Abstract
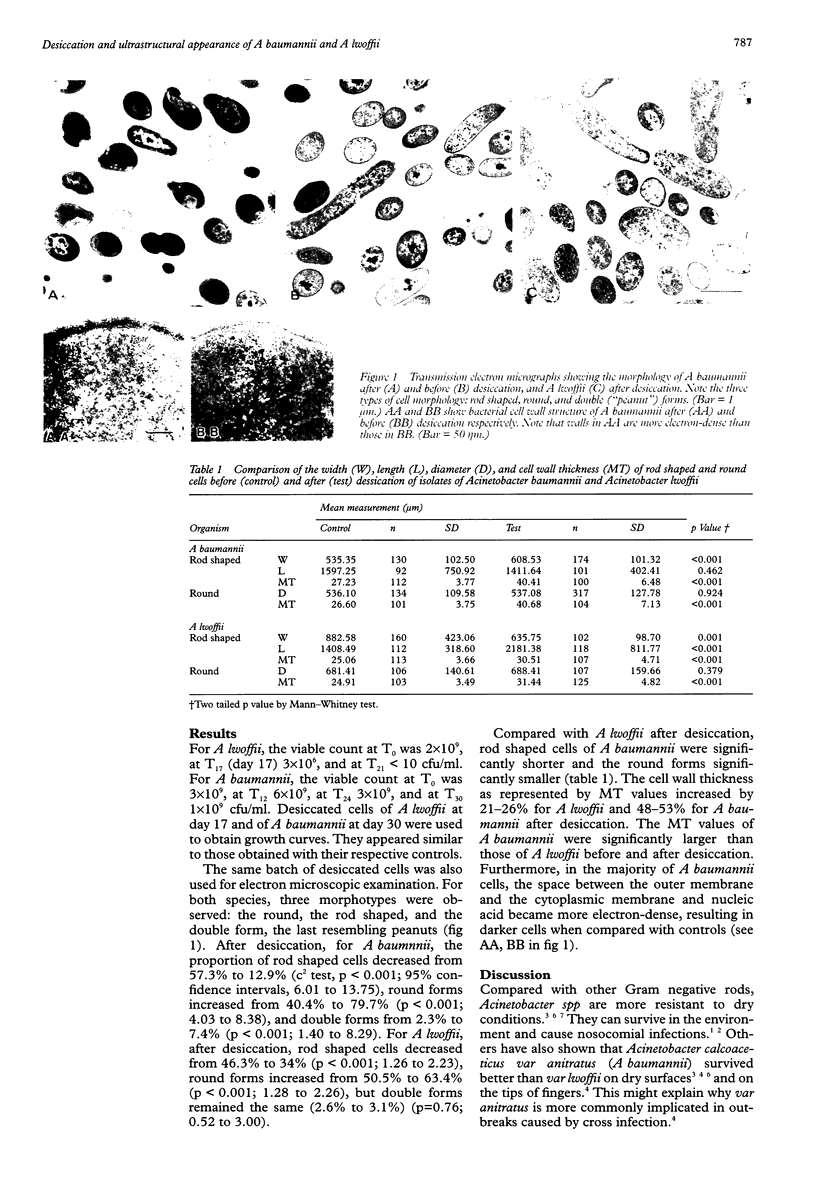
An Acinetobacter baumannii isolate survived desiccation beyond 30 days and an Acinetobacter lwoffii isolate up to 21 days. For both species, desiccation resulted in a significant increase in the proportion of round cells (A baumannii, 40% to 80%; A lwoffii, 51% to 63%) and a significant decrease in rod shaped cells (A baumannii, 58% to 13%; A lwoffii, 46% to 34%). Electronmicroscopic examination showed that there was also a corresponding significant increase in the cell wall thickness (A baumannii, up to 53%; A lwoffii, up to 26%). Desiccated A baumannii cells became more electron-dense and had significantly thicker cell walls (x1.3) than those of A lwoffii. Cell wall structures of A baumannii strains with different abilities to resist desiccation deserve further study.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergogne-Bérézin E., Towner K. J. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin Microbiol Rev. 1996 Apr;9(2):148–165. doi: 10.1128/cmr.9.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getchell-White S. I., Donowitz L. G., Gröschel D. H. The inanimate environment of an intensive care unit as a potential source of nosocomial bacteria: evidence for long survival of Acinetobacter calcoaceticus. Infect Control Hosp Epidemiol. 1989 Sep;10(9):402–407. doi: 10.1086/646061. [DOI] [PubMed] [Google Scholar]
- Hirai Y. Survival of bacteria under dry conditions; from a viewpoint of nosocomial infection. J Hosp Infect. 1991 Nov;19(3):191–200. doi: 10.1016/0195-6701(91)90223-u. [DOI] [PubMed] [Google Scholar]
- Hobot J. A., Carlemalm E., Villiger W., Kellenberger E. Periplasmic gel: new concept resulting from the reinvestigation of bacterial cell envelope ultrastructure by new methods. J Bacteriol. 1984 Oct;160(1):143–152. doi: 10.1128/jb.160.1.143-152.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James G. A., Korber D. R., Caldwell D. E., Costerton J. W. Digital image analysis of growth and starvation responses of a surface-colonizing Acinetobacter sp. J Bacteriol. 1995 Feb;177(4):907–915. doi: 10.1128/jb.177.4.907-915.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawad A., Heritage J., Snelling A. M., Gascoyne-Binzi D. M., Hawkey P. M. Influence of relative humidity and suspending menstrua on survival of Acinetobacter spp. on dry surfaces. J Clin Microbiol. 1996 Dec;34(12):2881–2887. doi: 10.1128/jcm.34.12.2881-2887.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musa E. K., Desai N., Casewell M. W. The survival of Acinetobacter calcoaceticus inoculated on fingertips and on formica. J Hosp Infect. 1990 Apr;15(3):219–227. doi: 10.1016/0195-6701(90)90029-n. [DOI] [PubMed] [Google Scholar]
- Thorne K. J. Regularly arranged protein on the surfaces of Gram-negative bacteria. Biol Rev Camb Philos Soc. 1977 May;52(2):219–234. doi: 10.1111/j.1469-185x.1977.tb01351.x. [DOI] [PubMed] [Google Scholar]
- Wendt C., Dietze B., Dietz E., Rüden H. Survival of Acinetobacter baumannii on dry surfaces. J Clin Microbiol. 1997 Jun;35(6):1394–1397. doi: 10.1128/jcm.35.6.1394-1397.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]



