Abstract
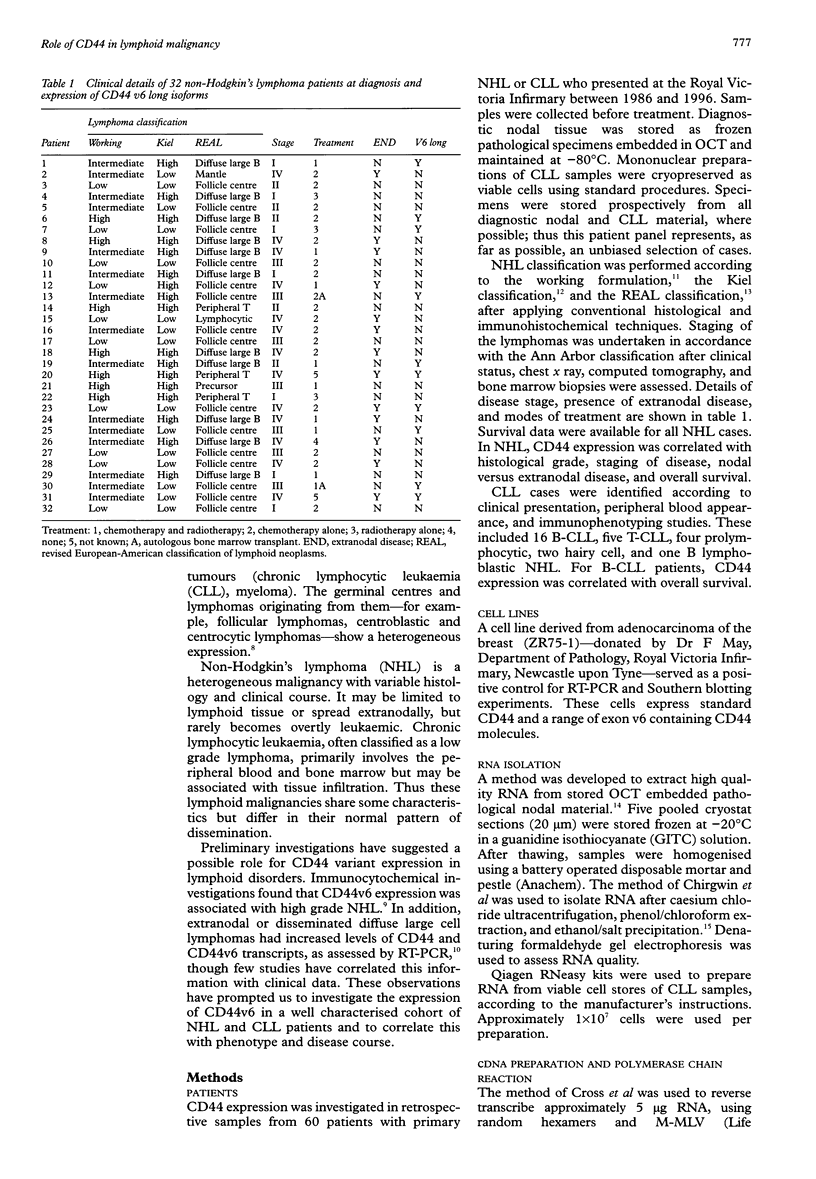
AIM: To investigate the expression of CD44 isoforms containing variant exon 6 (v6) in a well characterised cohort of patients with non-Hodgkin's lymphoma (NHL) and chronic lymphocytic leukaemia (CLL), and to correlate this with phenotype and disease course. METHODS: Cryostat sections of OCT embedded diagnostic nodal material from NHL patients and cryopreserved mononuclear preparations from CLL patients were used as sources of RNA. After reverse transcription, PCR was carried out with amplimers positioned at either side of the variant exon insertion site to amplify all possible CD44 isoforms. Those isoforms containing v6 were identified after Southern blotting and hybridisation with a radiolabelled oligonucleotide. RESULTS: Of 32 NHL samples analysed, 16 did not express CD44 isoforms containing v6, six expressed an isoform containing exon v6 alone, and 10 expressed v6 long isoforms which contained exon v6 in addition to other variant exons. These data did not correlate with lymphoma classification, disease staging, or the presence or absence of extranodal disease. However, those patients expressing v6 long CD44 isoforms had a worse overall survival than those that did not. The plateau of the survival curves was 50% compared with 82%. No v6 long isoforms were detected in the 21 CLL samples investigated. CONCLUSIONS: The expression of v6 long CD44 isoforms is associated with aggressive disease in NHL, independent of grade, stage, or presence of extranodal disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arch R., Wirth K., Hofmann M., Ponta H., Matzku S., Herrlich P., Zöller M. Participation in normal immune responses of a metastasis-inducing splice variant of CD44. Science. 1992 Jul 31;257(5070):682–685. doi: 10.1126/science.1496383. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cross N. C., Feng L., Bungey J., Goldman J. M. Minimal residual disease after bone marrow transplant for chronic myeloid leukaemia detected by the polymerase chain reaction. Leuk Lymphoma. 1993;11 (Suppl 1):39–43. doi: 10.3109/10428199309047861. [DOI] [PubMed] [Google Scholar]
- De Rossi G., Tenca C., Cerruti G., Favre A., Zarcone D., Tabilio A., Mauro F. R., Annino L., Grossi C. E. Adhesion molecule expression on B-cells from acute and chronic lymphoid leukemias. Leuk Lymphoma. 1994 Dec;16(1-2):31–36. doi: 10.3109/10428199409114137. [DOI] [PubMed] [Google Scholar]
- Eistere W., Hilbe W., Stauder R., Bechter O., Fend F., Thaler J. An aggressive subtype of B-CLL is characterized by strong CD44 expression and lack of CD11c. Br J Haematol. 1996 Jun;93(3):661–669. doi: 10.1046/j.1365-2141.1996.d01-1704.x. [DOI] [PubMed] [Google Scholar]
- Griffioen A. W., Horst E., Heider K. H., Wielenga V. J., Adolf G. R., Herrlich P., Pals S. T. Expression of CD44 splice variants during lymphocyte activation and tumor progression. Cell Adhes Commun. 1994 Jul;2(3):195–200. doi: 10.3109/15419069409004437. [DOI] [PubMed] [Google Scholar]
- Günthert U., Hofmann M., Rudy W., Reber S., Zöller M., Haussmann I., Matzku S., Wenzel A., Ponta H., Herrlich P. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991 Apr 5;65(1):13–24. doi: 10.1016/0092-8674(91)90403-l. [DOI] [PubMed] [Google Scholar]
- Harris N. L., Jaffe E. S., Stein H., Banks P. M., Chan J. K., Cleary M. L., Delsol G., De Wolf-Peeters C., Falini B., Gatter K. C. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994 Sep 1;84(5):1361–1392. [PubMed] [Google Scholar]
- Horst E., Meijer C. J., Radaskiewicz T., van Dongen J. J., Pieters R., Figdor C. G., Hooftman A., Pals S. T. Expression of a human homing receptor (CD44) in lymphoid malignancies and related stages of lymphoid development. Leukemia. 1990 May;4(5):383–389. [PubMed] [Google Scholar]
- Irving J. A., Cain G., Parr A., Howard M., Angus B., Cattan A. R. OCT embedded sections of pathological specimens as a source of high quality RNA for reverse transcriptase/polymerase chain reaction. J Clin Pathol. 1996 Mar;49(3):258–259. doi: 10.1136/jcp.49.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen N., Müller J., Giwercman A., Visfeldt J., Møller H., Skakkebaek N. E. DNA content and expression of tumour markers in germ cells adjacent to germ cell tumours in childhood: probably a different origin for infantile and adolescent germ cell tumours. J Pathol. 1995 Jul;176(3):269–278. doi: 10.1002/path.1711760309. [DOI] [PubMed] [Google Scholar]
- Kaufmann M., Heider K. H., Sinn H. P., von Minckwitz G., Ponta H., Herrlich P. CD44 variant exon epitopes in primary breast cancer and length of survival. Lancet. 1995 Mar 11;345(8950):615–619. doi: 10.1016/s0140-6736(95)90521-9. [DOI] [PubMed] [Google Scholar]
- Koopman G., Heider K. H., Horst E., Adolf G. R., van den Berg F., Ponta H., Herrlich P., Pals S. T. Activated human lymphocytes and aggressive non-Hodgkin's lymphomas express a homologue of the rat metastasis-associated variant of CD44. J Exp Med. 1993 Apr 1;177(4):897–904. doi: 10.1084/jem.177.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura Y., Hanbury D., Smith J., Tarin D. Non-invasive detection of malignancy by identification of unusual CD44 gene activity in exfoliated cancer cells. BMJ. 1994 Mar 5;308(6929):619–624. doi: 10.1136/bmj.308.6929.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura Y., Tarin D. Significance of CD44 gene products for cancer diagnosis and disease evaluation. Lancet. 1992 Oct 31;340(8827):1053–1058. doi: 10.1016/0140-6736(92)93077-z. [DOI] [PubMed] [Google Scholar]
- Salles G., Zain M., Jiang W. M., Boussiotis V. A., Shipp M. A. Alternatively spliced CD44 transcripts in diffuse large-cell lymphomas: characterization and comparison with normal activated B cells and epithelial malignancies. Blood. 1993 Dec 15;82(12):3539–3547. [PubMed] [Google Scholar]
- Screaton G. R., Bell M. V., Jackson D. G., Cornelis F. B., Gerth U., Bell J. I. Genomic structure of DNA encoding the lymphocyte homing receptor CD44 reveals at least 12 alternatively spliced exons. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12160–12164. doi: 10.1073/pnas.89.24.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansfeld A. G., Diebold J., Noel H., Kapanci Y., Rilke F., Kelényi G., Sundstrom C., Lennert K., van Unnik J. A., Mioduszewska O. Updated Kiel classification for lymphomas. Lancet. 1988 Feb 6;1(8580):292–293. doi: 10.1016/s0140-6736(88)90367-4. [DOI] [PubMed] [Google Scholar]
- Stauder R., Eisterer W., Thaler J., Günthert U. CD44 variant isoforms in non-Hodgkin's lymphoma: a new independent prognostic factor. Blood. 1995 May 15;85(10):2885–2899. [PubMed] [Google Scholar]
- Sy M. S., Guo Y. J., Stamenkovic I. Distinct effects of two CD44 isoforms on tumor growth in vivo. J Exp Med. 1991 Oct 1;174(4):859–866. doi: 10.1084/jem.174.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zöller M. CD44: physiological expression of distinct isoforms as evidence for organ-specific metastasis formation. J Mol Med (Berl) 1995 Sep;73(9):425–438. doi: 10.1007/BF00202261. [DOI] [PubMed] [Google Scholar]