). Absorption of a photon triggers the isomerization of the retinal to an all-trans form, a process that takes place on the order of picoseconds, which then activates the opsin (Hayward et al. 1981). Thus trans-retinal can be thought of as the endogenous ligand for the opsin and light as a stimulus capable of instantaneously converting the retinal from an inverse agonist to a full agonist. These considerations suggest that opsins could offer a powerful path for studying the temporal aspects of GPCR signaling in neurons.
Melanopsin couples to Gαq-11 to activate PLCβ, leading to the breakdown of phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2] and the elevation of intracellular Ca2+ levels (Panda et al. 2005). In this regard, melanopsin resembles other Gαq-11-coupled GPCRs of the rhodopsin family including 5-HT2 serotonergic, α1-adrenergic, and muscarinic M1 and M3 cholinergic receptors. In the present work we expressed melanopsin or melanopsin-5-HT2A chimeric receptors in cultured brain slices to test the feasibility of using the melanopsin backbone to build optogenetic tools for examining G protein signaling in CNS neurons.
METHODS
Ethical approval.
All procedures used for the experiments reported in the present work were approved by the Wayne State University animal investigation committee and are in accordance with the National Institutes of Health Office of Laboratory Animal Welfare Public Health Service Policy on Humane Care and Use of Laboratory Animals. The Wayne State University Institutional Animal Care and Use Committee is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International, and the laboratory animal care and use program conforms to the National Research Council's Guide for the Care and Use of Laboratory Animals (8th ed.).
Brain slice preparation.
All experiments were performed on Sprague-Dawley rats aged between postnatal day (p)7 and p26. Animals were procured from Hilltop Lab Animals (Scottdale, PA) as newborn pups in five- to seven-pup litters under the care of a single dam. These pups are presumed to have been born from multiple breeding pairs. A total of 64 rat pups from >10 litters were used to prepare acute as well as organotypic cultured cortical slices used for the present experiments. Rats were killed in accordance with the recommendations of the American Veterinary Medical Association guidelines on euthanasia with isoflurane followed by decapitation. Slices were prepared essentially as described previously (Beique et al. 2007). Briefly, the brain was removed and submerged in ice-cold Ringer solution containing (in mM) 119 NaCl, 2.5 KCl, 1.3 MgSO4, 2.5 CaCl2, 1 NaH2PO4, 10 HEPES, and 22 glucose, bubbled to saturation with 95% O2-5% CO2. The anterior forebrain was mounted onto a stage by application of cyanoacrylate glue, and coronal slices (300 μm thick) of the anterior cerebral cortex were prepared with a vibratome (Vibratome Series 1000 Sectioning System). Brain slices destined for acute experiments were cut and then transferred to a recovery chamber filled with prewarmed (33°C) Ringer solution (bubbled to saturation with 95% O2-5% CO2) for at least 1 h to allow for recovery. Slices destined for organotypic culture were cut under aseptic conditions, rinsed twice with filter-sterilized ice-cold Ringer solution, and placed in culture as outlined below.
Organotypic slice culture.
Slices were prepared for organotypic culture essentially as described previously (Beique et al. 2007; Stoppini et al. 1991). Briefly, slices were obtained under aseptic conditions, rinsed with sterile Ringer solution, and washed twice with ∼2 ml of culture medium [composition: 50% HMEM, 25% Hanks' balanced salt solution, 25% heat-inactivated horse serum, supplemented with l-glutamine (1 mM) and glucose (31 mM)] at 33°C and equilibrated with 5% CO2. Individual cortical slices were placed on cell culture inserts (Millicell, Millipore) and placed in individual wells in a six-well plate (Costar 3516) containing 1.25 ml of culture medium preequilibrated with 5% CO2. Plates were moved to an actively humidified incubator (5% CO2), where the slices were maintained at 33°C. Viable slices were clearly identifiable by their translucent appearance, the absence of signs of contamination, the large number of neurons visible under differential interference contrast (DIC) and, most importantly, the presence of fluorescent protein-expressing neurons 2 days after transfection.
Slice transfections.
Neuronal transfection was performed by particle-mediated gene transfer (biolistics) as previously described (Beique et al. 2007; Villalobos et al. 2005). Briefly, slices were transfected with a Bio-Rad Helios gene gun essentially as recommended by the manufacturer with the exception of an added “gene gun barrel” (O'Brien et al. 2001). Slices were transfected ∼30 min after cutting, following a brief stay in the incubator, and returned to the incubator immediately after transfection. Slices were maintained in culture for 2–6 days prior to use. This procedure results in transfection of a small number of neurons in all layers of cortex in what appeared to be a stochastic distribution.
cDNA constructs.
hMelanopsin (opsin 4, MGC:142118, Open Biosystems, untagged), mCherry, and tdTomato (kind gifts of Dr. R. Tsien) were subcloned into pcDNA3.1(+) (Invitrogen Life Technologies). For most experiments bullets were coated with a 4-to-1 ratio of melanopsin and fluorescent protein plasmids. We have previously shown that biolistic transfection allows for coexpression of two constructs in the vast majority of transfected neurons (Beique and Andrade 2003). The construct encoding the HA-tagged 5-HT2A-melanopsin chimera was generated step by step by several polymerase chain reaction (PCR) amplifications. All the intracellular loops and the carboxy-terminal tail of hMelanopsin were replaced with those of the 5-HT2A receptor (i1: nucleotides 295–330, i2: 514–573, i3: 763–972, Cter: 1153–1416). The construct was confirmed by DNA sequencing. The resulting opsin chimera was then subcloned into the pIRES-DsREd plamid (Clontech) with the XhoI/BamHI restriction sites. Bullets loaded with DNA coding for the chimera were also loaded with plasmid coding for a red fluorescent protein to facilitate visualization of the transfected neurons. In all cases, plasmids were coated onto gold particles (DNAdel Gold Carrier particles, Seashell Technology or Bio-Rad Biolistic 1.6 Micron Gold Microcarrier, Bio-Rad Laboratories) essentially as described in the manufacturer's protocol.
Slice imaging and recording.
Slices were transferred to a recording chamber on the stage of an upright microscope (E600FN, Nikon Instruments), where they were continuously perfused with Ringer solution (composition in mM: 119 NaCl, 2.5 KCl, 1.3 MgSO4, 2.5 CaCl2, 1 NaH2PO4, 26.2 NaHCO3, and 22 glucose) maintained at ∼31°C and bubbled to saturation with 95% O2-5% CO2. The Ringer solution was additionally supplemented with myo-inositol (300 μM; Sigma-Aldrich) to help preserve Gαq-11 signaling in the slices (Villalobos et al. 2011). Although melanopsin has been shown to function without the need for additional retinal supplementation (Panda et al. 2005; Qiu et al. 2005), as an extra precaution the culture medium was typically also supplemented with all-trans-retinal (10 μM; Sigma-Aldrich; dissolved in 70% ethanol, <0.1% of total medium) at least 30 min prior to recordings.
Neurons in slices were visualized with DIC imaging. For culture slices, transfected cells were first identified by the expression of the mCherry or tdTomato and then targeted for recording with DIC imaging. We used a red fluorescent protein as marker for transfection because these proteins are excited with green light (510–560 nm), well to the right of the excitation maxima of melanopsin (∼480 nm). However, because the melanopsin excitation spectrum exhibits a shallow tail extending into the green wavelengths (Bailes and Lucas 2013), fluorescent illumination during the initial identification was used conservatively to minimize the possibility of activation of melanopsin. Transfected cells did not exhibit noticeable responses to the relatively dim room lighting or even white-light DIC illumination. However, as an extra precaution, lighting was dimmed in the recording room and red-infrared light (instead of white light) was used for the DIC imaging.
Recordings were conducted with an EPC10 amplifier (HEKA Instruments) under the control of Patchmaster software (HEKA Instruments). Current and voltage were filtered at 2–5 kHz and sampled at 10–20 kHz. Recording pipettes were pulled from borosilicate glass (Sutter Instrument) with a horizontal Flaming/Brown micropipette puller (Sutter Instrument, model P-97). They were filled with a potassium-based internal solution (composition in mM: 120 KMeSO4, 5 KCl, 5 NaCl, 0.02 EGTA, 10 HEPES, 1 MgCl2, 10 myo-inositol, 10 Na2 phosphocreatine, 4 ATP Mg salt, 0.3 GTP Na salt, pH 7.4) and exhibited resistances ranging from 2.5 to 4 MΩ. Series resistance after breaking into the cell ranged from 5 to 20 MΩ, and cells that showed noticeable change in access resistance from the initial baseline were discarded. Only cells with stable membrane potentials negative to −60 mV and overshooting action potentials were used for experiments. During current-clamp recordings cells were stimulated every 20–30 s with constant-current injections (typically 70–200 pA for 0.6–1.5 s) to induce spiking and monitor excitability. During voltage-clamp recordings cells were held at −60 mV and for most experiments were depolarized to +10 or +30 mV for 100 ms every 20–30 s to allow calcium influx and activate the slow afterhyperpolarization current (IsAHP) and the slow afterdepolarization current (IsADP).
As outlined above, the biolistic transfection procedure resulted in stochastic transfection on neurons in the slice. To limit the heterogeneity of the cell population studied, we targeted morphologically identified pyramidal cells from layers II/III and V of the prelimbic, anterior cingulate, primary motor, and somatosensory cortices. While some heterogeneity likely remained, it is worth noting that in general the number of transfected pyramidal cells in any given slice was limited. This effectively randomized the cells used for each experiment. As an additional precaution, most experiments also avoided group comparisons and instead used an experimental design in which each cell served as its own control.
Light stimulation.
Activation of melanopsin was achieved with brief blue light flashes (460–500 nm, 5–20 ms, ∼1–3 mW/mm2) with a xenon arc lamp and a mechanical shutter (Lambda 10-2, Sutter Instrument). Alternatively, a small number of cells were stimulated with LEDs (470 or 447.5 nm; Luxeon, Quadica Developments) driven by a Mightex LED driver and attached to an optical fiber (730 μm, 0.48 NA; ThorLabs BFH48-400) (Campagnola et al. 2008). Total power at the tip of the fiber was ∼5 mW. Since no significant difference in response to illumination from the lamp and pole was noted, data from the two sources were pooled.
Drugs.
ACh (Sigma-Aldrich) was applied to acute slices by pressure application with a Picospritzer (General Valve). ACh (1 mM, dissolved in Ringer solution) was focally applied to pyramidal neurons from a patch pipette positioned ∼30 μm diagonally above the recorded cell with a Picospritzer (200–500 ms, 10 psi). Apamin (Sigma-Aldrich) was administered to block SK potassium channels and was present in the bath in many of the experiments aimed to study the slower light-activated currents. To reduce noise from spontaneous synaptic events, some experiments were conducted in the presence of 6,7-dinitroquinoxaline-2,3-dione disodium salt (DNQX; Abcam Biochemicals) and 6-cyano-7-nitroquinoxaline-2,3-dione disodium (CNQX; Sigma-Aldrich) to block AMPA receptor-mediated synaptic currents or in tetrodotoxin (TTX, 1 μM; Calbiochem) to block sodium channels and hence action potential-mediated synaptic activity.
Data analysis and assessment of statistical significance.
For assessment of light-induced currents or voltages, all values were baseline-subtracted. For the transient outward current the current amplitude was taken as the mean of a 100-ms segment at the highest point in the trace, baseline-subtracted using the mean of a 1-s segment from the start of each sweep. Onset latency was calculated from the start of the flash to the start of the rising phase of the current, whereas the rise time was measured from the start of the rising phase to the peak of the current. Average measurements are presented as means ± SE. Significance was assessed with Student's paired or unpaired t-test or ANOVA as appropriate. A value of P < 0.05 was taken as indicating statistical significance.
RESULTS
Electrophysiological effects of Gαq-11 activation in cortical pyramidal cells.
GPCRs coupled to heterotrimeric G proteins of the Gαq-11 class such as M1 muscarinic cholinergic, 5-HT2A serotonergic, or α1-adrenergic receptors regulate the excitability of cortical pyramidal cells by modulating the activity of multiple ion channels in parallel. These effects have been studied most thoroughly in the context of M1 muscarinic cholinergic responses, which can stand as representative for this class of responses. Activation of muscarinic receptors depolarizes and excites pyramidal cells (Andrade 1991; Constanti and Bagetta 1991; Egorov et al. 2003; Gloveli et al. 1999; Gulledge et al. 2009; Haj-Dahmane and Andrade 1996, 1998; Krnjevic et al. 1971; McCormick and Prince 1986; Metherate et al. 1992; Schwindt et al. 1988; Wang and McCormick 1993), although focal ACh administration also elicits an initial, transient inhibitory effect (Gulledge and Stuart 2005; McCormick and Prince 1986). These observations have led to the idea that muscarinic receptor-mediated responses consist of an initial “phasic” response that is followed by a “tonic” component and that both components coexist within a single neuron (Gulledge and Stuart 2005).
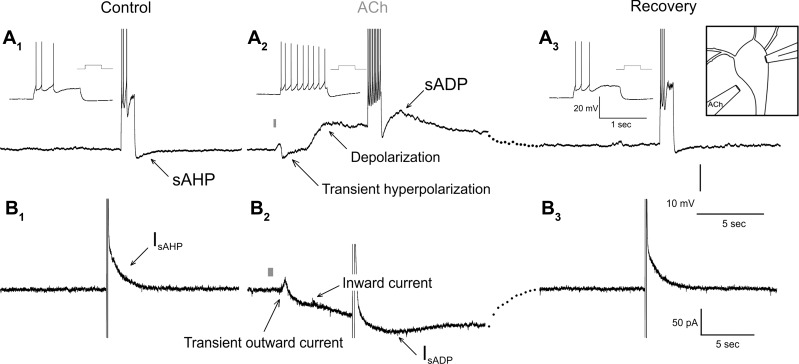
Figure 1 illustrates the effect of ACh on a layer V pyramidal cell of the medial prefrontal cortex. A brief “puff” of ACh elicits a transient “phasic” hyperpolarization followed by a “tonic” increase in excitability as evidenced by an increase in spiking in response to constant-current injection (Fig. 1A). In the anterior cerebral cortex and hippocampus these effects are solely mediated by the activation of muscarinic receptors, mostly of the M1 subtype (Dasari and Gulledge 2011; Gulledge et al. 2009; Haj-Dahmane and Andrade 1996, 1998), that signal these effects via Gαq-11 and PLCβ (Krause et al. 2002; Villalobos et al. 2011; Yan et al. 2009). At a mechanistic level the “phasic” hyperpolarization reflects activation of SK potassium channels secondary to intracellular calcium release. In contrast, the “tonic” increase in excitability involves the combined effect of a sustained membrane depolarization, the inhibition of the slow afterhyperpolarization (sAHP), and appearance of the slow afterdepolarization (sADP) (Fig. 1A). As a group these effects represent the canonical electrophysiological response to Gαq-11 activation observed in pyramidal cells. As illustrated in Fig. 1B, the ionic currents underlying each of these effects can be recorded in voltage clamp. The “phasic” hyperpolarization is recorded as a transient outward current, while the “tonic” effects are recorded as a sustained inward current, the inhibition of IsAHP, and the appearance of IsADP.
Fig. 1.
Electrophysiological effects elicited by Gαq-11 activation in cortical pyramidal neurons. A: response of a pyramidal cell to focal application of ACh (diagrammed in top right inset of A3) recorded in current clamp. A1: under control conditions a brief constant-current depolarizing pulse (150 pA, 1 s) triggers an accommodating burst of spikes that is followed by an afterhyperpolarization that includes a slow component, the sAHP. Inset: expanded view of the spiking response. A2: focal application of ACh from a patch pipette (1 mM, 200 ms, indicated by a gray bar) elicits a transient hyperpolarization that is followed by a more sustained depolarization. The same constant-current depolarizing pulse delivered after ACh application triggers sustained spiking and the replacement of the sAHP by a sADP. A3: recovery from the effect of ACh. B: response of a different neuron to focal application of ACh recorded in voltage clamp. B1: under control conditions, a brief depolarizing step (100 ms to +30 mV from a holding potential of −60 mV) elicits an outward aftercurrent that includes a slow component, the IsAHP. B2: focal application of ACh (1 mM, 500 ms) elicits a transient outward current that is followed by a more sustained inward current. After ACh, the IsAHP is inhibited and is replaced by a IsADP. B3: recovery from ACh. A and B show results obtained from layer V pyramidal neurons of the prelimbic cortex in acute brain slices prepared from rats aged p18 (A) and p21 (B).
Light-induced melanopsin-signaled responses in cortical pyramidal cells.
To gain optogenetic control over metabotropic signaling in cortical pyramidal cells, we transfected melanopsin into rat prefrontal cortex slices maintained in organotypic culture with a “gene gun” (biolistic transfection). Transfected cells were identified by the coexpression of a fluorescent protein (mCherry or tdTomato) (Fig. 2A), and transfected pyramidal neurons of layers II/III or V were targeted for whole cell electrophysiological recordings. We used brief light flashes on the order of 5–20 ms to activate melanopsin. Previous work has shown that the light-induced isomerization of retinal takes place within microseconds while the subsequent conformational changes that underlie the activation of the GPCR occur with a time course in the range of tens of milliseconds (Lohse et al. 2014). Thus the short light pulses used in the present study likely triggered, at the molecular level, a single opsin activation-deactivation cycle.
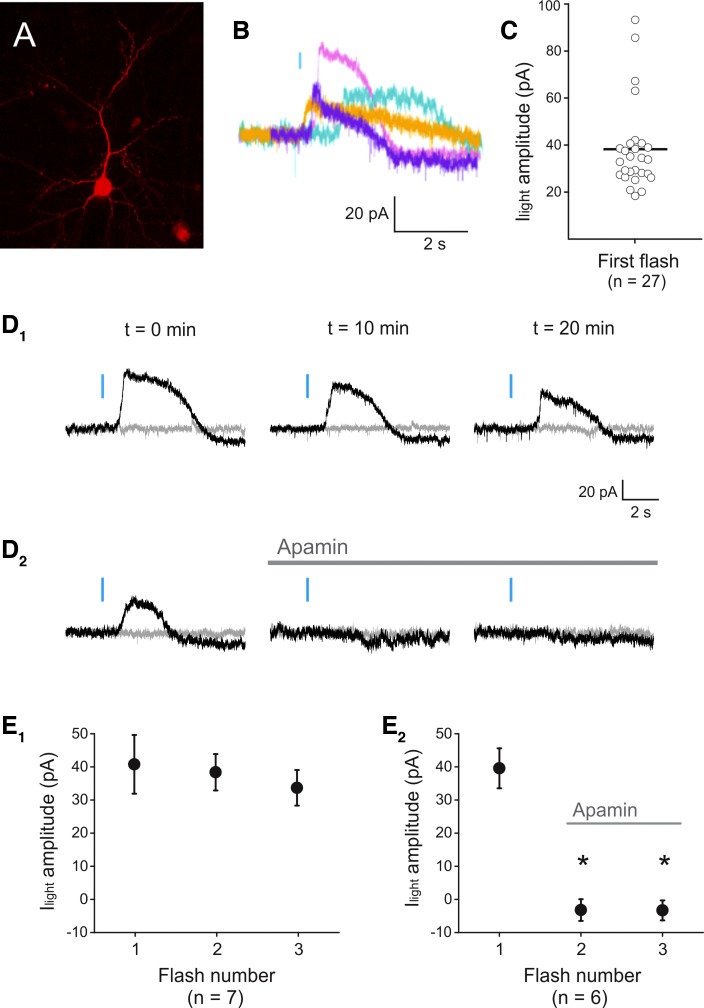
Fig. 2.
Light-induced activation of a phasic outward current in melanopsin-expressing pyramidal neurons. A: image derived from a flattened confocal z stack depicting a melanopsin-expressing pyramidal neuron identified by coexpression of a red fluorescent protein. B: light-evoked transient outward currents recorded in 4 different pyramidal cells. Blue bar denotes the light flash. C: graph plotting the amplitude of the light-evoked transient outward current (Ilight) amplitude observed in the present experiments. D1: light flashes delivered every 10 min could repeatedly evoke a transient outward current in a single pyramidal cell. D2: these light-evoked transient outward currents were blocked by administration of apamin (100 nM). Gray traces illustrate a sweep without light, collected immediately before the light sweep. E1: graph illustrating the relative stability of the light-evoked transient outward currents upon repeated light stimulation in 7 cells (P = 0.756, ANOVA). E2: graph summarizing the effect of apamin (100 nM) on the light-evoked transient outward currents (P = 3.48E-6, ANOVA, n = 6 cells). *Statistically significant inhibition.
As illustrated in Figs. 2 and 3, brief light pulses delivered to cells transfected with melanopsin qualitatively recapitulated the effects of muscarinic agonist delivered through conventional means. Approximately 45% (66/146) of the transfected pyramidal cells tested in the present experiments responded to brief light pulses in the millisecond range (see below). The light-induced responses recovered over time and could be evoked repeatedly within the same cell. None of the nontransfected pyramidal cells tested showed a response to light stimulation (0/12). We discuss the different components of the light-induced responses below.
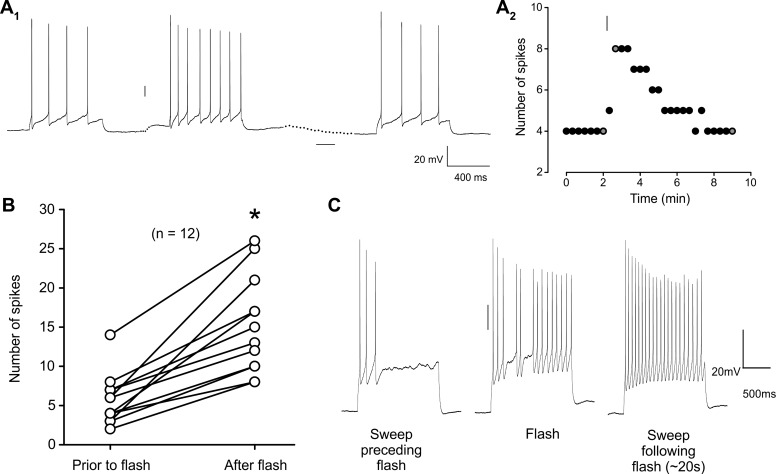
Fig. 3.
Brief light flashes caused prolonged effects on neuronal excitability in melanopsin-expressing cortical pyramidal neurons. A1: under current-clamp conditions, a brief flash of light (10 ms, blue bar) elicits a sustained depolarization [illustrated by the dots between the traces] and an increase in the spiking induced by constant depolarizing current injection (70 pA, 700 ms). A2: plot illustrating the time course of the increase in current-induced spiking of the cell shown in A1. Gray-filled circles correspond to the traces depicted in A1. B: summary plot illustrating the light-induced increase in spiking in the 11 pyramidal cells tested (paired t-test, P = 6.18E-6). C: traces from a cell receiving 5-ms light stimulation 50 ms prior to the current injection (250 pA, 1.5 s).
Induction of a transient hyperpolarization/outward current by light stimulation.
Brief flashes of blue light (as short as 5 ms) delivered to melanopsin-transfected cells recorded in voltage clamp triggered the appearance of robust transient outward currents in ∼20% of the recorded neurons. These light-induced transient outward currents exhibited an onset latency of 469 ± 68 ms and averaged 38.0 ± 3.6 pA in amplitude (Fig. 2, B and C; n = 27). When cells were recorded in current clamp, the light flashes could be seen to trigger the corresponding transient hyperpolarizations (n = 3 cells, not shown). Although only a subset of the recorded neurons exhibited these transient outward currents, the response of any individual cell was relatively consistent upon repeated light stimulation [Fig. 2, D1 and E1; ANOVA (P = 0.756), n = 7]. This allowed us to test whether the SK potassium channel blocker apamin would block this response and hence whether, at least mechanistically, these currents resemble the “phasic” effect signaled by ACh. As illustrated in Fig. 2, D2 and E2, administration of 100 nM apamin blocked the light-induced transient outward currents (P = 3.48E-6, ANOVA, n = 6). Thus expression of exogenous melanopsin in cortical pyramidal allows for light-induced metabotropic signaling in pyramidal neurons with a high degree of temporal precision.
Light-induced increase in excitability.
Implicit in the distinction between “phasic” and “tonic” muscarinic responses is the idea that “tonic” responses require prolonged, perhaps even nonphysiological, agonist exposure (Gulledge and Stuart 2005). To test this idea we next asked whether brief light flashes would be sufficient to elicit “tonic” increases in the excitability of pyramidal cells expressing melanopsin. For these experiments we monitored the spiking response to periodic (∼0.5 Hz) constant-current depolarizing stimuli (0.6–1.5 s long) as a broad measure of neuronal excitability. As illustrated in Fig. 3, a brief flash of light (5–20 ms) induced a membrane depolarization (amplitude of 3.2 ± 0.46 mV, n = 9/11 cells) that was accompanied by increases in spiking in response to current injection (Fig. 3B; 320 ± 48% increase in firing frequency; paired t-test, P = 6.18E-6, n = 11/11 cells). Both effects recovered over a time course of several minutes (depolarization recovery time: 3.8 ± 0.42 min, n = 9; spiking recovery time: 6.4 ± 1.25 min, n = 11). Remarkably, we observed that delivery of a light flash 50–100 ms prior to the onset of the current injection could still induce a robust increase in the spiking elicited by current injection (n = 3/3 cells). This suggested surprisingly short onset latency for this “tonic” response.
Light-induced depolarization/inward current.
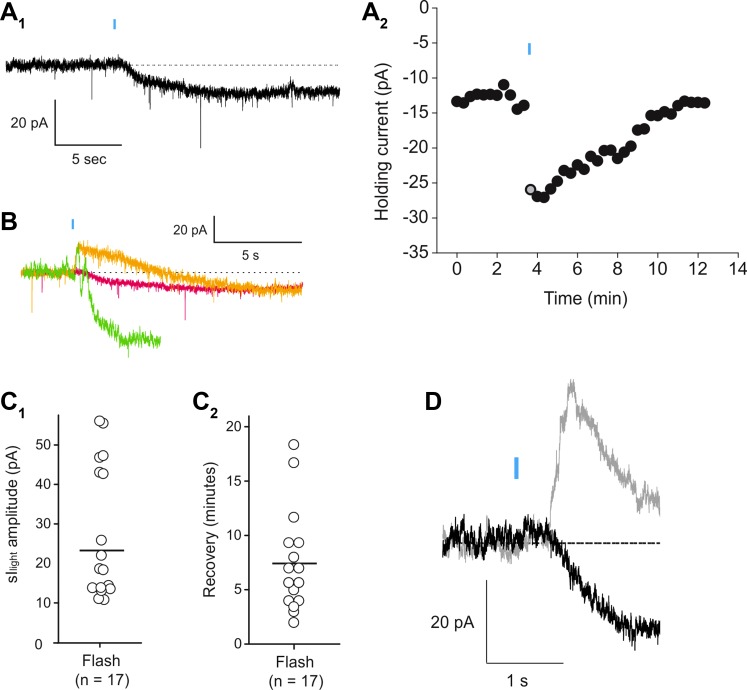
To better address the kinetics of the light-induced increase in excitability we next focused on the individual currents that are thought to contribute to the increase in excitability. As illustrated in Fig. 4, a brief flash of light was sufficient to trigger the development of a sustained inward current (Fig. 4A1), the counterpart of the membrane depolarization recorded in current clamp. To study this response we focused on a group of 14 cells exhibiting unambiguous, long-lasting, and stable recordings of light-induced inward currents (mean amplitude: 23.35 ± 4.05 pA; Fig. 4C1). To gain insight into the onset latency and rise time of the inward current, we either used cells that did not display a transient outward current or conducted the experiment in the presence of apamin (100–300 nM; Fig. 4D). Surprisingly, the light-induced inward current exhibited an onset latency comparable to that of the transient outward current (440.8 ± 65.6 ms, n = 3) but developed much more slowly (Fig. 4). In cells where the light flash induced both a transient outward and a more prolonged inward current it was apparent that the SK-mediated current overlapped with the onset of the inward current and altered its trajectory (Fig. 4, B and D). In contrast to the relatively fast onset of the sustained inward current, its recovery occurred over a time course of minutes (7.48 ± 1.32 min; Fig. 4, A2 and C2; n = 14).
Fig. 4.
Light-evoked sustained inward current in melanopsin-expressing pyramidal cells. A1: under voltage clamp a 20-ms flash of blue light causes the appearance of a slowly developing inward current. A2: graph plotting the time course for the light evoked inward current. Gray circle corresponds to the trace illustrated in A1. B: overlapped traces illustrating the variability in the early time course of the light-evoked resulting from the summation of variable light-induced transient outward and sustained inward currents in 3 cells. C1: graph plotting the amplitude of the light-induced inward current (sIlight) recorded in the present experiments. C2: graph plotting the recovery time for the light-induced inward current in the same 14 cells. D: overlapped traces depicting the light-evoked current observed under control conditions (gray trace) and after bath application of apamin (100 nM, black trace). Blockade of the transient outward current by apamin revealed the time course of the sustained inward current.
Light-induced inhibition of IsAHP.
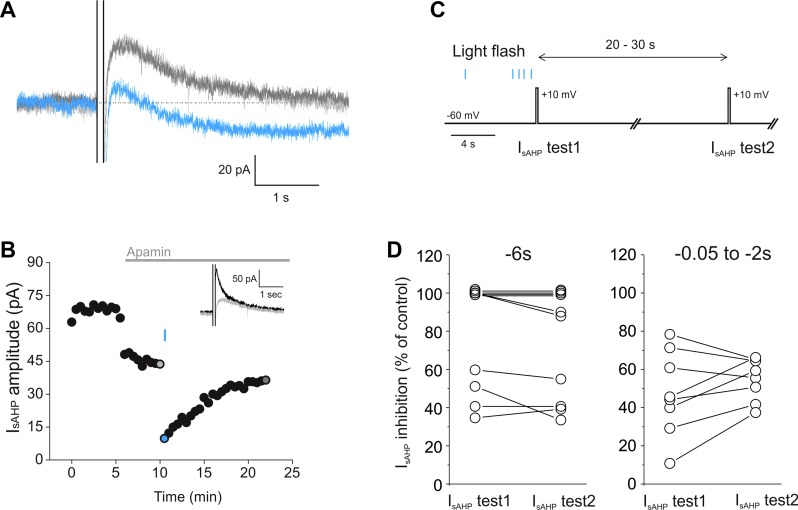
Light activation of melanopsin-transfected pyramidal cells also triggered the inhibition of the Ca2+-activated K+ current known as IsAHP. As illustrated in Fig. 5, a brief light flash delivered 6 s before the depolarizing step used to trigger IsAHP resulted in a robust and reversible suppression of this current (Fig. 5, A and B; n = 10 cells, mean time to recovery: 4.2 min ± 0.6 min, n = 4). Similar effects were seen under current-clamp conditions (data not shown; mean inhibition: 91 ± 6.9%, n = 4, mean time to recovery: 4.2 ± 0.6 min, n = 4). In terms of time course, the inhibition of IsAHP was near maximal when the light was flashed 6 s before the depolarizing step, compared with the inhibition seen after an additional 20–30 s [Fig. 5, B–D; 69.5 ± 11.2% (IsAHP test 1) vs. 65.2 ± 11.3% (IsAHP test 2), n = 7 cells, P = 0.79, Student's t-test]. These results again suggested a relatively fast onset for the inhibition of IsAHP. To further explore this issue, we repeated the experiment but delivered the light flash 50-2,000 ms before triggering IsAHP. As illustrated in Fig. 5D, despite the short interval between the flash and the testing of IsAHP, we still observed a robust inhibition of IsAHP. This inhibition was comparable to that seen after an additional 20–30 s had elapsed following the light flash (47.5 ± 4% vs. 55.1 ± 8%, n = 8 trials, P = 0.4, Student's t-test). These results suggest that the inhibition of IsAHP is near maximal shortly after the light pulse and thus again point to a rapid onset for the inhibition of this current.
Fig. 5.
Light-induced inhibition of the IsAHP in melanopsin-expressing cortical pyramidal cells. A: in voltage clamp, a depolarizing pulse (+30 mV, 100 ms) induced the appearance of a IsAHP (light gray trace) in a melanopsin-expressing pyramidal neuron. A brief (20 ms) flash of light caused prolonged inhibition of the IsAHP (blue trace), which recovered over time (dark gray trace). B: graph plotting the light-induced inhibition of the IsAHP in the cell pictured in A with colored circles corresponding to the individual traces. As shown in the inset, the IsAHP was initially isolated by bath administration of apamin (300 nM). (black trace is prior to apamin; light gray trace is after apamin and corresponds to the same color trace shown in A). C: 2-pulse protocol used to obtain information regarding the time course of the onset of the light-evoked IsAHP inhibition. D: when a brief flash of light was administered 6 s prior to the depolarizing pulse used to evoke IsAHP (−6 s), the extent of IsAHP inhibition (IsAHP test 1) (69.5 ± 11.2%) was not significantly different from that observed 20–30 s later (IsAHP test 2) (65.2 ± 11.3%) (n = 7, P = 0.79, Student's t-test). The extent of IsAHP inhibition was also not significantly different between IsAHP test 1 and IsAHP test 2 when the light stimulation for IsAHP test 1 occurred between −2 and −0.05 s prior to the pulse (47.5 ± 4% compared with 55.1 ± 8%, n = 8, P = 0.4, Student's t-test). For this analysis the amplitude of the IsAHP was taken as the mean of a 100-ms segment beginning ∼350 ms after the end of the depolarizing step and was baseline-subtracted.
Light-induced triggering of IsADP.
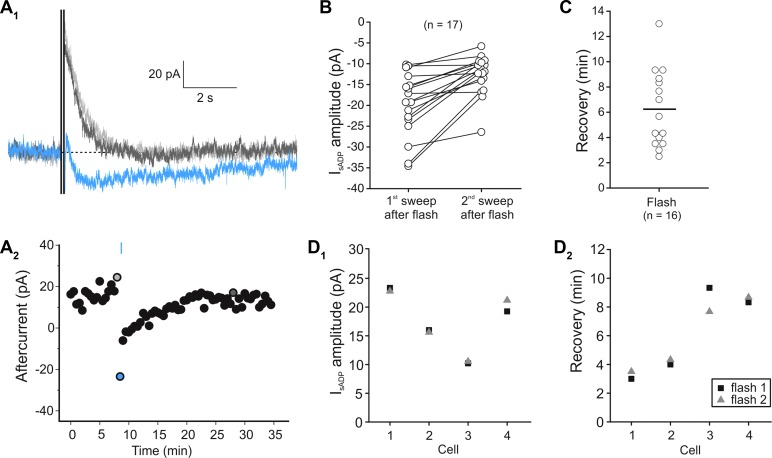
Light activation of melanopsin not only inhibits IsAHP but also triggers its replacement by IsADP. As illustrated in Fig. 6, brief light flashes delivered 6 s prior to the depolarizing step elicited a reduction in the amplitude of IsAHP that was accompanied by its replacement by a slow inward aftercurrent (IsADP; 19.9 ± 1.86 pA, n = 17; Fig. 6, A1 and B). This IsADP recovered over several minutes (6.15 ± 0.75 min, n = 16; Fig. 6, A2 and C) and could be evoked again in the same cell without a substantial change in amplitude (Fig. 6D1; n = 4) or recovery time (Fig. 6D2; n = 4). However, in contrast to what we observed for the inhibition of the IsAHP, the amplitude of the light-induced IsADP was consistently larger during the sweep immediately following the light flash (Fig. 6B; n = 17), a time course that was also seen when the experiments were conducted in current clamp (sADP amplitude for 1st sweep: 3.29 ± 0.57 mV, sADP amplitude for 2nd sweep: 1.95 ± 0.5 mV, n = 8). This made it difficult to investigate the onset kinetics. Nevertheless, in two cells we observed robust sADPs in current clamp following light flashes delivered 50 ms before the start of the current injection (which lasted 1.5 s). These results suggest that despite being classified as a “tonic” effect, the IsADP/sADP are likely signaled by a relatively early event following melanopsin activation.
Fig. 6.
Light-induced appearance of a IsADP in cortical pyramidal neurons expressing melanopsin. A1: a 5-ms long flash of light induced the inhibition of IsAHP and the appearance of a IsADP (blue trace) following a 100-ms-long depolarizing step to +30 mV (control: light gray trace, recovery: dark gray trace). A2: graph plotting the time course of the light-induced effects on the amplitude of the aftercurrent (IsAHP/IsADP). B: graph plotting the amplitude of IsADP after the 1st and 2nd depolarizing steps following a light flash. Note that in most cells tested the aftercurrent amplitude was largest after the pulse immediately following the light stimulation. C: graph plotting the time course of the light effect on IsADP in terms of the time required for the response to disappear (recovery).D1 and D2: there was significant cell-to-cell variation in the peak amplitude (B) and in the “lifetime” of IsADP. However, within a particular melanopsin-expressing neuron, these parameters remained relatively stable in response to subsequent flashes (n = 4).
Light-induced activation of Gαq-11 signaling by a 5-HT2A-melanopsin GPCR chimera.
Recent studies have emphasized the importance of intracellular receptor subtype-specific interacting components that participate in both spatially and temporally shaping the Gαq-11-mediated signal (Bockaert et al. 2010). Unfortunately, there are currently few experimental avenues available for studying the physiological consequences of such interactions. To test the idea that melanopsin could provide a backbone for studying the function of different receptor domains, we created a chimeric receptor that retained the light-sensitive extracellular and transmembrane portions of melanopsin but had the intracellular loops of the opsin replaced with those of the 5-HT2A receptor. We then performed whole cell electrophysiological recordings of 5-HT2A-melanopsin chimera-transfected cortical pyramidal cells.
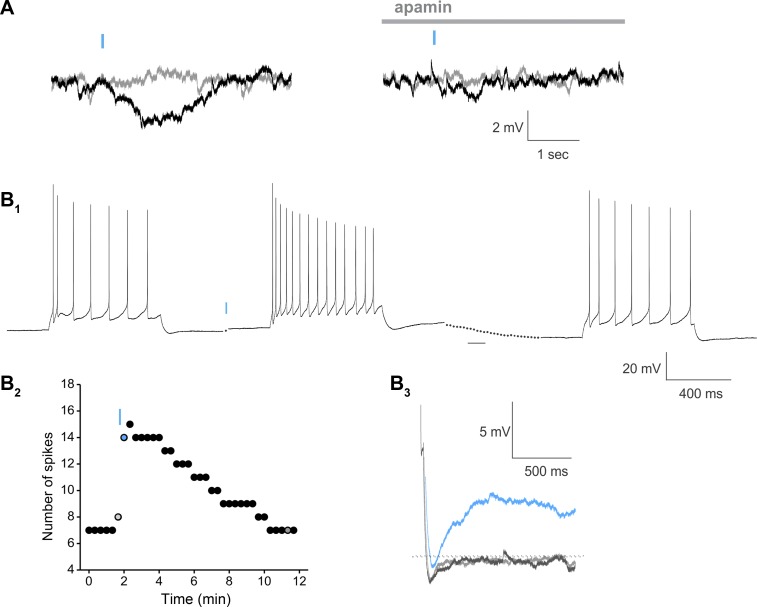
In pyramidal cells expressing the chimeric receptor, light stimulation successfully induced the Gαq-11-mediated biphasic responses that were qualitatively similar to those seen in cells expressing wild-type melanopsin, although the success rate was very low (∼6%, 3/48 cells tested). As illustrated in Fig. 7 for a cell recorded in current clamp, a brief flash of light to a cell expressing the receptor chimera elicited the appearance of a transient hyperpolarization that was blocked by bath application of apamin (300 nM; Fig. 7A). This effect was followed by a slow membrane depolarization (Fig. 7B1) that was associated with an increase in spiking in response to a constant depolarizing current injection (Fig. 7, B1 and B2) and the appearance of a sADP (Fig. 7B3). Similar effects were seen in two additional cells. The difficulty in getting reliable expression of the chimeric receptor, however, made it impractical to conduct a quantitative comparison of the effects elicited through the receptor chimera compared with the wild-type melanopsin.
Fig. 7.
Effects of light stimulation on pyramidal neurons expressing a 5-HT2A-melanopsin chimeric GPCR. A: a brief flash of light (10 ms) delivered to a pyramidal cell expressing the 5-HT2A-melanopsin chimera triggers the appearance of a transient hyperpolarization. This response was blocked by bath application of apamin (300 nM). B1: the same stimulation also evoked tonic changes in excitability in this neuron, as evidenced by a prolonged membrane depolarization and an increase in spiking in response to a constant depolarizing current injection (100 pA, 700 ms) and the appearance of a sADP. B2: graph plotting the effect of light on the current-induced spiking. B3: high-gain illustration of the afterpotential following a burst of spike portion of the traces shown for the experiment illustrated in B1 and B2. Light gray, control; blue, sweep immediately after the light flash; dark gray, recovery.
DISCUSSION
In the present work we have examined the feasibility of using the light-activated GPCR melanopsin to study temporal aspects of metabotropic signaling in CNS neurons. Specifically, we tested whether it would be possible to use melanopsin to activate endogenous G protein signaling, thereby allowing the use of light as an “agonist.” This approach offers the potential to harness the temporal precision of light stimulation and thus bypass some of the limitations associated with applying exogenous agonists to brain slices. We found that light flashes delivered to melanopsin-expressing pyramidal cells in organotypic brain slice cultures triggered the canonical electrophysiological effects associated with activation of Gαq-11 in cortical pyramidal neurons. Specifically, light stimulation elicited an initial transient hyperpolarization/outward current that was followed by a long-lasting increase in neuronal excitability that reflects the development of an inward current, the suppression of IsAHP, and its replacement by a IsADP. In the present experiments we found that pulses as short as 5 ms were sufficient to trigger all these electrophysiological effects. Thus melanopsin expression allows for the activation of G protein signaling in pyramidal cells with high temporal precision.
Methodological considerations.
For the present experiments we used biolistic (gene gun) transfection approaches to express melanopsin in cultured cortical brain slices. Overall we observed light-induced responses in about half of the transfected cells tested. The reasons for this variable functional expression are not clear. We supplemented the slices with trans-retinal and minimized exposure of the slices to light, but these precautions had no detectable effect. Thus neither lack of photopigment nor light desensitization appeared capable of accounting for the variable responses we found in transfected neurons. One possibility is that our focus on brief light pulses may have synergized with variable melanopsin expression to select only a subpopulation of the strongest melanopsin-expressing cells. Additionally, since we conducted experiments in a variety of pyramidal cell subclasses it is possible that cell background may have additionally contributed to the variability we observe. In practical terms this cell-to-cell variability made it difficult to compare responses between cells or groups of cells. Hence we focused predominantly on issues concerning the time course of the light-induced responses, where each cell could serve as its own control.
Light activation of melanopsin triggers a cluster of electrophysiological responses indistinguishable from those previously shown to be triggered by activation of GPCRs signaling via Gαq-11. This strongly suggests that exogenous melanopsin expressed in pyramidal cells under our experimental conditions signals predominantly, if not exclusively, via Gαq-11. Nevertheless, melanopsin has been reported to also couple to Gαi/o, albeit less efficiently than to Gαq-11 (Bailes and Lucas 2013; Spoida et al. 2016). The absence of a light-activated GIRK current in melanopsin-transfected cells in our sample argues against this possibility, although an involvement of Gαi/o cannot be ruled out. Unfortunately, as a result of the technical limitations outlined above, we have not been able to directly test the relative contributions of Gαq-11 and Gαi/o to the light responses we have studied. Therefore we discuss our results in the next few paragraphs in the context of Gαq-11 signaling, but we emphasize that this discussion must be regarded as tentative and model dependent.
Phasic and tonic components of the light response.
Previous studies have suggested that Gαq-11-operated responses can be separated into “phasic” and “tonic” components, with the transient hyperpolarization/outward current corresponding to the “phasic” component and the depolarization/inward current as well as the effects on the sAHP/IsAHP and sADP/IsADP corresponding to the “tonic” component. Implicit in the distinction between “phasic” and “tonic” effects is the idea that the “phasic” effects require fast, transient agonist exposure while the “tonic” effects require sustained agonist administration (Gulledge and Stuart 2005). We find that light flashes likely producing a single isomerization event (Hayward et al. 1981), and thus a single melanopsin activation-deactivation cycle, can elicit both the “phasic” and “tonic” components of the Gαq-11-mediated response. It seems likely that the distinction between “phasic” and “tonic” effects results from the unique characteristics of inositol 1,4,5-trisphosphate-mediated calcium release from intracellular stores that underlies the “phasic” hyperpolarization/outward current. Thus these results are inconsistent with the idea that the electrophysiological responses signaled by Gαq-11 can be separated into two classes based upon their activation requirements.
For these experiments we focused on the time course of the “tonic” electrophysiological effects since kinetic information can provide clues to the signaling mechanisms underlying these responses. In our recordings the latency from the light flash to the onset of the SK channel-mediated outward current was ∼500 ms, which provides an upper boundary estimate for the hypothesized breakdown of PtdIns(4,5)P2 under our experimental conditions. Remarkably, the slow inward current/membrane depolarization, the inhibition of IsAHP, and the appearance of the IsADP all exhibited latencies in this general time frame, suggesting a relatively tight coupling between the G protein activation by melanopsin and the triggering of these responses. This is consistent with our previous report that the inhibition of IsAHP following activation of Gαq-11 is signaled directly by the breakdown of PtdIns(4,5)P2 (Villalobos et al. 2011). This relatively fast time course also brackets the possible mechanisms signaling the slow inward current and the IsADP. We have previously reported that IsADP is mediated by nonselective cation/TRPC channels (Haj-Dahmane and Andrade 1996, 1998; Villalobos et al. 2011; Yan et al. 2009; but see Dasari et al. 2013). Since TRP channels appear to be gated by messengers downstream from the breakdown of PtdIns(4,5)P2 (Beech 2012), the short latency for the triggering of IsADP in the present experiments raises the possibility of scaffolding of key elements of the signaling cascade.
In a last set of experiments we created a chimeric GPCR comprised of a melanopsin and the intracellular domains of the 5-HT2A receptor, which, like melanopsin, couples to Gαq-11 and is highly expressed on pyramidal cells of the cortex (Weber and Andrade 2010). A similar approach has been used to investigate the interaction of the COOH tail of the 5-HT1A receptor with Gαi/o using vertebrate rhodopsin as a backbone (Oh et al. 2010). An advantage of using melanopsin for these experiments is that it is a bistable photopigment that expresses intrinsic isomerase activity. This allows it to autonomously regenerate 11-cis-retinal, a property that makes melanopsin a promising backbone for the generation of optogenetic tools (Bailes et al. 2012; Koyanagi and Terakita 2014). Unfortunately, in the present experiments the fraction of chimeric GPCR-transfected cells that responded to light was very low, dropping from about half in the case of wild-type melanopsin to ∼6% in the case of the chimera. These results demonstrate that melanopsin chimeric receptors represent a potentially viable optogenetic tool, although the strategy clearly will need to be optimized. While we do not know why the functional expression of the chimera was low, one possibility is that the extensive swapping of the intracellular domains may have interfered with expression of the opsin. This possibility can be addressed in future experiments, for example, by building a chimeric melanopsin receptor containing only the COOH-terminal tail of the target receptor. Such a chimera may not significantly disrupt the transmembrane organization of the wild-type opsin, thus preserving its high signaling efficiency, but could still provide valuable information on the physiological role of GPCR-associated protein scaffolds that are mainly recruited via their COOH-terminal tail.
In summary, the bistable nature of melanopsin and its ability to reversibly and repetitively manipulate G protein signaling on a millisecond timescale suggests its potential as a versatile optogenetic tool for the study of GPCR signaling. The present results demonstrate the feasibility of using melanopsin to obtain precise temporal control over GPCR signaling within semi-intact preparations and provide initial insight into the time course of electrophysiological responses downstream from the G protein. They also support the idea that melanopsin may provide a workable backbone for the functional study of the physiology of GPCR interacting proteins.
GRANTS
This work was supported by National Institute of Mental Health Grants MH-43985 and MH-100850 to R. Andrade and grants from Fondation pour la Recherche Médicale (FRM) (Equipe FRM2009) and Agence Nationale de la Recherche (ANR) (no. ANR-08-MNPS-0011) to C. Bécamel and P. Marin. K. M. McGregor was supported by a PhRMA Foundation Predoctoral Fellowship in Pharmacology/Toxicology.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
K.M.M. and R.A. conception and design of research; K.M.M. and C.B. performed experiments; K.M.M. analyzed data; K.M.M. and R.A. interpreted results of experiments; K.M.M. prepared figures; K.M.M. drafted manuscript; K.M.M., C.B., P.M., and R.A. edited and revised manuscript; K.M.M., C.B., P.M., and R.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Elaine Andrade for her technical work and the maintenance of our animal colony.
REFERENCES
- Andrade R. Cell excitation enhances muscarinic cholinergic responses in rat association cortex. Brain Res 548: 81–93, 1991. [DOI] [PubMed] [Google Scholar]
- Araneda R, Andrade R. 5-Hydroxytryptamine2 and 5-hydroxytryptamine1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience 40: 399–412, 1991. [DOI] [PubMed] [Google Scholar]
- Bailes HJ, Lucas RJ. Human melanopsin forms a pigment maximally sensitive to blue light (lambdamax approximately 479 nm) supporting activation of Gq/11 and Gi/o signalling cascades. Proc Biol Sci 280: 20122987, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailes HJ, Zhuang LY, Lucas RJ. Reproducible and sustained regulation of Galphas signalling using a metazoan opsin as an optogenetic tool. PloS One 7: e30774, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech DJ. Integration of transient receptor potential canonical channels with lipids. Acta Physiol 204: 227–237, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beique JC, Andrade R. PSD-95 regulates synaptic transmission and plasticity in rat cerebral cortex. J Physiol 546: 859–867, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beique JC, Imad M, Mladenovic L, Gingrich JA, Andrade R. Mechanism of the 5-hydroxytryptamine 2A receptor-mediated facilitation of synaptic activity in prefrontal cortex. Proc Natl Acad Sci USA 104: 9870–9875, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockaert J, Perroy J, Becamel C, Marin P, Fagni L. GPCR interacting proteins (GIPs) in the nervous system: roles in physiology and pathologies. Annu Rev Pharmacol Toxicol 50: 89–109, 2010. [DOI] [PubMed] [Google Scholar]
- Campagnola L, Wang H, Zylka MJ. Fiber-coupled light-emitting diode for localized photostimulation of neurons expressing channelrhodopsin-2. J Neurosci Methods 169: 27–33, 2008. [DOI] [PubMed] [Google Scholar]
- Constanti A, Bagetta G. Muscarinic receptor activation induces a prolonged post-stimulus afterdepolarization with a conductance decrease in guinea-pig olfactory cortex neurones in vitro. Neurosci Lett 131: 27–32, 1991. [DOI] [PubMed] [Google Scholar]
- Dasari S, Abramowitz J, Birnbaumer L, Gulledge AT. Do canonical transient receptor potential channels mediate cholinergic excitation of cortical pyramidal neurons? Neuroreport 24: 550–554, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasari S, Gulledge AT. M1 and M4 receptors modulate hippocampal pyramidal neurons. J Neurophysiol 105: 779–792, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorov AV, Angelova PR, Heinemann U, Muller W. Ca2+-independent muscarinic excitation of rat medial entorhinal cortex layer V neurons. Eur J Neurosci 18: 3343–3351, 2003. [DOI] [PubMed] [Google Scholar]
- Feuda R, Hamilton SC, McInerney JO, Pisani D. Metazoan opsin evolution reveals a simple route to animal vision. Proc Natl Acad Sci USA 109: 18868–18872, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson R, Lagerstrom MC, Lundin LG, Schioth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol 63: 1256–1272, 2003. [DOI] [PubMed] [Google Scholar]
- Gloveli T, Egorov AV, Schmitz D, Heinemann U, Muller W. Carbachol-induced changes in excitability and [Ca2+]i signalling in projection cells of medial entorhinal cortex layers II and III. Eur J Neurosci 11: 3626–3636, 1999. [DOI] [PubMed] [Google Scholar]
- Greene CC, Schwindt PC, Crill WE. Properties and ionic mechanisms of a metabotropic glutamate receptor-mediated slow afterdepolarization in neocortical neurons. J Neurophysiol 72: 693–704, 1994. [DOI] [PubMed] [Google Scholar]
- Gulledge AT, Bucci DJ, Zhang SS, Matsui M, Yeh HH. M1 receptors mediate cholinergic modulation of excitability in neocortical pyramidal neurons. J Neurosci 29: 9888–9902, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulledge AT, Stuart GJ. Cholinergic inhibition of neocortical pyramidal neurons. J Neurosci 25: 10308–10320, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haj-Dahmane S, Andrade R. Ionic mechanism of the slow afterdepolarization induced by muscarinic receptor activation in rat prefrontal cortex. J Neurophysiol 80: 1197–1210, 1998. [DOI] [PubMed] [Google Scholar]
- Haj-Dahmane S, Andrade R. Muscarinic activation of a voltage-dependent cation nonselective current in rat association cortex. J Neurosci 16: 3848–3861, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward G, Carlsen W, Siegman A, Stryer L. Retinal chromophore of rhodopsin photoisomerizes within picoseconds. Science 211: 942–944, 1981. [DOI] [PubMed] [Google Scholar]
- Koyanagi M, Terakita A. Diversity of animal opsin-based pigments and their optogenetic potential. Biochim Biophys Acta 1837: 710–716, 2014. [DOI] [PubMed] [Google Scholar]
- Krause M, Offermanns S, Stocker M, Pedarzani P. Functional specificity of Galphaq and Galpha11 in the cholinergic and glutamatergic modulation of potassium currents and excitability in hippocampal neurons. J Neurosci 22: 666–673, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjevic K, Pumain R, Renaud L. The mechanism of excitation by acetylcholine in the cerebral cortex. J Physiol 215: 247–268, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse MJ, Maiellaro I, Calebiro D. Kinetics and mechanism of G protein-coupled receptor activation. Curr Opin Cell Biol 27C: 87–93, 2014. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Prince DA. Mechanisms of action of acetylcholine in the guinea-pig cerebral cortex in vitro. J Physiol 375: 169–194, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melia TJ Jr, Cowan CW, Angleson JK, Wensel TG. A comparison of the efficiency of G protein activation by ligand-free and light-activated forms of rhodopsin. Biophys J 73: 3182–3191, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metherate R, Cox CL, Ashe JH. Cellular bases of neocortical activation: modulation of neural oscillations by the nucleus basalis and endogenous acetylcholine. J Neurosci 12: 4701–4711, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien JA, Holt M, Whiteside G, Lummis SC, Hastings MH. Modifications to the hand-held Gene Gun: improvements for in vitro biolistic transfection of organotypic neuronal tissue. J Neurosci Methods 112: 57–64, 2001. [DOI] [PubMed] [Google Scholar]
- Oh E, Maejima T, Liu C, Deneris E, Herlitze S. Substitution of 5-HT1A receptor signaling by a light-activated G protein-coupled receptor. J Biol Chem 285: 30825–30836, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S, Nayak SK, Campo B, Walker JR, Hogenesch JB, Jegla T. Illumination of the melanopsin signaling pathway. Science 307: 600–604, 2005. [DOI] [PubMed] [Google Scholar]
- Porter ML, Blasic JR, Bok MJ, Cameron EG, Pringle T, Cronin TW, Robinson PR. Shedding new light on opsin evolution. Proc Biol Sci 279: 3–14, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Kumbalasiri T, Carlson SM, Wong KY, Krishna V, Provencio I, Berson DM. Induction of photosensitivity by heterologous expression of melanopsin. Nature 433: 745–749, 2005. [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Spain WJ, Foehring RC, Chubb MC, Crill WE. Slow conductances in neurons from cat sensorimotor cortex in vitro and their role in slow excitability changes. J Neurophysiol 59: 450–467, 1988. [DOI] [PubMed] [Google Scholar]
- Spoida K, Eickelbeck D, Karapinar R, Eckhardt T, Mark MD, Jancke D, Ehinger BV, Konig P, Dalkara D, Herlitze S, Masseck OA. Melanopsin variants as intrinsic optogenetic on and off switches for transient versus sustained activation of G protein pathways. Curr Biol 26: 1206–1212, 2016. [DOI] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods 37: 173–182, 1991. [DOI] [PubMed] [Google Scholar]
- Villalobos C, Beique JC, Gingrich JA, Andrade R. Serotonergic regulation of calcium-activated potassium currents in rodent prefrontal cortex. Eur J Neurosci 22: 1120–1126, 2005. [DOI] [PubMed] [Google Scholar]
- Villalobos C, Foehring RC, Lee JC, Andrade R. Essential role for phosphatidylinositol 4,5-bisphosphate in the expression, regulation, and gating of the slow afterhyperpolarization current in the cerebral cortex. J Neurosci 31: 18303–18312, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, McCormick DA. Control of firing mode of corticotectal and corticopontine layer V burst-generating neurons by norepinephrine, acetylcholine, and 1S,3R-ACPD. J Neurosci 13: 2199–2216, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber ET, Andrade R. Htr2a gene and 5-HT2A receptor expression in the cerebral cortex studied using genetically modified mice. Front Neurosci 4: 36, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HD, Villalobos C, Andrade R. TRPC channels mediate a muscarinic receptor-induced afterdepolarization in cerebral cortex. J Neurosci 29: 10038–10046, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]