Abstract
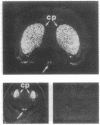
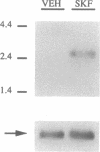
The existence of an activatable dopamine system within the hypothalamic suprachiasmatic nuclei (SCN), the site of a biological clock, was investigated in rats during fetal life. In situ hybridization studies revealed that D1-dopamine receptor mRNA was highly expressed in the fetal SCN and not expressed in other hypothalamic regions. Cocaine injected into pregnant rats or directly into rat fetuses on day 20 of gestation selectively activated c-fos gene expression in the fetal SCN; cocaine did not induce c-fos expression elsewhere in the fetal brain or in the maternal SCN. This cocaine-induced activation of c-fos expression in fetal SCN was mediated in part through D1-dopamine receptors, as the cocaine-induced activation was partially blocked by the D1-dopamine receptor antagonist SCH 23390. In addition, the selective D1-dopamine receptor agonist SKF 38393 induced high levels of c-fos expression in the fetal SCN. The presence of an activatable dopamine system within the fetal SCN provides a mechanism through which maternal signals could entrain the fetal biological clock and through which maternally administered psychotropic drugs could alter normal development of the circadian timing system.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe H., Rusak B., Robertson H. A. Photic induction of Fos protein in the suprachiasmatic nucleus is inhibited by the NMDA receptor antagonist MK-801. Neurosci Lett. 1991 Jun 10;127(1):9–12. doi: 10.1016/0304-3940(91)90881-s. [DOI] [PubMed] [Google Scholar]
- Aronin N., Sagar S. M., Sharp F. R., Schwartz W. J. Light regulates expression of a Fos-related protein in rat suprachiasmatic nuclei. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5959–5962. doi: 10.1073/pnas.87.15.5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasnoff I. J., Griffith D. R., MacGregor S., Dirkes K., Burns K. A. Temporal patterns of cocaine use in pregnancy. Perinatal outcome. JAMA. 1989 Mar 24;261(12):1741–1744. [PubMed] [Google Scholar]
- Davis F. C., Mannion J. Entrainment of hamster pup circadian rhythms by prenatal melatonin injections to the mother. Am J Physiol. 1988 Sep;255(3 Pt 2):R439–R448. doi: 10.1152/ajpregu.1988.255.3.R439. [DOI] [PubMed] [Google Scholar]
- Dubocovich M. L. Pharmacology and function of melatonin receptors. FASEB J. 1988 Sep;2(12):2765–2773. doi: 10.1096/fasebj.2.12.2842214. [DOI] [PubMed] [Google Scholar]
- Earnest D. J., Iadarola M., Yeh H. H., Olschowka J. A. Photic regulation of c-fos expression in neural components governing the entrainment of circadian rhythms. Exp Neurol. 1990 Sep;109(3):353–361. doi: 10.1016/s0014-4886(05)80027-5. [DOI] [PubMed] [Google Scholar]
- Frank D. A., Zuckerman B. S., Amaro H., Aboagye K., Bauchner H., Cabral H., Fried L., Hingson R., Kayne H., Levenson S. M. Cocaine use during pregnancy: prevalence and correlates. Pediatrics. 1988 Dec;82(6):888–895. [PubMed] [Google Scholar]
- Fremeau R. T., Jr, Duncan G. E., Fornaretto M. G., Dearry A., Gingrich J. A., Breese G. R., Caron M. G. Localization of D1 dopamine receptor mRNA in brain supports a role in cognitive, affective, and neuroendocrine aspects of dopaminergic neurotransmission. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3772–3776. doi: 10.1073/pnas.88.9.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garabal M. V., Arévalo R. M., Díaz-Palarea M. D., Castro R., Rodríguez M. Tyrosine availability and brain noradrenaline synthesis in the fetus: control by maternal tyrosine ingestion. Brain Res. 1988 Aug 9;457(2):330–337. doi: 10.1016/0006-8993(88)90703-2. [DOI] [PubMed] [Google Scholar]
- Graybiel A. M., Moratalla R., Robertson H. A. Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koe B. K. Molecular geometry of inhibitors of the uptake of catecholamines and serotonin in synaptosomal preparations of rat brain. J Pharmacol Exp Ther. 1976 Dec;199(3):649–661. [PubMed] [Google Scholar]
- Kornhauser J. M., Nelson D. E., Mayo K. E., Takahashi J. S. Photic and circadian regulation of c-fos gene expression in the hamster suprachiasmatic nucleus. Neuron. 1990 Aug;5(2):127–134. doi: 10.1016/0896-6273(90)90303-w. [DOI] [PubMed] [Google Scholar]
- Kornhauser J. M., Nelson D. E., Mayo K. E., Takahashi J. S. Regulation of jun-B messenger RNA and AP-1 activity by light and a circadian clock. Science. 1992 Mar 20;255(5051):1581–1584. doi: 10.1126/science.1549784. [DOI] [PubMed] [Google Scholar]
- Laemle L. K., Repke K. B., Hawkes R., Rice F. L. Synaptogenesis in the rat suprachiasmatic nucleus: a light microscopic immunocytochemical survey. Brain Res. 1991 Mar 22;544(1):108–117. doi: 10.1016/0006-8993(91)90891-x. [DOI] [PubMed] [Google Scholar]
- Lenn N. J., Beebe B., Moore R. Y. Postnatal development of the suprachiasmatic hypothalamic nucleus of the rat. Cell Tissue Res. 1977 Mar 24;178(4):463–475. doi: 10.1007/BF00219568. [DOI] [PubMed] [Google Scholar]
- Meijer J. H., Rietveld W. J. Neurophysiology of the suprachiasmatic circadian pacemaker in rodents. Physiol Rev. 1989 Jul;69(3):671–707. doi: 10.1152/physrev.1989.69.3.671. [DOI] [PubMed] [Google Scholar]
- Moore R. Y., Bernstein M. E. Synaptogenesis in the rat suprachiasmatic nucleus demonstrated by electron microscopy and synapsin I immunoreactivity. J Neurosci. 1989 Jun;9(6):2151–2162. doi: 10.1523/JNEUROSCI.09-06-02151.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. Y., Card J. P. Visual pathways and the entrainment of circadian rhythms. Ann N Y Acad Sci. 1985;453:123–133. doi: 10.1111/j.1749-6632.1985.tb11805.x. [DOI] [PubMed] [Google Scholar]
- Morgan J. I., Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- Rea M. A. Light increases Fos-related protein immunoreactivity in the rat suprachiasmatic nuclei. Brain Res Bull. 1989 Dec;23(6):577–581. doi: 10.1016/0361-9230(89)90204-9. [DOI] [PubMed] [Google Scholar]
- Reisert I., Schuster R., Zienecker R., Pilgrim C. Prenatal development of mesencephalic and diencephalic dopaminergic systems in the male and female rat. Brain Res Dev Brain Res. 1990 May 1;53(2):222–229. doi: 10.1016/0165-3806(90)90010-v. [DOI] [PubMed] [Google Scholar]
- Reppert S. M., Schwartz W. J. Maternal coordination of the fetal biological clock in utero. Science. 1983 May 27;220(4600):969–971. doi: 10.1126/science.6844923. [DOI] [PubMed] [Google Scholar]
- Reppert S. M., Weaver D. R., Rivkees S. A., Stopa E. G. Putative melatonin receptors in a human biological clock. Science. 1988 Oct 7;242(4875):78–81. doi: 10.1126/science.2845576. [DOI] [PubMed] [Google Scholar]
- Reppert S. M., Weaver D. R., Stehle J. H., Rivkees S. A. Molecular cloning and characterization of a rat A1-adenosine receptor that is widely expressed in brain and spinal cord. Mol Endocrinol. 1991 Aug;5(8):1037–1048. doi: 10.1210/mend-5-8-1037. [DOI] [PubMed] [Google Scholar]
- Rusak B., Robertson H. A., Wisden W., Hunt S. P. Light pulses that shift rhythms induce gene expression in the suprachiasmatic nucleus. Science. 1990 Jun 8;248(4960):1237–1240. doi: 10.1126/science.2112267. [DOI] [PubMed] [Google Scholar]
- Ugrumov M. V., Taxi J., Tixier-Vidal A., Thibault J., Mitskevich M. S. Ontogenesis of tyrosine hydroxylase-immunopositive structures in the rat hypothalamus. An atlas of neuronal cell bodies. Neuroscience. 1989;29(1):135–156. doi: 10.1016/0306-4522(89)90338-2. [DOI] [PubMed] [Google Scholar]
- Ugrumov M. V., Tixier-Vidal A., Taxi J., Thibault J., Mitskevich M. S. Ontogenesis of tyrosine hydroxylase-immunopositive structures in the rat hypothalamus. Fiber pathways and terminal fields. Neuroscience. 1989;29(1):157–166. doi: 10.1016/0306-4522(89)90339-4. [DOI] [PubMed] [Google Scholar]
- Weaver D. R., Namboodiri M. A., Reppert S. M. Iodinated melatonin mimics melatonin action and reveals discrete binding sites in fetal brain. FEBS Lett. 1988 Feb 8;228(1):123–127. doi: 10.1016/0014-5793(88)80599-4. [DOI] [PubMed] [Google Scholar]
- White M. P., Fisher L. J. Degree of light damage to the retina varies with time of day of bright light exposure. Physiol Behav. 1987;39(5):607–613. doi: 10.1016/0031-9384(87)90160-0. [DOI] [PubMed] [Google Scholar]
- Young S. T., Porrino L. J., Iadarola M. J. Cocaine induces striatal c-fos-immunoreactive proteins via dopaminergic D1 receptors. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1291–1295. doi: 10.1073/pnas.88.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zisapel N., Laudon M. Dopamine release induced by electrical field stimulation of rat hypothalamus in vitro: inhibition by melatonin. Biochem Biophys Res Commun. 1982 Feb 26;104(4):1610–1616. doi: 10.1016/0006-291x(82)91437-1. [DOI] [PubMed] [Google Scholar]
- Zuckerman B., Frank D. A., Hingson R., Amaro H., Levenson S. M., Kayne H., Parker S., Vinci R., Aboagye K., Fried L. E. Effects of maternal marijuana and cocaine use on fetal growth. N Engl J Med. 1989 Mar 23;320(12):762–768. doi: 10.1056/NEJM198903233201203. [DOI] [PubMed] [Google Scholar]
- van den Pol A. N., Tsujimoto K. L. Neurotransmitters of the hypothalamic suprachiasmatic nucleus: immunocytochemical analysis of 25 neuronal antigens. Neuroscience. 1985 Aug;15(4):1049–1086. doi: 10.1016/0306-4522(85)90254-4. [DOI] [PubMed] [Google Scholar]