Short-latency afferent inhibition (SAI) is a transcranial magnetic stimulation (TMS) paradigm that has previously been shown to be a reliable measure of cholinergic activity in the motor cortex. In the present study, we extended the SAI paradigm to the dorsolateral prefrontal cortex (DLPFC) using combined TMS-EEG. We identified that the optimal interstimulus interval of SAI from the DLPFC is N20 + 4 ms and its spatiotemporal profile is characterized by the modulation of N100 at the left frontal cortex.
Keywords: short-latency afferent inhibition, dorsolateral prefrontal cortex, combined TMS-EEG study, TMS-evoked potentials
Abstract
Combined transcranial magnetic stimulation and electroencephalography (TMS-EEG) enables noninvasive neurophysiological investigation of the human cortex. A TMS paradigm of short-latency afferent inhibition (SAI) is characterized by attenuation of the motor-evoked potential (MEP) and modulation of N100 of the TMS-evoked potential (TEP) when TMS is delivered to motor cortex (M1) following median nerve stimulation. SAI is a marker of cholinergic activity in the motor cortex; however, the SAI has not been tested from the prefrontal cortex. We aimed to explore the effect of SAI in dorsolateral prefrontal cortex (DLPFC). SAI was examined in 12 healthy subjects with median nerve stimulation and TMS delivered to M1 and DLPFC at interstimulus intervals (ISIs) relative to the individual N20 latency. SAI in M1 was tested at the optimal ISI of N20 + 2 ms. SAI in DLPFC was investigated at a range of ISI from N20 + 2 to N20 + 20 ms to explore its temporal profile. For SAI in M1, the attenuation of MEP amplitude was correlated with an increase of TEP N100 from the left central area. A similar spatiotemporal neural signature of SAI in DLPFC was observed with a marked increase of N100 amplitude. SAI in DLPFC was maximal at ISI N20 + 4 ms at the left frontal area. These findings establish the neural signature of SAI in DLPFC. Future studies could explore whether DLPFC-SAI is neurophysiological marker of cholinergic dysfunction in cognitive disorders.
NEW & NOTEWORTHY
Short-latency afferent inhibition (SAI) is a transcranial magnetic stimulation (TMS) paradigm that has previously been shown to be a reliable measure of cholinergic activity in the motor cortex. In the present study, we extended the SAI paradigm to the dorsolateral prefrontal cortex (DLPFC) using combined TMS-EEG. We identified that the optimal interstimulus interval of SAI from the DLPFC is N20 + 4 ms and its spatiotemporal profile is characterized by the modulation of N100 at the left frontal cortex.
transcranial magnetic stimulation (TMS) provides a noninvasive method to study human cortical neurophysiology. The integrity of various cortical circuits can be measured using different conditioning-test stimulus protocols. One such circuit involves an afferent conditioning input from peripheral nerve stimulation, which is conventionally characterized by inhibition of the amplitude of the motor response elicited by a TMS test pulse delivered 20–25 ms later (Mariorenzi et al. 1991; Tokimura et al. 2000). This inhibitory cortical circuit is known as short-latency afferent inhibition (SAI). SAI has been shown to be mediated by cholinergic and GABAAergic systems (Di Lazzaro et al. 2000, 2007). Growing evidence suggests that SAI may have clinical utility as a surrogate marker of central cholinergic activity in cognitive disorders (Cantone et al. 2014). Decreased SAI is thought to reflect cholinergic dysfunction in cognitive disorders such as Alzheimer's disease and mild cognitive impairment (Yarnall et al. 2013).
Recent technical advances allow for the concurrent recording of electroencephalographic (EEG) responses to TMS (Ilmoniemi and Kicić 2010; Komssi et al. 2004) extending its utility to non-motor areas (Kähkönen et al. 2005; Lioumis et al. 2009; Daskalakis et al. 2008). Indeed, recent TMS-EEG studies have also demonstrated neurophysiological cortical correlates of SAI in M1 (Bikmullina et al. 2009; Ferreri et al. 2012) but have not investigated this measure in prefrontal areas that more directly involve cognition (Julkunen et al. 2011). In M1, SAI is most robustly associated with modulation of the prominent negative deflection of the TMS-evoked potential (TEP) at a latency of 100 ms following TMS (N100) (Bikmullina et al. 2009; Ferreri et al. 2012).
We hypothesized that SAI can be demonstrated in prefrontal areas by extending this paradigm to the dorsolateral prefrontal cortex (DLPFC). Ascending sensory input is relayed via the ventral nuclei of the thalamus to sensory and prefrontal regions (Draganski et al. 2008). In addition, non-human primate studies have shown complex projections and interconnections between the somatosensory cortex and the posterior parietal cortex area 7b and the ventral rim of the principal sulcus in the DLPFC (Goldman-Rakic 1988; Neal et al. 1990). In human studies, the first cortical component of the somatosensory-evoked potential (SSEP) evoked by median nerve stimulation is a negative deflection at a latency of ∼20 ms (N20) over somatosensory areas (Verroust et al. 1990), followed shortly thereafter by a negative potential over the contralateral frontal region with a 24-ms latency (N24) (Valeriani et al. 1998), and thereafter by a prominent negative deflection at 30-ms latency (N30) (Desmedt and Cheron 1981). The N24 shows maximal amplitude at the F3 electrode, which typically corresponds to the DLPFC (Valeriani et al. 1998), whereas N30 may have a more diffuse distribution involving broader sensorimotor integration (Cebolla et al. 2011). SAI in M1 is maximal at median nerve stimulation and a TMS interstimulus intervals (ISIs) 2 ms longer than the latency of N20 (N20 + 2 ms), which is thought to allow sufficient time for conduction from sensory to motor cortex (Fischer and Orth 2011; Tokimura et al. 2000). However, the optimal interval for SAI in DLPFC has not been determined.
We investigated the existence, neurophysiological correlates, and spatiotemporal profile of SAI using TMS applied to M1 (M1-SAI) and DLPFC (DLPFC-SAI), with simultaneous measurement of EEG. Based on the latency of prefrontal SSEP components, we hypothesized that DLPFC-SAI would be maximal at a latency of approximating N24 or N30. Based on prior studies of N100 (Bikmullina et al. 2009; Ferreri et al. 2012), we anticipated that TEP N100 amplitude would be modulated for both M1- and DLPFC-SAI in the stimulated area (Premoli et al. 2014a). Other TEPs and regions of interest (ROIs) were also explored on an exploratory basis.
MATERIALS AND METHODS
Participants
Twelve right-handed adults (6 female; aged 22–57 yr; mean age 39 ± 12 yr) participated in the study. Written informed consent was obtained from each participant. Participants between the ages of 18 and 59 who met the following criteria were eligible to participate in the study: 1) no history of neurological disorders including seizure or stroke, 2) no history of neuropsychiatric disorders, 3) normal cognitive function, 4) no history of alcohol or other drug abuse/dependence, and 5) did not smoke, use recreational substances, or use prescription medications. All participants were screened with the Structured Clinical Interview for DSM-IV Axis I Disorders before the study. The experiment was conducted in accordance with the Declaration of Helsinki and was approved by the Research Ethics Board of the Centre for Addiction and Mental Health.
Experimental Design and Procedures
During the experiment, participants sat in a chair and were instructed to keep their eyes open and relax throughout the study.
Surface Electromyography Recording
Electromyography (EMG) was recorded from the relaxed right first dorsal interosseous of the dominant hand using surface electrodes placed in a belly-tendon arrangement. The EMG signal was amplified (Intronix Technologies Model 2024F, Bolton, ON, Canada), filtered (band pass 2 Hz-5 kHz), digitized at 5 kHz (Micro 1401; Cambridge Electronics Design, Cambridge, UK), and monitored on a computer screen with stored in a laboratory computer for offline analysis.
Transcranial Magnetic Stimulation
Monophasic TMS pulses were administered to the left M1 using a 70-mm figure-of-eight coil and two Magstim 200 stimulators (Magstim, UK) connected via a Bistim module, with an intertrial interval of 5 s. The motor-evoked potential (MEP) data were collected using the commercially available software Signal (Cambridge Electronics, UK).
Median Nerve Stimulation
The median nerve was stimulated at the wrist with a standard bar electrode, with cathode positioned proximally using a constant current stimulator (Digitimer model DS7A; Digitimer). The conditioning stimulus (pulse width 200 μs) intensity was adjusted to three times sensory threshold. The intensity required for SAI evokes a small direct muscle response. Throughout the experiment, the amplitude of median nerve stimulation evoked EMG activity was monitored online to ensure a consistent level of stimulation (Cash et al. 2015). In the rare event that amplitudes induced by median nerve stimulation decreased, sensory threshold was checked and stimulation intensity adjusted if necessary
Determination of N20 Latency in the SSEP
ISIs between median nerve stimulation and TMS were based on the latency of SSEP N20 to account for individual differences in conduction time of sensory afferent input to the cortex (Alle et al. 2009; Cash et al. 2015; Ferreri et al. 2012; Fischer and Orth 2011). To obtain the individual N20 latency for each participant, SSEPs evoked by median nerve stimulation were recorded before the SAI protocol (200 stimuli delivered at 3 Hz and 3 times sensory threshold). Analysis was performed using Neuroscan software (Curry 7; Compumedics Neuroscan) as follows: channels were referenced to the reference electrode, epoched −80 to +150 ms, baseline corrected −80 to −50 ms, and averaged and the latency of N20 peak was determined at C3/CP3 electrodes.
Measurement of SAI
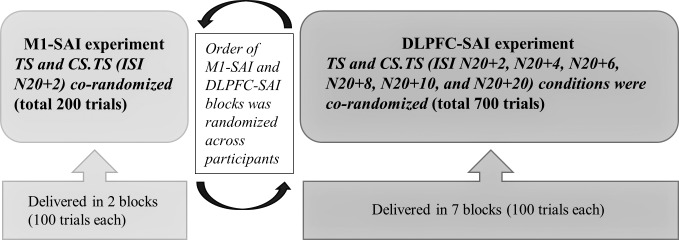
SAI was studied according to established methods (3× sensory threshold; TMS delivered at an intensity that generated 1-mV peak-to-peak MEP amplitude in the first dorsal interosseous muscle) (Tokimura et al. 2000). For SAI in M1, TMS was delivered to the hotspot corresponding to the first dorsal interosseous muscle and the ISI between median nerve stimulation and TMS was set to be 2 ms longer than the latency of N20 (referred to as N20 + 2 ms), as SAI is maximal at this ISI (Fischer and Orth 2011; Tokimura et al. 2000). DLPFC stimulation was performed with the coil centred on the F5 electrode, which provides the most accurate estimation of left DLPFC (border of BA9 and BA46) and low intersubject variability in the absence of neuronavigational equipment (Rusjan et al. 2010), and placed at an angle of 45° to the sagittal plane. A range of TMS ISIs between median nerve stimulation and TMS was used to identify the optimal interval for SAI in DLPFC and explore its time course: N20 + 2, N20 + 4, N20 + 6, N20 + 8, N20 + 10, N20 + 20 ms, and the TMS test stimulus (TS). For each condition in M1 and DLPFC, 100 trials were collected. Thus, for M1, 200 pulses and for DLPFC 700 pulses were delivered. The order of M1 and DLPFC stimulation was randomized across participants (Fig. 1). Stimuli were delivered in blocks of 100 trials (∼8 min each) in which all conditions (i.e., TS alone and all MNS.TS ISIs) were corandomized.
Fig. 1.
Short-latency afferent inhibition (SAI) protocol: The order of motor cortex (M1)-SAI and dorsolateral prefrontal cortex (DLPFC)-SAI paradigms was randomized across participants (see bottom). Blocks consisted of 100 trials with test stimulus (TS) and interstimulus intervals (ISIs) (MNS.TS conditions) corandomized in each block.
EEG RECORDING
To evaluate TEPs, EEG was recorded concurrently with EMG. EEG was acquired through a TMS-compatible 64-channel Neuroscan Synamps 2 system with Ag/AgCl ring electrodes by using EEG cap (Compumedics Neuroscan). All electrodes were referenced to an electrode placed on the vertex positioned posterior to the Cz electrode, and recording electrodes impedance was lowered to ≤5 kΩ. EEG signals were recorded at DC and an online low-pass filter of 200 Hz at a 20-kHz sampling rate, which was shown to avoid high-frequency noise and minimize the TMS related artifacts (Daskalakis et al. 2008).
EEG Signal Processing
EEG data were processed offline using MATLAB (R2014a; The MathWorks) and EEGLAB toolbox (version 13.4.3b, Swartz Center for Computational Neuroscience, University of California at San Diego) (Delorme and Makeig 2004). EEG signal processing was performed in line with previous methodology outlined in Rogasch et al. (2014). EEG data were downsampled to 1,000 Hz and epoched from −1,000 to 2,000 ms relative to the TMS test pulse, and baseline corrected with respect to the prestimulus interval −500 to −110 ms. To avoid TMS artifacts, we resegmented from 10 to 2,000 ms post-TMS for TEP analysis. EEG data were visually inspected to eliminate trials and channels that were highly contaminated with noise (muscle activity, electrode artifacts). More than 80% of trials and 95% of channels survived artifact rejection. Before applying the independent component analysis (ICA), each condition of EEG data was concatenated to avoid the bias of ICA cleaning. Subsequently, the ICA (EEGLAB toolbox; Infomax algorithm) was performed to remove eye-related artifacts (blinks and movements), muscle artifacts, and other remaining noise in the EEG data. In each subject, the number of ICA components that were removed from original 62 ICA components was no greater than 20%. Then, the Butterworth, zero-phase shift 1–55 Hz band pass filter (24 dB/Oct) and notch filter were applied. Finally, data was re-referenced to the average for further analysis (Bell and Sejnowski 1995; Delorme and Makeig 2004).
Data Analysis
EMG analysis.
First, trials were visually inspected and those with preceding EMG activity were excluded from the analysis. Then, MEPs following the single and paired pulse (conditioned-test) stimulations for each subject were averaged in each condition (100 trials per condition). EMG measures of SAI were calculated as the ratio of attenuation of peak-to-peak MEP amplitude of conditioned MEP (i.e., median nerve stimulation + TMS) over unconditioned MEP (i.e., TMS alone) as follows: SAI-MEP = conditioned MEP (median nerve stimulation + TMS)/unconditioned MEP (TMS alone).
EEG analysis.
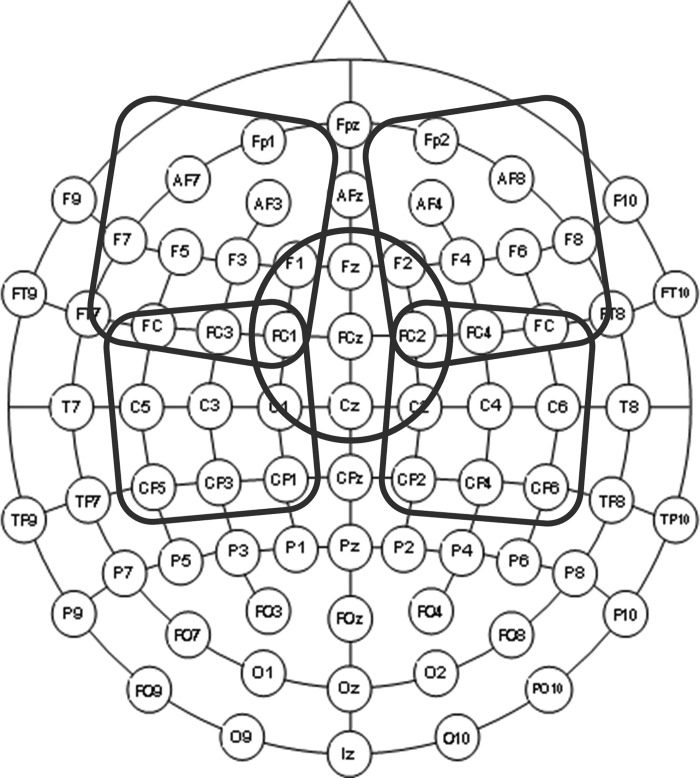
We analyzed the SAI data in two steps separately. First, to see the overall SAI effect on TEP by each condition for each ROI, the TEP power, defined as the amplitude envelope of the TEP curve (time window: 20–200 ms), was computed using the Hilbert transform method (Kortelainen and Vayrynen 2015; Zhu et al. 2015). Here, the Hilbert transform was applied to the TEP time series to calculate the TEP power to identify the cortical region (i.e., ROI; see Fig. 2) in which the TEP power changes was greatest. We have set the time window from 20 to 200 ms, since this time of interest could encompass all components of TEP in the SAI paradigm. Second, the influence of SAI on the amplitude of individual TEP components (P30, N45, P60, N100, and P180) was subsequently computed at each TMS condition and ROI for the M1- and DLPFC-SAI experiments. Here, to evaluate the SAI effect on TEPs, we calculated the ratio for each TEP component in the M1-SAI and DLPFC-SAI, which means the degree of modulation by SAI paradigm. The TEP ratio was calculated as follows: TEP ratio = conditioned TEP (median nerve stimulation + TMS)/unconditioned TEP (TMS alone)
Fig. 2.
The schema of regions of interest (ROI). The schema of the 64 EEG electrode topographical distribution is displayed. Five ROI were generated to cover the left frontal, right frontal, left central, right central, and midline central areas.
Of note, to correct the effect of median nerve stimulation-evoked SSEP on TEPs, we applied a subtraction method individually (Bikmullina et al. 2009; Premoli et al. 2014b) before the SAI-TEP analysis. Further, SAI data were also analyzed without SSEP subtraction.
In addition, to determine whether SAI remained stable over time, SAI was calculated in bins of ten stimuli from the beginning to end of M1 stimulation.
Statistical analyses.
SPSS version 19.0 was used for statistical analysis. The following assessments were performed: 1) SAI effects on MEP amplitude, 2) SAI effects on EEG power and amplitude, and 3) correlation between TEP and MEP changes. The MEP amplitudes were analyzed with paired t-tests comparing MEPs with and without preceding median nerve stimulation (TMS TS alone vs. ISI of N20 + 2) (significant α-level = 0.05). ANOVA were applied to TEP powers and amplitudes to examine the effects of SAI on TEPs with factors described below. Specifically, we performed a three-way repeated measure ANOVA for TEP amplitudes with ROI (i.e., 5 levels: the left frontal, right frontal, midline central, left central, and right central ROIs), TEP components (i.e., 5 levels: P30, N45, P60, N100, and P180), and TMS conditions (i.e., 2 levels for M1-SAI: TS alone and ISI N20 + 2; 7 levels for DLPFC-SAI: TS alone, ISI N20 + 2, N20 + 4, N20 + 6, N20 + 8, N20 + 10, and N20 + 20) as within-subject factors in both M1- and DLPFC-SAI paradigms separately. Bonferroni correction was applied to adjust the confidence intervals in these ANOVA models. Significant results in the ANOVA were further examined with Bonferroni corrected post hoc t-tests. The correlation between SAI-MEP and TEP N100 changes in the M1-SAI paradigm at the left central ROI was examined using Pearson's correlation coefficient (α = 0.05). Furthermore, we also performed the partial correlation analysis between the two with controlled for age. In addition, we explored the correlations between SAI-MEP and TEP N100 changes in the DLPFC-SAI as well as between TEP N100 changes in the M1-SAI and DLPFC-SAI.
Sample size.
Based on mean effect size derived from Nardone et al. (2006) (Cohen's d = 1.72; α = 0.05; β = 0.80) (Nardone et al. 2006), the estimated minimal sample size required was eight subjects. In a previous TMS-EEG study on the effect of SAI from the M1, eight subjects were used for their analysis (Ferreri et al. 2012). We have extended the number of subjects to 12 to minimize Type II error.
RESULTS
SSEP Latencies
The latency of the N20 peak at the C3/CP3 electrode was 20.8 ± 1.6 ms and the latency of N24 peak at the F3/F5 electrode was 24.9 ± 1.2 ms.
SAI-MEP
Mean intensity to induce 1-mV peak-to-peak MEP amplitude was 80.3 ± 11.5%. With M1 stimulation, SAI was significant with TS. MEP amplitude attenuated by 41.2 ± 8.0% (P < 0.01). Analysis of SAI-MEP amplitude indicated that the level of SAI remained stable during the course of the experiment (main effect of time: F9,99 = 0.728, P = 0.682).
Power and Amplitude Changes of the TMS-Evoked Potentials
M1-SAI.
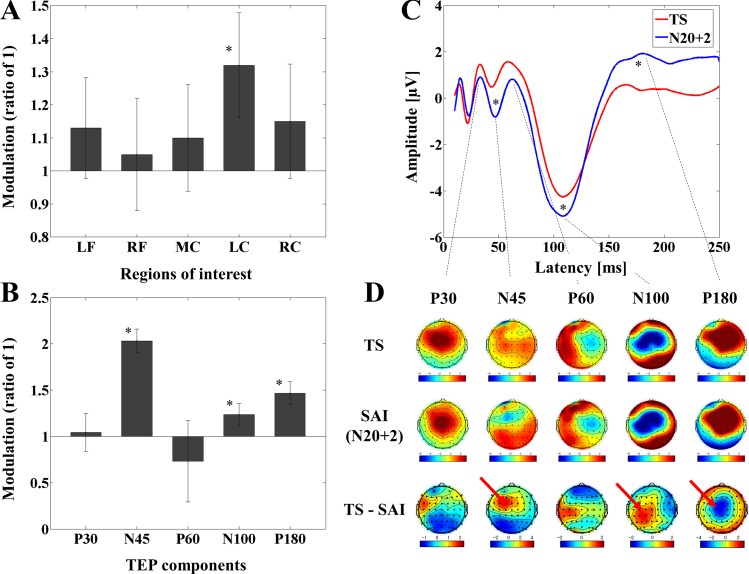
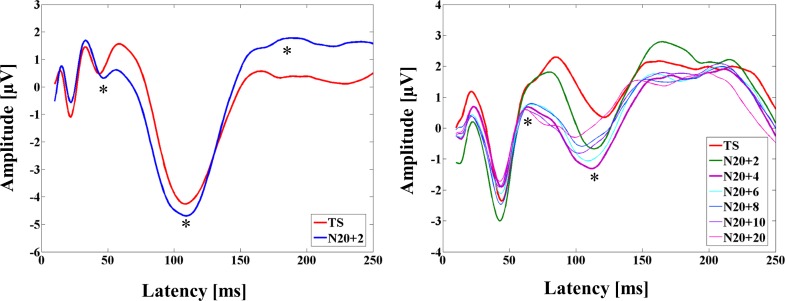
Results of the modulation of the TEP power for all ROIs and TEP amplitude at the left central ROI with the M1-SAI are depicted in Fig. 3. Furthermore, averaged TEP traces of all subjects for the unconditioned stimulus (TS, TMS alone) and the optimal conditioned stimulus (median nerve stimulation + TMS, ISI N20 + 2) obtained from the M1-SAI paradigm are shown in Fig. 3C.
Fig. 3.
Modulation of cortical activity by SAI with transcranial magnetic stimulation (TMS) delivered to M1 (M1-SAI). A: modulation of TMS-evoked potential (TEP) power by SAI: TEP power ratio for the time window of 20–200 ms for SAI (ISI N20 + 2 ms) compared with TS alone (test stimulus) at the ROIs in M1-SAI paradigm. The ANOVA and post hoc analysis indicated a significant TEP power increase at the ISI N20 + 2 over the left central ROI (*P < 0.05). LF, left frontal; RF, right frontal; MC, midline central; LC, left central; RC, right central. B: modulation of the amplitude of TEP components (left central ROI): TEP amplitude ratio for the ISI N20 + 2 compared with TS over the left central ROI with respect to each TEP component in M1-SAI paradigm. The ANOVA and post hoc analysis demonstrated significant TEP ratio increases at the ISI N20 + 2 on TEP N45, P60, and P180 (*P < 0.05). Note that the ratio here represents the degree of modulation for each TEP component by M1-SAI paradigm. C: modulation of TEP traces: TEP traces following subtraction of individual somatosensory-evoked potential (SSEP) components. The graphs depict TEP traces averaged across all subjects for unconditioned TMS (TS; TMS alone) and conditioned TMS (median nerve stimulation + TMS; ISI N20 + 2 ms) at the left central ROI. TMS was delivered at a time equivalent to 0 ms. The ANOVA and post hoc analysis indicated significant modulation of TEP components N45, N100, and P180 over the left central ROI (*P < 0.05). D: topographical plots: TMS-EEG topographical plots for all TEP components in M1-SAI paradigm. The TEP topoplots are shown for 1) TS delivered alone, 2) conditioned TS at an ISI of N20 + 2 ms, and 3) the difference between TS alone and conditioned TS (SAI) at ISI N20 + 2 ms. Each vertical column depicts the TEP topoplots for P30, N45, P60, N100 and P180 component from left to right, respectively. The areas showing significant TEP changes are shown with red arrows.
A two-way ANOVA for the TEP power demonstrated significant main effects of ROI and TMS condition as well as a significant ROI-by-TMS condition interaction. Further, subeffect tests of the two-way ANOVA (i.e., multivariate ANOVA) indicated significant main effects of ROI and TMS condition as well as significant interaction effects of ROI-by-TMS condition for factors of TS, TMS condition, and the left central ROI. The TEP power was significantly increased at ISI N20 + 2 ms compared with TS at the left central ROI (see Fig. 3A and Table 1).
Table 1.
Summary of significant results of M1-SAI
| Analysis | Results |
|---|---|
| Two-way ANOVA | |
| Main effects | |
| ROI | F4,44 = 4.536, P = 0.004 |
| ISI | F1,11 = 11.331, P = 0.006 |
| Interactions | |
| ROI-by-ISI | F4,44 = 4.403, P = 0.004 |
| MANOVA | |
| Simple main effects | |
| ROI | Pillai's Trace; F4,8 = 6.272, P = 0.014 |
| ISI | Pillai's Trace; F1,11 = 11.331, P = 0.006 |
| Simple interactions | |
| ROI-by-ISI; TS | Pillai's Trace; F4,8 = 4.732, P = 0.030 |
| ROI-by-ISI; ISI N20 + 2 | Pillai's Trace; F4,8 = 6.374, P = 0.013 |
| ROI-by-ISI; the left central ROI | Pillai's Trace; F1,11 = 6.015, P = 0.032 |
| Post hoc paired t-test | |
| TS > ISI N20 + 2; at the left central ROI | t11 = −2.449, P = 0.032 |
| Three-way ANOVA | |
| Main effects | |
| ROI | F4,44 = 3.243, P = 0.020 |
| ISI | F4,44 = 277.516, P < 0.0001 |
| Interactions | |
| ROI-by-ISI | F4,44 = 5.856, P = 0.001 |
| TEP component-by-ISI | F4,44 = 16.159, P < 0.0001 |
| ROI-by-TEP component-by-ISI | F16,176 = 2.198, P = 0.007 |
| MANOVA | |
| Simple main effects | |
| TEP component | Pillai's Trace; F4,8 = 116.908, P < 0.0001 |
| Simple interactions | |
| ROI-by-ISI; ISI N20 + 2 | Pillai's Trace; F4,8 = 3.871, P = 0.049 |
| ROI-by-ISI; the left central ROI | Pillai's Trace; F1,11 = 6.958, P = 0.023 |
| TEP component-by-ISI; TS | Pillai's Trace; F4,8 = 113.534, P < 0.0001 |
| TEP component-by-ISI; ISI N20 + 2 | Pillai's Trace; F4,8 = 108.429, P < 0.0001 |
| TEP component-by-ISI; TEP N45 | Pillai's Trace; F1,11 = 6.748, P = 0.025 |
| TEP component-by-ISI; TEP N100 | Pillai's Trace; F1,11 = 23.864, P < 0.0001 |
| TEP component-by-ISI; TEP P180 | Pillai's Trace; F1,11 = 23.910, P < 0.0001 |
| ROI-by-TEP component-by-ISI; TEP N45-by-TS | Pillai's Trace; F4,8 = 14.253, P = 0.001 |
| ROI-by-TEP component-by-ISI; TEP N45-by-ISI N20 + 2 | Pillai's Trace; F4,8 = 8.279, P = 0.006 |
| ROI-by-TEP component-by-ISI; TEP N100-by-TS | Pillai's Trace; F4,8 = 18.161, P < 0.0001 |
| ROI-by-TEP component-by-ISI; TEP N100-by-ISI N20 + 2 | Pillai's Trace; F4,8 = 4.659, P = 0.031 |
| ROI-by-TEP component-by-ISI; TEP P180-by-TS | Pillai's Trace; F4,8 = 32.693, P < 0.0001 |
| ROI-by-TEP component-by-ISI; TEP P180-by-ISI N20 + 2 | Pillai's Trace; F4,8 = 26.273, P < 0.0001 |
| ROI-by-TEP component-by-ISI; the left central ROI-by-TEP N45 | Pillai's Trace; F1,11 = 82.796, P < 0.0001 |
| ROI-by-TEP component-by-ISI; the left central ROI-by-TEP N100 | Pillai's Trace; F1,11 = 6.239, P = 0.030 |
| ROI-by-TEP component-by-ISI; the left central ROI-by-TEP P180 | Pillai's Trace; F1,11 = 6.143, P = 0.031 |
| Post hoc paired t-test | |
| TS > ISI N20 + 2; TEP N45 at the left central ROI | t11 = 9.099, P < 0.0001 |
| TS > ISI N20 + 2; TEP N100 at the left central ROI | t11 = 2.498, P = 0.030 |
| TS < ISI N20 + 2; TEP P180 at the left central ROI | t11 = −2.479, P = 0.031 |
Significant results of two-way and three-way ANOVA for the transcranial magnetic stimulation (TMS)-evoked potential (TEP) powers in motor cortex (M1)-short-latency afferent inhibition (SAI). ROI, regions of interest; TS, test stimulation; ISI, interstimulus interval.
Furthermore, a three-way ANOVA and post hoc analyses for TEP values obtained from the M1-SAI revealed significant differences between TS and ISI N20 + 2 over the left central ROI: 1) TEP N45 (TS > ISI N20 + 2), 2) TEP N100 (TS > ISI N20 + 2), and 3) TEP P180 (TS < ISI N20 + 2) (α = 0.05). (See Table 1). The effect size (Cohen's d) for the modulation of N100 component in M1-SAI was 0.74 (α = 0.05, β = 0.80).
Figure 3D displays the EEG topographical plots for conditions of TS alone, ISI of N20 + 2, and the difference between TS and ISI N20 + 2 obtained from M1-SAI experiment. The data reveal that TEP N45 and N100 amplitudes are significantly increased at the ISI N20 + 2 ms and TEP P180 amplitude is significantly attenuated at the ISI N20 + 2 ms for M1-SAI at the left central ROI. Figure 3B illustrates the TEP ratio changes for all TEP components over the left central ROI.
DLPFC-SAI.
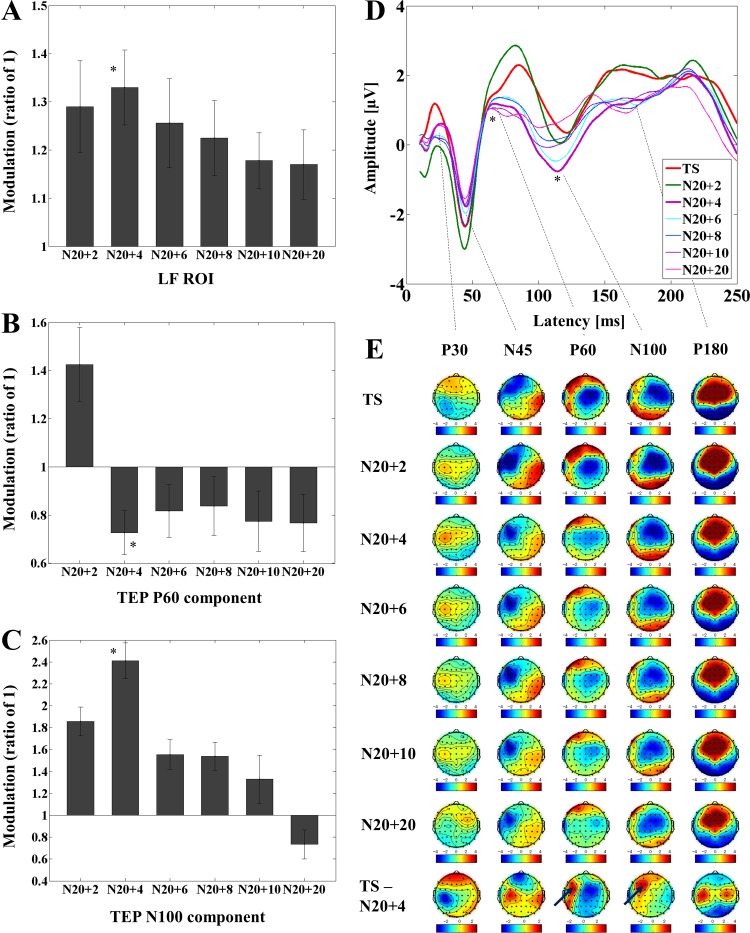
The modulation of TEP power with DLPFC-SAI at the 5 ROIs and TEP amplitude at the left frontal ROI is depicted in Fig. 4. Averaged TEP traces of all subjects for the unconditioned stimulus (TS, TMS alone) and the optimal conditioned stimulus (median nerve stimulation + TMS, ISI N20 + 4) obtained from the DLPFC-SAI paradigm are shown in Fig. 4D.
Fig. 4.
Modulation of cortical activity by SAI with TMS delivered to DLPFC (DLPFC-SAI). A: modulation of TEP power by SAI: TEP power ratio for the time window of 20–200 ms for each ISI compared with TS alone at the left frontal ROI in DLPFC-SAI paradigm. The ANOVA and post hoc analysis indicated a significant TEP power increase at the ISI N20 + 4 over the left frontal ROI (*P < 0.05). B and C: modulation of the amplitude of TEP components (left frontal ROI): TEP amplitude ratio for each ISI compared with TS over the left frontal ROI in DLPFC-SAI paradigm. The ANOVA and post hoc analysis indicated a significant attenuation of TEP P60 amplitude as well as a significant increase of TEP N100 amplitude at the ISI N20 + 4 over the left frontal ROI (*P < 0.05). D: modulation of TEP traces: TEP traces following subtraction of individual SSEP components. The graphs depict TEP traces averaged across all subjects for unconditioned TMS (TS) and conditioned TMS (median nerve stimulation + TMS; ISI N20 + 2 to N20 + 20 ms) at the left frontal ROI. The ANOVA and post hoc analysis showed a significant attenuation of TEP P60 and a significant increase of TEP N100 at the ISI N20 + 4 over the left frontal ROI (*P < 0.05). E: topographical plots: TEP topographical plots for DLPFC-SAI paradigm. Each line of the TEP topoplots indicates TS delivered alone, conditioned TS at the ISI N20 + 2, N20 + 4, N20 + 6, N20 + 8, N20 + 10, N20 + 20 ms, and the difference between TS alone and conditioned TS (SAI) at ISI N20 + 4 ms, respectively Each vertical column of the topoplots depicts TEP P30, N45, P60, N100, and P180 component from left to right, respectively. The areas showing significant TEP changes are shown with blue arrows.
A Bonferroni corrected two-way ANOVA and post hoc analyses for the TEP powers of the DLPFC-SAI demonstrated a significant difference in the ISI N20 + 4 (TS < ISI N20 + 4; significant α-level: 0.05/6 = 0.0083 here) at the left frontal ROI (see Fig. 4A and Table 2).
Table 2.
Summary of significant results of the DLPFC-SAI
| Analysis | Results |
|---|---|
| Two-way ANOVA | |
| Interactions | |
| ROI-by-ISI | F24,264 = 2.194, P = 0.001 |
| MANOVA | |
| Simple main effects | |
| ROI | Pillai's Trace; F4,8 = 9.702, P = 0.004 |
| ISI | Pillai's Trace; F6,6 = 7.223, P = 0.015 |
| Simple interactions | |
| ROI-by-ISI; TS | Pillai's Trace; F4,8 = 8.831, pcorrected = 0.005 |
| ROI-by-ISI; ISI N20 + 2 | Pillai's Trace; F4,8 = 11.832, pcorrected = 0.002 |
| ROI-by-ISI; the left frontal ROI | Pillai's Trace; F6,6 = 4.626, pcorrected = 0.042 |
| Post hoc paired t-test | |
| TS < ISI N20 + 4; at the left frontal ROI | t11 = −3.401, P = 0.006 (α-level = 0.05/6) |
| Three-way ANOVA | |
| Main effects | |
| ROI | F4,44 = 3.319, pcorrected = 0.018 |
| TEP component | F4,44 = 160.056, pcorrected < 0.0001 |
| ISI | F6,66 = 2.539, pcorrected = 0.028 |
| Interactions | |
| ROI-by-TEP component | F16,176 = 20.460, pcorrected < 0.0001 |
| ROI-by-ISI | F24,264 = 1.754, pcorrected = 0.018 |
| TEP component-by-ISI | F24,264 = 2.312, pcorrected = 0.001 |
| ROI-by-TEP component-by-ISI | F96,1056 = 1.314, pcorrected = 0.027 |
| MANOVA | |
| Simple main effects | |
| TEP component | Pillai's Trace; F4,8 = 108.366, pcorrected <0.0001 |
| Simple interactions | |
| ROI-by-TEP component; TEP P60 | Pillai's Trace; F4,8 = 8.642, pcorrected = 0.005 |
| ROI-by-TEP component; TEP N100 | Pillai's Trace; F4,8 = 24.119, pcorrected < 0.0001 |
| ROI-by-TEP component; the left frontal ROI | Pillai's Trace; F4,8 = 63.408, pcorrected < 0.0001 |
| ROI-by-ISI; ISI N20 + 4 | Pillai's Trace; F4,8 = 5.052, pcorrected = 0.025 |
| ROI-by-ISI; the left frontal ROI | Pillai's Trace; F6,6 = 8.095, pcorrected = 0.011 |
| TEP component-by-ISI; ISI N20 + 4 | Pillai's Trace; F4,8 = 106.217, pcorrected < 0.0001 |
| TEP component-by-ISI; TEP P60 | Pillai's Trace; F6,6 = 22.129, pcorrected = 0.001 |
| TEP component-by-ISI; TEP N100 | Pillai's Trace; F6,6 = 6.034, pcorrected = 0.023 |
| ROI-by-TEP component-by-ISI; TEP P60-by-ISI N20 + 4 | Pillai's Trace; F4,8 = 12.962, pcorrected = 0.001 |
| ROI-by-TEP component-by-ISI; TEP N100-by-ISI N20 + 4 | Pillai's Trace; F4,8 = 6.239, pcorrected = 0.014 |
| ROI-by-TEP component-by-ISI; the left frontal ROI-by-ISI N20 + 4 | Pillai's Trace; F4,8 = 73.114, pcorrected < 0.0001 |
| ROI-by-TEP component-by-ISI; the left frontal ROI-by-TEP P60 | Pillai's Trace; F6,6 = 19.826, pcorrected = 0.001 |
| ROI-by-TEP component-by-ISI; the left frontal ROI-by-TEP N100 | Pillai's Trace; F6,6 = 9.007, pcorrected = 0.009 |
| Post hoc paired t-test | |
| TS > ISI N20 + 4; TEP P60 at the left frontal ROI | t11 = 4.218, P = 0.001 (α-level = 0.05/6) |
| TS > ISI N20 + 4; TEP N100 at the left frontal ROI | t11 = 4.039, P = 0.002 (α-level = 0.05/6) |
Significant results of two-way and three-way ANOVA for the TEP powers in dorsolateral prefrontal cortex (DLPFC)-SAI.
In addition, a Bonferroni corrected three-way ANOVA and post hoc analyses for TEP values of the DLPFC-SAI indicated significant differences at the ISI N20 + 4 on the TEP P60 (TS < ISI N20 + 4) and at the ISI N20 + 4 on the N100 (TS > ISI N20 + 4) over the left frontal ROI (α = 0.05/6 = 0.0083) (See Table 2). The effect size (Cohen's d) for the modulation of N100 component in the DLPFC-SAI was 1.11 (α = 0.0083, β = 0.80).
EEG topographical plots for conditions of TS, ISI N20 + 2 to N20 + 20, and the difference between TS and ISI N20 + 4 obtained from DLPFC-SAI experiment are displayed in Fig. 4E. The TEP ratio changes for P60 and N100 components over the left frontal ROI are shown in Fig. 4, B and C. TEP P60 amplitude attenuation was maximal at the ISI N20 + 4 ms while the N100 increase was maximal at the ISI N20 + 4 ms over the left frontal ROI in DLPFC-SAI paradigm.
Influence of SSEP Subtraction
To ensure the robustness of results, analysis was also performed without SSEP subtraction. A Bonferroni corrected three-way ANOVA with ROI (5 ROIs), TEP component (P30, N45, P60, N100, and P180), and TMS condition (TS alone and ISI of N20 + 2) as within-subject factors and post hoc analyses for TEP values in the M1-SAI showed significant modulations in TEP N45 [t11 = 7.960, P < 0.0001; TS > SAI (ISI of N20 + 2)], N100 [t11 = 2.324, P = 0.040; TS > SAI (ISI of N20 + 2)], and P180 [t11 = −2.808, P = 0.017; TS < SAI (ISI of N20 + 2)] component at the ISI of N20 + 2, compared with TS condition, over the left central ROI. These significant modulations by M1-SAI were consistent with our original M1-SAI results with SSEP subtraction. Again, for the DLPFC-SAI paradigm, we conducted a Bonferroni corrected three-way ANOVA with ROI (5 ROIs), TEP component (P30, N45, P60, N100, and P180), and TMS condition (TS alone, ISI of N20 + 2, N20 + 4, N20 + 6, N20 + 8, N20 + 10, and N20 + 20) as within-subject factors and post hoc analyses for TEP values demonstrated significant modulations in TEP P60 [t11 = 3.376, P = 0.006; TS > SAI (ISI of N20 + 4)] and N100 [t11 = 5.821, P < 0.0001; TS > SAI (ISI of N20 + 4)] component at the ISI of N20 + 4 compared with TS condition over the left frontal ROI (α = 0.05/6 = 0.0083). Even for the calculation method without SSEP subtraction, we observed significant modulations in TEP P60 and N100 components by DLPFC-SAI, which were also identical to our original DLPFC-SAI results with SSEP subtraction. Thus results remained consistent regardless of whether SSEP was subtracted (see Fig. 5).
Fig. 5.
TEP traces in SAI paradigm for both M1 and DLPFC stimulation without the SSEP subtraction. TEP traces are displayed in the SAI paradigm for stimulation of M1 (left) and DLPFC (right) stimulation without SSEP subtraction. The appearance of SAI-TEPs looks similar to those with the SSEP subtraction.
CorrelationalAnalysis
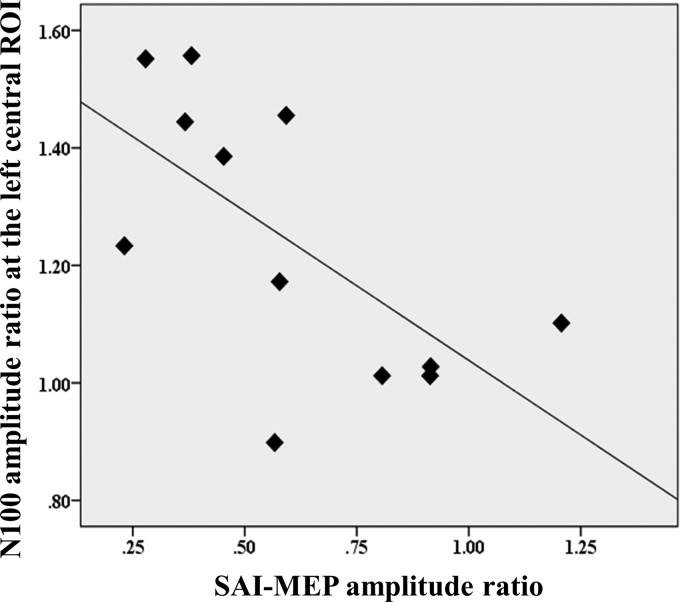
There was a significant negative correlation between the ratios of MEP amplitude attenuation and TEP N100 amplitude increase at the left central ROI (r = −0.649, P = 0.023, n = 12; Fig. 6). This association was investigated based on the previously described correlation between N100 and SAI (Bikmullina et al. 2009). Furthermore, when we controlled for the effect of age with this association, the correlation between the two remains significant (rSAI-MEP*TEP-N100 = −0.576, P = 0.032, df = 9). In addition, we did not observe any correlation between SAI-MEP and TEP N100 modulation in the SAI-DLPFC (r = −0.091, P = 0.777, n = 12) or between TEP N100 modulations in the M1-SAI and DLPFC-SAI (r = 0.009, P = 0.977, n = 12).
Fig. 6.
M1-SAI correlation between MEP and TEP N100 amplitude changes. Scatter plot depicting the correlation between ratios of MEP amplitude attenuation and TEP N100 amplitude increase at the ISI N20 + 2 over the left central ROI in the M1-SAI experiment (r = −0.649, P = 0.023, n = 12). Here, the ratio represents the degree of modulation of SAI-MEP or TEP in M1-SAI. The ratio was calculated as follows: TEP ratio = conditioned TEP (median nerve stimulation + TMS)/unconditioned TEP (TMS alone).
DISCUSSION
Previous work has shown that SAI in M1 is characterized by attenuation of EMG response amplitude modulation of the TEP P60 and N100 components (Bikmullina et al. 2009; Ferreri et al. 2012). In the present study, we demonstrate for the first time that SAI in DLPFC is similarly associated with a similar neural signature for SAI in M1 in the prefrontal area. The detection of SAI in the DLPFC on TEP P60 and N100 is a unique finding. The change in TEP power was greatest in the left central ROI for M1-SAI and left frontal ROI for DLPFC-SAI, suggesting that overall the changes in neural activity were primarily localized to the area of stimulation. In addition, the temporal profile of SAI in DLPFC, examined using various ISIs, indicated that modulation of neural activity was maximal at an ISI of N20 + 4 ms, thus approximating the latency of the prefrontal N24 deflection.
The first aim of the present study, before investigating DLPFC-SAI, was to confirm the modulatory effect of M1-SAI on TEP N100 component (Bikmullina et al. 2009; Ferreri et al. 2012). Consistent with previous studies, we found that M1-SAI was associated with modulation of N100 amplitude and that this modulation correlated with the attenuation of MEP amplitude demonstrating a direct relationship between TMS-EEG and TMS-EMG measures of SAI (Bikmullina et al. 2009; Ferreri et al. 2012). The second aim of the study was to identify SAI in the DLPFC and determine its temporal profile. We anticipated that TEPs in the DLPFC would be modulated by sensory input at ISIs corresponding to the negative SSEP deflections N24 or N30. Interestingly, DLPFC-SAI evoked a prominent modulation of N100 amplitude at an ISI of N20 + 4 ms (Fig. 4C). This latency corresponds approximately to the latency of the SSEP N24 that localizes in the DLPFC (Valeriani et al. 1998). Such temporal characteristics of SAI in DLPFC are reminiscent of its profile in M1, where the optimal latency of SAI depends on the latency of sensory input at the cortex (Fischer and Orth 2011). Our data suggest that the maximal effect of DLPFC-SAI is concurrent with the SSEP N24.
The N100 deflection is considered to be the most prominent and robust TMS-EEG component and is understood to have the greatest interindividual and intersession reproducibility compared with other TEPs (Lioumis et al. 2009). In addition, N100 is one TEP component that is reliably evoked by both frontal and motor cortical stimulation (Lioumis et al. 2009). Furthermore, relative to other TEP components, N100 is considered to have a high degree of sensitivity to small changes in cortical excitability (Nikulin et al. 2003). These factors enhance the value of N100 as a marker of cortical processing in basic and clinical research and make it ideal for exploration of motor and prefrontal SAI in the present study (Ilmoniemi and Kicić 2010; Lioumis et al. 2009). A number of studies indicated that N100 is related to cortical inhibitory processes (Farzan et al. 2013; Nikulin et al. 2003; Premoli et al. 2014b; Rogasch et al. 2013). In particular, N100 amplitude has been shown to correlate with cortical silent period duration (Kimiskidis et al. 2008) and is reduced by long interval intracortical inhibition (Premoli et al. 2014b), which are GABAB-receptor-mediated TMS measures of cortical inhibition (McDonnell et al. 2006). A recent pharmacological TMS-EEG study suggested that N100 likely reflects the long-lasting GABAB-receptor mediated inhibitory postsynaptic potential but may also be influenced by benzodiazepines which are positive allosteric modulators of the GABAA-receptor (Premoli et al. 2014a). Since SAI is known to decrease the long interval intracortical inhibition (Udupa et al. 2009), the most parsimonious explanation for the relationship between SAI and the modulation of N100 amplitude is the GABAA-receptor-mediated modulation of longer lasting GABAB-receptor mediated inhibition (Bikmullina et al. 2009). GABAA-receptor-modulated GABAB-receptor mediated inhibition has also been demonstrated elsewhere (Inghilleri et al. 1996) and may be mediated by changes in electrochemical gradients or interneuronal inhibitory input (Crunelli et al. 1988; Lopantsev and Schwartzkroin 1999; Stanford et al. 1995; Thomson and Destexhe 1999). The finding that N100 amplitude was significantly modulated for both M1- and DLPFC-SAI would be consistent with shared physiological mechanisms in both domains related to inhibition. Future pharmacological TMS-EEG studies may help to clarify the relationship between GABA and cholinergically mediated effects on N100 in the SAI paradigm.
While our primary hypothesis focused on N100 as a marker of SAI (Bikmullina et al. 2009; Ferreri et al. 2012), we also explored the influence of SAI on other TEP components. While N100 is consistently evoked by frontal and motor cortical stimulation, the representation of other TEPs may differ depending on the site of stimulation (Lioumis et al. 2009) and, perhaps consistent with this, we found that their modulation also differed for M1- and DLPFC-SAI. M1-SAI was associated with an increase in N45 and attenuation of P180. N45 has been linked to the GABAA-receptor-mediated inhibitory postsynaptic potential (Premoli et al. 2014a) and SAI is known to be mediated by this receptor (Di Lazzaro et al. 2000, 2007). The origin of P180 is less clear; however, diazepam, an allosteric modulator of the GABAA-receptor, has been found to induce opposite effects on N100 compared with P180 (Premoli et al. 2014b). Here, we found that SAI induced opposite effects on these two components and this may be related to the GABAAergic basis of SAI although confirming this would require further research.
There are several limitations of the study. The DLPFC was localized using EEG approximation, while neuronavigation allows for more accurate localization. Mitigating this limitation is previous work demonstrating that a scalp position for the left DLPFC between electrodes F3 and F5 in standard space was closest to electrode F5 in individual space (Rusjan et al. 2010) and further this position is assumed to provide the most accurate estimation of DLPFC (border of BA46/9) when neuronavigation is not used (Fitzgerald et al. 2009; Rogasch et al. 2015). Furthermore, we applied the ROI analysis method by setting the left frontal ROI to include the entire left DLPFC. Another potential limitation is the lack of noise masking to address auditory-evoked potentials (AEPs) induced by the TMS ”click.“ However, in this paradigm the median nerve stimulation input does not generate additional AEPs, i.e., the SAI and TS conditions are matched. SAI in the present study demonstrated a similar neural spatiotemporal signature to that observed in previous studies in which masking noise was used (Ferreri et al. 2011a) or not used (Bikmullina et al. 2009), and the results are therefore not suggestive of interference by an AEP, which could occur with both continuous noise or event related noise (Farzan et al. 2009; Lam et al. 1999; Rogasch et al. 2014). In addition, the amplitude of the AEP has been shown to be negligible compared with TMS-EEG components (Nikulin et al. 2003). Furthermore, the precise temporal acuity of SAI in DLPFC, with its maximum at ISI N20 + 4 ms, is not suggestive of interference by the AEP, which has a considerably broader spanning temporal component. Moreover, although we confirmed that the level of SAI-MEP remained stable during the experiment, there might be a potential influence of the fluctuation of alertness given the length of the experiment.
One further methodological point warrants discussion: in our study we subtracted SSEP waveforms from conditioned TS (median nerve stimulation + TMS) data for each individual. One previous study looked at SAI in M1 comparing ISI N20 + 3 ms and N20 + 10 ms (Ferreri et al. 2012); however, it should be noted that SAI at an ISI of N20 + 10 ms may result in facilitation of MEP amplitude (Fischer and Orth 2011) and therefore TS alone (used in the present study) may possibly provide a better baseline. Bikmullina et al. (2009) may have subtracted group average rather than individual SSEP waveforms from the group TEP trace; however, this was unclear (Bikmullina et al. 2009). Our findings appeared robust regardless of whether SSEP was subtracted or not. Our findings appear to be supported by the correlation between TEP N100 increase (i.e., more inhibitory modulation) and MEP amplitude reduction (i.e., more inhibition) in SAI paradigm in M1. Nonetheless, overall these studies are in agreement that the modulation of N100 is the most robust correlate of SAI.
DLPFC-SAI was also associated with a decrease in P60 amplitude at ISI N20 + 4 ms. This result is consistent with a previous study that found reduced P60 amplitude with M1-SAI (Ferreri et al. 2012), although we did not observe a significant change in P60 with M1-SAI in the present study, possibly due to higher variability in this component (Lioumis et al. 2009) (see also Fig. 3B). P60 amplitude was previously shown to be negatively correlated with N100 amplitude (Rogasch et al. 2013), and its modulation by long interval intracortical inhibition was found to be opposite to that of N100 (Premoli et al. 2014b), showing a possible association with inhibitory mechanisms (Rogasch et al. 2013).
It is worth noting that while modulation of N100 amplitude was a robust finding for SAI in M1 and DLPFC, there was no significant correlation between the degree of N100 amplitude modulation for motor and DLPFC. Previous studies have similarly reported that the level of TEP modulation was not correlated for M1 and DLPFC stimulation (Farzan et al. 2009, 2010, 2013). This may suggest that these measures provide unique information in DLPFC compared with M1, for example, due to differences in cholinergic or GABAergic tone.
In the present study, we found that the SAI paradigm is not limited to M1. Our findings, demonstrating the SAI paradigm in the DLPFC, have significant potential for clinical research, as further work may result in the development of SAI as a biomarker of cholinergic dysfunction in a cortical region related to cognition. Previous studies of SAI, using TMS-EMG, have found decreased SAI in a number of cognitive disorders involving dementia (Celebi et al. 2012; Di Lazzaro et al. 2002; Nardone et al. 2006, 2008). Modulation of SAI has been shown to predict long-term response to cholinesterase inhibitor in Alzheimer's disease (Di Lazzaro et al. 2005b), and is able to distinguish between non-amnestic patients and amnestic patients with mild cognitive impairment, suggesting that SAI may have potential use in helping to identify patients at increased risk of dementia (Nardone et al. 2012). Cognitive dysfunction is related, at least in part, with loss of cortical cholinergic innervation (Coyle et al. 1983; Ruberg et al. 1982). Muscarinic acetylcholine receptors, which regulate SAI, are the primary cholinergic receptors involved in learning and memory (Nathanson 1987). The functional effects of acetylcholine derive from its powerful neuromodulatory role in synaptic transmission, neuronal excitability, and plasticity (González et al. 2011). It has been suggested that SAI may involve cholinergically modulated GABAergic basket cells, explaining the high sensitivity of this circuit to cholinergic tone (Teo et al. 2009). Future confirmation of the sensitivity of DLPFC-SAI to cholinergic modulation will enhance its relevance as a potential biomarker of cholinergic dysfunction in cognitive disorders. In addition, the study of patients with conditions that have underlying cholinergic dysfunction warrant further study with this approach.
GRANTS
This research was supported by the Temerty Centre for Therapeutic Brain Intervention and the Campbell Family Research Institute through the Centre for Addiction and Mental Health (CAMH) Foundation and Canada Foundation for Innovation. Y. Noda received a Postdoctoral Fellowship from the CAMH Foundation. R. F. Cash was supported by a Canadian Institutes of Health Research (CIHR)-Dystonia Medical Research Foundation Fellowship Award. F. Farzan received funding from NARSAD, Slaight Family Centre for Youth in Transition at the CAMH, Natural Sciences and Engineering Research Council of Canada (NSERC), and the Ontario Brain Institute (OBI). T. K. Rajji received research support from Brain Canada, Brain and Behavior Research Foundation, Canada Foundation for Innovation, the CIHR, Ontario Ministry of Health and Long-Term Care, Ontario Ministry of Research and Innovation, the US National Institutes of Health (NIH), and the W. Garfield Weston Foundation. M. S. Barr receives research support from the Brain and Behavior Research Foundation (Formerly NARSAD) Young Investigator Grant and Schizophrenia Junior Faculty Grant from the CAMH Foundation. Z. J. Daskalakis has received research support from the Ontario Mental Health (OMH) Foundation, the CIHR, the Brain and Behaviour Research Foundation (Formerly NARSAD), and the Temerty family and Grant family through the CAMH Foundation and the Campbell Institute. Z. J. Daskalakis received research and equipment in kind support for an investigator-initiated study through Brainsway and a travel allowance through Merck. Z. J. Daskalakis has also received speaker funding through Sepracor and AstraZeneca, served on advisory boards for Hoffmann-La Roche Limited and Merck, and received speaker support from Eli Lilly. D. M. Blumberger has received research support from the CIHR, US NIH, Brain Canada, and the Temerty family through the CAMH Foundation and the Campbell Research Institute. He receives research support and in-kind equipment support for an investigator-initiated study from Brainsway Limited, and he is the site principal investigator for three sponsor-initiated studies for Brainsway Limited. He receives in-kind equipment support from Magventure for an investigator-initiated study. He receives medication supplies for an investigator-initiated trial from Invidior.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Y.N., R.F.H.C., R.C., Z.J.D., and D.M.B. conception and design of research; Y.N. and R.F.H.C. performed experiments; Y.N., R.Z., and L.G.D. analyzed data; Y.N., R.F.H.C., R.Z., F.F., T.K.R., R.C., Z.J.D., and D.M.B. interpreted results of experiments; Y.N. prepared figures; Y.N. and R.F.H.C. drafted manuscript; Y.N., R.F.H.C., R.Z., F.F., T.K.R., M.S.B., R.C., Z.J.D., and D.M.B. edited and revised manuscript; Y.N., R.F.H.C., R.Z., L.G.D., F.F., T.K.R., M.S.B., R.C., Z.J.D., and D.M.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Stacey Shim and Felicity Backhouse for support in the recruitment of participants.
REFERENCES
- Alle H, Heidegger T, Kriváneková L, Ziemann U. Interactions between short-interval intracortical inhibition and short-latency afferent inhibition in human motor cortex. J Physiol 587: 5163–5176, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural Comput 7: 1129–1159, 1995. [DOI] [PubMed] [Google Scholar]
- Bikmullina R, Kicić D, Carlson S, Nikulin VV. Electrophysiological correlates of short-latency afferent inhibition: a combined EEG and TMS study. Exp Brain Res 194: 517–526, 2009. [DOI] [PubMed] [Google Scholar]
- Cantone M, Di Pino G, Capone F, Piombo M, Chiarello D, Cheeran B, Pennisi G, Di Lazzaro V. The contribution of transcranial magnetic stimulation in the diagnosis and in the management of dementia. Clin Neurophysiol 125: 1509–1532, 2014. [DOI] [PubMed] [Google Scholar]
- Cash RF, Gunraj CA, Isayama R, Ni Z, Chen R. The influence of sensory afferent input on local motor cortical excitatory circuitry in human. J Physiol 593: 1667–1684, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebolla AM, Palmero-Soler E, Dan B, Cheron G. Frontal phasic and oscillatory generators of the N30 somatosensory evoked potential. Neuroimage 54: 1297–1306, 2011. [DOI] [PubMed] [Google Scholar]
- Celebi O, Temuçin CM, Elibol B, Saka E. Short latency afferent inhibition in Parkinson's disease patients with dementia. Mov Disord 27: 1052–1055, 2012. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Price DL, DeLong MR. Alzheimer's disease: a disorder of cortical cholinergic innervation. Science 219: 1184–1190, 1983. [DOI] [PubMed] [Google Scholar]
- Crunelli V, Haby M, Jassik-Gerschenfeld D, Leresche N, Pirchio M. Cl−- and K+-dependent inhibitory postsynaptic potentials evoked by interneurones of the rat lateral geniculate nucleus. J Physiol 399: 153–176, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis ZJ, Farzan F, Barr MS, Maller JJ, Chen R, Fitzgerald PB. Long-interval cortical inhibition from the dorsolateral prefrontal cortex: a TMS-EEG study. Neuropsychopharmacology 33: 2860–2869, 2008. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134: 9–21, 2004. [DOI] [PubMed] [Google Scholar]
- Desmedt JE, Cheron G. Non-cephalic reference recording of early somatosensory potentials to finger stimulation in adult or aging normal man: differentiation of widespread N18 and contralateral N20 from the prerolandic P22 and N30 components. Electroencephalogr Clin Neurophysiol 52: 553–570, 1981. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Tonali PA, Marra C, Daniele A, Profice P, Saturno E, Pilato F, Masullo C, Rothwell JC. Noninvasive in vivo assessment of cholinergic cortical circuits in AD using transcranial magnetic stimulation. Neurology 59: 392–397, 2002. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Saturno E, Dileone M, Pilato F, Nardone R, Ranieri F, Musumeci G, Fiorilla T, Tonali PA. Effects of lorazepam on short latency afferent inhibition and short latency intracortical inhibition in humans. J Physiol 564: 661–668, 2005b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Pennisi MA, Di Giovanni S, Zito G, Tonali PA, Rothwell JC. Muscarinic receptor blockade has differential effects on the excitability of intracortical circuits in the human motor cortex. Exp Brain Res 135: 455–461, 2000. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Profice P, Ranieri F, Ricci V, Bria P, Tonali PA, Ziemann U. Segregating two inhibitory circuits in human motor cortex at the level of GABAA receptor subtypes: a TMS study. Clin Neurophysiol 118: 2207–2214, 2007. [DOI] [PubMed] [Google Scholar]
- Draganski B, Kherif F, Klöppel S, Cook PA, Alexander DC, Parker GJ, Deichmann R, Ashburner J, Frackowiak RS. Evidence for segregated and integrative connectivity patterns in the human basal ganglia. J Neurosci 28: 7143–7152, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzan F, Barr MS, Wong W, Chen R, Fitzgerald PB, Daskalakis ZJ. Suppression of gamma-oscillations in the dorsolateral prefrontal cortex following long interval cortical inhibition: a TMS-EEG study. Neuropsychopharmacology 34: 1543–1551, 2009. [DOI] [PubMed] [Google Scholar]
- Farzan F, Barr MS, Levinson AJ, Chen R, Wong W, Fitzgerald PB, Daskalakis ZJ. Evidence for gamma inhibition deficits in the dorsolateral prefrontal cortex of patients with schizophrenia. Brain 133: 1505–1514, 2010. [DOI] [PubMed] [Google Scholar]
- Farzan F, Barr MS, Hoppenbrouwers SS, Fitzgerald PB, Chen R, Pascual-Leone A, Daskalakis ZJ. The EEG correlates of the TMS-induced EMG silent period in humans. Neuroimage 83: 120–134, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreri F, Pasqualetti P, Maatta S, Ponzo D, Ferrarelli F, Tononi G, Mervaala E, Miniussi C, Rossini PM. Human brain connectivity during single and paired pulse transcranial magnetic stimulation. Neuroimage 54: 90–102, 2011a. [DOI] [PubMed] [Google Scholar]
- Ferreri F, Ponzo D, Hukkanen T, Mervaala E, Könönen M, Pasqualetti P, Vecchio F, Rossini PM, Määttä S. Human brain cortical correlates of short-latency afferent inhibition: a combined EEG-TMS study. J Neurophysiol 108: 314–323, 2012. [DOI] [PubMed] [Google Scholar]
- Fischer M, Orth M. Short-latency sensory afferent inhibition: conditioning stimulus intensity, recording site, and effects of 1 Hz repetitive TMS. Brain Stimul 4: 202–209, 2011. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Maller JJ, Hoy KE, Thomson R, Daskalakis ZJ. Exploring the optimal site for the localization of dorsolateral prefrontal cortex in brain stimulation experiments. Brain Stimul 2: 234–237, 2009. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Topography of cognition: parallel distributed networks in primate association cortex. Annu Rev Neurosci 11: 137–156, 1988. [DOI] [PubMed] [Google Scholar]
- González JC, Albiñana E, Baldelli P, García AG, Hernández-Guijo JM. Presynaptic muscarinic receptor subtypes involved in the enhancement of spontaneous GABAergic postsynaptic currents in hippocampal neurons. Eur J Neurosci 33: 69–81, 2011. [DOI] [PubMed] [Google Scholar]
- Ilmoniemi RJ, Kicić D. Methodology for combined TMS and EEG. Brain Topogr 22: 233–248, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inghilleri M, Berardelli A, Marchetti P, Manfredi M. Effects of diazepam, baclofen and thiopental on the silent period evoked by transcranial magnetic stimulation in humans. Exp Brain Res 109: 467–472, 1996. [DOI] [PubMed] [Google Scholar]
- Julkunen P, Jauhiainen AM, Könönen M, Pääkkönen A, Karhu J, Soininen H. Combining transcranial magnetic stimulation and electroencephalography may contribute to assess the severity of Alzheimer's disease. Int J Alzheimers Dis 2011: 654794, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kähkönen S, Komssi S, Wilenius J, Ilmoniemi RJ. Prefrontal transcranial magnetic stimulation produces intensity-dependent EEG responses in humans. Neuroimage 24: 955–960, 2005. [DOI] [PubMed] [Google Scholar]
- Kimiskidis VK, Papagiannopoulos S, Kazis DA, Vasiliadis G, Oikonomidi A, Sotirakoglou K, Pseftogianni D, Anogianakis G, Vlaikidis N. Silent period (SP) to transcranial magnetic stimulation: the EEG substrate. Brain Stimulation Abstracts from the Thirrd International Conference on Transcranial Magnetic Stimulation and Direct Current Stimulation 1: 315–316, 2008. [Google Scholar]
- Komssi S, Kähkönen S, Ilmoniemi RJ. The effect of stimulus intensity on brain responses evoked by transcranial magnetic stimulation. Hum Brain Mapp 21: 154–164, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortelainen J, Vayrynen E. Assessing EEG slow wave activity during anesthesia using Hilbert-Huang transform. Conf Proc IEEE Eng Med Biol Soc 2015: 117–120, 2015. [DOI] [PubMed] [Google Scholar]
- Lam K, Kakigi R, Kaneoke Y, Naka D, Maeda K, Suzuki H. Effects of visual and auditory stimulation on somatosensory evoked magnetic fields. Clin Neurophysiol 110: 295–304, 1999. [DOI] [PubMed] [Google Scholar]
- Lioumis P, Kicić D, Savolainen P, Mäkelä JP, Kähkönen. Reproducibility of TMS-Evoked EEG responses. Hum Brain Mapp 30: 1387–1396, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopantsev V, Schwartzkroin PA. GABAA-Dependent chloride influx modulates GABAB-mediated IPSPs in hippocampal pyramidal cells. J Neurophysiol 82: 1218–1223, 1999. [DOI] [PubMed] [Google Scholar]
- Mariorenzi R, Zarola F, Caramia MD, Paradiso C, Rossini PM. Non-invasive evaluation of central motor tract excitability changes following peripheral nerve stimulation in healthy humans. Electroencephalogr Clin Neurophysiol 81: 90–101, 1991. [DOI] [PubMed] [Google Scholar]
- McDonnell MN, Orekhov Y, Ziemann U. The role of GABA(B) receptors in intracortical inhibition in the human motor cortex. Exp Brain Res 173: 86–93, 2006. [DOI] [PubMed] [Google Scholar]
- Nardone R, Marth R, Ausserer H, Bratti A, Tezzon F. Reduced short latency afferent inhibition in patients with Down syndrome and Alzheimer-type dementia. Clin Neurophysiol 117: 2204–2210, 2006. [DOI] [PubMed] [Google Scholar]
- Nardone R, Bergmann J, Kronbichler M, Kunz A, Klein S, Caleri F, Tezzon F, Ladurner G, Golaszewski S. Abnormal short latency afferent inhibition in early Alzheimer's disease: a transcranial magnetic demonstration. J Neural Transm 115: 1557–1562, 2008. [DOI] [PubMed] [Google Scholar]
- Nardone R, Bergmann J, Christova M, Caleri F, Tezzon F, Ladurner G, Trinka E, Golaszewski S. Short latency afferent inhibition differs among the subtypes of mild cognitive impairment. J Neural Transm 119: 463–471, 2012. [DOI] [PubMed] [Google Scholar]
- Nathanson NM. Molecular properties of the muscarinic acetylcholine receptor. Annu Rev Neurosci 10: 195–236, 1987. [DOI] [PubMed] [Google Scholar]
- Neal JW, Pearson RC, Powell TP. The ipsilateral corticocortical connections of area 7 with the frontal lobe in the monkey. Brain Res 509: 31–40, 1990. [DOI] [PubMed] [Google Scholar]
- Nikulin VV, Kicić D, Kähkönen S, Ilmoniemi RJ. Modulation of electroencephalographic responses to transcranial magnetic stimulation: evidence for changes in cortical excitability related to movement. Eur J Neurosci 18: 1206–1212, 2003. [DOI] [PubMed] [Google Scholar]
- Premoli I, Castellanos N, Rivolta D, Belardinelli P, Bajo R, Zipser C, Espenhahn S, Heidegger T, Müller-Dahlhaus F, Ziemann U. TMS-EEG signatures of GABAergic neurotransmission in the human cortex. J Neurosci 34: 5603–5612, 2014a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premoli I, Rivolta D, Espenhahn S, Castellanos N, Belardinelli P, Ziemann U, Müller-Dahlhaus F. Characterization of GABAB-receptor mediated neurotransmission in the human cortex by paired-pulse TMS-EEG. Neuroimage 103: 152–162, 2014b. [DOI] [PubMed] [Google Scholar]
- Rogasch NC, Daskalakis ZJ, Fitzgerald PB. Mechanisms underlying long-interval cortical inhibition in the human motor cortex: a TMS-EEG study. J Neurophysiol 109: 89–98, 2013. [DOI] [PubMed] [Google Scholar]
- Rogasch NC, Thomson RH, Farzan F, Fitzgibbon BM, Bailey NW, Hernandez-Pavon JC, Daskalakis ZJ, Fitzgerald PB. Removing artefacts from TMS-EEG recordings using independent component analysis: importance for assessing prefrontal and motor cortex network properties. Neuroimage 101: 425–439, 2014. [DOI] [PubMed] [Google Scholar]
- Rogasch NC, Daskalakis ZJ, Fitzgerald PB. Cortical inhibition of distinct mechanisms in the dorsolateral prefrontal cortex is related to working memory performance: a TMS-EEG study. Cortex 64: 68–77, 2015. [DOI] [PubMed] [Google Scholar]
- Ruberg M, Ploska A, Javoy-Agid F, Agid Y. Muscarinic binding and choline acetyltransferase activity in Parkinsonian subjects with reference to dementia. Brain Res 232: 129–139, 1982. [DOI] [PubMed] [Google Scholar]
- Rusjan PM, Barr MS, Farzan F, Arenovich T, Maller JJ, Fitzgerald PB, Daskalakis ZJ. Optimal transcranial magnetic stimulation coil placement for targeting the dorsolateral prefrontal cortex using novel magnetic resonance image-guided neuronavigation. Hum Brain Mapp 31: 1643–1652, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford IM, Wheal HV, Chad JE. Bicuculline enhances the late GABAB receptor-mediated paired-pulse inhibition observed in rat hippocampal slices. Eur J Pharmacol 277: 229–234, 1995. [DOI] [PubMed] [Google Scholar]
- Teo JT, Terranova C, Swayne O, Greenwood RJ, Rothwell JC. Differing effects of intracortical circuits on plasticity. Exp Brain Res 193: 555–563, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM, Destexhe A. Dual intracellular recordings and computational models of slow inhibitory postsynaptic potentials in rat neocortical and hippocampal slices. Neuroscience 92: 1193–1215, 1999. [DOI] [PubMed] [Google Scholar]
- Tokimura H, Di Lazzaro V, Tokimura Y, Oliviero A, Profice P, Insola A, Mazzone P, Tonali PA, Rothwell JC. Short latency inhibition of human hand motor cortex by somatosensory input from the hand. J Physiol 2: 503–513, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udupa K, Ni Z, Gunraj C, Chen R. Interactions between short latency afferent inhibition and long interval intracortical inhibition. Exp Brain Res 199: 177–183, 2009. [DOI] [PubMed] [Google Scholar]
- Valeriani M, Restuccia D, Di Lazzaro V, Le Pera D, Barba C, Tonali PA, Mauguiere F. Dipolar sources of the early scalp somatosensory evoked potentials to upper limb stimulation. Effect of increasing stimulus rates. Exp Brain Res 120: 306–315, 1998. [DOI] [PubMed] [Google Scholar]
- Verroust J, Blinowska A, Vilfrit R, Couperie D, Malapert D, Perrier M. Somatosensory evoked potentials from median nerve; normative data. Electromyogr Clin Neurophysiol 30: 35–39, 1990. [PubMed] [Google Scholar]
- Yarnall AJ, Rochester L, Baker MR, David R, Khoo TK, Duncan GW, Galna B, Burn DJ. Short latency afferent inhibition: a biomarker for mild cognitive impairment in Parkinson's disease? Mov Disord 28: 1285–1288, 2013. [DOI] [PubMed] [Google Scholar]
- Zhu JD, Lin CF, Chang SH, Wang JH, Peng TI, Chien YY. Analysis of spike waves in epilepsy using Hilbert-Huang transform. J Med Syst 39: 170, 2015. [DOI] [PubMed] [Google Scholar]