Abstract
Background
Schizophrenia is associated with cognitive impairment and brain network dysconnectivity. Recent efforts have explored brain circuits underlying cognitive dysfunction in schizophrenia and documented altered activation of large-scale brain networks, including the task-positive network (TPN) and the task-negative default mode network (DMN) in response to cognitive demands. However, to what extent TPN and DMN dysfunction reflect overlapping mechanisms and are dependent on cognitive state remain to be determined.
Methods
In the current study, we investigated the recruitment of TPN and DMN using independent component analysis in patients with schizophrenia spectrum disorders (n = 29) and healthy controls (n = 21) during two different executive tasks probing planning/problem-solving and spatial working memory.
Results
We found reduced load-dependent DMN deactivation across tasks in patients compared to controls. Furthermore, we observed only moderate associations between the TPN and DMN activation across groups, implying that the two networks reflect partly independent mechanisms. Additionally, whereas TPN activation was associated with task performance in both tasks, no such associations were found for DMN.
Conclusion
These results support a general load-dependent DMN dysfunction in schizophrenia spectrum disorder across two demanding executive tasks that is not merely an epiphenomenon of cognitive dysfunction.
Keywords: Schizophrenia spectrum disorder, Across tasks, Task-positive network, Default mode network, Functional magnetic resonance imaging, Independent component analysis
Highlights
-
•
SZ patients have reduced load-dependent DMN deactivation compared to controls.
-
•
TPN activation is associated with task performance, whereas DMN deactivation is not.
-
•
There are only moderate associations between the TPN and DMN activation.
1. Introduction
Schizophrenia (SZ) is a psychotic disorder characterized by positive and negative symptoms, accompanied by cognitive dysfunction (Bleuler, 1950, Kahn and Keefe, 2013, Schneider, 1959), and converging neuroimaging evidence has implicated brain network dysfunction (Insel, 2010, Uhlhaas and Singer, 2006). Recent efforts have conceptualized the brain as a never-resting organ, of which complex functions are enabled by the continuous cross-talk between different networks rather than simple increases or decreases in activation of modular brain systems (Fox et al., 2005, Raichle and Snyder, 2007, Whitfield-Gabrieli and Ford, 2012). A subset of these brain networks is referred to as task-positive and task-negative networks as they are associated with task-related activations or deactivations in functional magnetic resonance imaging (fMRI) studies (Fox et al., 2005). Task-positive networks (TPN) are activated by effort demanding tasks (Fox et al., 2005), and comprise several sub-networks, including the dorsal attention network (DAN) and the central executive network (CEN). DAN is involved in top-down goal directed processing requiring mental effort and includes the superior parietal lobe, the inferior parietal sulcus, the posterior parietal cortex, and the frontal eye field (Alnaes et al., 2015, Fox et al., 2005, Szczepanski et al., 2013, Toro et al., 2008). CEN is involved in executive processes such as sustained attention, working memory and decision making, and includes the dorsolateral prefrontal cortex and the posterior parietal cortex (Seeley et al., 2007). In contrast, the default mode network (DMN), encompassing medial prefrontal, lateral parietal, and the posterior cingulate cortices and precuneus (Buckner et al., 2008, Garrity et al., 2007), is a task-negative network more active in absence of specific task demands (Raichle and Snyder, 2007). The DMN is active when individuals are engaged in internal processes not directly attributed to a specific external task, such as recall of the past and imagining of the future, autobiographical memory, and conceiving the perspective of others (Buckner et al., 2008, Ostby et al., 2012).
In line with the notion that cognition is enabled by the reciprocal regulation of various brain networks (Fox et al., 2005), altered temporal synchronization between several brain networks has been reported in all stages of SZ, including high genetic risk, ultra-high risk, early onset schizophrenia, first episode and in chronic SZ (Pettersson-Yeo et al., 2011, Zhou et al., 2015). This is evident both during cognitive task performance (Brandt et al., 2015, Repovs and Barch, 2012) and rest (Alonso-Solis et al., 2012, Kaufmann et al., 2015, Manoliu et al., 2014), providing support for the brain dysconnectivity hypothesis of SZ (Stephan et al., 2009). Optimal task performance depends on efficient suppression of the DMN (Buckner et al., 2008, Harrison et al., 2007), in accordance with reports of reduced task-related suppression of the DMN in SZ (Guerrero-Pedraza et al., 2012, Kim et al., 2009, Nygard et al., 2012, Schneider et al., 2011, Whitfield-Gabrieli et al., 2009, Williamson and Allman, 2012). Both task-positive and task-negative networks are modulated by cognitive load (Alnaes et al., 2015, Fryer et al., 2013, Newton et al., 2011, Repovs and Barch, 2012), and it has been suggested that SZ patients show less flexible resource allocation during the dynamic transitions between rest and task, partly due to an hyperactive DMN (Nygard et al., 2012). Yet, more knowledge is needed to clarify whether SZ spectrum disorders are primarily associated with DMN increases or decreases compared to controls (Fryer et al., 2013, Newton et al., 2011, Repovs and Barch, 2012). It also remains to be determined if altered task-related suppression of the DMN generalizes across tasks (Brandt et al., 2015, Repovs and Barch, 2012). Finally, it is unclear whether TPN and DMN dysfunction in SZ spectrum disorders represents independent markers.
Thus, in order to answer these questions, our main aims were to compare load-dependent recruitment of TPN and DMN between patients with SZ spectrum disorders and healthy controls (HC) in two tasks designed to probe executive functions. Secondly, in order to assess if TPN and DMN recruitment reflects independent mechanisms, we tested for associations between load-dependent activation in TPN and DMN across tasks. Based on previous research and the pervasiveness of the clinical symptoms of psychotic disorders we hypothesized that patients with a schizophrenia spectrum diagnosis would show reduced load-dependent DMN deactivation in both tasks, reflecting a generalized dysfunction. Further, based on previous work on the neurocognitive specificity of various brain networks, we hypothesized that degree of task-related activation of the TPN and DMN are only weakly associated, and therefore may represent two independent markers of brain function.
2. Methods and materials
2.1. Participants
Twenty-nine patients diagnosed with SZ spectrum disorders in the early phase of illness, defined as less than five years since starting their first adequate treatment (14 SZ, 4 schizophreniform, 5 schizoaffective disorder, and 6 psychosis not otherwise specified; NOS), and 21 HC participants were included. The HC group was randomly selected from the same catchment area as the patients using population records, and was matched on age and sex at a group level. The study is part of the ongoing Thematically Organized Psychosis (TOP) Study at the University of Oslo and Oslo University hospital, and is approved by the Regional Committee for Medical Research Ethics and the Norwegian Data Protection Authority. All participants gave written informed consent.
Diagnostic assessment was based on the Structured Clinical Interview for DSM-IV Axis I disorders (SCID-I; (First et al., 1995)) and symptom assessment on the Positive and Negative Syndrome Scale (PANSS; (Kay et al., 1987)). Physicians or clinical psychologists administered the clinical interviews. Symptoms were assessed within 2 weeks prior to MRI (mean, 12 days; SD, 10), and patients were asked on the day of scanning if they had experienced recent changes in symptoms. IQ was assessed using the vocabulary and matrix reasoning subtests from Wechsler Abbreviated Scale of Intelligence (WASI, (Wechsler, 2007)).
Age at onset was calculated as the age of the first SCID-verified psychotic episode. Duration of untreated psychosis (DUP) was calculated as the time in weeks from first psychotic symptoms (the first week with PANSS score of four or above on at last one item of the Positive Scale items 1, 3, 5, 6 or general item 9; mean: 21 years; SD: 4.0) until start of first adequate treatment (antipsychotic medication in sufficient amount and duration, or hospitalization for the treatment of psychotic symptoms). Medication use is reported as current usage of one or more antipsychotic drugs, and defined daily dose (DDD, (http://www.whocc.no/)). Alcohol and drug usage was reported using the Alcohol Use Disorders Identification Test and Drug Use Disorders Identification Test (AUDIT/DUDIT, (Berman et al., 1986, Saunders et al., 1993)).
Common exclusion criteria were neurological disorders, traumatic brain injury, IQ < 70, and MRI contraindications. HC were screened with the Primary Care Evaluation of Mental Disorders for depressive symptoms (Spitzer et al., 1994) and questionnaires for drug and alcohol use. Those with a history of severe substance use the last year, a lifetime history of psychosis or major depression, or a first-degree relative with mental illness were excluded.
Fifty-six participants were initially included. Six participants were excluded from the analyses; 3 (2 patients, 1 control) due to excess head motion in the scanner (defined as 3 SD > group mean), 1 patient due to performance below chance level (defined as < 25% accuracy in the Tower of London task), 1 patient due to missing behavioral data, and 1 control due to incidental neuroradiological findings.
2.2. Experimental designs measuring executive performance
2.2.1. Tower of London
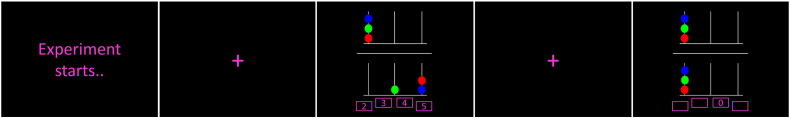
We used a computerized version of the Tower of London task (Shallice, 1982), which is widely used to assess aspects of planning and problem-solving (see Fig. 1 for details). Briefly, the task involves movement of colored balls on three pegs, and the subjects were instructed to calculate the minimum number of moves from an initial state to a goal configuration. The task was designed as a blocked design with two conditions, one problem-solving Tower of London (ToL) condition (problems involving 2–5 moves) and one control task (CT) condition (upper and lower halves of the image were identical). There were six repetitions of each condition, and the duration of each block was 32 s, with continuous problems to solve in the ToL condition. The CT condition comprised 4 tasks, each of 8 s duration. In between every block there was a baseline rest condition (fixation cross) of 16 s duration.
Fig. 1.
Schematic representation of a typical task timeline. The upper half of the screen contains a goal configuration of three different colored balls on three pegs. The participant was instructed to mentally calculate the minimum number of moves required to reach the goal configuration, given the distribution of balls in the lower half of the image, moving one ball at a time. The session consisted of problems involving 2 to 5 moves, interleaved with control trials in which the upper and lower halves of the image were identical (“zero move”). There were four alternative answers, and participants indicated their response by button presses of thumb and index fingers on both hands. In the control task, the participants were instructed to indicate the location of the zero blinks with a button press. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.2.2. Spatial working memory
Fig. 2 provides a description of the spatial working memory task (SWM). Briefly, the task comprised 4 different load conditions, with 1, 3, 4 or 5 black and white drawings of neutral objects (Snodgrass and Vanderwart, 1980) presented consecutively at one of eight possible locations on screen. Task instruction was to remember every drawing-by-location presented, followed by a test indicating by button press if a target drawing was presented in the same location during encoding. Load 1 was the CT condition with one presented drawing - and always in the same central positions. We included 18 blocks per load condition, in total 72 blocks, each followed by a fixation cross of 3 to 5 s duration (jittered). The task block duration was between 6.05 to 9.65 s, depending on the number of drawings presented (load).
Fig. 2.
The task instruction was to remember every drawing-by-location presented along in an encoding phase, followed by a test where participants were instructed to respond either yes or no to whether a particular drawing was presented in the same location as during the encoding phase. Load conditions involving 3–5 drawings were presented randomly in sequential order along with a control condition in which the drawings were always the same (load 1). Answers (right, wrong) were indicated by button presses of right and left index finger.
The experimental paradigms were designed using the E-Prime software (Psychology Software Tools Inc., Pittsburgh, Pennsylvania, USA), and presented in the scanner using video goggles, while responses were collected using response grips (VisualSystem and ResponseGrip, Nordic Imaging Lab, Bergen, Norway). The participants were given task instructions and training prior to the scanning session to ensure that they understood the task properly. All participants completed the same amount of training for both tasks, yet they did not receive any feedback as to how well they performed. In addition, participants practiced the use of the response grips inside the scanner and received a brief recapitulation of the task instruction.
2.3. MRI acquisition
Imaging was performed on a 3T General Electric Sigma HDxt scanner (GE Healthcare, Milwaukee, WI, USA). For functional imaging, 36 contiguous axial 3.5 mm thick slices, with a 0.5 mm gap, covering the whole brain were acquired using a T2*-sensitive echo-planar imaging (EPI) sequence (TR = 2000 ms; TE = 25 ms; FA = 90° (ToL)/78° (SWM); FOV = 256 mm; matrix size 64 × 64). Three volumes were discarded prior to analyses. For registration purposes, T1-weighted FSPGR BRAVO data (248 contiguous axial 1.2 mm thick slices; TR = 10.9 s; TE = 4.6 s; FA = 13°; FOV: 240 × 240 mm; matrix size 352 × 224) were collected.
2.4. MRI processing and voxel-wise analysis
FreeSurfer (http://surfer.nmr.mgh.harvard.edu) was used to process the T1-weighted images, including surface reconstruction and full brain segmentation (Fischl et al., 2002) to obtain precise brain extracted volumes for registration.
Functional images were processed using FEAT, part of FSL (FMRIB's Software Library, http://www.fmrib.ox.ac.uk/fsl; (Jenkinson et al., 2012)). fMRI processing included motion correction (MCFLIRT, (Jenkinson et al., 2002)) non-brain removal (Smith, 2002), temporal high pass filtering (ToL: 128 s; SWM: 100 s), spatial smoothing with a Gaussian kernel with full width at half maximum (FWHM) of 8 mm. FMRIB's Nonlinear Image Registration Tool (FNIRT, (Andersson et al., 2007a, Andersson et al., 2007b)) was used to align the functional volumes to the Montreal Neurological Institute (MNI) 152 standard space, using the T1-weighted scan as an intermediate. In addition, we used boundary based registration (BBR, (Greve and Fischl, 2009)) on all datasets to improve the functional to structural space registrations, except in 2 cases where we performed the registration without the BBR option due to a technical failure.
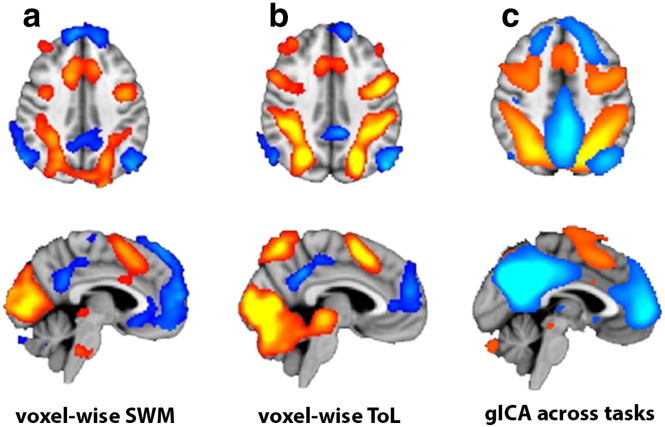
GLMs modeling task-related activation and deactivation for the experimental conditions, with the fixation periods as implicit baselines, were specified for each subject and independently for ToL and SWM. The models included independent regressors for each of the experimental conditions (ToL: CT and 2–5 moves; SWM: CT and 3–5 images) and the subject specific motion parameters and their derivatives and squares were included as nuisance regressors. For both tasks, contrasts estimating average task load versus the control conditions were calculated (ToL: 2–5 moves > rest; SWM: 3/4/5 images [0.33, 0.33, 0.33] > rest). Whereas the main hypothesis targeted the independent component time series (see below), we also performed voxel-wise analyses in order to enable a direct comparison between the relevant components and the voxel-wise main effects of load in the two tasks. In order to compare with the ICA spatial maps, the individual level contrast parameter estimates (COPEs) were submitted to higher-level whole brain random effects analyses, testing for main effects of high load versus fixation across subjects using mixed effects FLAME 1 + 2 and automatic outliers de-weighting (Beckmann et al., 2003, Woolrich, 2008).
2.5. Independent component analysis (ICA and dual regression)
Group ICA was performed using Multivariate Exploratory Linear Optimized Decomposition into Independent Components (MELODIC, (Beckmann and Smith, 2004)) using a temporal concatenation approach. To avoid a possible bias in the decomposition due to uneven sample sizes, an age and gender matched sub-sample comprising SWM and ToL runs from 20 patients and 20 controls were used for the initial decomposition.
The model order was set to 40, of which spatial maps and time frequency characteristics were inspected. Two components reflecting the canonical DMN and TPN, respectively, were selected for further analyses (Fig. 1c). Next, we used dual regression (Filippini et al., 2009) to generate subject specific maps and associated time series from the group average spatial maps. Dual regression time series were submitted to time series modeling using the same individual level GLM design matrices as used for the voxel-wise analysis. Individual contrast parameter estimates were calculated for both networks in high (ToL (2–5) - rest, SWM (3–5) - rest) and low (ToL CT - rest, SWM CT - rest) load task conditions in the two runs. Also, difference scores were calculated reflecting load-dependent activation (high - low load) of TPN and DMN, respectively.
2.6. Statistical analysis
Statistical analyses were conducted using the Statistical Package for the Social Science (SPSS) for Windows, version 20.0 (SPSS Inc., Chicago, IL, USA) and MATLAB (R2014b, MathWorks, Inc., Natrick, MA, US). Chi-square analysis compared groups on categorical variables, and group differences on continuous variables were investigated using t-tests, analyses of variance (ANOVA), or analyses of co-variance (ANCOVA).
Task accuracy and reaction time (RT) were investigated using repeated measures ANOVAs within each task, with load (high, low) as within-subject factor and group (patient, control) as between-subject factor. Group differences in accuracy and RT were further investigated in the different load conditions using independent sample t-tests.
Our main analysis tested for effects of group on load-dependent activation (difference scores: high - low load) of each of the networks using repeated measures ANCOVAs with task (ToL, SWM) as within-subject factor, and group (patient, control) as between subject factor, and age and sex as covariates. The statistical threshold was set to p < 0.025, corresponding to a Bonferroni alfa level correction for two tests (DMN, TPN). For components showing main effect of group in the repeated measures analysis, ANCOVAs covarying for sex and age were performed within tasks (ToL, SWM).
In subsequent descriptive analyses we used one-sample t-tests within groups to estimate the IC parameters model fit with the task design (high, low loads, see Supplementary). Effects of load were estimated in the two networks using one-sample t-tests on the difference score (see Supplementary).
To address possible confounding effects and their influence on the main results, additional ANCOVAs were performed, including in-scanner motion (displacement from one timepoint to the next) task performance (ACC, RT), IQ and education level as covariates in the model (one at the time) in addition to age and sex. Further, we tested for associations between clinical variables and network activation within patients using ANCOVAs with the difference score as dependent variable, adding PANSS total score (composite of positive, negative, and general score), DDD, AUDIT and DUDIT on at the time as covariate, in addition to and age and sex. For all post hoc analyses, the statistical threshold was set to a nominal p ≤ 0.05.
Finally, in order to assess the generalizability of the activation patterns we used Pearson correlation analysis to investigate associations between the parameter estimates in the two networks and tasks. The statistical threshold was set to p < 0.0125, corresponding to a Bonferroni correction for four tests. For transparency, and in order to facilitate comparisons with previous and future studies, both uncorrected p values and Bonferroni corrected p values will be reported throughout the manuscript.
3. Results
3.1. Demographics
Table 1 summarizes demographic and clinical characteristics. Briefly, there were no group differences in age (t(48) = 1.0 p = 0.343) or sex distribution (χ2[1, n = 184] p = 0.668). There were significant differences in IQ (t(44) = 2.4, p = 0.02) and education (t(47) = 2.7, p = 0.011), with lower levels in patients.
Table 1.
Demographics and clinical characteristics.
| Demographics | SZ | HC | Group comparison |
|---|---|---|---|
| Age | 25.0(5.2a) | 26.5 (5.6a) | t(48) = 0.957 p = 0.343 |
| Education (n) | 12.2 | 13.8 (19) | t(47) = 2.663 p = 0.011 |
| Hand n (% right) | 28 (96.6) | 19 (90.5) | |
| IQ (n) | 101.4 (27) | 110.5 (19) | t(44) = 2.422 p = 0.020 |
| Gender (male) n (%) | 22(75.9) | 17 (81.0) | χ2(1, n = 184) p = 0.668 |
| Clinical characteristics | |||
| Age at onset | 21 (4.0a) | ||
| DUP weeks (n25) | 10b (88c) | ||
| Diagnoses | |||
| Schizophrenia | 14 | ||
| Schizophreniform | 4 | ||
| Schizoaffective | 5 | ||
| Other psychosis | 6 | ||
| Comorbid disorders; n (%) | |||
| Depression | 3 (10.3) | ||
| Substance abuse | 3 (10.3) | ||
| Current symptoms n (%) | |||
| PANSS positive score | 11.9 (4.4a) | ||
| PANSS negative score | 13.1 (4.9a) | ||
| PANSS g score | 26.6 (6.7) | ||
| PANSS total | 52.0 (13.5) | ||
| Medication | |||
| Antipsychotic n (%) | 26 (89.7) | ||
| Months on antipsychotic medication | 5.7(5.1) | ||
| DDD; mean | 1.3 (0.6a) | ||
| Antidepressant n (%) | 6 (20.1) | ||
| DDD; mean | 1.3 (0.4a) | ||
| Anxiolytics n (%) | 1(3.4) | ||
| DDD; mean | 0.9 | ||
| Antiepileptics n (%) | 2(6.9) | ||
| DDD; mean | 0.3 (0.1a) | ||
| Current drug usage (DUDIT, n) | 4.8 (7.7) | ||
| Current alcohol abuse (AUDIT, n) | 5.5 (6.1) | ||
Note, SZ: schizophrenia; HC: healthy controls; PANSS: positive and negative syndrome scale; DUP: duration of untreated psychosis; DDD: defined daily dose; AUDIT/DUDIT: alcohol/drug use disorders identification test.
Standard deviation.
Median.
Interquartile range.
3.2. Task performance
We found significant effects of load both on accuracy and RT (ToL accuracy: F(1,48) = 93.3, p < 0.001; ToL RT: F(1,44) = 412.6, p < 0. 001; SWM accuracy: F(1,48) = 161.2, p < 0.001; SWM RT: F(1,48) = 696.9, p < 0.001), indicating decreasing accuracy and increasing RT with increasing load. Compared to controls, patients revealed significantly reduced accuracy (F(1,48) = 11.6, p = 0.001) but no differences in RT (F(1,48) = 0.0, p = 0.848) in SWM. In ToL, patients showed increased RT compared to controls (F(1,44) = 12.4, p = 0.001), but no group differences were observed in accuracy (F(1,48) = 0.2, p = 0.665). In addition, we found a significant load x group interaction effect on accuracy in SWM (F(1,48) = 7.6, p = 0.008), indicating decreasing accuracy in patients with increasing load in comparison to the control group. We also observed a load x group interaction effect on RT in both tasks (ToL RT: F(1,44) = 14.7, p < 0.001; SWM RT: F(1,48) = 8.2, p = 0.006) (Table A.1a,b).
3.3. Voxel-wise analyses – main effects of load conditions
Fig. 3 (uncorrected t > 2) and Table A.2 show the main effects for the contrast high load > rest in the two tasks. Briefly, we observed highly overlapping task-related activation patterns across tasks including the paracingulate gyrus, precentral gyrus, middle frontal gyrus, superior frontal gyrus, and the superior parietal lobe and lateral occipital cortex. We found overlapping deactivation in the lateral parietal cortices, temporal lobe, posterior cingulate gyrus, precuneus, and dorso- and ventromedial frontal regions including the paracingulate and cingulate gyri.
Fig. 3.
Main effects of task conditions a) Results from the voxel-wise GLM analysis showing task activations and deactivations in SWM (a) and ToL (b) (uncorrected t-stats, | t | > 2). c) Group ICA spatial maps reflecting the TPN (hot colors) and DMN (cold colors). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.4. ICA analysis
Fig. 3c shows the group ICA spatial maps representing the TPN and DMN. Fig. S1 summarizes the IC parameter estimates obtained from the two tasks for each group. Briefly, one-sample t-tests revealed significant (0.05/16 = p < 0.003, Bonferroni corrected) main effects of all conditions in both tasks except for the DMN in the low load condition in ToL in controls (t = − 1.54, p = 0.140), and in low SWM load in patients (t = − 2.0, p = 0.054) and controls (t = 3.0, p = 0.007). As expected, the marginal means were negative for the DMN and positive for the TPN, suggesting negative and positive task-related modulations, respectively (Fig. A.1).
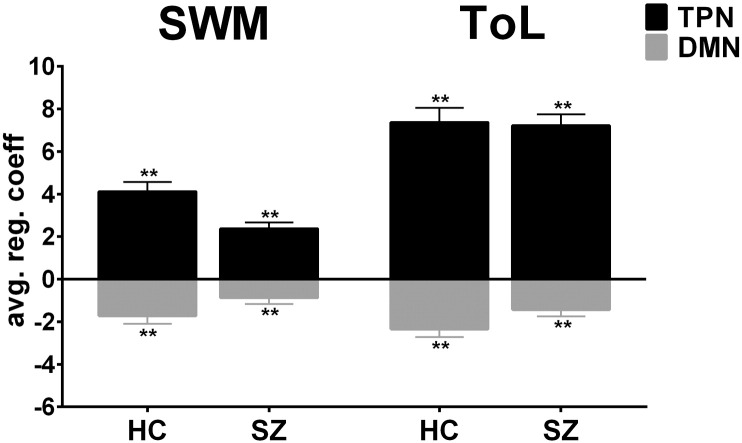
3.5. Load-dependent activation in TPN and DMN
Fig. 4 shows the load-dependent activation of TPN and DMN for both groups and tasks, as measured using the difference scores (high - low load). One-sample t-tests revealed significant (0.05/8 = p < 0.006, Bonferroni corrected) main effects of load on the parameter estimates in both groups, tasks and networks, indicating robust load-modulation across brain networks, tasks, and groups.
Fig. 4.
Main effects on large-scale brain networks revealed by ICA. Regression coefficients difference between high and low (high - low) load conditions within groups in TPN and DMN. One-sample t-tests revealed significant main effects (p < 0.006, Bonferroni corrected) of load on the parameter estimates within groups, tasks and networks.
Repeated measures ANOVA testing for load-dependent associations (high - low load) in DMN across tasks (TOL and SWM, within factor) and group (patients and controls, between factor) revealed no effects of task (F(1,48) = 3.7, p = 0.060, ηp2 = 0.08), and no interaction (F(1,48) = 0.2, p = 0.697, ηp2 = 0.0), but a main effect of group (F(1,48) = 5.6, p = 0.022, ηp2 = 0.11, significant after Bonferroni correction, alpha level of p < 0.025), indicating an overall reduced load-dependent deactivation in patients compared to controls across tasks. Post hoc ANCOVAs for DMN within task revealed group differences in ToL (t(46) = − 2.2, p = 0.034), and trend effects in SWM (t(46) = − 1.8, p = 0.088).
For TPN, repeated measures ANOVA revealed a trend effect of task (F(1,48) = 4.1, p = 0.049, ηp2 = 0.08) and a trend group by task interaction (F(1,48) = 4.9, p = 0.031, ηp2 = 0.10) (not significant after Bonferroni correction alpha level of p < 0.025), and no main effects of group (F(1,48) = 3.1, p = 0.085, ηp2 = 0.06), indicating a trend for stronger load-dependent TPN activation in ToL compared to SWM. Explorative post hoc ANCOVAs revealed group differences in SWM (t(46) = 3.0, p = 0.005), and no differences in ToL (t(46) = − 0.1, p = 0.936) in TPN.
3.6. Confounding effects related to age, sex, motion, behavioral performance, IQ and education
There was no effect of age or sex on the main group effects. Including in-scanner head motion in the model did not influence the results, and there were no unique effects of motion on load-dependent activation in either task or network. Task accuracy showed moderate unique associations with TPN activation in ToL (t = 2.0, p = 0.053) and SWM (t = 3.2, p = 0.002), indicating higher accuracy with stronger load-dependent activation. There was no unique effect of RT on load-dependent activation. There were no unique effects of IQ or education on load dependent activation in either network or task, but the effect of group on DMN deactivation disappears when controlling for IQ in ToL (t = − 1.4, p = 0.181). Furthermore, the trend level group effect on DMN deactivation in SWM becomes weaker when controlling for IQ (t = − 0.8, p = 0.437) and education (t = − 1.4, p = 0.182). (Table A.3a,b).
3.7. Clinical confounders
DMN deactivation showed a trend association with medication dosage in ToL (t = 1.9, p = 0.075, ηp2 = 0.12), but not in SWM (t = 0.1, p = 0.910), indicating a tendency for decreased activation with increased medication use in ToL. There were no association between DMN activation and symptom severity (as measured with the PANSS total score) in ToL (t = − 1.2, p = 0.245) or in SWM (t = 0.5, p = 0.656), nor any association between DMN activation and substance use (ToL: t = − 1.3, p = 0.194; SWM: t = − 1.0, p = 0.318) or alcohol use (ToL: t = − 0.4, p = 0.720; SWM: t = − 1.3, p = 0.197). For TPN there were no associations with medication dosage (ToL: t = − 0.4, p = 0.684; SWM: t = − 0.6, p = 0.528), symptom severity (ToL: t = − 0.3, p = 0.736; SWM: t = − 1.3, p = 0.205) drug (ToL: t = − 0.3, p = 0.795; SWM: t = − 0.3, p = 0.753) or alcohol use (ToL: t = − 0.7, p = 0.525; SWM: t = 0.2, p = 0.825).
3.8. Correlations within and between components across tasks and groups
We found moderate correlations between load-dependent activation (high - low load) of the TPN and the DMN both in ToL (r = − 0.28, p = 0.051,), and SWM (r = − 0.27, p = 0.054), indicating that subjects with a strong load-dependent activation in TPN only to a moderate extent showed a strong load-dependent deactivation of the DMN within tasks. The correlation was slightly stronger in patients in SWM (r = − 0.442, p = 0.016), while in ToL the correlation was strongest in the controls (r = − 0.479, p = 0.028).
For DMN, we observed a positive correlation between tasks (r = 0.41, p = 0.003, significant after Bonferroni correction, alpha level of p < 0.0125), indicating that subjects with strong load-dependent deactivation during ToL tended to show strong deactivation during SWM. For TPN, similar analysis revealed no relationship across task and group (r = 0.19, p = 0.183), but a moderate positive correlation was observed within SZ (r = 0.42), indicating that patients with a strong load-dependent activation in ToL tended to show a strong load-depended activation in SWM.
4. Discussion
We investigated cognitive load-dependent activations and deactivations of two canonical task-related brain networks in patients with SZ spectrum disorders and HC during two executive tasks. There are three main findings. First, we found reduced deactivation of DMN in patients across tasks. Second, load-dependent TPN activation was reduced in SZ compared to HC in SWM. Lastly, whereas DMN deactivation was relatively reliable across tasks, we found only moderate associations between load-dependent activation of TPN and DMN, indicating that they reflect partly independent mechanisms.
4.1. Load-dependent activation across tasks and networks
Overall, our findings are in agreement with previous reports showing reduced deactivation of the DMN in SZ (Anticevic et al., 2011, Guerrero-Pedraza et al., 2012, Pomarol-Clotet et al., 2008, Schneider et al., 2011, Whitfield-Gabrieli et al., 2009). Yet, few studies have tested the generalizability across tasks in the same sample. Schneider et al. (2011) reported stronger DMN deactivation during low load and weaker in high load for SZ compared to controls. They were, however, unable to replicate this pattern across different goal-directed tasks, concluding that patients show decreased differentiation between reference (low loads) and experimental state in task-specific brain regions. We found reduced load-dependent deactivation of DMN in SZ across tasks, with significant effects in ToL and trend effects in SWM. However, our findings of increased deactivation in the low load condition in ToL and decreased deactivation in the high load condition in SWM in SZ (see Appendices section) may indicate a more complex pattern including some task-dependency, which may partly explain the heterogeneity in previous reports (Harrison et al., 2007, Hasenkamp et al., 2011, Mannell et al., 2010, Repovs and Barch, 2012, Schneider et al., 2011, Whitfield-Gabrieli et al., 2009). In addition, task difficulty and length of block could potentially influence the degree of deactivation. It has previously been shown that task-induced deactivation increases with longer response time and task difficulty (Harrison et al., 2007, McKiernan et al., 2006, McKiernan et al., 2003, Weissman et al., 2006). We observed increased DMN deactivation in the low ToL load in patients, which may indicate that SZ patients allocate more cognitive resources during low load conditions, possibly yielding a reduced potential for additional deactivation when increasing load (Fryer et al., 2013, Schneider et al., 2011), reflecting less efficient and flexible shifts in cognitive resources (Weissman et al., 2006).
Nygard et al. (2012) suggested that patients with SZ show both reduced up-regulation of TPN and reduced down-regulation of the DMN. This is partly in line with our findings of reduced load-dependent TPN activation in the SWM task. Compared to HC, patients with SZ showed reduced load-dependent activation in TPN, and decreased performance during SWM. In contrast, we observed no activation or accuracy differences in ToL. Hence, across-task generalizability of TPN differences between SZ and HC may partly depend on behavioral differences.
Several studies have investigated brain function during working memory tasks in SZ, generally reporting reduced task performance, and both prefrontal hypoactivation as well as medial and temporal hyperactivation (Callicott et al., 2000, Callicott et al., 2003, Glahn et al., 2005, Landin-Romero et al., 2015, Pomarol-Clotet et al., 2008). Similar findings of hypo- and hyper-activity in specific areas have been observed in ToL (Beauchamp et al., 2003, Liemburg et al., 2015, Rasser et al., 2005). Along this line, prefrontal hypo- and hyper activation in SZ might partly reflect altered recruitment and connectivity of large-scale task-positive and task-negative brain networks. Yet, few studies have investigated TPN dysfunction in SZ across rest and different tasks (Repovs and Barch, 2012). ToL is a complex task implicating several cognitive domains, including working memory, which is required for holding and counting moves ahead, in addition to planning and problem solving. Our current lack of group effects in ToL could therefore also represent involvement of multiple cognitive processes in extended TPN areas (Cole et al., 2014).
4.2. Association with possible confounders
Previous studies have reported an association between DMN deactivation and task performance in healthy individuals (Anticevic et al., 2012, Anticevic et al., 2011) and in patients with SZ (Whitfield-Gabrieli et al., 2009), and it has been argued that reduced deactivation of DMN may be confined to tasks where performance is impaired in patients (Schneider et al., 2011). We found no association between load-dependent deactivation of the DMN and task accuracy. For TPN we observed that task accuracy had a unique effect on load-dependent activation in both tasks, indicating that higher accuracy is associated with stronger load-dependent activation in these tasks. Also, the observed group differences in brain activation mirrored the performance differences in SWM, i.e. SZ patients exhibited both reduced accuracy and load-dependent activation of TPN compared to HC. This is in accordance with one previous finding of associations between reduced activations in TPN regions, including the dorsolateral prefrontal cortex, and impaired performance in patients (Pomarol-Clotet et al., 2008). These results indicate that task performance is associated with load-dependent activation, and suggest that performance differences may partly explain the reduction in load-dependent activation in patients.
Furthermore, as expected the group effects on DMN become weaker when controlling for IQ and education. However, the inherent associations between severe mental illness, cognitive functioning and brain activation, respectively, make it very difficult to disentangle one from the other.
Several previous studies have demonstrated influence of in-scanner motion, age, and gender on network activation and connectivity (Filippi et al., 2013, Mowinckel et al., 2012, Zeng et al., 2014). We found no significant associations between brain network activation and any of these variables. Further, there were no associations between load-dependent DMN or TPN activation and symptom severity as measured with PANSS in our patient sample, which is in line with several previous studies (Guerrero-Pedraza et al., 2012, Landin-Romero et al., 2015, Nygard et al., 2012). Also, whereas others have reported relationships between medication dosage and task-induced deactivations (Schneider et al., 2011) and connectivity (Brandt et al., 2015) in patients, and usage of antipsychotic medication has been related to modulation of DMN connectivity (Sambataro et al., 2010), we found no significant relationship between network activation and medication dosage, alcohol or drug use.
Summing up, these results indicate that load-dependent activation of TPN and DMN is not substantially affected by clinical characteristics within patients, nor by in-scanner subject motion or any demographic variables including IQ within the total sample. Task accuracy was, however, associated with load-dependent activation of TPN, implying that cognitive performance and SZ might be mediated by overlapping mechanisms. In contrast, the reductions in DMN deactivations in SZ were seen independently of task performance differences, implying that the observed effect is not merely an epiphenomenon of reduced task performance in patients.
4.3. Association between task-positive and default mode network
Strong task-related activation and deactivation of the TPN and DMN, respectively, have often been associated with the functioning of a healthy brain (Fox et al., 2005), yet the interdependencies between these metrics have rarely been explicitly tested (Dørum et al., 2016). Therefore, the final aim of the current study was to assess the relative independence of the activation patterns of the two large-scale brain networks by testing for relationships between load-dependent activation of the TPN and DMN. We found only moderate correlations between load-dependent activation of the TPN and DMN within tasks in accordance with Dørum et al. (2016) finding in healthy individuals. This may be due to the task difficulty (McKiernan et al., 2003) or it could relate to variable performance within the sample. It has been shown that stronger negative correlation between TPN and DMN is associated with less variable behavior in healthy individuals (Kelly et al., 2008), implying that TPN connectivity may partly contribute to the modulation of DMN efficiency during cognitive performance (Mannell et al., 2010). Further, we found no relationship in the level of TPN activation between tasks across groups. For DMN we found a positive relationship between tasks, both across and within groups, implying that the recruitment of the DMN is relatively generalizable across different executive tasks, supporting its use as an intermediate neuroimaging phenotype.
4.4. Study limitations
Limitations of the current study include that most patients were on antipsychotic medication, and that the groups were unevenly matched on IQ and education. The potential effects of antipsychotic medications on network activity are difficult to disentangle using the present study design because of the inherent collinearity between the different types, dosage, symptom severity, and diagnosis. Reduced cognitive function likely reflects pathophysiological mechanisms, which complicates statistical corrections for such differences since it is difficult to statistically isolate any unique associations with IQ and education from disease processes.
5. Conclusions
In summary, our analysis revealed reduced load-dependent DMN deactivation across two executive tasks in patients with SZ compared to healthy controls. Further, we found that load-dependent TPN activation was reduced in SZ compared to HC in SWM, and associated with task performance in both tasks. Finally, whereas DMN deactivation was relatively reliable across tasks, we found only moderate associations between the degree of network-level activation of TPN and DMN, implying that the two brain networks reflect partly independent mechanisms.
Acknowledgements and funding
We are very thankful for the patients and volunteers who participated in this study. In addition, our recognitions go to the clinicians and psychologists who contributed with patient recruitment. Special thanks go to Thomas Bjella, Seyran Khalili, and Jeanette Haatveit for technical assistance, Anne-Hilde Farstad and the staff at the Department of Radiology and Nuclear Medicine, and Kristina Skåtun, Siren Tønnesen, June Lystad, and Marte Tandberg for recruitment. Funding was provided by the Research Council of Norway (#421716, #223273, #204966/F20), the South-East Norway Health Authority (#N1, #2011085, #2013123, #52026, #2014-097), and the Kristian Gerhard Jebsen Foundation (#SKGJ-2011-36).
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2016.08.012.
Appendix A. Supplementary data
Supplementary material.
References
- Alnaes D., Kaufmann T., Richard G., Duff E.P., Sneve M.H., Endestad T., Nordvik J.E., Andreassen O.A., Smith S.M., Westlye L.T. Attentional load modulates large-scale functional brain connectivity beyond the core attention networks. NeuroImage. 2015;109:260–272. doi: 10.1016/j.neuroimage.2015.01.026. [DOI] [PubMed] [Google Scholar]
- Alonso-Solis A., Corripio I., de Castro-Manglano P., Duran-Sindreu S., Garcia-Garcia M., Proal E., Nunez-Marin F., Soutullo C., Alvarez E., Gomez-Anson B., Kelly C., Castellanos F.X. Altered default network resting state functional connectivity in patients with a first episode of psychosis. Schizophr. Res. 2012;139(1–3):13–18. doi: 10.1016/j.schres.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J.L., Jenkinson M., Smith S. FMRIB Technical Report TR07JA1. FMRIB Centre; Oxford (UK): 2007. Non-linear op misa on. [Google Scholar]
- Andersson J.L., Jenkinson M., Smith S. FMRIB Technical Report TR07JA2. Group of the University of Oxford; FMRIB Analysis: 2007. Non-linear registra on, aka Spa al normalisa on. [Google Scholar]
- Anticevic A., Repovs G., Barch D.M. Schizophr. Bull. 2011. Working memory encoding and maintenance deficits in schizophrenia: neural evidence for activation and deactivation abnormalities. (sbr107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A., Cole M.W., Murray J.D., Corlett P.R., Wang X.J., Krystal J.H. The role of default network deactivation in cognition and disease. Trends Cogn. Sci. 2012;16(12):584–592. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp M.H., Dagher A., Aston J.A., Doyon J. Dynamic functional changes associated with cognitive skill learning of an adapted version of the Tower of London task. NeuroImage. 2003;20(3):1649–1660. doi: 10.1016/j.neuroimage.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Beckmann C.F., Smith S.M. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans. Med. Imaging. 2004;23(2):137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Beckmann C.F., Jenkinson M., Smith S.M. General multilevel linear modeling for group analysis in FMRI. NeuroImage. 2003;20(2):1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Berman K.F., Zec R.F., Weinberger D.R. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. II. Role of neuroleptic treatment, attention, and mental effort. Arch. Gen. Psychiatry. 1986;43(2):126–135. doi: 10.1001/archpsyc.1986.01800020032005. [DOI] [PubMed] [Google Scholar]
- Bleuler E. 9 International Universities Press; 1950. Dementia Praecox or the Group of Schizophrenias; p. 1961. English version. [Google Scholar]
- Brandt C.L., Kaufmann T., Agartz I., Hugdahl K., Jensen J., Ueland T., Haatveit B., Skatun K.C., Doan N.T., Melle I., Andreassen O.A., Westlye L.T. Cognitive effort and schizophrenia modulate large-scale functional brain connectivity. Schizophr. Bull. 2015;41(6):1360–1369. doi: 10.1093/schbul/sbv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain's default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Callicott J.H., Bertolino A., Mattay V.S., Langheim F.J., Duyn J., Coppola R., Goldberg T.E., Weinberger D.R. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb. Cortex. 2000;10(11):1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- Callicott J.H., Mattay V.S., Verchinski B.A., Marenco S., Egan M.F., Weinberger D.R. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am. J. Psychiatr. 2003;160(12):2209–2215. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- Cole M.W., Repovš G., Anticevic A. The frontoparietal control system a central role in mental health. Neuroscientist. 2014;20(6):652–664. doi: 10.1177/1073858414525995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dørum E.S., Alnæs D., Kaufmann T., Richard G., Lund M.J., Tønnesen S., Sneve M.H., Mathiesen N.C., Rustan Ø.G., Gjertsen Ø., Vatn S., Fure B., Andreassen O.A., Nordvik J., Westlye L.T. Age-related differences in brain network activation and co-activation during multiple object tracking. Brain Behav. 2016 doi: 10.1002/brb3.533. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi M., Valsasina P., Misci P., Falini A., Comi G., Rocca M.A. The organization of intrinsic brain activity differs between genders: a resting-state fMRI study in a large cohort of young healthy subjects. Hum. Brain Mapp. 2013;34(6):1330–1343. doi: 10.1002/hbm.21514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini N., MacIntosh B.J., Hough M.G., Goodwin G.M., Frisoni G.B., Smith S.M., Matthews P.M., Beckmann C.F., Mackay C.E. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc. Natl. Acad. Sci. U. S. A. 2009;106(17):7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M., Spitzer R., Gibbon M., Williams J.B.W. 2nd ed. New York State Psychiatric Institute: Biometrics Research; New York, NY: 1995. Structured Clinical Interview for DSM-IV Axis I Disorders: Patient Edition (SCID-P) [Google Scholar]
- Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C., van der Kouwe A., Killiany R., Kennedy D., Klaveness S., Montillo A., Makris N., Rosen B., Dale A.M. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U. S. A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer S.L., Woods S.W., Kiehl K.A., Calhoun V.D., Pearlson G.D., Roach B.J., Ford J.M., Srihari V.H., McGlashan T.H., Mathalon D.H. Deficient suppression of default mode regions during working memory in individuals with early psychosis and at clinical high-risk for psychosis. Front Psychiatry. 2013;4:92. doi: 10.3389/fpsyt.2013.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity A.G., Pearlson G.D., McKiernan K., Lloyd D., Kiehl K.A., Calhoun V.D. Aberrant “default mode” functional connectivity in schizophrenia. Am. J. Psychiatry. 2007;164(3):450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- Glahn D.C., Ragland J.D., Abramoff A., Barrett J., Laird A.R., Bearden C.E., Velligan D.I. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum. Brain Mapp. 2005;25(1):60–69. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve D.N., Fischl B. Accurate and robust brain image alignment using boundary-based registration. NeuroImage. 2009;48(1):63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Pedraza A., McKenna P.J., Gomar J.J., Sarro S., Salvador R., Amann B., Carrion M.I., Landin-Romero R., Blanch J., Pomarol-Clotet E. First-episode psychosis is characterized by failure of deactivation but not by hypo- or hyperfrontality. Psychol. Med. 2012;42(1):73–84. doi: 10.1017/S0033291711001073. [DOI] [PubMed] [Google Scholar]
- Harrison B.J., Yucel M., Pujol J., Pantelis C. Task-induced deactivation of midline cortical regions in schizophrenia assessed with fMRI. Schizophr. Res. 2007;91(1–3):82–86. doi: 10.1016/j.schres.2006.12.027. [DOI] [PubMed] [Google Scholar]
- Hasenkamp W., James G.A., Boshoven W., Duncan E. Altered engagement of attention and default networks during target detection in schizophrenia. Schizophr. Res. 2011;125(2–3):169–173. doi: 10.1016/j.schres.2010.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T.R. Rethinking schizophrenia. Nature. 2010;468(7321):187–193. doi: 10.1038/nature09552. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E., Woolrich M.W., Smith S.M. Fsl. Neuroimage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Kahn R.S., Keefe R.S. Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psychiatry. 2013;70(10):1107–1112. doi: 10.1001/jamapsychiatry.2013.155. [DOI] [PubMed] [Google Scholar]
- Kaufmann T., Skatun K.C., Alnaes D., Doan N.T., Duff E.P., Tonnesen S., Roussos E., Ueland T., Aminoff S.R., Lagerberg T.V., Agartz I., Melle I.S., Smith S.M., Andreassen O.A., Westlye L.T. Disintegration of sensorimotor brain networks in schizophrenia. Schizophr. Bull. 2015 doi: 10.1093/schbul/sbv060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The positive and negative symptome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kelly A.M.C., Uddin L.Q., Biswal B.B., Castellanos F.X., Milham M.P. Competition between functional brain networks mediates behavioral variability. NeuroImage. 2008;39(1):527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kim D.I., Manoach D.S., Mathalon D.H., Turner J.A., Mannell M., Brown G.G., Ford J.M., Gollub R.L., White T., Wible C., Belger A., Bockholt H.J., Clark V.P., Lauriello J., O'Leary D., Mueller B.A., Lim K.O., Andreasen N., Potkin S.G., Calhoun V.D. Dysregulation of working memory and default-mode networks in schizophrenia using independent component analysis, an fBIRN and MCIC study. Hum. Brain Mapp. 2009;30(11):3795–3811. doi: 10.1002/hbm.20807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landin-Romero R., McKenna P.J., Salgado-Pineda P., Sarro S., Aguirre C., Sarri C., Compte A., Bosque C., Blanch J., Salvador R., Pomarol-Clotet E. Failure of deactivation in the default mode network: a trait marker for schizophrenia? Psychol. Med. 2015;45(6):1315–1325. doi: 10.1017/S0033291714002426. [DOI] [PubMed] [Google Scholar]
- Liemburg E.J., Dlabac-De Lange J.J., Bais L., Knegtering H., van Osch M.J., Renken R.J., Aleman A. Neural correlates of planning performance in patients with schizophrenia—relationship with apathy. Schizophr. Res. 2015;161(2–3):367–375. doi: 10.1016/j.schres.2014.11.028. [DOI] [PubMed] [Google Scholar]
- Mannell M.V., Franco A.R., Calhoun V.D., Canive J.M., Thoma R.J., Mayer A.R. Resting state and task-induced deactivation: a methodological comparison in patients with schizophrenia and healthy controls. Hum. Brain Mapp. 2010;31(3):424–437. doi: 10.1002/hbm.20876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoliu A., Riedl V., Zherdin A., Muhlau M., Schwerthoffer D., Scherr M., Peters H., Zimmer C., Forstl H., Bauml J., Wohlschlager A.M., Sorg C. Aberrant dependence of default mode/central executive network interactions on anterior insular salience network activity in schizophrenia. Schizophr. Bull. 2014;40(2):428–437. doi: 10.1093/schbul/sbt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan K.A., Kaufman J.N., Kucera-Thompson J., Binder J.R. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J. Cogn. Neurosci. 2003;15(3):394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- McKiernan K.A., D'Angelo B.R., Kaufman J.N., Binder J.R. Interrupting the “stream of consciousness”: an fMRI investigation. NeuroImage. 2006;29(4):1185–1191. doi: 10.1016/j.neuroimage.2005.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowinckel A.M., Espeseth T., Westlye L.T. Network-specific effects of age and in-scanner subject motion: a resting-state fMRI study of 238 healthy adults. NeuroImage. 2012;63(3):1364–1373. doi: 10.1016/j.neuroimage.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Newton A.T., Morgan V.L., Rogers B.P., Gore J.C. Modulation of steady state functional connectivity in the default mode and working memory networks by cognitive load. Hum. Brain Mapp. 2011;32(10):1649–1659. doi: 10.1002/hbm.21138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygard M., Eichele T., Loberg E.M., Jorgensen H.A., Johnsen E., Kroken R.A., Berle J.O., Hugdahl K. Patients with schizophrenia fail to up-regulate task-positive and down-regulate task-negative brain networks: an fMRI study using an ICA analysis approach. Front. Hum. Neurosci. 2012;6:149. doi: 10.3389/fnhum.2012.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostby Y., Walhovd K.B., Tamnes C.K., Grydeland H., Westlye L.T., Fjell A.M. Mental time travel and default-mode network functional connectivity in the developing brain. Proc. Natl. Acad. Sci. U. S. A. 2012;109(42):16800–16804. doi: 10.1073/pnas.1210627109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson-Yeo W., Allen P., Benetti S., McGuire P., Mechelli A. Dysconnectivity in schizophrenia: where are we now? Neurosci. Biobehav. Rev. 2011;35(5):1110–1124. doi: 10.1016/j.neubiorev.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Pomarol-Clotet E., Salvador R., Sarro S., Gomar J., Vila F., Martinez A., Guerrero A., Ortiz-Gil J., Sans-Sansa B., Capdevila A., Cebamanos J.M., McKenna P.J. Failure to deactivate in the prefrontal cortex in schizophrenia: dysfunction of the default mode network? Psychol. Med. 2008;38(8):1185–1193. doi: 10.1017/S0033291708003565. [DOI] [PubMed] [Google Scholar]
- Raichle M.E., Snyder A.Z. A default mode of brain function: a brief history of an evolving idea. NeuroImage. 2007;37(4):1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. (discussion 1097–1089) [DOI] [PubMed] [Google Scholar]
- Rasser P.E., Johnston P., Lagopoulos J., Ward P.B., Schall U., Thienel R., Bender S., Toga A.W., Thompson P.M. Functional MRI BOLD response to Tower of London performance of first-episode schizophrenia patients using cortical pattern matching. NeuroImage. 2005;26(3):941–951. doi: 10.1016/j.neuroimage.2004.11.054. [DOI] [PubMed] [Google Scholar]
- Repovs G., Barch D.M. Working memory related brain network connectivity in individuals with schizophrenia and their siblings. Front. Hum. Neurosci. 2012;6:137. doi: 10.3389/fnhum.2012.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambataro F., Blasi G., Fazio L., Caforio G., Taurisano P., Romano R., Di Giorgio A., Gelao B., Lo Bianco L., Papazacharias A., Popolizio T., Nardini M., Bertolino A. Treatment with olanzapine is associated with modulation of the default mode network in patients with schizophrenia. Neuropsychopharmacology. 2010;35(4):904–912. doi: 10.1038/npp.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J.B., Aasland O.G., Babor T.F., De la Fuente J.R., Grant M. Development of the alcohol use disorders identification test (AUDIT). WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addict. Abingdon. 1993;88:791. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Schneider K. Grune & Stratton; New York: 1959. Clinical Psychopathology. [Google Scholar]
- Schneider F.C., Royer A., Grosselin A., Pellet J., Barral F.G., Laurent B., Brouillet D., Lang F. Modulation of the default mode network is task-dependant in chronic schizophrenia patients. Schizophr. Res. 2011;125(2–3):110–117. doi: 10.1016/j.schres.2010.11.013. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T. Specific impairments of planning. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1982;298(1089):199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- Smith S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass J.G., Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J. Exp. Psychol. Hum. Learn. 1980;6(2):174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Spitzer R.L., Williams J.B., Kroenke K., Linzer M., deGruy F.V., III, Hahn S.R., Brody D., Johnson J.G. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA. 1994;272(22):1749–1756. [PubMed] [Google Scholar]
- Stephan K.E., Friston K.J., Frith C.D. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr. Bull. 2009;35(3):509–527. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepanski S.M., Pinsk M.A., Douglas M.M., Kastner S., Saalmann Y.B. Functional and structural architecture of the human dorsal frontoparietal attention network. Proc. Natl. Acad. Sci. U. S. A. 2013;110(39):15806–15811. doi: 10.1073/pnas.1313903110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro R., Fox P.T., Paus T. Functional coactivation map of the human brain. Cereb. Cortex. 2008;18(11):2553–2559. doi: 10.1093/cercor/bhn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas P.J., Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52(1):155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Wechsler D. In: Wechsler Abbreviated Scale of Intelligence (WASI), Norwegian Manual Supplement. Company H.B., editor. Pearson Assessment; Stockholm: 2007. [Google Scholar]
- Weissman D.H., Roberts K.C., Visscher K.M., Woldorff M.G. The neural bases of momentary lapses in attention. Nat. Neurosci. 2006;9(7):971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Ford J.M. Default mode network activity and connectivity in psychopathology. Annu. Rev. Clin. Psychol. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Thermenos H.W., Milanovic S., Tsuang M.T., Faraone S.V., McCarley R.W., Shenton M.E., Green A.I., Nieto-Castanon A., LaViolette P., Wojcik J., Gabrieli J.D., Seidman L.J. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 2009;106(4):1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson P.C., Allman J.M. A framework for interpreting functional networks in schizophrenia. Front. Hum. Neurosci. 2012;6:184. doi: 10.3389/fnhum.2012.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich M. Robust group analysis using outlier inference. NeuroImage. 2008;41(2):286–301. doi: 10.1016/j.neuroimage.2008.02.042. [DOI] [PubMed] [Google Scholar]
- Zeng L.L., Wang D., Fox M.D., Sabuncu M., Hu D., Ge M., Buckner R.L., Liu H. Neurobiological basis of head motion in brain imaging. Proc. Natl. Acad. Sci. U. S. A. 2014;111(16):6058–6062. doi: 10.1073/pnas.1317424111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Fan L., Qiu C., Jiang T. Prefrontal cortex and the dysconnectivity hypothesis of schizophrenia. Neurosci. Bull. 2015;31(2):207–219. doi: 10.1007/s12264-014-1502-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.