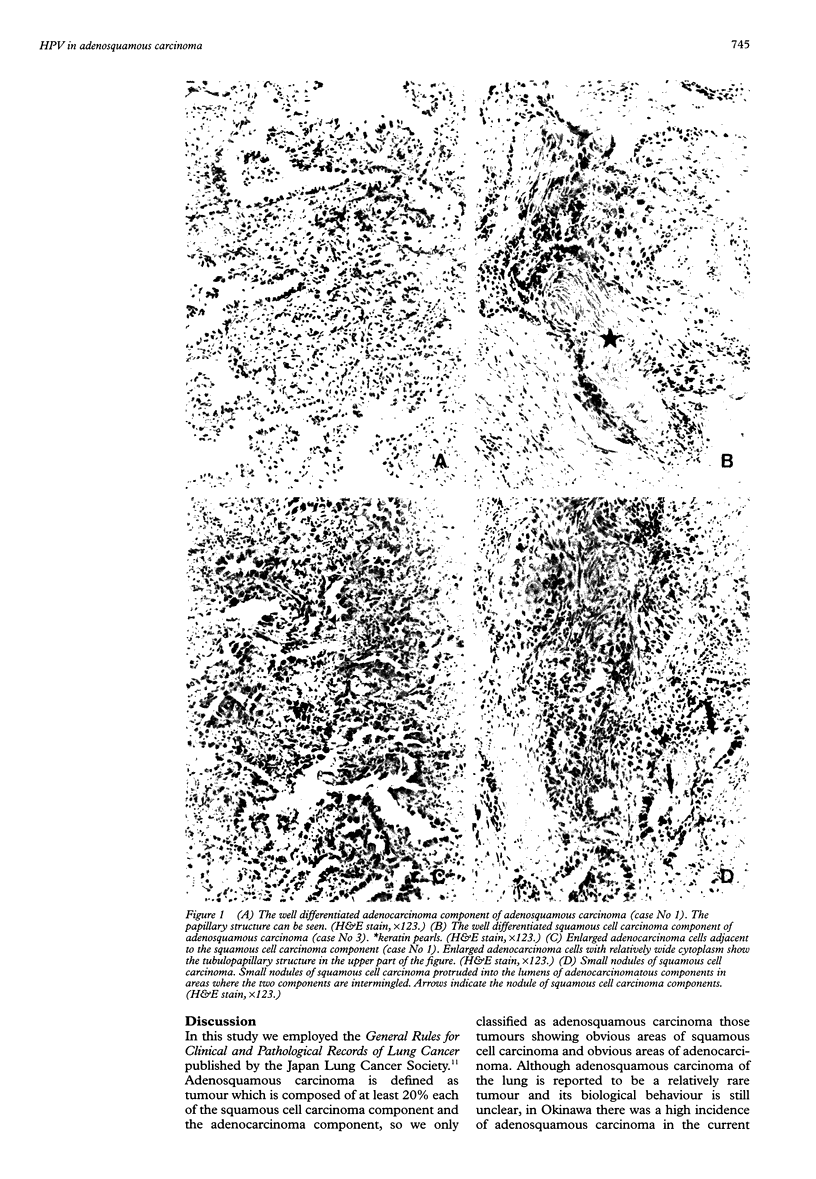
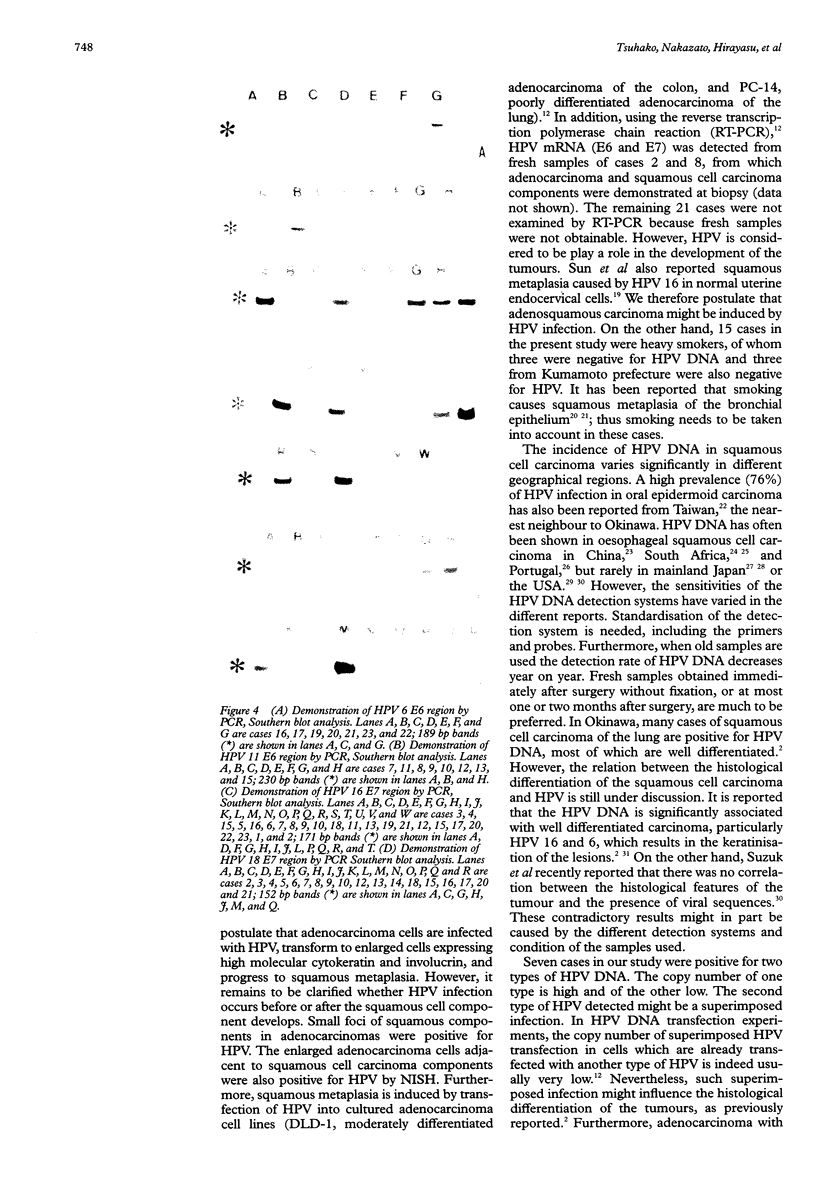
Abstract
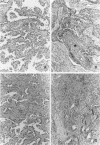
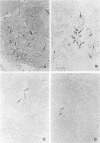
AIM: To investigate the presence of human papillomavirus (HPV) DNA in adenosquamous carcinoma of the lung--which is relatively common in Okinawa but not in mainland Japan--and examine its histological features. METHODS: Of 207 cases where primary lung cancers were surgically removed between January 1995 and June 1997 in Okinawa, 23 were adenosquamous carcinoma. HPV was detected by non-isotopic in situ hybridisation (NISH) and polymerase chain reaction (PCR) amplification with primers specific for E6 and E7 regions of the HPV genome. PCR products were analysed by Southern blotting. Immunohistochemical determination of high molecular weight cytokeratin (HMC) and involucrin was also carried out. RESULTS: 18 cases were positive for HPV DNA by PCR and NISH. HPV types 6, 11, 16, and 18 were found. Seven cases were dual positive for different types of HPV. Using NISH, HPV was also found in the squamous cell components and in neighbouring enlarged adenocarcinoma cells. The HMC and involucrin were demonstrated immunohistochemically in the same areas. CONCLUSIONS: HPV DNA was found in a high proportion (78.3%) of adenosquamous carcinomas in Okinawa, a region where HPV has previously been shown to be prevalent in squamous cell carcinoma of the lung. The adenocarcinoma cells adjacent to the squamous cell carcinoma component were enlarged and positive for HPV, HMC, and involucrin. This is thought to indicate the transition from adenocarcinoma to squamous cell carcinoma.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang K. W., Chang C. S., Lai K. S., Chou M. J., Choo K. B. High prevalence of human papillomavirus infection and possible association with betel quid chewing and smoking in oral epidermoid carcinomas in Taiwan. J Med Virol. 1989 May;28(1):57–61. doi: 10.1002/jmv.1890280113. [DOI] [PubMed] [Google Scholar]
- Chen B., Yin H., Dhurandhar N. Detection of human papillomavirus DNA in esophageal squamous cell carcinomas by the polymerase chain reaction using general consensus primers. Hum Pathol. 1994 Sep;25(9):920–923. doi: 10.1016/0046-8177(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Cole S. T., Danos O. Nucleotide sequence and comparative analysis of the human papillomavirus type 18 genome. Phylogeny of papillomaviruses and repeated structure of the E6 and E7 gene products. J Mol Biol. 1987 Feb 20;193(4):599–608. doi: 10.1016/0022-2836(87)90343-3. [DOI] [PubMed] [Google Scholar]
- Cooper K., Herrington C. S., Stickland J. E., Evans M. F., McGee J. O. Episomal and integrated human papillomavirus in cervical neoplasia shown by non-isotopic in situ hybridisation. J Clin Pathol. 1991 Dec;44(12):990–996. doi: 10.1136/jcp.44.12.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidalgo P. O., Cravo M. L., Chaves P. P., Leitão C. N., Mira F. C. High prevalence of human papillomavirus in squamous cell carcinoma and matched normal esophageal mucosa: assessment by polymerase chain reaction. Cancer. 1995 Nov 1;76(9):1522–1528. doi: 10.1002/1097-0142(19951101)76:9<1522::aid-cncr2820760904>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Fitzgibbons P. L., Kern W. H. Adenosquamous carcinoma of the lung: a clinical and pathologic study of seven cases. Hum Pathol. 1985 May;16(5):463–466. doi: 10.1016/s0046-8177(85)80083-6. [DOI] [PubMed] [Google Scholar]
- Hirayasu T., Iwamasa T., Kamada Y., Koyanagi Y., Usuda H., Genka K. Human papillomavirus DNA in squamous cell carcinoma of the lung. J Clin Pathol. 1996 Oct;49(10):810–817. doi: 10.1136/jcp.49.10.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T., Kaneko S., Yokoyama H., Inoue T., Sugio K., Sugimachi K. Adenosquamous carcinoma of the lung. Clinicopathologic and immunohistochemical features. Am J Clin Pathol. 1992 May;97(5):678–685. doi: 10.1093/ajcp/97.5.678. [DOI] [PubMed] [Google Scholar]
- Kinoshita I., Dosaka-Akita H., Shindoh M., Fujino M., Akie K., Kato M., Fujinaga K., Kawakami Y. Human papillomavirus type 18 DNA and E6-E7 mRNA are detected in squamous cell carcinoma and adenocarcinoma of the lung. Br J Cancer. 1995 Feb;71(2):344–349. doi: 10.1038/bjc.1995.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyabu M. T., Shibata D., Arnheim N., Martin W. J., Fitzgibbons P. L. Detection of human papillomavirus in formalin-fixed, invasive squamous carcinomas using the polymerase chain reaction. Am J Surg Pathol. 1989 Mar;13(3):221–224. doi: 10.1097/00000478-198903000-00007. [DOI] [PubMed] [Google Scholar]
- McNicol P., Paraskevas M., Guijon F. Variability of polymerase chain reaction-based detection of human papillomavirus DNA is associated with the composition of vaginal microbial flora. J Med Virol. 1994 Jun;43(2):194–200. doi: 10.1002/jmv.1890430218. [DOI] [PubMed] [Google Scholar]
- Mitra R. S., Wrone-Smith T., Simonian P., Foreman K. E., Nunez G., Nickoloff B. J. Apoptosis in keratinocytes is not dependent on induction of differentiation. Lab Invest. 1997 Jan;76(1):99–107. [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Nakazato I., Hirayasu T., Kamada Y., Tsuhako K., Iwamasa T. Carcinoma of the lung in Okinawa, Japan: with special reference to squamous cell carcinoma and squamous metaplasia. Pathol Int. 1997 Oct;47(10):659–672. doi: 10.1111/j.1440-1827.1997.tb04439.x. [DOI] [PubMed] [Google Scholar]
- Peters E. J., Morice R., Benner S. E., Lippman S., Lukeman J., Lee J. S., Ro J. Y., Hong W. K. Squamous metaplasia of the bronchial mucosa and its relationship to smoking. Chest. 1993 May;103(5):1429–1432. doi: 10.1378/chest.103.5.1429. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sun Q., Tsutsumi K., Kelleher M. B., Pater A., Pater M. M. Squamous metaplasia of normal and carcinoma in situ of HPV 16-immortalized human endocervical cells. Cancer Res. 1992 Aug 1;52(15):4254–4260. [PubMed] [Google Scholar]
- Suzuk L., Noffsinger A. E., Hui Y. Z., Fenoglio-Preiser C. M. Detection of human papillomavirus in esophageal squamous cell carcinoma. Cancer. 1996 Aug 15;78(4):704–710. doi: 10.1002/(SICI)1097-0142(19960815)78:4<704::AID-CNCR2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Takamori S., Noguchi M., Morinaga S., Goya T., Tsugane S., Kakegawa T., Shimosato Y. Clinicopathologic characteristics of adenosquamous carcinoma of the lung. Cancer. 1991 Feb 1;67(3):649–654. doi: 10.1002/1097-0142(19910201)67:3<649::aid-cncr2820670321>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Togawa K., Jaskiewicz K., Takahashi H., Meltzer S. J., Rustgi A. K. Human papillomavirus DNA sequences in esophagus squamous cell carcinoma. Gastroenterology. 1994 Jul;107(1):128–136. doi: 10.1016/0016-5085(94)90070-1. [DOI] [PubMed] [Google Scholar]
- Toh Y., Kuwano H., Tanaka S., Baba K., Matsuda H., Sugimachi K., Mori R. Detection of human papillomavirus DNA in esophageal carcinoma in Japan by polymerase chain reaction. Cancer. 1992 Nov 1;70(9):2234–2238. doi: 10.1002/1097-0142(19921101)70:9<2234::aid-cncr2820700903>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Travis W. D., Lubin J., Ries L., Devesa S. United States lung carcinoma incidence trends: declining for most histologic types among males, increasing among females. Cancer. 1996 Jun 15;77(12):2464–2470. doi: 10.1002/(SICI)1097-0142(19960615)77:12<2464::AID-CNCR8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Vincent R. G., Pickren J. W., Lane W. W., Bross I., Takita H., Houten L., Gutierrez A. C., Rzepka T. The changing histopathology of lung cancer: a review of 1682 cases. Cancer. 1977 Apr;39(4):1647–1655. doi: 10.1002/1097-0142(197704)39:4<1647::aid-cncr2820390439>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Wilczynski S. P., Bergen S., Walker J., Liao S. Y., Pearlman L. F. Human papillomaviruses and cervical cancer: analysis of histopathologic features associated with different viral types. Hum Pathol. 1988 Jun;19(6):697–704. doi: 10.1016/s0046-8177(88)80176-x. [DOI] [PubMed] [Google Scholar]
- Williamson A. L., Jaskiesicz K., Gunning A. The detection of human papillomavirus in oesophageal lesions. Anticancer Res. 1991 Jan-Feb;11(1):263–265. [PubMed] [Google Scholar]