Abstract
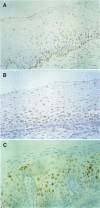
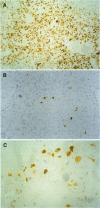
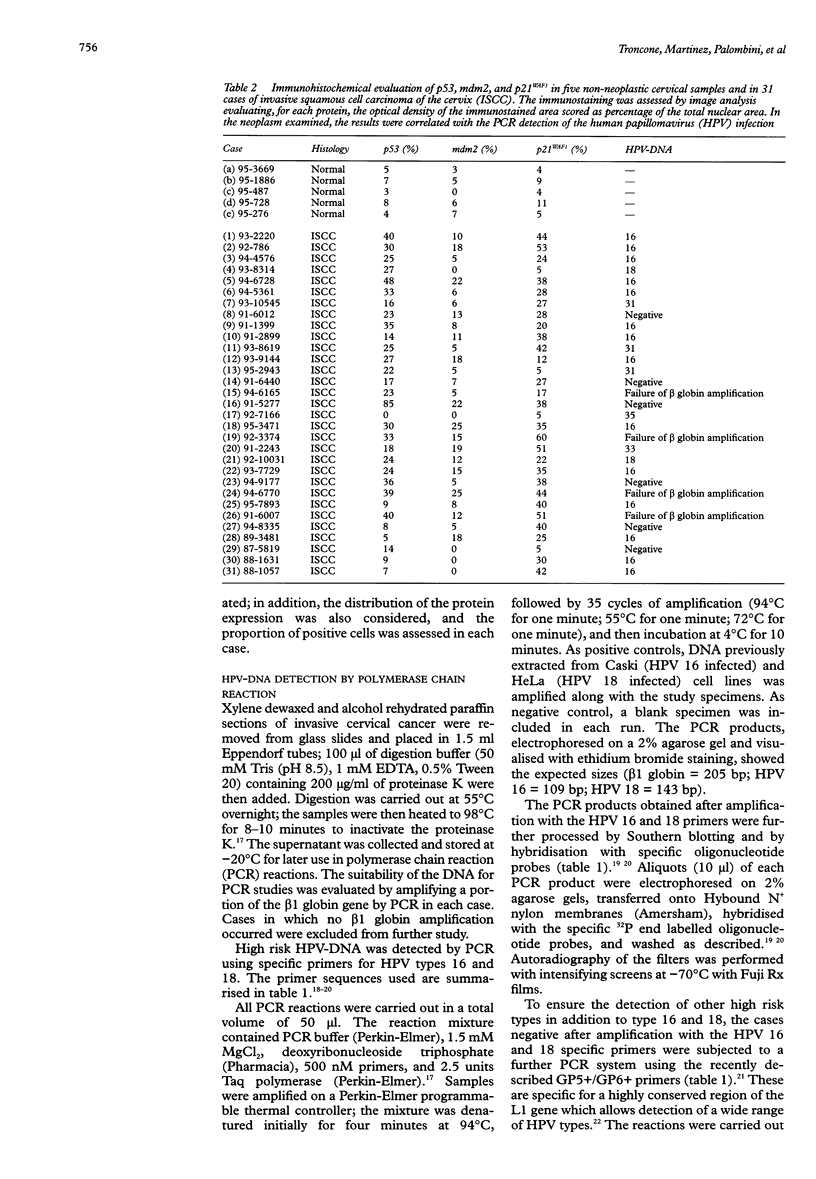
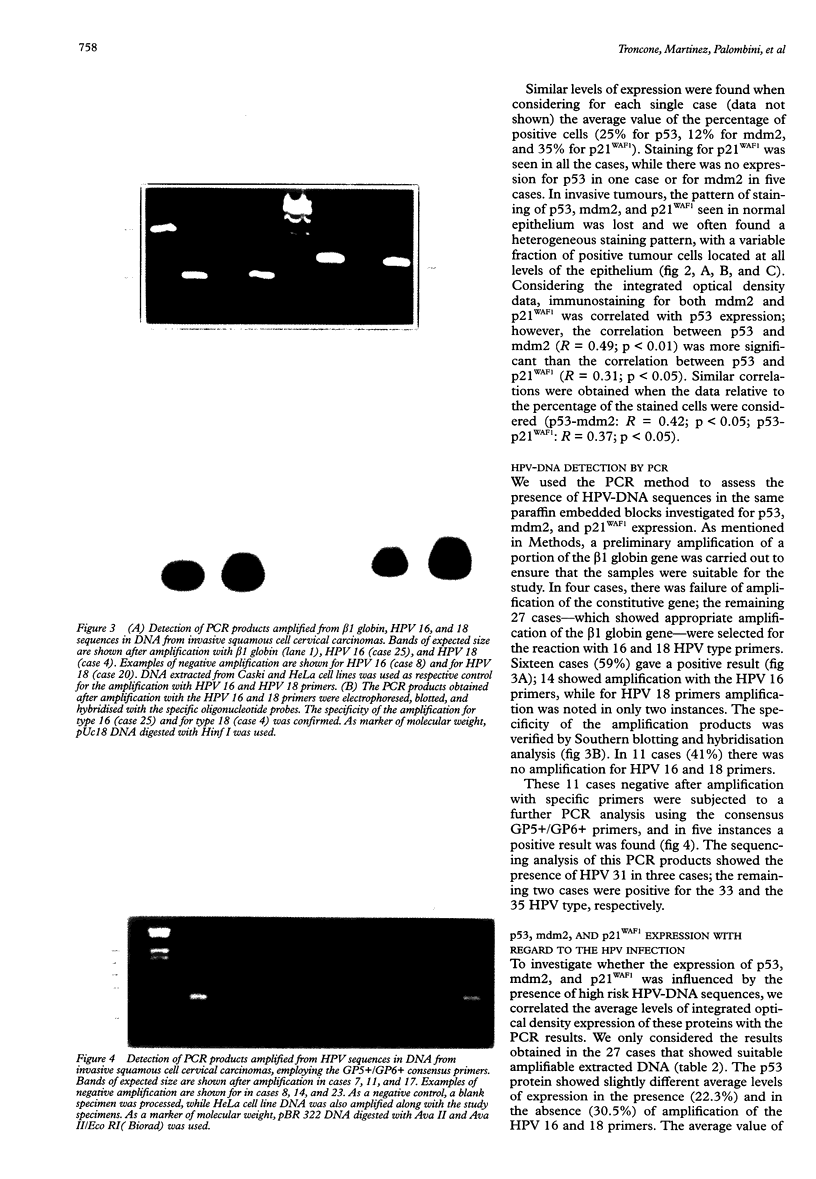
AIM: To investigate the immunocytochemical staining pattern of mdm2 and p21WAF1 proteins in invasive cervical cancer and to determine its relation with the expression of p53 and with the high risk HPV infection. METHODS: Immunocytochemistry for p53, mdm2, and p21WAF1 was performed in 31 paraffin embedded sections of invasive cervical cancer. The results were assessed by image analysis, evaluating for each protein the optical density of the immunostained area, scored as percentage of the total nuclear area. The presence of high risk human papillomavirus (HPV) infection was detected by using the polymerase chain reaction. RESULTS: Immunostaining for both mdm2 and p21WAF1 was correlated with p53 expression; however, the correlation between p53 and mdm2 (R = 0.49; p < 0.01) was more significant than between p53 and p21WAF1 (R = 0.31; p < 0.05); the less stringent correlation between p53 and p21WAF1 might reflect the p53 independent mechanisms of p21WAF1 induction. Similar average levels of p53, mdm2, and p21WAF1 immunostaining were found in the presence or absence of high risk HPV-DNA, without significant differences between the two groups. CONCLUSIONS: These data suggest that mdm2 and p21WAF1 proteins are expressed in invasive cervical cancer and that their immunocytochemical staining pattern is not abrogated by the presence of high risk HPV genomic sequences.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bosari S., Roncalli M., Viale G., Bossi P., Coggi G. p53 immunoreactivity in inflammatory and neoplastic diseases of the uterine cervix. J Pathol. 1993 Apr;169(4):425–430. doi: 10.1002/path.1711690407. [DOI] [PubMed] [Google Scholar]
- Butz K., Shahabeddin L., Geisen C., Spitkovsky D., Ullmann A., Hoppe-Seyler F. Functional p53 protein in human papillomavirus-positive cancer cells. Oncogene. 1995 Mar 2;10(5):927–936. [PubMed] [Google Scholar]
- DiGiuseppe J. A., Redston M. S., Yeo C. J., Kern S. E., Hruban R. H. p53-independent expression of the cyclin-dependent kinase inhibitor p21 in pancreatic carcinoma. Am J Pathol. 1995 Oct;147(4):884–888. [PMC free article] [PubMed] [Google Scholar]
- Fujita M., Inoue M., Tanizawa O., Iwamoto S., Enomoto T. Alterations of the p53 gene in human primary cervical carcinoma with and without human papillomavirus infection. Cancer Res. 1992 Oct 1;52(19):5323–5328. [PubMed] [Google Scholar]
- Greer C. E., Peterson S. L., Kiviat N. B., Manos M. M. PCR amplification from paraffin-embedded tissues. Effects of fixative and fixation time. Am J Clin Pathol. 1991 Feb;95(2):117–124. doi: 10.1093/ajcp/95.2.117. [DOI] [PubMed] [Google Scholar]
- Gu Z., Pim D., Labrecque S., Banks L., Matlashewski G. DNA damage induced p53 mediated transcription is inhibited by human papillomavirus type 18 E6. Oncogene. 1994 Feb;9(2):629–633. [PubMed] [Google Scholar]
- Helland A., Holm R., Kristensen G., Kaern J., Karlsen F., Trope C., Nesland J. M., Børresen A. L. Genetic alterations of the TP53 gene, p53 protein expression and HPV infection in primary cervical carcinomas. J Pathol. 1993 Oct;171(2):105–114. doi: 10.1002/path.1711710207. [DOI] [PubMed] [Google Scholar]
- Herrington C. S. Human papillomaviruses and cervical neoplasia. II. Interaction of HPV with other factors. J Clin Pathol. 1995 Jan;48(1):1–6. doi: 10.1136/jcp.48.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M. V., de Roda Husman A. M., van den Brule A. J., Snijders P. J., Meijer C. J., Walboomers J. M. Group-specific differentiation between high- and low-risk human papillomavirus genotypes by general primer-mediated PCR and two cocktails of oligonucleotide probes. J Clin Microbiol. 1995 Apr;33(4):901–905. doi: 10.1128/jcm.33.4.901-905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffers M. D., Richmond J., Farquharson M., McNicol A. M. p53 immunoreactivity in cervical intraepithelial neoplasia and non-neoplastic cervical squamous epithelium. J Clin Pathol. 1994 Dec;47(12):1073–1076. doi: 10.1136/jcp.47.12.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambkin H. A., Mothersill C. M., Kelehan P. Variations in immunohistochemical detection of p53 protein overexpression in cervical carcinomas with different antibodies and methods of detection. J Pathol. 1994 Jan;172(1):13–18. doi: 10.1002/path.1711720105. [DOI] [PubMed] [Google Scholar]
- Lambkin H. A., Mothersill C. M., Kelehan P. Variations in immunohistochemical detection of p53 protein overexpression in cervical carcinomas with different antibodies and methods of detection. J Pathol. 1994 Jan;172(1):13–18. doi: 10.1002/path.1711720105. [DOI] [PubMed] [Google Scholar]
- Miwa K., Miyamoto S., Kato H., Imamura T., Nishida M., Yoshikawa Y., Nagata Y., Wake N. The role of p53 inactivation in human cervical cell carcinoma development. Br J Cancer. 1995 Feb;71(2):219–226. doi: 10.1038/bjc.1995.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noffsinger A. E., Suzuk L., Hui Y. Z., Gal A. A., Fenoglio-Preiser C. M. Differential sensitivities of E6 type-specific and L1 consensus primers in the detection of human papillomavirus in anal carcinoma. Mod Pathol. 1995 Jun;8(5):509–514. [PubMed] [Google Scholar]
- Park D. J., Wilczynski S. P., Paquette R. L., Miller C. W., Koeffler H. P. p53 mutations in HPV-negative cervical carcinoma. Oncogene. 1994 Jan;9(1):205–210. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffner M., Huibregtse J. M., Vierstra R. D., Howley P. M. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993 Nov 5;75(3):495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- Slebos R. J., Lee M. H., Plunkett B. S., Kessis T. D., Williams B. O., Jacks T., Hedrick L., Kastan M. B., Cho K. R. p53-dependent G1 arrest involves pRB-related proteins and is disrupted by the human papillomavirus 16 E7 oncoprotein. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5320–5324. doi: 10.1073/pnas.91.12.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tron V. A., Tang L., Yong W. P., Trotter M. J. Differentiation-associated overexpression of the cyclin-dependent kinase inhibitor p21waf-1 in human cutaneous squamous cell carcinoma. Am J Pathol. 1996 Oct;149(4):1139–1146. [PMC free article] [PubMed] [Google Scholar]
- Troncone G., Anderson S. M., Herrington C. S., de Angelis M. L., Noell H., Chimera J. A., O'D McGee J. Comparative analysis of human papillomavirus detection by dot blot hybridisation and non-isotopic in situ hybridisation. J Clin Pathol. 1992 Oct;45(10):866–870. doi: 10.1136/jcp.45.10.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troncone G., Herrington C. S., Cooper K., de Angelis M. L., McGee J. O. Detection of human papillomavirus in matched cervical smears and biopsy specimens by non-isotopic in situ hybridisation. J Clin Pathol. 1992 Apr;45(4):308–313. doi: 10.1136/jcp.45.4.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg W. C., Azzoli C. G., Chapman K., Levine A. J., Yuspa S. H. p53-mediated transcriptional activity increases in differentiating epidermal keratinocytes in association with decreased p53 protein. Oncogene. 1995 Jun 15;10(12):2271–2279. [PubMed] [Google Scholar]
- Werness B. A., Wang H. Q., Chance J., Goldstein D. J. p53-independent expression of p21waf1/cip1 in preinvasive and invasive squamous neoplasms of the uterine cervix. Mod Pathol. 1997 Jun;10(6):578–584. [PubMed] [Google Scholar]
- de Roda Husman A. M., Walboomers J. M., van den Brule A. J., Meijer C. J., Snijders P. J. The use of general primers GP5 and GP6 elongated at their 3' ends with adjacent highly conserved sequences improves human papillomavirus detection by PCR. J Gen Virol. 1995 Apr;76(Pt 4):1057–1062. doi: 10.1099/0022-1317-76-4-1057. [DOI] [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Waldman T., Oliner J. D., Velculescu V. E., Burrell M., Hill D. E., Healy E., Rees J. L., Hamilton S. R. Topological control of p21WAF1/CIP1 expression in normal and neoplastic tissues. Cancer Res. 1995 Jul 1;55(13):2910–2919. [PubMed] [Google Scholar]