Abstract
Altered mitochondrial metabolism is the underlying basis for the increased sensitivity in the aged heart to stress. The aged heart exhibits impaired metabolic flexibility, with a decreased capacity to oxidize fatty acids and enhanced dependence on glucose metabolism. Aging impairs mitochondrial oxidative phosphorylation, with a greater role played by the mitochondria located between the myofibrils, the interfibrillar mitochondria. With aging, there is a decrease in activity of complexes III and IV, which account for the decrease in respiration. Furthermore, aging decreases mitochondrial content among the myofibrils. The end result is that in the interfibrillar area there is an approximate 50% decrease in mitochondrial function, affecting all substrates. The defective mitochondria persist in the aged heart, leading to enhanced oxidant production and oxidative injury and the activation of oxidant signaling for cell death. Aging defects in mitochondria represent new therapeutic targets, whether by manipulation of the mitochondrial proteome, modulation of electron transport, activation of biogenesis or mitophagy, or the regulation of mitochondrial fission and fusion. These mechanisms provide new ways to attenuate cardiac disease in elders by preemptive treatment of age-related defects, in contrast to the treatment of disease-induced dysfunction.
Keywords: oxidative phosphorylation, electron transport chain, reactive oxygen species, cardiolipin, fatty acid oxidation, ubiquinol:cytochrome c oxidoreductase
Subject Terms: Aging, Metabolism, Oxidant Stress, Ischemia, Pathophysiology, Myocardial Biology
1. Introduction
With age, the heart exhibits a decrease in the number of myocytes with a concomitant increase in the size of each cardiomyocyte and an increased accumulation of lipid and areas of fibrosis. There is increased reactive oxygen species (ROS), which are considered to be of mitochondrial origin and lead to increased cell loss. The uptake of fatty acids through the sarcolemma is augmented because of an increase in the amount of transport protein, CD36, but fatty acid oxidation is decreased.1 Additionally, the aged heart has a reduced tolerance for stress, as exemplified by the greater injury than in the adult following ischemia/reperfusion. The heart relies on continuous production of energy and mitochondrial oxidative phosphorylation as the predominant source of this energy. Cardiac mitochondria generate 90% of the ATP in heart, but their importance is not restricted to bioenergetics, as they also act as a metabolic waystation, which contributes to the balance between glucose and fatty acid oxidation. The structure of the heart is intimately connected to the presence of two populations of mitochondria, one under the sarcolemmal membrane (SSM) and the other between the myofibrils (IFM); these two populations have different activities.
The mitochondrial defect in aging has been localized to the IFM population. Furthermore, this population of mitochondria in the aged heart generates the increased ROS. The former defect and its ROS consequence in mitochondrial energy metabolism are considered to be the underlying factors in aging, which affects an ever-growing segment of our population. Furthermore, impaired mitochondrial energy metabolism contributes to myocardial injury during stress such as ischemia and reperfusion. Defects in mitochondrial energy production in the heart during aging are now recognized as central players in impaired cellular and organ function. Extending beyond oxidative metabolism, recently described mitochondrial responses including mitochondrial dynamics of fission and fusion; the interrelated potential clearance of dysfunctional mitochondria are appreciated to potentially contribute to the aging cardiac phenotype. In this review of mitochondrial metabolism in the aged heart, we will discuss these issues and address areas where additional research is required.
2. Metabolism and Metabolic Flexibility in the Aging Heart
Mitochondrial oxidative phosphorylation (OXPHOS) is the main energy source for the myocardium. In cardiac mitochondria, fatty acids and carbohydrate are the two main classes of substrates used to generate ATP through OXPHOS.2 Under physiologic conditions, the heart receives approximately 70% of its energy from fatty acid oxidation and 30% from metabolism of carbohydrates via pyruvate and lactate. Under different conditions, the ratio of energy supply from fatty acid and carbohydrate is dynamically adjusted, indicating that heart has a flexibility of substrate usage.3 The impact of aging on substrate flexibility is incompletely understood.
Glycolysis yields pyruvate in the cytoplasm. To enter the mitochondria, pyruvate, first traverses the mitochondrial outer membrane via the voltage-dependent anion channel (VDAC) and then the inner membrane via monocarboxylate transporter system.4, 5 Once in the mitochondrial matrix, pyruvate dehydrogenase (PDH) metabolizes pyruvate and generates NADH and acetyl-CoA. From acetyl-CoA, citrate synthase produces citrate using oxaloacetate from the tricarboxylic acid cycle (TCA) located in the matrix space.4 In addition to pyruvate, other TCA substrates are regulated by the action of inner membrane transporters. Pyruvate dehydrogenase complex (PDC), a gatekeeper for mitochondrial glucose oxidation, is a key enzyme complex that modulates the competition between fatty acid and carbohydrate metabolism. PDH activity is regulated by pyruvate dehydrogenase kinases (PDKs) that respond to allosteric modifiers derived from glycolysis and fatty acid oxidation.5 NADH and acetyl-CoA derived from fatty acid oxidation (and pyruvate oxidation) activate PDKs, which leads to PDH inhibition, whereas pyruvate generated from glycolysis deactivates the PDKs, in turn activating PDH. PDK activity is regulated via post-translational modifications including phosphorylation and lysine acetylation.6 PDC regulation is critical to regulation of the metabolic flux of carbohydrate in pathophysiologic states.5 In aging, post-translational modification by PDK via altered phosphorylation increases flux through PDC, favoring mitochondrial metabolism that leads to enhanced glucose oxidation.7 Thus, there is increased capacity for glucose oxidation by mitochondria via enhanced PDC-mediated pyruvate metabolism. Also there is increased capacity for glucose oxidation by mitochondria via enhanced PDC-mediated pyruvate metabolism. When PDH activity is measured without inhibiting PDK during the assay, apparent PDH activity is decreased in aged rat hearts, but the total activity was not measured.8
Fatty acids are oxidized mainly within mitochondria. Beta oxidation of fatty acids occurs in the matrix following activation and transport of fatty acids into the matrix via contact sites.3 Beta oxidation also produces NADH and FADH2. Fatty acid oxidation is decreased in the aging heart.9, 10 PDK4 content is decreased in the aged heart,9 favoring increased glucose oxidation at the expense of fatty acid oxidation.
In contrast, the enzymes involved in the regulated transport of long-chain fatty acids into mitochondria and oxidation are unaltered despite decreases in PPARγ activity.9 The long-chain fatty acyl-CoA is first converted to the long-chain acylcarnitine through carnitine palmitoyltransferase I (CPT-I).11 The long-chain acylcarnitine is transported into the mitochondrial matrix across the inner mitochondrial membrane by carnitine-acylcarnitine translocase. Then the long-chain acylcarnitine is converted back to matrix long-chain acyl-CoA by carnitine palmitoyltransferase II (CPT-II).12 Among these enzymes, CPT-I is the rate-limiting step in regulating fatty acid oxidation. There are two isoforms of CPT-I: liver type isoform and muscle type isoform. The dominant CPT-I isoform in adult heart is the muscle type isoform, which is very sensitive to malonyl-CoA inhibition. Malonyl-CoA is produced through carboxylation of acetyl-CoA by acetyl-CoA carboxylase and is converted back to acetyl CoA by malonyl-CoA decarboxylase. The increased pyruvate oxidation results in inhibition of fatty acid oxidation by increasing acetyl-CoA and subsequent malonyl-CoA generation.3, 13 In contrast, increased fatty acid oxidation can inhibit pyruvate oxidation by generation of acetyl-CoA. Thus, there is interaction between fatty acids oxidation and glucose metabolism. Aging does not alter CPT-I content nor phosphorylation, nor is there a shift of isoform.3
We found no change in CPT-I velocity or sensitivity to malonyl-CoA in either population of heart mitochondria. In contrast, the Hagen laboratory described a decrease in palmitoyl-CoA oxidation and CPT-I activity only in IFM. We believe the decreased palmitoyl-CoA oxidation occurs as a result of the IFM ETC defects; nagarse was used to isolate IFM14 and that is associated with a decrease in CPT-I activity, whereas we employ trypsin without an effect on CPT-I.15 Acetyl-L-carnitine supplementation reverses the age-related decline in CPT1 activity in interfibrillar mitochondria without changing the L-carnitine content in the rat heart.14
Fatty acid metabolism is altered in disease conditions that could interact with age-induced defects. In an in vitro working model, fatty acid oxidation is decreased with compromised cardiac function in aged mouse hearts exposed to high afterload.16 Ischemia leads to decreased malonyl CoA content and increased FAO by increasing CPT-I activity. The circulating fatty acid concentration also is increased following ischemia.17
The harnessing of chemical energy by mitochondria as an inner membrane potential requires the inner membrane to have tightly-controlled permeability. The inner membrane contains ion channels and transporters that regulate proton and ion fluxes as well as the uptake of substrates and the translocation of small molecules into and out of the matrix.2
The phosphorylation apparatus harnesses the electrochemical energy stored in the inner membrane proton gradient to phosphorylate ADP by complex V.2 Uncoupling of electron flux from ADP phosphorylation occurs with dissipation of the electrochemical gradient. Mechanisms to dissipate the electrochemical gradient under physiologic circumstances include activation of uncoupling proteins that span the inner membrane18 and regulated “proton slip” within electron transport chain (ETC) complexes.19 Membrane potential controls the rate of respiration since in complexes I and III electron flow with the complex includes a segment where electrons move counter to the membrane potential. In disease settings, dissipation of the electrochemical gradient occurs due to breaches in the inner membrane or damage to complex V that allow back-diffusion of protons down their electrochemical gradient not coupled to phosphorylation. As discussed below, damage to inner membrane phospholipids, especially cardiolipin,20 or activation of the mitochondrial permeability transition pore are mechanisms 21, 22 that lead to pathologic uncoupling of respiration.
Respiratory defects present in mitochondria are reflected in the rate of global respiration in permeablilized muscle fibers.23 Even though only IFM mitochondria are affected by age, global respiration in the fibers is decreased. Decreases in the rate of oxidative phosphorylation with substrates that selectively donate to complex III or complex IV are observed, supporting the impact on global respiration in the myocyte of the age-induced decrease in respiration in IFM.23
Interfibrillar mitochondria (IFM) isolated from 24 and 28 month aged Fischer 344 rat hearts exhibit decreased OXPHOS, whereas respiration in SSM is unaffected.24 Although the ADP-stimulated rates of respiration are decreased in IFM, oxygen consumption remains tightly coupled to ADP phosphorylation.24 The decrease in uncoupled respiration localizes the defect to the ETC.24 The decrease in respiration using selective donors to complex IV localizes a defect at least to cytochrome oxidase, the most distal complex,24 as discussed below. Controversy regarding the presence of age-related defects was based on the fact that in some laboratories no defect was found when isolation techniques yielded only SSM25,26 whereas combined mitochondrial populations were studied in different laboratories27,28, 29 and in the later case, the IFM defect in fact is masked to a great extent by the presence of unaffected SSM. Age-related decreases in fatty acid oxidation 28 and cytochrome oxidase activity 30–32 were localized to IFM.24 Previous studies of mitochondrial populations containing only SSM found, as expected, that ROS production 33 and protein import 34 were unaltered by age. The finding of defects localized to IFM is not unique to aging and is also observed in diabetes35 and cardiomyopathy.36 Decreased OXPHOS capacity has been observed in a variety of rat strains 37 as well as murine models of aging.38 The study of IFM, in which key aging-related alterations in OXPHOS reside, without the confounding effects of admixed, unaltered SSM, has facilitated the study of the contributions of mitochondria to age-related dysfunction in the heart.39
The identification of each population is based upon location in the myocyte and the documentation of selective isolation based upon electron microscopy. Molecular markers for each population remain elusive. Unfortunately, there have not been studies of the global mitochondrial proteome to compare SSM and IFM in adult and aged hearts, unlike in the diabetic heart.35 Nonetheless, responses at the molecular level differ between the two populations. In a proteomic study of the acetylated proteins in each population, differences between SSM and IFM were observed. The differences included outcomes from both endogenous acetylation/deacetylation as well as responses to chemical/non-enzymatic acetylation.40 Of note, SIRT3 content was decreased in SSM but remained unaltered in IFM. 40
It remains a possibility that the alterations in each population arise as a result of differences in regional environments within the cardiac myocyte. Global ATP content in adult and aged hearts are similar.41 The ATP content in the subsarcolemmal region where SSM reside could differ from that in the remainder of the myocyte. IFM are in proximity to t-tubules in the sarcomeres, which may increase exposure of the IFM to calcium. The relative interaction of SSM versus IFM with sarcoplasmic reticulum via mitochondria-associated membranes also is unknown. The regional environment may provide a milieu for selective defects observed in IFM in genetic36 and diabetic35 cardiomyopathies as well as with aging due to the impairment of the removal of damaged mitochondria from this region. The regional differences in mitochondrial function, either as a result of damage to the mitochondria by external mechanisms or regional impairment in function, may be attenuated by the presence of connections between mitochondria in subsarcolemmal and interfibrillar regions.42, 43
The role of regional differences within the myocyte due to genetic deletion or overexpression of nuclear encoded mitochondrial proteins has not been considered. It would initially seem that both SSM and IFM populations would be affected by the absence or overexpression of a nuclear-encoded mitochondrial component protein to a similar extent. Thus, the study of alteration in mitochondrial function that involved only SSM or IFM in response to gain of function or loss of function of a mitochondrial protein encoded by the nuclear genome would seem to be irrelevant. However, in light of the potential for mitochondrial damage due to regional-specific stresses, or, possibly, defective generation or removal mitochondria specific to one region of the myocyte, it is plausible that despite the similar gain or loss of a mitochondrial component, that dysfunction may be manifest in only one subpopulation of mitochondria.
3. Mitochondrial Morphology in the Aging Heart
3A. Subsarcolemmal vs. Interfibrillar mitochondria
Using transmission electron microscopy (TEM) to examine heart ventricular tissue, the presence of two spatially distinct populations of mitochondria is apparent. Using a Polytron homogenizer, skinned fibers are produced: these lack surface mitochondria, but retain mitochondria trapped between the myofibrils. This observation provides morphological evidence of the two disparate types of mitochondria: one type clustered beneath the sarcolemma (SSM), the other type, “imprisoned” by myofibrils are the interfibrillar mitochondria (IFM).44 The content of IFM is decreased in the aged heart.24 As supported by rigorous studies of the recovery of mitochondrial marker enzymes, the decreased content of IFM isolated from aged hearts reflects a true decrement present in myocardium.24 In contrast, the content of SSM is unaltered with age. Thus, the decreased content of IFM magnifies the respiratory defect present in IFM. Regional differences in mitochondrial function have been observed in multiple species including mouse, hamster, guinea pig, rabbit, and dog. Aging defects in mitochondrial function have been observed in mouse and human, although studies have not yet addressed if defects are present in only one subpopulation.
3B. Mitochondrial morphology in the aging heart: Cristae and contact sites
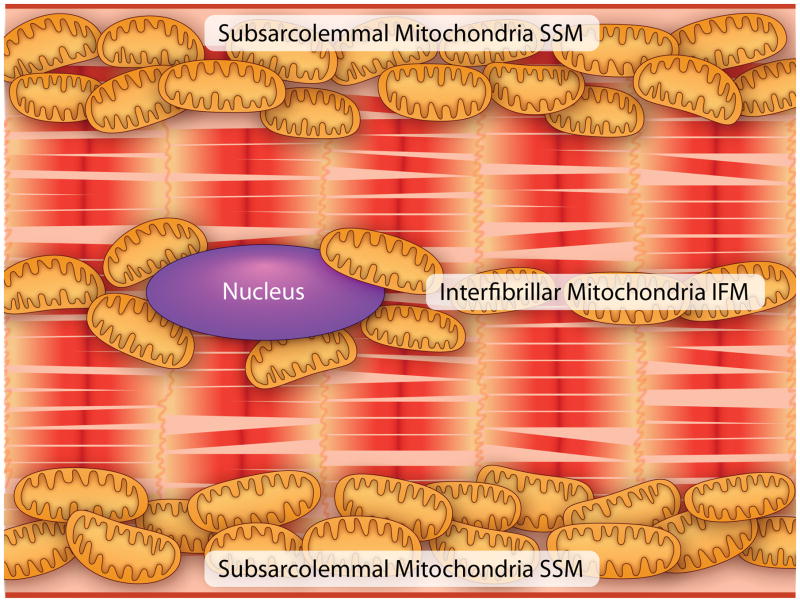
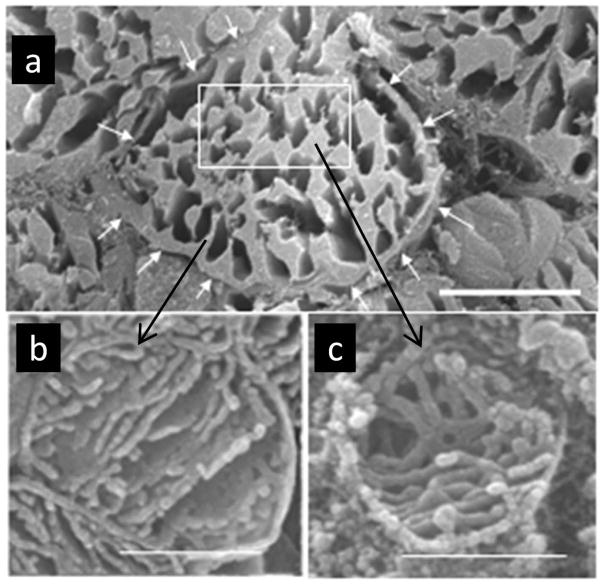
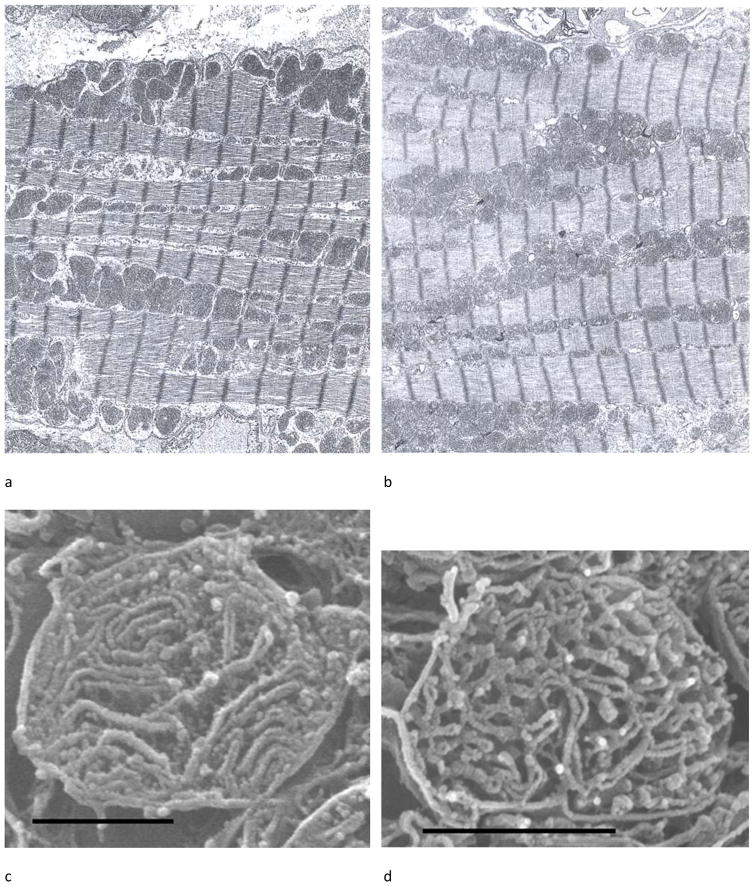
The cartoon in Figure 3 depicts a cardiomyocyte to illustrate the location of SSM under the sarcolemmal membrane and IFM between the myofibrils (Figure 3). Structural differences of the two populations of mitochondria in adult and aged Fischer 344 rat hearts using TEM are not apparent in in situ or in isolated organelles (Figure 4a, b).24, 44, 45 However, osmium-extracted cardiomyocytes examined by high resolution scanning electron microscopy (HRSEM) revealed differences in the three-dimensional structure of cristae. As shown in Figure 5a for adult rat heart, the sarcolemma is clearly observed, immediately beneath the sarcolemma are the SSM (shown by the arrow to Figure 5b). The empty spaces within the fiber represent areas previously containing myofibrils, which have been extracted by the osmium process. Around these voids are the organelles, the IFM, which are stacked as shown in Figure 5a. The arrow points to an IFM at greater magnification (Figure 5c). The SSM contain largely lamelliform cristae (Figure 5b), but in contrast the IFM have mostly tubular cristae (Figure 5c). Using HRSEM, in the 24-month old Fischer 344 rat the isolated SSM are mainly lamelliform (Fig. 4c) whereas the IFM contain predominantly tubular cristae (Fig. 4d).46, 47 There are no age-related alterations in crista morphology that accompany the biochemical defects. In this study, isolated mitochondria show relatively more plasticity with rearrangement of the cristae morphology, but again there are no structural differences between the two ages for either population of mitochondria. However, a recent study of 24 mo. aged Wistar and OXYS rats using TEM described alterations in cristae structure with age.48 The different results could be due to the different strains of rat using different fixation.24
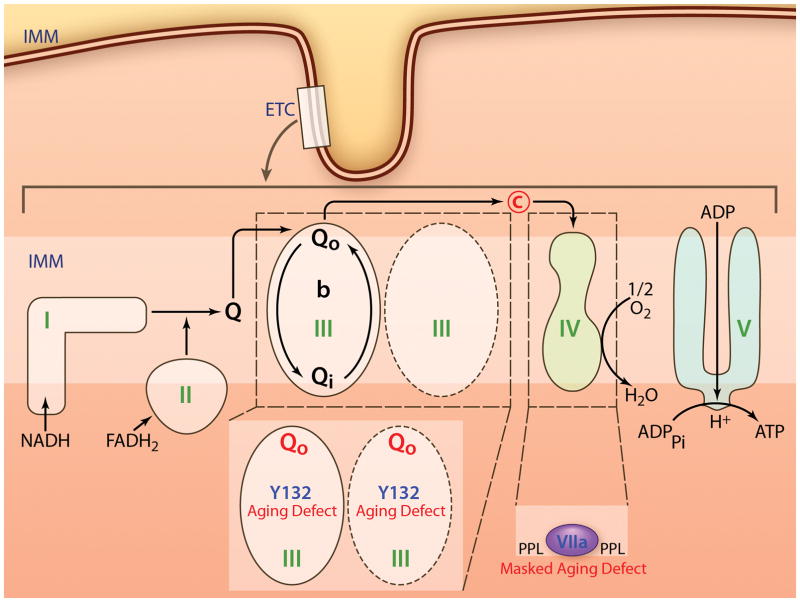
Figure 3.
A schematic of a cardiomyocyte to highlight the location of subsarcolemmal (SSM) and interfibrillar mitochondria (IFM). (Illustration Credit: Ben Smith).
Figure 4.
Ultrastructure of young and aged Fischer 344 rat hearts. (a) Electron micrograph of myocardium of a 6-month rat. (b) Myocardium of a 28-month rat. SSM are clustered in groups under the sarcolemmal membrane; IFM are between the myofibrils. There are no structural differences noted between the two ages.24 (c) Isolated SSM from a 24-month rat, osmium-extracted. The cristae morphology is mixed between lamelliform and tubular. (d) Isolated IFM from a 24-month rat, osmium-extracted. Cristae are primarily tubular.47 Magnification in (a) and (b) is 6000 and the bar in (c) and (d) is 1 μm.
Figure 5.
(a) Osmium-extracted cardiomyocyte in situ from an adult rat heart observed in cross-section by HRSEM. The sarcolemma is pointed out by the series of white arrows. The SSM are under the sarcolemma; the black arrow to Fig. 1b where a typical SSM is shown at higher magnification. The box identifies the more central area where the myofibrils have been extracted by the osmium treatment exposing the IFM. The arrow to Fig. 1c shows an IFM in higher magnification. (b) A SSM containing lamelliform cristae exclusively. (c) An IFM with tubular cristae forming a lattice. Scale line for (a) = 4 μm and for (b) and (c) = 0.5 μm.
The images are taken from Figure 1a and Figure 2a and 2d from reference 46.
3C. Cardiolipin: Membrane building block and signaling component
Cardiolipin (CL) is a diphosphatidylglycerol lipid found largely in the mitochondrial inner membrane and that is required for optimal energy generation.20, 49 CL is highly enriched in oxidatively-sensitive linoleic acid (C18:2).50 Aging did not alter the content or composition of CL in SSM or IFM from elderly 24 month Fischer 344 rats compared to adult.51 CL content was documented by analytic phosphate assay with recovery studies.51 The acyl-group composition and molecular species of CL also was unaltered in aged hearts. In contrast, the Paradies group has described a decrease in CL content with aging estimated by UV absorbance using a mixed population of cardiac mitochondria.30, 32 However, in our hands CL content using this approach also was unchanged in IFM with aging.51 Thus, although others propose decreases in CL content with aging leading to altered membrane fluidity in the inner membrane,52, 53 the role of CL content as a mechanism of age-induced defects in electron transport remains controversial.
CL undergoes age-specific modification in response to the stress of ischemia in the aged heart. 54, 55 Consistent with a lipid peroxide mechanism, the new molecular species involves the addition of 48 daltons to a single linoleic acid acyl-group, consistent with the formation of a hydroxyl-lipid peroxide. Products of linoleic acid with molecular weight increased by 48 daltons are formed during the mitochondria-driven peroxidation of linoleic acid.56, 57 CL with a molecular weight also increased by 48 daltons is present during in situ oxidative stress in other systems.58 The oxidation of CL provides strong support for the presence of age-enhanced oxidative damage to mitochondria during ischemic stress in the aged heart.
The formation of a single product, rather than mixture of oxygenated linoleic residues,54 suggests that a specific, perhaps enzymatic, mechanism of peroxidation occurs in contrast to stochastic chemical peroxidation. Cytochrome c can alter its interaction with CL59 leading to a conformational change that favors the oxidation of a key methionine ligand of the cytochrome c heme, resulting in formation of a new peroxidase activity. CL is oxidized by this newly generated cytochrome c peroxidase.58,60 The key oxidative modification of cytochrome c required for acquisition of peroxidase activity occurs during ischemia,54, 55,61 targeting oxidative modification as a plausible mechanism.
Alteration in CL species often results from defective remodeling of CL.60 Under oxidative conditions, monolysocardiolipin (MLCL) is generated via hydrolysis of an oxidized C18:2 residue The MLCL then undergoes reacylation via a process of salvage remodeling.60 The enzyme, tafazzin, remodels nascent CL during de novo synthesis to the required (C18:2)4 species.62, 63 Tafazzin, however, appears to contribute a minor, if any, step in salvage remodeling of CL in the mature heart in response to disease-induced CL oxidation and damage.64 In contrast, acyl-CoA:lysocardiolipin acyltransferase-1 (ALCAT-1) is a major contributor to the salvage remodeling of CL.60, 65, 66 In ALCAT-1 mediated remodeling, MLCL is usually reacylated using non-linoleate acyl-residues.60, 66, 67 including very long chain fatty acids, such as docosahexanoic acid (C22:6), creating higher mass CL species.60, 68 Substitution of docosahexanoic acid occurs in heart failure and diabetes.68 Thus, an alternative mechanism of formation of the new molecular species is that a higher molecular weight acyl-group has been substituted for a C18:2. However, the impact of higher molecular weight acyl residues on OXPHOS is unclear, since mitochondria with increased docosahexaenoic and arachidonic content in CL displayed unaltered rates of OXPHOS.69 The presence of non-linoleic acyls disrupts the compact configuration of (C18:2)4-CL, paradoxically increasing the oxidative sensitivity of the asymmetric CL.20 ALCAT1 is located in the endoplasmic reticulum,70 suggesting that secondary CL remodeling may require mitochondria-endoplasmic reticulum interactions. Since secondary remodeling of CL by ALCAT1 is extramitochondrial, it is unlikely that regional defects would be present. Thus, the lack of altered remodeling in IFM from the age heart is consistent with ALCAT1 as the major mechanism of remodeling.
Oxidation of CL decreases the function of ETC complexes,71 disrupts the bilayer arrangement of the inner membrane,20, 60 and favors the release of cytochrome c to initiate cell death.58, 72 CL oxidation likely will continue to emerge as a key mechanism of the enhanced mitochondrial-dependent injury that occurs in the aged heart in response to pathologic stress, and is a potential mechanism of the augmented age-enhanced susceptibility to superimposed cardiac disease.
SS-31, (a synthetic peptide), can directly bind to CL on the inner membrane and improve the efficiency of the ETC. SS-31 inhibits the function of the cytochrome c cardiolipin peroxidase.73 SS-31 treatment increases mitochondrial efficiency in aged skeletal muscle, decreases cardiac injury during ischemia-reperfusion, and improves mitochondrial function in heart failure.73 These actions support the pathogenic role of cardiolipin oxidation by the cytochrome c peroxidase in the heart under stress.
4. Age-Induced defects in Electron Transport
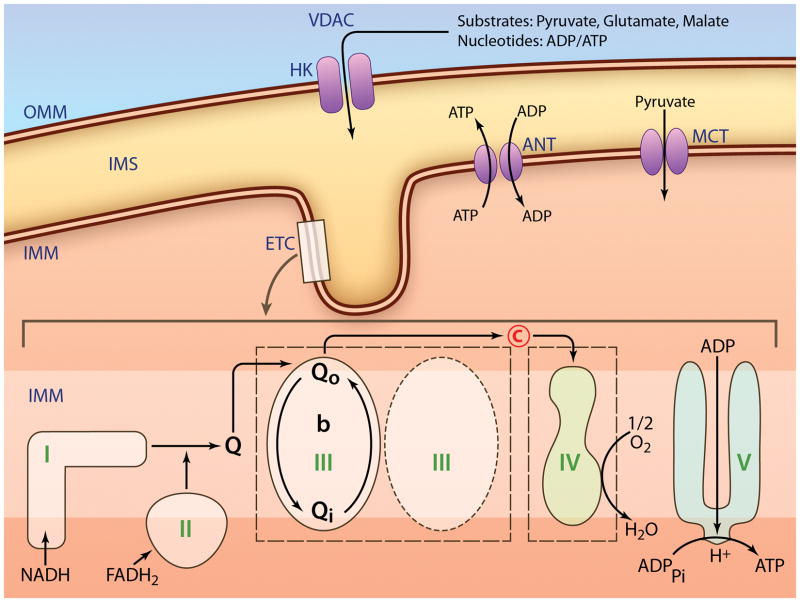
The ETC is composed of four multisubunit enzyme complexes, complexes I-IV (Figure 1). Electron transfer down the redox potential gradient from NADH or FADH2 to oxygen is coupled to the active transport of hydrogen ions from the matrix to the cytosolic side of the inner membrane by complexes I, III, and IV.74 Complex I oxidizes NADH, with sequential electron flow to coenzyme Q, complex III, cytochrome c, and ultimately to cytochrome oxidase (complex IV), the latter reducing oxygen to water. The complexes of the ETC are organized into larger respirasomes (“supercomplexes”),75, 76 to optimize channeling of reducing equivalents between the components. The organization of respirasomes is altered in the aged heart,77 which can result in functional defects in electron transport in the absence of post-translational modification or decreased content of an individual ETC complex. For example, although OXPHOS of substrates that enter through complex I is decreased, the site of ETC defects in complexes III and IV (discussed below) account for the decrease. Complex I enzyme activity remained unaltered by age in rat24 and mouse hearts.78 Functional decreases in complex I might be related to alteration in complex I containing respirasomes,77 potentially related to oxidative damage to CL.79 Such alterations in supercomplex organization are observed following the onset of congestive heart failure.80
Figure 1.
Schematic of mitochondrial metabolism. OM, mitochondrial outer membrane; HK, hexokinase; VDAC, voltage-dependent anion channel; IMS, intermembrane space; IM, mitochondrial inner membrane; MCT, monocarboxylate transporter; ANT, adenine nucleotide translocase; IMM, mitochondrial inner membrane; ETC, electron transport chain; Q, ubiquinone; C, cytochrome c. (Illustration Credit: Ben Smith).
4A. Cytochrome oxidase (complex IV)
The aging defect in cytochrome oxidase activity occurs as a result of an altered inner membrane environment of the complex.24, 31, 32 Activity is recovered by the addition of phospholipid liposomes,24 including CL.32 The finding of unaltered CL content and composition in IFM, despite the presence of a functional defect, supports the presence of a probable defect in CL in the immediate environment of cytochrome oxidase. Such a defect likely impacts the organization of cytochrome oxidase into supercomplexes,81 with subsequent decreased enzyme activity. The altered membrane environment leads to a decrease in fluidity of the inner membrane.52, 53 Altered phase behavior of the inner membrane with age may occur due to the depletion of n-3 acyl-groups 82 or to oxidative damage in membrane phospholipids.83
In addition to the phospholipid environment defect, there is a functional defect regarding the subunits of cytochrome oxidase. The content of subunits are unaltered with age, as identified by analytical immunoblotting.84, 85 In contrast, immunoelectron microscopy revealed a 25% reduction in cytochrome oxidase subunit VIIa selective to IFM in the aged heart. The absence of immunoreaction of the VIIa subunit in situ assessed by immunoelectron microscopy was not due to a reduction in protein, but rather by a masking phenomenon or by an in situ change in protein structure affecting cytochrome oxidase.
Decreased cytochrome oxidase activity not only has the potential to impair energy production, but also to enhance cytotoxicity. Attenuated flux through cytochrome oxidase increases the relative reduction of “upstream” redox centers in complexes I and III, favoring ROS production as discussed below. Additionally, decreased cytochrome oxidase activity activates cell stress responses,86 in part via the activation of endoplasmic reticulum stress.87 Cytochrome oxidase content and activity are decreased in the aged human heart.84, 85
4B. Complex III
Complex III activity is decreased only in IFM in the aged heart.88 Mammalian complex III is composed of two 11 subunit monomers, with three of these subunits, cytochrome b, cytochrome c1, and the iron-sulfur protein (ISP) involved in electron transfer.89, 90 Following oxidation of the substrate, ubiquinol, there is a concerted transfer of electrons to cytochromes b and c1 in a bifurcated fashion mediated by motion of the ISP.89, 90 Based upon structure-functional relationships of electron flow within complex III, 91–94 the study of mutant complex III phenotypes in bacteria,91, 94–96 and the three-dimensional crystal structure, 89, 90 the study of partial reactions of complex III was utilized to localize the defect to the ubiquinol binding site (Qo) within cytochrome b. The use of specific chemical inhibitors further localizes the defect to the proximal domain of the Qo site. By comparison to phenotypes that result from site-directed mutagenesis in the bacterium Rhodobacter spheroids (bacteria allow straightforward site-directed mutagenesis of cytochrome b), the region of the age-induced defect can be deduced. The aging phenotype of complex III consists of decreased maximal activity,88 preserved EPR signal,88 an increased leak through myxothiazol blockade to reduce cytochrome b, and preserved inhibition by stigmatellin.97 Amino acids in the Qo site are highly conserved from R. spheroides to mammalian cytochrome b.95 Point mutations with resistance to myxothiazol but not to stigmatellin (A126, Y132, V133, M139, G143, F275)95 are potential sites of the age-related defect. In addition to myxothiazol resistance, mutation at Y132 also decreases complex III activity, yet maintains the EPR signal.95 For this reason, Y132 can be considered as a potential site of aging-induced oxidative modification to the Qo site, a mechanism of the aging phenotype of complex III. Proteomic evaluation of the Qo site, including Y132, is limited by the difficultly of proteomic approaches to this most hydrophobic region of the markedly hydrophobic cytochrome b subunit. Current proteomic techniques should provide approaches to study this key region of age-induced alteration in electron transport. Cytochrome b is encoded by mitochondrial DNA, so mitochondrial DNA mutations with age could alter the composition of the Qo site with subsequent alteration in complex III activity and age-induced pathophysiology. Unfortunately, because it is a mitochondrial-encoded protein, direct molecular manipulation to test the role of Y132 is not feasible due to the multiple copies of mtDNA present.
4C. Complex V
The activity of complex V decreases with age,98, 99 potentially decreasing the efficiency or coupling of OXPHOS. Localization of the site of the defect within complex V remains unknown. Age-induced alteration of complex V is of contemporary interest since complex V, recently identified as the likely locus of the mitochondrial permeability transition pore,100 has an increased propensity to open, even in the baseline state, in the aged heart.101 Nonetheless, uncoupled respiration is decreased by age in IFM in aged hearts, localizing the defects that limit the rate of OXPHOS to the ETC rather than to complex V.24 The ADP:O ratio declines with age in the rat,24 perhaps reflecting a defect in this complex that modestly decreases the efficiency of phosphorylation.
5. Mitochondrial DNA with age and impact on OXPHOS
Accumulation of mtDNA deletions in cells leads to respiratory chain deficiency.102 Mitochondrial DNA copy number is decreased in aged skeletal muscle and liver compared to adults.103 However, mitochondrial DNA copy number is not altered in the aged heart,103,104 including SSM and IFM.105 However, analysis of transcripts of catalytic mtDNA encoded subunits in aged hearts is suggestive of increased mtDNA mutation with age.104 The downregulation of the 8-oxodeoxyguanosine glycosylase mouse model with defective base excision repair in mitochondria clearly supports the accumulation of oxidative damage to mtDNA with age.106, 107 The accumulation of oxidative damage to mtDNA, likely in part from mitochondria themselves as discussed below, may be a contributing mechanism to age-induced impairment of mitochondrial metabolism. Oxidative damage to mtDNA leads to point mutations and eventual double strand breaks, resulting in large deletions.108, 109 However, the accumulation of point mutations with age or in mice heterozygous for a proofreading-defective mitochondrial polymerase gamma (“mitochondrial mutator” heterozygotes) did not affect lifespan or cardiac function.108 In contrast, mice homozygous for proofreading defective polymerase gamma (Polga mut/mut) exhibit a marked increase in mtDNA mutation burden and cardiac dysfunction with an increase in the prevalence of cytochrome oxidase negative myocytes109 that are likely to exhibit defective mitochondrial respiration. Of interest, aged human hearts have an increased frequency of cytochrome oxidase negative myocytes.84, 85 Damage and deletion of mtDNA can give rise to non-homogeneous mtDNA composition between mitochondrial populations, thus increasing mitochondrial heteroplasmy.
Accumulation of mtDNA mutations with age has functional consequences for mitochondria and the heart. There is increased protein oxidation in heart tissue and increased programmed cell death.110 The finding of an age-related defect in IFM localized to mtDNA encoded cytochrome b could imply mtDNA mutation. Altered mtDNA transcription with age may also indicate defects in mtDNA regulation at levels above the mitochondrial genome itself. Recently, STAT1 localized to mitochondria was found to impact mtDNA replication.111 Potential defects in TFAM transcription, nucleoid (the mtDNA in aggregate) function may also impact mtDNA replication and stability. Although previous studies support the lack of change in mitochondrial DNA copy number, the impact of mtDNA number and mutation burden, especially differences between the two populations of mitochondria, are worthy of reassessment in the current era of deep sequencing.
6. Production of ROS by Mitochondria in the Aged Heart
6A. Sites of Production of Oxidants within the ETC
Aging increases ROS production from mitochondria in multiple cardiac models.112–114 Complexes I, II, and III are potential sources for ROS production by the ETC,115–117 with complex I producing ROS by both forward and reverse electron transport.118–120 Two mechanisms combine to increase ROS production from mitochondria in aged tissues.121 First, decreased flux through the ETC increases the reduction of upstream complexes, especially complexes I and III, increasing the probability that reduced redox centers will directly react with molecular oxygen to generate ROS.122, 123 Thus, the fraction of electron flux that forms ROS is greater during state 4 ADP-limited respiration than during state 3 ADP-stimulated respiration.112, 118 Dissipation of the inner membrane potential decreases the efficiency of phosphorylation; the increased rate of electron flux through the ETC decreases the probability that a reduced redox center will directly react with molecular oxygen. ROS production from complex III is especially responsive to modulation of inner membrane potential.124, 125 Thus, the decrease in respiration through cytochrome oxidase present in IFM from the aged heart favors the relative reduction of complexes I and III with greater ROS production. Second, aging-induced modification of individual ETC complexes alter the function of the complex to directly favor ROS production as seen with complex III in IFM.97
Complex III is a major site for net ROS release from mitochondria.115,122 ROS produced at the quinol oxidation site (Qo center) are generated at the cytosolic face of the inner membrane115, 125 and are released into the intermembrane space, ultimately favoring release from mitochondria.115, 126, 127 The aging defect at cytochrome b in the Qo site of complex III in IFM 97 predicts the increase in net production and release of ROS from IFM isolated from aged hearts. The net release of H2O2 was increased in IFM isolated from aged rat hearts compared to adult controls, whereas SSM, without the aging defect in complex III, showed no increase in ROS production.128 In line with increased ROS production within IFM, indicators of oxidative stress increased in these organelles during aging.128, 129 The increased oxidative stress to mitochondria may underlie the apparent tendency of mitochondria from the aged heart to undergo permeability transition leading to cytochrome c release as discussed below, with subsequent initiation of programmed cell death.128–130 The pathway of release of ROS generated by the Qo site of complex III is the mitochondrial contact site.115, 126, 127 Contact sites are in close association with sites of mitochondria-endoplasmic reticulum interaction.131 Thus, superoxide directed outward from mitochondria is likely to impact mitochondria-associated membranes and endoplasmic reticulum as the proximate target.132
ROS production from complex I can increase in two ways: by a blockade of forward electron flow from NADH-linked substrates via inhibition of enzyme activity or via blockade at distal sites in the ETC,112, 122, 127, 133–135 including cytochrome oxidase. Alternatively, the induction of reverse electron flow from complex II to complex I using succinate as a substrate leads to ROS production.120, 122, 136–138 Reverse electron flow requires mitochondrial membrane potential.137 Reverse flow can occur in the presence of simultaneously available complex I and complex II substrates, thus is of probable physiologic relevance.120
In addition to the ETC, p66shc and monoamine oxidase (MAO) are sources that generate ROS in mitochondria. The p66shc is a ubiquitously expressed adaptor protein139 located within the mitochondrial intermembrane space. Molecular oxygen is reduced to H2O2 in a redox center of p66Shc coupled with the oxidation of cytochrome c.140 Ablation of p66shc increases the resistance to oxidative stress and extends the life span of mice.139 Inhibition of p66shc also decreases cell injury during ischemia-reperfusion through a reduction of oxidative stress.141–143 However, knockout of p66shc increases cell injury in the mouse heart subjected to reperfusion after a short period of ischemia.144
MAO is a flavoenzyme located in the outer mitochondrial membrane. MAO includes two isoforms (MAO-A and B) that generate H2O2 when MAO oxidatively breaks down neurotransmitters such as norepinephrine, epinephrine, and dopamine.145 Oxidative stress induced by MAO contributes to cardiac injury146 and to muscle dystrophy.147 MAO-B, but not MAO-A, is increased in aged mouse hearts.148 Interestingly, only inhibition of MAO-A but not MAO-B can decrease MAO-mediated H2O2 generation in aged heart.149 MAO is a source of oxidative stress during aging
The increased production of ROS by mitochondria leads to greater oxidative damage within mitochondria, including protein sulfhydryl oxidation, lipid peroxidation, and mtDNA damage.129, 150 A relative increase in the uncoupling of respiration, 118, 119, 151 as well as an increase in antioxidant enzyme contents, provides complimentary mechanisms to decrease oxidative damage. Thus, the initial target of mitochondrial ROS production is the mitochondria themselves. Next, ROS, including superoxide can be released by traversing anion channels and contact sites, 115, 126, 127 possibly targeting structures adjacent to mitochondria. Recent work has highlighted key effectors of oxidants produced by the ETC, rather than merely stochastic oxidant release from mitochondria. Examples of oxidant dependent targeted injury include activation of mitochondrial permeability transition pore opening, generation of the cytochrome c cardiolipin peroxidase, and activation of the oxidant-dependent ASK-1 signaling cascade.
Overexpression of catalase within mitochondria attenuates cardiac aging, providing direct evidence that ROS generated from mitochondria is detrimental to aged hearts.152 Although this is not always the case is best documented in invertebrate species153 including Caenorhabditis elegans. Multiple genotypes that exhibit either decreased production or enhanced disposition of ROS exhibit increased lifespan.154,155 In mammalian systems, clear evidence of the potentially protective low levels of signaling ROS production is more difficult to demonstrate. In fact, signaling ROS are critical for the mechanism of classic ischemic preconditioning, yet, as discussed below, the aged heart is relatively refractory to the protection of ischemic preconditioning, perhaps due to the excessive tonic production of ROS.
Nrf2 (nuclear factor-erythroid 2-related factor 2) is a redox-sensitive transcription factor. Nrf2 is activated in response to oxidative stress and leads to subsequent up-regulation of antioxidants.156 Surf1 (surfeit locus protein 1) is a nuclear encoded chaperone protein and aids the assembly of the subunits of Complex IV. Knockout of the Surf1 leads to decreased activity of complex IV and the OXPHOS rate. The defect of complex IV in Surf1 knockout mice activates mitochondrial biogenesis and Nrf2 expression,157 in line with a cytochrome oxidase defect that impairs energy production and enhances ROS production. Expression of Nrf2 is decreased in elderly patients.158 Strategies to stimulate Nrf2 expression and enhance endogenous antioxidants may decrease the severity of cardiovascular disease in the elderly.159 Thus, the lack of endogenous activation of this redox sensitive transcription factor does not support an upregulation in cytoprotective ROS signaling in the aged heart.
6B. MPTP Opening in the Aged Heart: Relationship to ROS Production
The susceptibility to mitochondrial permeability transition pore opening is increased in the aged heart101 increasing the potential of augmenting disease-enhanced permeability transition. Although the precise identity of the permeability transition pore remains a matter of debate, emerging data supports complex V as a key component;100 and contributions of the phosphate carrier 160 and contact sites 161 still deserve consideration.
In contrast to the severe metabolic stress of ischemia-reperfusion with attendant calcium overload and oxidative storm, the role of enhanced permeability transition pore opening in the aged heart during baseline metabolism is possibly related to reversible, transient opening (“flickering”) of the pore. This may participate in the regulation of mitochondrial calcium content 162 and can affect membrane potential. Calcium overload in the mitochondria does not appear to be a primary mechanism of pore opening during basal metabolism, although transient calcium release from sarcoplasmic reticulum via mitochondria-associated membranes, especially in response to mitochondrial driven oxidant production as discussed above, deserves consideration. A well-known stimulus for pore opening is oxidative stress,163 especially that from the ETC. ROS production from complex I 164 and possibly complex III 165 favors pore opening in line with the sites of its production in the aged heart. Furthermore, blockade of the ETC proximal to complex III decreases the likelihood of pore opening.166 Thus, increased susceptibility to pore opening is a potential effector of ETC-mediated enhanced ROS production.
6C. Mitochondria-Metabolism Based Signaling in Response to ROS Production
Mitochondria serve as important signaling nodes for cell survival and death. In addition to serving as stochastic sources of ROS production, mitochondrial homeostasis is important for cell survival and death. Oxidant-mediated damage to proteins within mitochondria leads to activation of new, reinforcing signaling in response to cell stress. The potential of ETC-mediated mitochondrial oxidative damage increasing the susceptibility to permeability transition pore opening was discussed above. Signaling-mediated readouts of mitochondrial metabolism and oxidant production are critical to cardiomyocyte survival in the aged heart. Enhanced ROS production from IFM may indeed drive regional redox signaling. The combination of increased ROS production with relative blockade at cytochrome oxidase would support the preferential activation of the cytochrome c cardiolipin peroxidase in IFM from aged hearts.
Cytochrome c, a carrier component of the ETC, can undergo oxidative modification and activate oxidant-dependent signaling for cell death. It contains binding sites for CL.62 In the normal state, all the coordination positions of the heme iron are occupied. The heme iron has two axial ligands, His18 and Met80. Oxidation of Met80 to a methionine sulfoxide opens the axial coordination site allowing acquisition of a peroxidase function of electron transfer from H2O2 to CL.167 The peroxidation and depletion of CL signals the activation of cell death programs.58 An increase in intra-mitochondrial ROS generation coupled with the electron flow through the cytochrome c segment of the ETC oxidizes cytochrome c, resulting in depletion of CL.61 The oxidative stress of ischemia-reperfusion increases cytochrome c methionine sufoxide, indicative of peroxidase formation in the in situ heart.61 The role of cytochrome c peroxidase formation in the CL alterations that occur with aging requires further study.
The activation of oxidant-sensing signaling systems for cell survival includes the redox-sensitive protein kinase apoptosis signal-regulating kinase 1 (ASK-1). ASK1 is present in an inactive complex with the reduced form of thioredoxin (Trx). When oxidative stress is increased, ASK1 dissociates from Trx, resulting in ASK1 activation.168 ASK1 activates the mitogen-activated protein kinase cascade. In liver, aging leads to activation of ASK-1 in the baseline state.169 In response to stress, there is evidence in brain of age-enhanced activation of ASK-1.170 ASK-1 is a mediator of cell death in the heart in response to ischemia-reperfusion,171 but its role in the aging heart in response to baseline stress remains uncertain. ASK-1 activation in response to oxidative stress can impact OXPHOS and susceptibility to permeability transition.172 Thus, oxidant production from mitochondria might activate ASK-1, which interacts with cardiac stress responses,171 and potentially further alters mitochondrial function.
7. Regulation of Mitochondrial Metabolism by Transcription Factors
The ability to acutely regulate oxidative metabolism concomitant with a redirection of transcriptional programs is an appealing mechanism for a concerted cell stress response, especially for aging tissues susceptible to age-enhanced disease. Transcription factors have been identified within mitochondria.173 Some transcriptional factors localize to the outer membrane and regulate outer membrane permeability and susceptibility to cell death.173 Modulation of mtDNA expression by “nuclear” transcription factors is an elegant expression of mitochondria-nucleus cross-talk,111, 173 and likely contributes to the regulation and stability of mtDNA as discussed above. Regulation of mtDNA can impact metabolism via regulation of mtDNA-encoded catalytic subunits of complexes I, III, IV, and V. A more direct modulation of ETC activity and OXPHOS is evident for STAT3 174 and perhaps for p53.175 These latter two peptides are incorporated into mitochondria.
STAT3 provides a central contribution to stress response176 with a cardioprotective role.177 In addition to its canonical actions as a transcription factor, a pool of STAT3 resides within the mitochondria111, 174, 178 in the matrix of both SSM and IFM.179 STAT3 binds to GRIM-19, a subunit of complex I.180 Mitochondria-localized STAT3 modulates activity of the ETC, although it clearly is not present in a 1:1 stoichiometry with metabolic targets.181, 182 In STAT3-null hearts the activities of complex I and II are decreased, leading to decreased OXPHOS.174 Reconstitution of STAT3-null cells with mitochondria-targeted STAT3 containing an inactive DNA-binding domain restored deficits in OXPHOS.174 STAT3 deletion and inhibition mitochondria are more sensitive to calcium-induced opening of the MPTP,178 likely reflecting the association of STAT3 with cyclophilin D.178 The role of STAT3 mediated processes in relationship to age-related OXPHOS defects as well as the response to exogenous stress of mitochondria from aged hearts remain areas of active investigation.
Stress-induced translocation of p53 to the mitochondria involves mono-ubiquitination of a distinct cytoplasmic pool of p53 by the E3 ligase Mdm2.183 The translocation of p53 to the outer membrane modulates members of the bcl-2 family and impacts susceptibility to programmed cell death.184 p53 also accesses the matrix compartment..185 p53 is activated in senescent tissues, including endothelial cells, and thus may affect vascular function and perhaps even metabolism in the aged heart.
8. Mitochondrial Fission and Fusion
A number of excellent reviews have highlighted the importance of fission and fusion processes in cardiac injury during ischemia-reperfusion and subsequent heart failure development.186–190 Mitochondrial dynamic processes are proposed to be involved in the aging process.191 The blockade of fission or promotion of fusion contributes to cell senescence. Knockout of fis1 in cultured cells results in elongation of mitochondria accompanied by cell morphological changes of senescence including enlargement and flattening as well as staining for senescence-associated acidic β-galactosidase activity and increased expression of Mitofusin-1.192, 193 Cell senescence is observed in dysfunctional endothelial cells during aging.194 However, the impact for cardiac myocytes, which do not exhibit a classic senescent transcriptional program including β-galactosidase activity, is less clear. Nonetheless, this body of work supports the notion that enhanced mitochondrial fission may attenuate age-induced cellular dysfunction, likely via isolation and removal of dysfunctional mitochondria as discussed below. In fact, fission-associated autophagy191 is emerging as an important mechanism of mitochondrial homeostasis in the heart.
Post-translational modification of mitofusin-1 modulates cell responses during oxidative stress.195 In antimycin A-treated cells, the Mitofusin-1 level is markedly increased with augmented cell death. Antimycin-A is a complex III inhibitor that is used to increase ROS production from from the Qo site of complex III,122 favoring ROS production in a similar manner to the age-related defect discussed above.97 Proper regulation of Mitofusin-1 content leading to a balance of mitochondrial fission and fusion needs to be maintained to promote cell survival during oxidative stress. Mitofusin- ubiquitination is increased in antimycin-A treated cells, leading to proteasome degradation and decreased fusion in response to oxidative stress. Inhibition of Mitofusin-1 degradation increases cell death in antimycin-A-treated cells. These studies indicate a net protective role of mitochondrial fission in cell senescence and aging.193
The content of Mitofusin-1 and Mitofusin-2 is increased in skeletal muscle in aged mice whereas the Fis1 level is decreased. Beclin 1 content is also decreased. These results suggest that aging increases mitochondrial fusion and attenuates mitophagy.196 The findings in skeletal muscle again support the concept that fission favors protection against oxidative stress. Manipulation of mitochondrial dynamics is a new strategy to decrease cardiac injury during ischemia-reperfusion.197, 198 Thus, the enhanced oxidant production present in the aged heart may impair protective mitochondria fission-based responses to superimposed disease. Modulation of mitochondrial dynamic changes may be a strategy to attenuate the deleterious impact of age-related defects in the ETC. Decreased fission in the baseline state may predispose to an increased susceptibility of the aged heart to injury.
9. Mitochondrial Biogenesis, Dynamics and Removal
Age-related decreases in mitochondrial yield and OXPHOS are limited to IFM. The decrease in IFM may reflect a population with content decreased due to impaired production or a more rapid turnover due to increased removal. Mitochondrial biogenesis and removal are altered by age.199, In contrast to the neonatal heart, the activation of PCG1 alpha mediated mitochondrial biogenesis in the adult heart did not lead to evident region specific proliferation within the myocyte.200 The differential responses in adult and neonatal hearts to activation of a constitutively active PGC1alpha transgene might be related to the preexisting developed cardiomyocyte cellular architecture in the adult. Targeted removal of IFM may be enhanced in the aged heart. The defective IFM, with increased ROS production97 and potential for membrane depolarization due to enhanced susceptibility to permeability transition,101 should be targeted for elimination by mitophagy.201 Such removal would attenuate the oxidative-driven injury mechanisms discussed above. Membrane depolarization may activate Parkin-mediated mitophagy,202, 203 or, alternatively, appropriate activation of fission may activate fission-driven autophagy.191 However, the localization of age-related defects to IFM suggests that due to their siting among the myofibrils, that removal of these mitochondria may be impaired. In this case, reduced removal would point to decreased mitochondrial biogenesis as the mechanism of the decreased content of IFM with age. In fact, rates of replacement and half-lives of the two populations in vivo is a key question relevant to the aged heart, and can be addressed by stable isotope approaches and the analysis of mitochondrial proteins and phospholipids.204 It is critical to understand the activation of mitochondrial removal mechanisms in vivo in the aged wild-type heart, both at baseline and in disease states. Transgenic models provide key insights into mechanisms of removal, but may be less informative in identifying the pathway of activation for removal of dysfunctional mitochondria in aging with or without superimposed disease. An impaired response of mitophagy could contribute to the increased susceptibility of the aged heart to cardiac stress, and impaired biogenesis of mitochondria could enhance susceptibility of the aged heart to superimposed stress.
Proteolytic enzyme mediated protein degradation impacts cardiovascular dysfunction in acquired heart disease.201, 205, 206 An increase of Omi/HtrA2 (an intermembrane space protease) content enhances cardiac injury in aged hearts compared to adult hearts.207 In contrast, loss of HtrA2/Omi causes premature aging.208 In aged rat hearts, Lon protease activity is decreased even despite an increase in protein content.209 However, the downregulation of Lon can decrease cell injury during hypoxia-reoxygenation.210. The decreased Lon activity may contribute to suppressed autophagy during aging.211
10. Harnessing the Mitochondria: Treatment of Age-Enhanced Cardiac Disease by Modulation of Aging-Induced Defects
Mitochondria-directed strategies to limit cardiac injury are highly relevant to the protection of the aged heart and to the ultimate improvement of clinical outcomes in high-risk elderly patients. Relevant to mitochondrial metabolism in the aged heart and treatment of cardiac disease in elders is the goal to modulate or mitigate age-related defects in mitochondrial metabolism in order to reduce injury from subsequent cardiac disease. Novel therapies are required because classical approaches widely used in experimental studies in the adult heart are of limited benefit when applied to the aged heart. Activation of cytoprotective signaling cascades that center on mitochondria in ischemic preconditioning and postconditioning are clearly less effective in the aged heart.212–217 It remains unclear if the ineffectiveness of signaling-based cytoprotection in the aged heart is at the level of the signaling cascades upstream of the mitochondria or the inability of dysfunctional mitochondria to respond to the cytoprotective modulation. It appears that mitochondria in the aged heart retain the capacity to respond to cytoprotective modulation since endogenous protective mechanisms can be restored in aging hearts by treatments including caloric restriction (CR)213 and exercise.218
Compared to young rats, the rate of oxidation is decreased in permeabilized cardiac fibers isolated from aged rat hearts using pyruvate-/malate and succinate as complex I and complex II substrates.219 CR improves OXPHOS with complex I but not complex II substrates. CR also improves complex I activity in aged hearts through induction of ND6 expression (a complex I subunit encoded by mitochondrial DNA).219 CR does not affect ETC enzyme activity nor mitochondrial respiration in young rats.219 CR can improve mitochondrial bioenergetics through reduction of oxidative stress and activation of sirtutins.220 Resveratrol supplementation also provides benefit in delaying aging-mediated cardiovascular dysfunction. CR, exercise, and resveratrol all activate sirtuin 1 and 3. The details can be found in many excellent reviews.221–223
In Fisher 344 rats, wheel running exercise decreases mitochondrial production of H2O2 with reduced MnSOD activity in both SSM and IFM from aged rat hearts.129 However, acute prolonged exercise paradoxically may impair OXPHOS and lead to decreased respiratory coupling.224 Thus, proper exercise may provide a beneficial effect in improvement of mitochondrial oxidative metabolism.224
Furthermore, isolated mitochondria from aged hearts respond to pharmacologic effectors including direct agonists of the mitochondrial potassium-ATP channel.214, 225 Thus, the potential for direct mitochondria-targeted intervention remains. The aged heart exhibits a major clinical challenge for protective therapy. First, not only myocardial injury increases compared to younger hearts for similar ischemic stress, but the ability to muster endogenous protective pathways is impaired. This challenge in the aged heart makes a strong case for the consideration of novel, direct mitochondrial-centered treatments to mitigate cardiac damage during acute and chronic cardiac stress.
If the aging defect in mitochondrial respiration could be diminished or removed, would the aged heart now sustain less injury? Acetylcarnitine treatment improved aging-induced decreases in OXPHOS, complex III and complex IV.226,227 The content of cytochrome b in IFM from aged hearts was increased.227 In the aged heart, acetylcarnitine treatment improved tolerance to ischemia-reperfusion injury to that observed in adult controls, with decreased infarction and improved contractile recovery.227 Acetylcarnitine increased transcription of mtDNA linked to a greater content of ETC subunits,228 indicating stimulation of mitochondrial protein synthesis.229 Increased transcriptional responses may occur via activation of mitochondrial sirtuins 230, 231 or alternatively, via increases in intracellular acetyl-CoA. The latter may activate nuclear transcription via modulation of nuclear epigenetic responses. The acetylcarnitine studies provide proof of concept that modulation of aging-induced defects in mitochondrial metabolism can reduce cardiac injury from cardiac disease. The approach to rejuvenate mitochondrial function in order to directly address age-enhanced cardiac disease remains an attractive and potentially useful clinical strategy.
In addition to the defect in the ETC of the aged hearts, post-translational modification can also contribute to the altered mitochondrial metabolism. Sirtuins can affect mitochondrial metabolism through reversible lysine-residue acetylation.221, 223, 232 In female Wistar rats, the activities of complex I-IV are decreased in middle and aged hearts compared to young rats. The NAD+ content in middle and aged hearts is decreased compared to young, whereas NADH content is increased in middle and aged hearts. The sirtuin1 activity is also decreased in aged hearts.233 Thus, the altered sirtuins may contribute to altered metabolism in aged hearts. As an alternative, therapy to directly increase NAD+ content remains an option, having been utilized in skeletal muscle and brain, but the impact on heart remains to be seen.234–236
Supplemental coenzyme Q10 in PUFA (polyunsaturated fatty acids) diet extends life span in 24 month old rats with decreased H2O2 generation and increased catalase activity.237 The coenzyme Q10 supplementation in PUFA diet also improves the activity of cytochrome oxidase in 24 month aged mouse heart mitochondria.238 However, addition of coenzyme Q10 does not affect the respiratory chain activities, superoxide generation, and antioxidants in C57BL/6 mice.239 Coenzyme Q10 may attenuated aging defect in aged heart mitochondria.
Rapamycin is known to extend life span in animal species.240 Feeding of rapamycin in late life extends life span in C57BL/6 mice with decreased metabolite rate.241 In aged female C57BL/6 mice, rapamycin feeding for 10 weeks transiently induces autophagy, increases mitochondrial biogenesis, improves energy metabolism, especially fatty acid metabolism, and alters the myocardial metabolome.10 Rapamycin therapy started even late in life exerted beneficial effects in cardiac function and cardiac gene and protein expression.242, 243 The response to superimposed stress of ischemia-reperfusion or heart failure remains an area of major interest.
11. Summary and Conclusions
Aging results in defects in cardiac mitochondrial metabolism centered on the electron transport chain. IFM, located amongst the myofibrils, sustain the greatest alteration with age, exhibiting a decrease in both number and mass and a depressed capacity for OXPHOS because of defects in ETC complexes III and IV. The presence of dysfunctional mitochondria reflects age-induced alterations in mitochondrial dynamics involving altered mitochondrial fission and. Age-altered mitochondria produce greater fluxes of ROS, leading to damage of mitochondrial components, injury to adjacent organelles, and finally and fatally to the oxidant-activation of signaling pathways for cell death. Reduced electron transport may in part be due to age-related dysregulation of transcription factors that regulates mitochondrial metabolism, an understudied area. Impaired metabolism of fatty acids, dysregulated glucose metabolism, and mitochondrial defects are critical in metabolic responses of the elderly heart to disease-imposed stress. Aging defects in mitochondria represent new therapeutic targets, whether by manipulation of the mitochondrial proteome, modulation of electron transport, activation of biogenesis or mitophagy, or the regulation of fission and fusion. The study of aging defects in cardiac metabolism is not a sterile one, rather an area that presents an opportunity for developing novel therapies that could benefit cardiac disease, and perhaps slow the pace of age-induced changes In the heart, with the happy result of lengthening life spans.
Supplementary Material
Figure 2.
Schematic of the interfibrillar mitochondrial defects in the aged heart. Abbreviations similar to Figure 1. Qo site, ubiquinol binding site on cytochrome bl with proposed defect in Y132; PPL, phospholipid, proposed defect affecting subunit VIIa in complex IV. (Illustration Credit: Ben Smith).
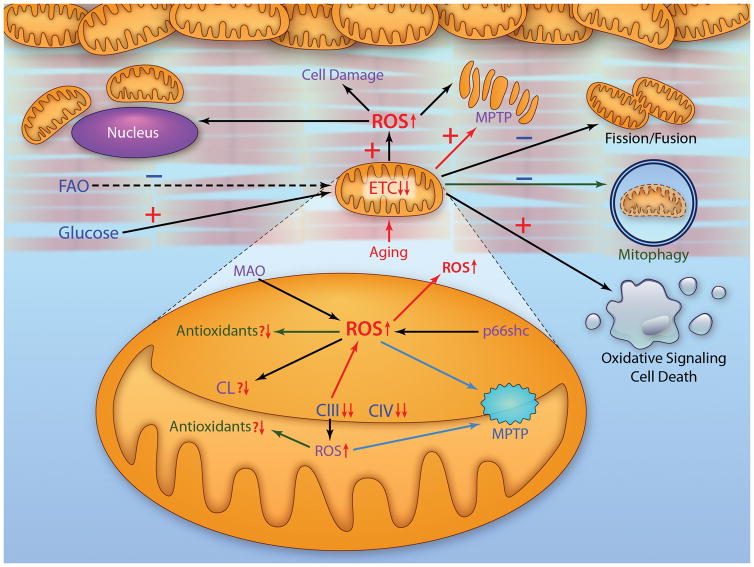
Figure 6.
A schematic of a cardiomyocyte to show defects present in global and mitochondrial metabolism with age in concert with mechanisms of mitochondria-driven cellular injury. The aged heart exhibits impaired fatty acid oxidation (FAO) and preserved oxidation of glucose. Aging leads to defects in the electron transport chain that involve complexes III and IV. Monoamine oxidase (MAO) and p66shc are significant sources of oxidants. There is a decrease in cardiolipin (CL). Antioxidant contents are relatively unchanged, although a decrease in matrix antioxidant capacity leads to mitochondrial damage. Age-related damage enhances the production of ROS that lead to mitochondrial and cell damage. Additionally, effector mechanisms of age-induced mitochondrial damage discussed include an increased susceptibility to mitochondrial permeability transition pore opening (MPTP) (Section 6B); impaired mitochondrial dynamics favoring fusion over fission (Section 8); likely impaired mitophagy (Section 9); and enhanced oxidant derived signaling to activate cell death programs (Section 6C). (Illustration Credit: Ben Smith).
Acknowledgments
The editorial assistance of Dr. Bernard Tandler is greatly appreciated.
Sources of Funding
This work was supported by a R21AG049461 from the National Institute on Aging, and a Scientist Development Grant (11SDG5120011, QC) and Grant-in-aids (15GRNT24480123, QC) and (12GRNT20510024, CLH) from the American Heart Association, VCU’s CTSA (UL1TR000058 from the National Institutes of Health’s National Center for Advancing Translational Science) and the CCTR Endowment Fund of the Virginia Commonwealth University (QC), the Office of Research and Development, Medical Research Service Merit Review Award (1IO1BX001355-01A1), Department of Veterans Affairs (EJL), and the Pauley Heart Center, Virginia Commonwealth University (QC, EJL).
Abbreviations
- ALCAT1, Acyl-CoA
lysocardiolipin acyltransferase-1
- ASK-1
Activation of apoptosis signal-regulating kinase 1
- CL
Cardiolipin
- CPT-I
Carnitine palmitoyltransferase I
- CPT-II
Carnitine palmitoyltransferase II
- FAO
Fatty acid oxidation
- HRSEM
High resolution scanning electron microscopy
- IFM
interfibrillar mitochondria
- ISP
Iron-sulfur Protein
- MAO
Monoamine oxidase
- MLCL
Monolysocardiolipin
- MPTP
Mitochondrial permeability transition pore
- OXPHOS
Mitochondrial oxidative phosphorylation
- PDH
Pyruvate dehydrogenase
- PDC
Pyruvate dehydrogenase complex
- PDK
Pyruvate dehydrogenase kinase
- ROS
Reactive oxygen species
- SSM
subsarcolemmal mitochondria
- TEM
transmission electron microscopy
- Trx
thioredoxin
- VDAC
Voltage-dependent anion channel
Footnotes
Disclosures:
The authors state they have no conflict of interest or financial interests to disclose.
References
- 1.Koonen DP, Febbraio M, Bonnet S, Nagendran J, Young ME, Michelakis ED, Dyck JR. Cd36 expression contributes to age-induced cardiomyopathy in mice. Circulation. 2007;116:2139–2147. doi: 10.1161/CIRCULATIONAHA.107.712901. [DOI] [PubMed] [Google Scholar]
- 2.Lesnefsky EJ, Moghaddas S, Tandler B, Kerner J, Hoppel CL. Mitochondrial dysfunction in cardiac disease: Ischemia-reperfusion, aging, and heart failure. J Mol Cell Cardiol. 2001;33:1065–1089. doi: 10.1006/jmcc.2001.1378. [DOI] [PubMed] [Google Scholar]
- 3.Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90:207–258. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 4.Calvani M, Reda E, Arrigoni-Martelli E. Regulation by carnitine of myocardial fatty acid and carbohydrate metabolism under normal and pathological conditions. Basic Res Cardiol. 2000;95:75–83. doi: 10.1007/s003950050167. [DOI] [PubMed] [Google Scholar]
- 5.Sugden MC, Langdown ML, Harris RA, Holness MJ. Expression and regulation of pyruvate dehydrogenase kinase isoforms in the developing rat heart and in adulthood: Role of thyroid hormone status and lipid supply. Biochem J. 2000;352(Pt 3):731–738. [PMC free article] [PubMed] [Google Scholar]
- 6.Vadvalkar SS, Baily CN, Matsuzaki S, West M, Tesiram YA, Humphries KM. Metabolic inflexibility and protein lysine acetylation in heart mitochondria of a chronic model of type 1 diabetes. Biochem J. 2013;449:253–261. doi: 10.1042/BJ20121038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moreau R, Heath SH, Doneanu CE, Harris RA, Hagen TM. Age-related compensatory activation of pyruvate dehydrogenase complex in rat heart. Biochem Biophys Res Commun. 2004;325:48–58. doi: 10.1016/j.bbrc.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Chuffa LG, Seiva FR. Combined effects of age and diet-induced obesity on biochemical parameters and cardiac energy metabolism in rats. Indian J Biochem Biophys. 2013;50:40–47. [PubMed] [Google Scholar]
- 9.Hyyti OM, Ledee D, Ning XH, Ge M, Portman MA. Aging impairs myocardial fatty acid and ketone oxidation and modifies cardiac functional and metabolic responses to insulin in mice. Am J Physiol Heart Circ Physiol. 2010;299:H868–875. doi: 10.1152/ajpheart.00931.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiao YA, Kolwicz SC, Basisty N, Gagnidze A, Zhang J, Gu H, Djukovic D, Beyer RP, Raftery D, MacCoss M, Tian R, Rabinovitch PS. Rapamycin transiently induces mitochondrial remodeling to reprogram energy metabolism in old hearts. Aging (Albany NY) 2016;8:314–327. doi: 10.18632/aging.100881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomec RJ, Hoppel CL. Carnitine palmitoyltransferase in bovine fetal heart mitochondria. Arch Biochem Biophys. 1975;170:716–723. doi: 10.1016/0003-9861(75)90169-1. [DOI] [PubMed] [Google Scholar]
- 12.Hoppel CL, Kerner J, Turkaly P, Turkaly J, Tandler B. The malonyl-coa-sensitive form of carnitine palmitoyltransferase is not localized exclusively in the outer membrane of rat liver mitochondria. J Biol Chem. 1998;273:23495–23503. doi: 10.1074/jbc.273.36.23495. [DOI] [PubMed] [Google Scholar]
- 13.Lopaschuk GD, Folmes CD, Stanley WC. Cardiac energy metabolism in obesity. Circ Res. 2007;101:335–347. doi: 10.1161/CIRCRESAHA.107.150417. [DOI] [PubMed] [Google Scholar]
- 14.Gomez LA, Heath SH, Hagen TM. Acetyl-l-carnitine supplementation reverses the age-related decline in carnitine palmitoyltransferase 1 (cpt1) activity in interfibrillar mitochondria without changing the l-carnitine content in the rat heart. Mech Ageing Dev. 2012;133:99–106. doi: 10.1016/j.mad.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kashfi K, Cook GA. Proteinase treatment of intact hepatic mitochondria has differential effects on inhibition of carnitine palmitoyltransferase by different inhibitors. Biochem J. 1992;282(Pt 3):909–914. doi: 10.1042/bj2820909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ledee D, Portman MA, Kajimoto M, Isern N, Olson AK. Thyroid hormone reverses aging-induced myocardial fatty acid oxidation defects and improves the response to acutely increased afterload. PLoS One. 2013;8:e65532. doi: 10.1371/journal.pone.0065532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fillmore N, Mori J, Lopaschuk GD. Mitochondrial fatty acid oxidation alterations in heart failure, ischaemic heart disease and diabetic cardiomyopathy. Br J Pharmacol. 2013;171:2080–2090. doi: 10.1111/bph.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLeod CJ, Aziz A, Hoyt RF, Jr, McCoy JP, Jr, Sack MN. Uncoupling proteins 2 and 3 function in concert to augment tolerance to cardiac ischemia. J Biol Chem. 2005;280:33470–33476. doi: 10.1074/jbc.M505258200. [DOI] [PubMed] [Google Scholar]
- 19.Papa S, Guerrieri F, Capitanio N. A possible role of slips in cytochrome c oxidase in the antioxygen defense system of the cell. Biosci Rep. 1997;17:23–31. doi: 10.1023/a:1027331116866. [DOI] [PubMed] [Google Scholar]
- 20.Hoch FL. Cardiolipins and biomembrane function. Biochim Biophys Acta. 1992;1113:71–133. doi: 10.1016/0304-4157(92)90035-9. [DOI] [PubMed] [Google Scholar]
- 21.Petronilli V, Penzo D, Scorrano L, Bernardi P, Di Lisa F. The mitochondrial permeability transition, release of cytochrome c and cell death. Correlation with the duration of pore openings in situ. J Biol Chem. 2001;276:12030–12034. doi: 10.1074/jbc.M010604200. Epub 12000 Dec 12027. [DOI] [PubMed] [Google Scholar]
- 22.Weiss JN, Korge P, Honda HM, Ping P. Role of the mitochondrial permeability transition in myocardial disease. Circ Res. 2003;93:292–301. doi: 10.1161/01.RES.0000087542.26971.D4. [DOI] [PubMed] [Google Scholar]
- 23.Lemieux H, Vazquez EJ, Fujioka H, Hoppel CL. Decrease in mitochondrial function in rat cardiac permeabilized fibers correlates with the aging phenotype. J Gerontol A Biol Sci Med Sci. 2010;65:1157–1164. doi: 10.1093/gerona/glq141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fannin SW, Lesnefsky EJ, Slabe TJ, Hassan MO, Hoppel CL. Aging selectively decreases oxidative capacity in rat heart interfibrillar mitochondria. Arch Biochem Biophys. 1999;372:399–407. doi: 10.1006/abbi.1999.1508. [DOI] [PubMed] [Google Scholar]
- 25.Bratic I, Trifunovic A. Mitochondrial energy metabolism and ageing. Biochim Biophys Acta. 2010;1797:961–967. doi: 10.1016/j.bbabio.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Takasawa M, Hayakawa M, Sugiyama S, Hattori K, Ito T, Ozawa T. Age-associated damage in mitochondrial function in rat hearts. Exp Gerontol. 1993;28:269–280. doi: 10.1016/0531-5565(93)90034-b. [DOI] [PubMed] [Google Scholar]
- 27.Chen JC, Warshaw JB, Sanadi DR. Regulation of mitochondrial respiration in senescence. J Cell Physiol. 1972;80:141–148. doi: 10.1002/jcp.1040800115. [DOI] [PubMed] [Google Scholar]
- 28.Hansford RG. Lipid oxidation by heart mitochondria from young adult and senescent rats. Biochem J. 1978;170:285–295. doi: 10.1042/bj1700285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manzelmann MS, Harmon HJ. Lack of age-dependent changes in rat heart mitochondria. Mech Ageing Dev. 1987;39:281–288. doi: 10.1016/0047-6374(87)90067-4. [DOI] [PubMed] [Google Scholar]
- 30.Paradies G, Ruggiero FM, Dinoi P, Petrosillo G, Quagliariello E. Age-dependent decrease in the cytochrome c oxidase activity and changes in phopsholiids in rat-heart mitochondria. Arch Gerontol Geriatr. 1993;16:262–272. doi: 10.1016/0167-4943(93)90037-i. [DOI] [PubMed] [Google Scholar]
- 31.Paradies G, Ruggiero FM, Petrosillo G, Gadaleta MN, Quagliariello E. Effect of aging and acetyl-l-carnitine on the activity of cytochrome oxidase and adenine nucleotide translocase in rat heart mitochondria. FEBS Lett. 1994;350:213–215. doi: 10.1016/0014-5793(94)00763-2. [DOI] [PubMed] [Google Scholar]
- 32.Paradies G, Ruggiero FM, Petrosillo G, Quagliariello E. Age-dependent decline in the cytochrome c oxidase activity in rat heart mitochondria: Role of cardiolipin. FEBS Lett. 1997;406:136–138. doi: 10.1016/s0014-5793(97)00264-0. [DOI] [PubMed] [Google Scholar]
- 33.Muscari C, Frascaro M, Guarnieri C, Caldarera CM. Mitochondrial function and superoxide generation from submitochondrial particles of aged rat hearts. Biochim Biophys Acta. 1990;1015:200–204. doi: 10.1016/0005-2728(90)90021-u. [DOI] [PubMed] [Google Scholar]
- 34.Craig EE, Hood DA. Influence of aging on protein import into cardiac mitochondria. Am J Physiol. 1997;272:H2983–2988. doi: 10.1152/ajpheart.1997.272.6.H2983. [DOI] [PubMed] [Google Scholar]
- 35.Hollander JM, Thapa D, Shepherd DL. Physiological and structural differences in spatially distinct subpopulations of cardiac mitochondria: Influence of cardiac pathologies. Am J Physiol Heart Circ Physiol. 2014;307:H1–14. doi: 10.1152/ajpheart.00747.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoppel CL, Tandler B, Parland W, Turkaly JS, Albers LD. Hamster cardiomyopathy. A defect in oxidative phosphorylation in the cardiac interfibrillar mitochondria. J Biol Chem. 1982;257:1540–1548. [PubMed] [Google Scholar]
- 37.Tatarkova Z, Kuka S, Racay P, Lehotsky J, Dobrota D, Mistuna D, Kaplan P. Effects of aging on activities of mitochondrial electron transport chain complexes and oxidative damage in rat heart. Physiol Res. 2011;60:281–289. doi: 10.33549/physiolres.932019. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Mi SL, Hu N, Doser TA, Sun A, Ge J, Ren J. Mitochondrial aldehyde dehydrogenase 2 accentuates aging-induced cardiac remodeling and contractile dysfunction: Role of ampk, sirt1, and mitochondrial function. Free Radic Biol Med. 2014;71:208–220. doi: 10.1016/j.freeradbiomed.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoppel CL, Moghaddas S, Lesnefsky EJ. Interfibrillar cardiac mitochondrial complex iii defects in the aging rat heart. Biogerontology. 2002;3:41–44. doi: 10.1023/a:1015251212039. [DOI] [PubMed] [Google Scholar]
- 40.Kerner J, Yohannes E, Lee K, Virmani A, Koverech A, Cavazza C, Chance MR, Hoppel C. Acetyl-l-carnitine increases mitochondrial protein acetylation in the aged rat heart. Mech Ageing Dev. 2015;145:39–50. doi: 10.1016/j.mad.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramani K, Lust WD, Whittingham TS, Lesnefsky EJ. Atp catabolism and adenosine generation during ischemia in the aging heart. Mech Ageing Dev. 1996;89:113–124. doi: 10.1016/0047-6374(96)01732-0. [DOI] [PubMed] [Google Scholar]
- 42.Skulachev VP, Anisimov VN, Antonenko YN, Bakeeva LE, Chernyak BV, Erichev VP, Filenko OF, Kalinina NI, Kapelko VI, Kolosova NG, Kopnin BP, Korshunova GA, Lichinitser MR, Obukhova LA, Pasyukova EG, Pisarenko OI, Roginsky VA, Ruuge EK, Senin II, Severina II, Skulachev MV, Spivak IM, Tashlitsky VN, Tkachuk VA, Vyssokikh MY, Yaguzhinsky LS, Zorov DB. An attempt to prevent senescence: A mitochondrial approach. Biochim Biophys Acta. 2009;1787:437–461. doi: 10.1016/j.bbabio.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 43.Vays VB, Eldarov CM, Vangely IM, Kolosova NG, Bakeeva LE, Skulachev VP. Antioxidant skq1 delays sarcopenia-associated damage of mitochondrial ultrastructure. Aging (Albany NY) 2014;6:140–148. doi: 10.18632/aging.100636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palmer JW, Tandler B, Hoppel CL. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J Biol Chem. 1977;252:8731–8739. [PubMed] [Google Scholar]
- 45.Palmer JW, Tandler B, Hoppel CL. Biochemical differences between subsarcolemmal and interfibrillar mitochondria from rat cardiac muscle: Effects of procedural manipulations. Arch Biochem Biophys. 1985;236:691–702. doi: 10.1016/0003-9861(85)90675-7. [DOI] [PubMed] [Google Scholar]
- 46.Riva A, Tandler B, Loffredo F, Vazquez E, Hoppel C. Structural differences in two biochemically defined populations of cardiac mitochondria. Am J Physiol Heart Circ Physiol. 2005;289:H868–872. doi: 10.1152/ajpheart.00866.2004. [DOI] [PubMed] [Google Scholar]
- 47.Riva A, Tandler B, Lesnefsky EJ, Conti G, Loffredo F, Vazquez E, Hoppel CL. Structure of cristae in cardiac mitochondria of aged rat. Mech Ageing Dev. 2006;127:917–921. doi: 10.1016/j.mad.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.El’darov Ch M, Vays VB, Vangeli IM, Kolosova NG, Bakeeva LE. Morphometric examination of mitochondrial ultrastructure in aging cardiomyocytes. Biochemistry (Mosc) 2015;80:604–609. doi: 10.1134/S0006297915050132. [DOI] [PubMed] [Google Scholar]
- 49.Houtkooper RH, Vaz FM. Cardiolipin, the heart of mitochondrial metabolism. Cell Mol Life Sci. 2008;65:2493–2506. doi: 10.1007/s00018-008-8030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lesnefsky EJ, Stoll MS, Minkler PE, Hoppel CL. Separation and quantitation of phospholipids and lysophospholipids by high-performance liquid chromatography. Anal Biochem. 2000;285:246–254. doi: 10.1006/abio.2000.4783. [DOI] [PubMed] [Google Scholar]
- 51.Moghaddas S, Stoll MS, Minkler PE, Salomon RG, Hoppel CL, Lesnefsky EJ. Preservation of cardiolipin content during aging in rat heart interfibrillar mitochondria. J Gerontol A Biol Sci Med Sci. 2002;57:B22–28. doi: 10.1093/gerona/57.1.b22. [DOI] [PubMed] [Google Scholar]
- 52.Lee J, Yu BP, Herlihy JT. Modulation of cardiac mitochondrial membrane fluidity by age and calorie intake. Free Radic Biol Med. 1999;26:260–265. doi: 10.1016/s0891-5849(98)00195-6. [DOI] [PubMed] [Google Scholar]
- 53.Paradies G, Ruggiero FM, Petrosillo G, Gadaleta MN, Quagliariello E. The effect of aging and acetyl-l-carnitine on the function and on the lipid composition of rat heart mitochondria. Ann N Y Acad Sci. 1994;717:233–243. doi: 10.1111/j.1749-6632.1994.tb12093.x. [DOI] [PubMed] [Google Scholar]
- 54.Lesnefsky EJ, Hoppel CL. Cardiolipin as an oxidative target in cardiac mitochondria in the aged rat. Biochim Biophys Acta. 2008;1777:1020–1027. doi: 10.1016/j.bbabio.2008.05.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lesnefsky EJ, Minkler P, Hoppel CL. Enhanced modification of cardiolipin during ischemia in the aged heart. J Mol Cell Cardiol. 2009;46:1008–1015. doi: 10.1016/j.yjmcc.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 56.Imagawa T, Kasai S, Matsui K, Nakamura T. Methyl hydroperoxy-epoxy-octadecenoate as an autoxidation product of methyl linoleate: A new inhibitor-uncoupler of mitochondrial respiration. J Biochem. 1982;92:1109–1121. doi: 10.1093/oxfordjournals.jbchem.a134027. [DOI] [PubMed] [Google Scholar]
- 57.Iwase H, Takatori T, Nagao M, Iwadate K, Nakajima M. Monoepoxide production from linoleic acid by cytochrome c in the presence of cardiolipin. Biochem Biophys Res Commun. 1996;222:83–89. doi: 10.1006/bbrc.1996.0701. [DOI] [PubMed] [Google Scholar]
- 58.Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, Amoscato AA, Osipov AN, Belikova NA, Kapralov AA, Kini V, Vlasova II, Zhao Q, Zou M, Di P, Svistunenko DA, Kurnikov IV, Borisenko GG. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol. 2005;1:223–232. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- 59.Belikova NA, Vladimirov YA, Osipov AN, Kapralov AA, Tyurin VA, Potapovich MV, Basova LV, Peterson J, Kurnikov IV, Kagan VE. Peroxidase activity and structural transitions of cytochrome c bound to cardiolipin-containing membranes. Biochemistry. 2006;45:4998–5009. doi: 10.1021/bi0525573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sparagna GC, Lesnefsky EJ. Cardiolipin remodeling in the heart. J Cardiovasc Pharmacol. 2009;53:290–301. doi: 10.1097/FJC.0b013e31819b5461. [DOI] [PubMed] [Google Scholar]
- 61.Aluri HS, Simpson DC, Allegood JC, Hu Y, Szczepanek K, Gronert S, Chen Q, Lesnefsky EJ. Electron flow into cytochrome c coupled with reactive oxygen species from the electron transport chain converts cytochrome c to a cardiolipin peroxidase: Role during ischemia-reperfusion. Biochim Biophys Acta. 2014;1840:3199–3207. doi: 10.1016/j.bbagen.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schlame M. Cardiolipin remodeling and the function of tafazzin. Biochim Biophys Acta. 2013;1831:582–588. doi: 10.1016/j.bbalip.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 63.Xu Y, Malhotra A, Ren M, Schlame M. The enzymatic function of tafazzin. J Biol Chem. 2006;281:39217–39224. doi: 10.1074/jbc.M606100200. [DOI] [PubMed] [Google Scholar]
- 64.Szczepanek K, Allegood J, Aluri H, Hu Y, Chen Q, Lesnefsky EJ. Acquired deficiency of tafazzin in the adult heart: Impact on mitochondrial function and response to cardiac injury. Biochim Biophys Acta. 2015;1861:294–300. doi: 10.1016/j.bbalip.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 65.Cao J, Shen W, Chang Z, Shi Y. Alcat1 is a polyglycerophospholipid acyltransferase potently regulated by adenine nucleotide and thyroid status. Am J Physiol Endocrinol Metab. 2009;296:E647–653. doi: 10.1152/ajpendo.90761.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu X, Ye B, Miller S, Yuan H, Zhang H, Tian L, Nie J, Imae R, Arai H, Li Y, Cheng Z, Shi Y. Ablation of alcat1 mitigates hypertrophic cardiomyopathy through effects on oxidative stress and mitophagy. Mol Cell Biol. 2012;32:4493–4504. doi: 10.1128/MCB.01092-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shi Y. Emerging roles of cardiolipin remodeling in mitochondrial dysfunction associated with diabetes, obesity, and cardiovascular diseases. J Biomed Res. 2010;24:6–15. doi: 10.1016/S1674-8301(10)60003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sparagna GC, Chicco AJ, Murphy RC, Bristow MR, Johnson CA, Rees ML, Maxey ML, McCune SA, Moore RL. Loss of cardiac tetralinoleoyl cardiolipin in human and experimental heart failure. J Lipid Res. 2007;48:1559–1570. doi: 10.1194/jlr.M600551-JLR200. [DOI] [PubMed] [Google Scholar]
- 69.Khairallah RJ, Kim J, O’Shea KM, O’Connell KA, Brown BH, Galvao T, Daneault C, Des Rosiers C, Polster BM, Hoppel CL, Stanley WC. Improved mitochondrial function with diet-induced increase in either docosahexaenoic acid or arachidonic acid in membrane phospholipids. PLoS One. 2012;7:e34402. doi: 10.1371/journal.pone.0034402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baile MG, Whited K, Claypool SM. Deacylation on the matrix side of the mitochondrial inner membrane regulates cardiolipin remodeling. Mol Biol Cell. 2013;24:2008–2020. doi: 10.1091/mbc.E13-03-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paradies G, Ruggiero FM, Petrosillo G, Quagliariello E. Peroxidative damage to cardiac mitochondria: Cytochrome oxidase and cardiolipin alterations. FEBS Lett. 1998;424:155–158. doi: 10.1016/s0014-5793(98)00161-6. [DOI] [PubMed] [Google Scholar]
- 72.Ott M, Robertson JD, Gogvadze V, Zhivotovsky B, Orrenius S. Cytochrome c release from mitochondria proceeds by a two-step process. Proc Natl Acad Sci U S A. 2002;99:1259–1263. doi: 10.1073/pnas.241655498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Szeto HH, Birk AV. Serendipity and the discovery of novel compounds that restore mitochondrial plasticity. Clin Pharmacol Ther. 2014;96:672–683. doi: 10.1038/clpt.2014.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mitchell P. Vectorial chemistry and the molecular mechanics of chemiosmotic coupling: Power transmission by proticity. Biochem Soc Trans. 1976;4:399–430. doi: 10.1042/bst0040399. [DOI] [PubMed] [Google Scholar]
- 75.Schagger H, de Coo R, Bauer MF, Hofmann S, Godinot C, Brandt U. Significance of respirasomes for the assembly/stability of human respiratory chain complex i. J Biol Chem. 2004;279:36349–36353. doi: 10.1074/jbc.M404033200. [DOI] [PubMed] [Google Scholar]
- 76.Schagger H, Pfeiffer K. Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. Embo J. 2000;19:1777–1783. doi: 10.1093/emboj/19.8.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gomez LA, Monette JS, Chavez JD, Maier CS, Hagen TM. Supercomplexes of the mitochondrial electron transport chain decline in the aging rat heart. Arch Biochem Biophys. 2009;490:30–35. doi: 10.1016/j.abb.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kwong LK, Sohal RS. Age-related changes in activities of mitochondrial electron transport complexes in various tissues of the mouse. Arch Biochem Biophys. 2000;373:16–22. doi: 10.1006/abbi.1999.1495. [DOI] [PubMed] [Google Scholar]
- 79.Paradies G, Petrosillo G, Pistolese M, Di Venosa N, Federici A, Ruggiero FM. Decrease in mitochondrial complex i activity in ischemic/reperfused rat heart: Involvement of reactive oxygen species and cardiolipin. Circ Res. 2004;94:53–59. doi: 10.1161/01.RES.0000109416.56608.64. [DOI] [PubMed] [Google Scholar]
- 80.Rosca MG, Vazquez EJ, Kerner J, Parland W, Chandler MP, Stanley W, Sabbah HN, Hoppel CL. Cardiac mitochondria in heart failure: Decrease in respirasomes and oxidative phosphorylation. Cardiovasc Res. 2008;80:30–39. doi: 10.1093/cvr/cvn184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mejia EM, Cole LK, Hatch GM. Cardiolipin metabolism and the role it plays in heart failure and mitochondrial supercomplex formation. Cardiovasc Hematol Disord Drug Targets. 2014;14:98–106. doi: 10.2174/1871529x14666140505123753. [DOI] [PubMed] [Google Scholar]
- 82.Hansford RG, Tsuchiya N, Pepe S. Mitochondria in heart ischaemia and aging. Biochem Soc Symp. 1999;66:141–147. doi: 10.1042/bss0660141. [DOI] [PubMed] [Google Scholar]
- 83.Paradies G, Petrosillo G, Pistolese M, Ruggiero FM. The effect of reactive oxygen species generated from the mitochondrial electron transport chain on the cytochrome c oxidase activity and on the cardiolipin content in bovine heart submitochondrial particles. FEBS Lett. 2000;466:323–326. doi: 10.1016/s0014-5793(00)01082-6. [DOI] [PubMed] [Google Scholar]
- 84.Muller-Hocker J. Cytochrome-c-oxidase deficient cardiomyocytes in the human heart--an age-related phenomenon. A histochemical ultracytochemical study. Am J Pathol. 1989;134:1167–1173. [PMC free article] [PubMed] [Google Scholar]
- 85.Fujioka H, Moghaddas S, Murdock DG, Lesnefsky EJ, Tandler B, Hoppel CL. Decreased cytochrome c oxidase subunit viia in aged rat heart mitochondria: Immunocytochemistry. Anat Rec (Hoboken) 2011;294:1825–1833. doi: 10.1002/ar.21486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schull S, Gunther SD, Brodesser S, Seeger JM, Tosetti B, Wiegmann K, Pongratz C, Diaz F, Witt A, Andree M, Brinkmann K, Kronke M, Wiesner RJ, Kashkar H. Cytochrome c oxidase deficiency accelerates mitochondrial apoptosis by activating ceramide synthase 6. Cell Death Dis. 2015;6:e1691. doi: 10.1038/cddis.2015.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leadsham JE, Sanders G, Giannaki S, Bastow EL, Hutton R, Naeimi WR, Breitenbach M, Gourlay CW. Loss of cytochrome c oxidase promotes ras-dependent ros production from the er resident nadph oxidase, yno1p, in yeast. Cell Metab. 2013;18:279–286. doi: 10.1016/j.cmet.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 88.Lesnefsky EJ, Gudz TI, Moghaddas S, Migita CT, Ikeda-Saito M, Turkaly PJ, Hoppel CL. Aging decreases electron transport complex iii activity in heart interfibrillar mitochondria by alteration of the cytochrome c binding site. J Mol Cell Cardiol. 2001;33:37–47. doi: 10.1006/jmcc.2000.1273. [DOI] [PubMed] [Google Scholar]
- 89.Iwata S, Lee JW, Okada K, Lee JK, Iwata M, Rasmussen B, Link TA, Ramaswamy S, Jap BK. Complete structure of the 11-subunit bovine mitochondrial cytochrome bc1 complex. Science. 1998;281:64–71. doi: 10.1126/science.281.5373.64. [DOI] [PubMed] [Google Scholar]
- 90.Xia D, Yu CA, Kim H, Xia JZ, Kachurin AM, Zhang L, Yu L, Deisenhofer J. Crystal structure of the cytochrome bc1 complex from bovine heart mitochondria. Science. 1997;277:60–66. doi: 10.1126/science.277.5322.60. [DOI] [PubMed] [Google Scholar]
- 91.Ghosh M, Wang Y, Ebert CE, Vadlamuri S, Beattie DS. Substituting leucine for alanine-86 in the tether region of the iron-sulfur protein of the cytochrome bc1 complex affects the mobility of the [2fe2s] domain. Biochemistry. 2001;40:327–335. doi: 10.1021/bi001708t. [DOI] [PubMed] [Google Scholar]
- 92.Matsuno-Yagi A, Hatefi Y. Ubiquinol:Cytochrome c oxidoreductase. The redox reactions of the bis-heme cytochrome b in unenergized and energized submitochondrial particles. Journal of Biological Chemistry. 1997;272:16928–16933. doi: 10.1074/jbc.272.27.16928. [DOI] [PubMed] [Google Scholar]
- 93.Snyder CH, Denke E, Trumpower BL. Aromatic amino acids in the rieske iron-sulfur protein do not form an obligatory conduit for electron transfer from the iron-sulfur cluster to the heme of cytochrome c1 in the cytochrome bc1 complex. Biochimica Et Biophysica Acta. 1999;1410:237–247. doi: 10.1016/s0005-2728(99)00002-x. [DOI] [PubMed] [Google Scholar]
- 94.Snyder CH, Gutierrez-Cirlos EB, Trumpower BL. Evidence for a concerted mechanism of ubiquinol oxidation by the cytochrome bc1 complex. Journal of Biological Chemistry. 2000;275:13535–13541. doi: 10.1074/jbc.275.18.13535. [DOI] [PubMed] [Google Scholar]
- 95.Crofts AR, Guergova-Kuras M, Huang L, Kuras R, Zhang Z, Berry EA. Mechanism of ubiquinol oxidation by the bc(1) complex: Role of the iron sulfur protein and its mobility. Biochemistry. 1999;38:15791–15806. doi: 10.1021/bi990961u. [DOI] [PubMed] [Google Scholar]
- 96.Guergova-Kuras M, Kuras R, Ugulava N, Hadad I, Crofts AR. Specific mutagenesis of the rieske iron-sulfur protein in rhodobacter sphaeroides shows that both the thermodynamic gradient and the pk of the oxidized form determine the rate of quinol oxidation by the bc(1) complex. Biochemistry. 2000;39:7436–7444. doi: 10.1021/bi992491+. [DOI] [PubMed] [Google Scholar]
- 97.Moghaddas S, Hoppel CL, Lesnefsky EJ. Aging defect at the qo site of complex iii augments oxyradical production in rat heart interfibrillar mitochondria. Arch Biochem Biophys. 2003;414:59–66. doi: 10.1016/s0003-9861(03)00166-8. [DOI] [PubMed] [Google Scholar]
- 98.Guerrieri F, Capozza G, Fratello A, Zanotti F, Papa S. Functional and molecular changes in fof1 atp-synthase of cardiac muscle during aging. Cardioscience. 1993;4:93–98. [PubMed] [Google Scholar]
- 99.Barogi S, Baracca A, Parenti Castelli G, Bovina C, Formiggini G, Marchetti M, Solaini G, Lenaz G. Lack of major changes in atpase activity in mitochondria from liver, heart, and skeletal muscle of rats upon ageing. Mech Ageing Dev. 1995;84:139–150. doi: 10.1016/0047-6374(95)01640-6. [DOI] [PubMed] [Google Scholar]
- 100.Giorgio V, von Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M, Glick GD, Petronilli V, Zoratti M, Szabo I, Lippe G, Bernardi P. Dimers of mitochondrial atp synthase form the permeability transition pore. Proc Natl Acad Sci U S A. 2013;110:5887–5892. doi: 10.1073/pnas.1217823110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hafner AV, Dai J, Gomes AP, Xiao CY, Palmeira CM, Rosenzweig A, Sinclair DA. Regulation of the mptp by sirt3-mediated deacetylation of cypd at lysine 166 suppresses age-related cardiac hypertrophy. Aging (Albany NY) 2010;2:914–923. doi: 10.18632/aging.100252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Baris OR, Ederer S, Neuhaus JF, von Kleist-Retzow JC, Wunderlich CM, Pal M, Wunderlich FT, Peeva V, Zsurka G, Kunz WS, Hickethier T, Bunck AC, Stockigt F, Schrickel JW, Wiesner RJ. Mosaic deficiency in mitochondrial oxidative metabolism promotes cardiac arrhythmia during aging. Cell Metab. 2015;21:667–677. doi: 10.1016/j.cmet.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 103.Barazzoni R, Short KR, Nair KS. Effects of aging on mitochondrial dna copy number and cytochrome c oxidase gene expression in rat skeletal muscle, liver, and heart. J Biol Chem. 2000;275:3343–3347. doi: 10.1074/jbc.275.5.3343. [DOI] [PubMed] [Google Scholar]
- 104.Marin-Garcia J, Ananthakrishnan R, Goldenthal MJ. Mitochondrial gene expression in rat heart and liver during growth and development. Biochem Cell Biol. 1997;75:137–142. [PubMed] [Google Scholar]
- 105.Frahm T, Mohamed SA, Bruse P, Gemund C, Oehmichen M, Meissner C. Lack of age-related increase of mitochondrial dna amount in brain, skeletal muscle and human heart. Mech Ageing Dev. 2005;126:1192–1200. doi: 10.1016/j.mad.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 106.de Souza-Pinto NC, Eide L, Hogue BA, Thybo T, Stevnsner T, Seeberg E, Klungland A, Bohr VA. Repair of 8-oxodeoxyguanosine lesions in mitochondrial dna depends on the oxoguanine dna glycosylase (ogg1) gene and 8-oxoguanine accumulates in the mitochondrial dna of ogg1-defective mice. Cancer Res. 2001;61:5378–5381. [PubMed] [Google Scholar]
- 107.Klungland A, Rosewell I, Hollenbach S, Larsen E, Daly G, Epe B, Seeberg E, Lindahl T, Barnes DE. Accumulation of premutagenic dna lesions in mice defective in removal of oxidative base damage. Proc Natl Acad Sci U S A. 1999;96:13300–13305. doi: 10.1073/pnas.96.23.13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vermulst M, Bielas JH, Kujoth GC, Ladiges WC, Rabinovitch PS, Prolla TA, Loeb LA. Mitochondrial point mutations do not limit the natural lifespan of mice. Nat Genet. 2007;39:540–543. doi: 10.1038/ng1988. [DOI] [PubMed] [Google Scholar]
- 109.Vermulst M, Wanagat J, Kujoth GC, Bielas JH, Rabinovitch PS, Prolla TA, Loeb LA. DNA deletions and clonal mutations drive premature aging in mitochondrial mutator mice. Nat Genet. 2008;40:392–394. doi: 10.1038/ng.95. [DOI] [PubMed] [Google Scholar]
- 110.Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Van Remmen H, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla TA. Mitochondrial dna mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- 111.Sisler JD, Morgan M, Raje V, Grande RC, Derecka M, Meier J, Cantwell M, Szczepanek K, Korzun WJ, Lesnefsky EJ, Harris TE, Croniger CM, Larner AC. The signal transducer and activator of transcription 1 (stat1) inhibits mitochondrial biogenesis in liver and fatty acid oxidation in adipocytes. PLoS One. 2015;10:e0144444. doi: 10.1371/journal.pone.0144444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Barja G. Mitochondrial free radical production and aging in mammals and birds. Ann N Y Acad Sci. 1998;854:224–238. doi: 10.1111/j.1749-6632.1998.tb09905.x. [DOI] [PubMed] [Google Scholar]
- 113.Kwong LK, Sohal RS. Substrate and site specificity of hydrogen peroxide generation in mouse mitochondria. Arch Biochem Biophys. 1998;350:118–126. doi: 10.1006/abbi.1997.0489. [DOI] [PubMed] [Google Scholar]
- 114.Skulachev VP. The programmed death phenomena, aging, and the samurai law of biology. Exp Gerontol. 2001;36:995–1024. doi: 10.1016/s0531-5565(01)00109-7. [DOI] [PubMed] [Google Scholar]
- 115.Han D, Antunes F, Canali R, Rettori D, Cadenas E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem. 2003;278:5557–5563. doi: 10.1074/jbc.M210269200. [DOI] [PubMed] [Google Scholar]
- 116.Sugioka K, Nakano M, Totsune-Nakano H, Minakami H, Tero-Kubota S, Ikegami Y. Mechanism of o2- generation in reduction and oxidation cycle of ubiquinones in a model of mitochondrial electron transport systems. Biochim Biophys Acta. 1988;936:377–385. doi: 10.1016/0005-2728(88)90014-x. [DOI] [PubMed] [Google Scholar]
- 117.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brand MD, Esteves TC. Physiological functions of the mitochondrial uncoupling proteins ucp2 and ucp3. Cell Metab. 2005;2:85–93. doi: 10.1016/j.cmet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 119.Brand MD, Pakay JL, Ocloo A, Kokoszka J, Wallace DC, Brookes PS, Cornwall EJ. The basal proton conductance of mitochondria depends on adenine nucleotide translocase content. Biochem J. 2005;392:353–362. doi: 10.1042/BJ20050890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ross T, Szczepanek K, Bowler E, Hu Y, Larner A, Lesnefsky EJ, Chen Q. Reverse electron flow-mediated ros generation in ischemia-damaged mitochondria: Role of complex i inhibition vs. Depolarization of inner mitochondrial membrane. Biochim Biophys Acta. 2013;1830:4537–4542. doi: 10.1016/j.bbagen.2013.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen YR, Zweier JL. Cardiac mitochondria and reactive oxygen species generation. Circ Res. 2014;114:524–537. doi: 10.1161/CIRCRESAHA.114.300559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: Central role of complex iii. J Biol Chem. 2003;278:36027–36031. doi: 10.1074/jbc.M304854200. [DOI] [PubMed] [Google Scholar]
- 123.Qian T, Nieminen AL, Herman B, Lemasters JJ. Mitochondrial permeability transition in ph-dependent reperfusion injury to rat hepatocytes. Am J Physiol. 1997;273:C1783–1792. doi: 10.1152/ajpcell.1997.273.6.C1783. [DOI] [PubMed] [Google Scholar]
- 124.Demin OV, Kholodenko BN, Skulachev VP. A model of o2.-generation in the complex iii of the electron transport chain. Mol Cell Biochem. 1998;184:21–33. [PubMed] [Google Scholar]
- 125.Demin OV, Westerhoff HV, Kholodenko BN. Mathematical modelling of superoxide generation with the bc1 complex of mitochondria. Biochemistry (Mosc) 1998;63:634–649. [PubMed] [Google Scholar]
- 126.Gille L, Nohl H. The ubiquinol/bc1 redox couple regulates mitochondrial oxygen radical formation. Arch Biochem Biophys. 2001;388:34–38. doi: 10.1006/abbi.2000.2257. [DOI] [PubMed] [Google Scholar]
- 127.St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem. 2002;277:44784–44790. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- 128.Suh JH, Heath SH, Hagen TM. Two subpopulations of mitochondria in the aging rat heart display heterogenous levels of oxidative stress. Free Radic Biol Med. 2003;35:1064–1072. doi: 10.1016/s0891-5849(03)00468-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Judge S, Jang YM, Smith A, Selman C, Phillips T, Speakman JR, Hagen T, Leeuwenburgh C. Exercise by lifelong voluntary wheel running reduces subsarcolemmal and interfibrillar mitochondrial hydrogen peroxide production in the heart. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1564–1572. doi: 10.1152/ajpregu.00396.2005. [DOI] [PubMed] [Google Scholar]
- 130.Phaneuf S, Leeuwenburgh C. Cytochrome c release from mitochondria in the aging heart: A possible mechanism for apoptosis with age. Am J Physiol Regul Integr Comp Physiol. 2002;282:R423–430. doi: 10.1152/ajpregu.00296.2001. [DOI] [PubMed] [Google Scholar]
- 131.Paillard M, Tubbs E, Thiebaut PA, Gomez L, Fauconnier J, Da Silva CC, Teixeira G, Mewton N, Belaidi E, Durand A, Abrial M, Lacampagne A, Rieusset J, Ovize M. Depressing mitochondria-reticulum interactions protects cardiomyocytes from lethal hypoxia-reoxygenation injury. Circulation. 2013;128:1555–1565. doi: 10.1161/CIRCULATIONAHA.113.001225. [DOI] [PubMed] [Google Scholar]
- 132.Eisner V, Csordas G, Hajnoczky G. Interactions between sarco-endoplasmic reticulum and mitochondria in cardiac and skeletal muscle - pivotal roles in Ca2+ and reactive oxygen species signaling. J Cell Sci. 2013;126:2965–2978. doi: 10.1242/jcs.093609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Turrens JF, Boveris A. Generation of superoxide anion by the nadh dehydrogenase of bovine heart mitochondria. Biochem J. 1980;191:421–427. doi: 10.1042/bj1910421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Genova ML, Ventura B, Giuliano G, Bovina C, Formiggini G, Parenti Castelli G, Lenaz G. The site of production of superoxide radical in mitochondrial complex i is not a bound ubisemiquinone but presumably iron-sulfur cluster n2. FEBS Lett. 2001;505:364–368. doi: 10.1016/s0014-5793(01)02850-2. [DOI] [PubMed] [Google Scholar]
- 135.Ohnishi ST, Ohnishi T, Muranaka S, Fujita H, Kimura H, Uemura K, Yoshida K, Utsumi K. A possible site of superoxide generation in the complex i segment of rat heart mitochondria. J Bioenerg Biomembr. 2005;37:1–15. doi: 10.1007/s10863-005-4117-y. [DOI] [PubMed] [Google Scholar]
- 136.Heinen A, Aldakkak M, Stowe DF, Rhodes SS, Riess ML, Varadarajan SG, Camara AK. Reverse electron flow-induced ros production is attenuated by activation of mitochondrial Ca2+-sensitive k+ channels. Am J Physiol Heart Circ Physiol. 2007;293:H1400–1407. doi: 10.1152/ajpheart.00198.2007. [DOI] [PubMed] [Google Scholar]
- 137.Miwa S, Brand MD. Mitochondrial matrix reactive oxygen species production is very sensitive to mild uncoupling. Biochem Soc Trans. 2003;31:1300–1301. doi: 10.1042/bst0311300. [DOI] [PubMed] [Google Scholar]
- 138.Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Ischemic defects in the electron transport chain increase the production of reactive oxygen species from isolated rat heart mitochondria. Am J Physiol Cell Physiol. 2008;294:C460–466. doi: 10.1152/ajpcell.00211.2007. [DOI] [PubMed] [Google Scholar]
- 139.Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 140.Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, Pelliccia G, Luzi L, Minucci S, Marcaccio M, Pinton P, Rizzuto R, Bernardi P, Paolucci F, Pelicci PG. Electron transfer between cytochrome c and p66shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221–233. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 141.Yan H, Jihong Y, Feng Z, Xiaomei X, Xiaohan Z, Guangzhi W, Zhenhai M, Dongyan G, Xiaochi M, Qing F, Kexin L, Xiaofeng T. Sirtuin 1-mediated inhibition of p66shc expression alleviates liver ischemia/reperfusion injury. Crit Care Med. 2014;42:e373–381. doi: 10.1097/CCM.0000000000000246. [DOI] [PubMed] [Google Scholar]
- 142.Spescha RD, Klohs J, Semerano A, Giacalone G, Derungs RS, Reiner MF, Rodriguez Gutierrez D, Mendez-Carmona N, Glanzmann M, Savarese G, Krankel N, Akhmedov A, Keller S, Mocharla P, Kaufmann MR, Wenger RH, Vogel J, Kulic L, Nitsch RM, Beer JH, Peruzzotti-Jametti L, Sessa M, Luscher TF, Camici GG. Post-ischaemic silencing of p66shc reduces ischaemia/reperfusion brain injury and its expression correlates to clinical outcome in stroke. Eur Heart J. 2015;36:1590–1600. doi: 10.1093/eurheartj/ehv140. [DOI] [PubMed] [Google Scholar]
- 143.Carpi A, Menabo R, Kaludercic N, Pelicci P, Di Lisa F, Giorgio M. The cardioprotective effects elicited by p66(shc) ablation demonstrate the crucial role of mitochondrial ros formation in ischemia/reperfusion injury. Biochim Biophys Acta. 2009;1787:774–780. doi: 10.1016/j.bbabio.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 144.Akhmedov A, Montecucco F, Braunersreuther V, Camici GG, Jakob P, Reiner MF, Glanzmann M, Burger F, Paneni F, Galan K, Pelli G, Vuilleumier N, Belin A, Vallee JP, Mach F, Luscher TF. Genetic deletion of the adaptor protein p66shc increases susceptibility to short-term ischaemic myocardial injury via intracellular salvage pathways. Eur Heart J. 2015;36:516–526a. doi: 10.1093/eurheartj/ehu400. [DOI] [PubMed] [Google Scholar]
- 145.Kaludercic N, Mialet-Perez J, Paolocci N, Parini A, Di Lisa F. Monoamine oxidases as sources of oxidants in the heart. J Mol Cell Cardiol. 2014;73:34–42. doi: 10.1016/j.yjmcc.2013.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kaludercic N, Carpi A, Menabo R, Di Lisa F, Paolocci N. Monoamine oxidases (mao) in the pathogenesis of heart failure and ischemia/reperfusion injury. Biochim Biophys Acta. 2011;1813:1323–1332. doi: 10.1016/j.bbamcr.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Menazza S, Blaauw B, Tiepolo T, Toniolo L, Braghetta P, Spolaore B, Reggiani C, Di Lisa F, Bonaldo P, Canton M. Oxidative stress by monoamine oxidases is causally involved in myofiber damage in muscular dystrophy. Hum Mol Genet. 2010;19:4207–4215. doi: 10.1093/hmg/ddq339. [DOI] [PubMed] [Google Scholar]
- 148.Saura J, Richards JG, Mahy N. Age-related changes on mao in bl/c57 mouse tissues: A quantitative radioautographic study. J Neural Transm Suppl. 1994;41:89–94. doi: 10.1007/978-3-7091-9324-2_11. [DOI] [PubMed] [Google Scholar]
- 149.Maurel A, Hernandez C, Kunduzova O, Bompart G, Cambon C, Parini A, Frances B. Age-dependent increase in hydrogen peroxide production by cardiac monoamine oxidase a in rats. Am J Physiol Heart Circ Physiol. 2003;284:H1460–1467. doi: 10.1152/ajpheart.00700.2002. [DOI] [PubMed] [Google Scholar]
- 150.Floyd RA, West M, Hensley K. Oxidative biochemical markers; clues to understanding aging in long-lived species. Exp Gerontol. 2001;36:619–640. doi: 10.1016/s0531-5565(00)00231-x. [DOI] [PubMed] [Google Scholar]
- 151.Bevilacqua L, Ramsey JJ, Hagopian K, Weindruch R, Harper ME. Long-term caloric restriction increases ucp3 content but decreases proton leak and reactive oxygen species production in rat skeletal muscle mitochondria. Am J Physiol Endocrinol Metab. 2005;289:E429–438. doi: 10.1152/ajpendo.00435.2004. [DOI] [PubMed] [Google Scholar]
- 152.Dai DF, Santana LF, Vermulst M, Tomazela DM, Emond MJ, MacCoss MJ, Gollahon K, Martin GM, Loeb LA, Ladiges WC, Rabinovitch PS. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation. 2009;119:2789–2797. doi: 10.1161/CIRCULATIONAHA.108.822403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang Y, Hekimi S. Mitochondrial dysfunction and longevity in animals: Untangling the knot. Science. 2015;350:1204–1207. doi: 10.1126/science.aac4357. [DOI] [PubMed] [Google Scholar]
- 154.Van Raamsdonk JM, Hekimi S. Deletion of the mitochondrial superoxide dismutase SOD-2 extends lifespan in caenorhabditis elegans. PLoS Genet. 2009;5:e1000361. doi: 10.1371/journal.pgen.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Feng J, Bussiere F, Hekimi S. Mitochondrial electron transport is a key determinant of life span in caenorhabditis elegans. Dev Cell. 2001;1:633–644. doi: 10.1016/s1534-5807(01)00071-5. [DOI] [PubMed] [Google Scholar]
- 156.Imhoff BR, Hansen JM. Extracellular redox status regulates NRF2 activation through mitochondrial reactive oxygen species. Biochem J. 2009;424:491–500. doi: 10.1042/BJ20091286. [DOI] [PubMed] [Google Scholar]
- 157.Pulliam DA, Deepa SS, Liu Y, Hill S, Lin AL, Bhattacharya A, Shi Y, Sloane L, Viscomi C, Zeviani M, Van Remmen H. Complex iv-deficient surf1(−/−) mice initiate mitochondrial stress responses. Biochem J. 2014;462:359–371. doi: 10.1042/BJ20140291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Safdar A, deBeer J, Tarnopolsky MA. Dysfunctional nrf2-keap1 redox signaling in skeletal muscle of the sedentary old. Free Radic Biol Med. 2010;49:1487–1493. doi: 10.1016/j.freeradbiomed.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 159.Silva-Palacios A, Konigsberg M, Zazueta C. Nrf2 signaling and redox homeostasis in the aging heart: A potential target to prevent cardiovascular diseases? Ageing Res Rev. 2016;26:81–95. doi: 10.1016/j.arr.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 160.Kwong JQ, Davis J, Baines CP, Sargent MA, Karch J, Wang X, Huang T, Molkentin JD. Genetic deletion of the mitochondrial phosphate carrier desensitizes the mitochondrial permeability transition pore and causes cardiomyopathy. Cell Death Differ. 2014;21:1209–1217. doi: 10.1038/cdd.2014.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Halestrap AP, Pereira GC, Pasdois P. The role of hexokinase in cardioprotection - mechanism and potential for translation. Br J Pharmacol. 2015;172:2085–2100. doi: 10.1111/bph.12899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Elrod JW, Wong R, Mishra S, Vagnozzi RJ, Sakthievel B, Goonasekera SA, Karch J, Gabel S, Farber J, Force T, Brown JH, Murphy E, Molkentin JD. Cyclophilin d controls mitochondrial pore-dependent ca(2+) exchange, metabolic flexibility, and propensity for heart failure in mice. J Clin Invest. 2010;120:3680–3687. doi: 10.1172/JCI43171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion--a target for cardioprotection. Cardiovasc Res. 2004;61:372–385. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 164.Gharib A, De Paulis D, Li B, Augeul L, Couture-Lepetit E, Gomez L, Angoulvant D, Ovize M. Opposite and tissue-specific effects of coenzyme q2 on mptp opening and ros production between heart and liver mitochondria: Role of complex i. J Mol Cell Cardiol. 2012;52:1091–1095. doi: 10.1016/j.yjmcc.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 165.Giorgio V, Petronilli V, Ghelli A, Carelli V, Rugolo M, Lenaz G, Bernardi P. The effects of idebenone on mitochondrial bioenergetics. Biochim Biophys Acta. 2012;1817:363–369. doi: 10.1016/j.bbabio.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Teixeira G, Abrial M, Portier K, Chiari P, Couture-Lepetit E, Tourneur Y, Ovize M, Gharib A. Synergistic protective effect of cyclosporin a and rotenone against hypoxia-reoxygenation in cardiomyocytes. J Mol Cell Cardiol. 2013;56:55–62. doi: 10.1016/j.yjmcc.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 167.Kagan VE, Borisenko GG, Tyurina YY, Tyurin VA, Jiang J, Potapovich AI, Kini V, Amoscato AA, Fujii Y. Oxidative lipidomics of apoptosis: Redox catalytic interactions of cytochrome c with cardiolipin and phosphatidylserine. Free Radic Biol Med. 2004;37:1963–1985. doi: 10.1016/j.freeradbiomed.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 168.Ebrahimian T, Sairam MR, Schiffrin EL, Touyz RM. Cardiac hypertrophy is associated with altered thioredoxin and ask-1 signaling in a mouse model of menopause. Am J Physiol Heart Circ Physiol. 2008;295:H1481–1488. doi: 10.1152/ajpheart.00163.2008. [DOI] [PubMed] [Google Scholar]
- 169.Hsieh CC, Papaconstantinou J. Thioredoxin-ask1 complex levels regulate ros-mediated p38 mapk pathway activity in livers of aged and long-lived snell dwarf mice. Faseb J. 2006;20:259–268. doi: 10.1096/fj.05-4376com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Minn Y, Cho KJ, Kim HW, Kim HJ, Suk SH, Lee BI, Kim GW. Induction of apoptosis signal-regulating kinase 1 and oxidative stress mediate age-dependent vulnerability to 3-nitropropionic acid in the mouse striatum. Neurosci Lett. 2008;430:142–146. doi: 10.1016/j.neulet.2007.10.042. [DOI] [PubMed] [Google Scholar]
- 171.Liu Q, Sargent MA, York AJ, Molkentin JD. Ask1 regulates cardiomyocyte death but not hypertrophy in transgenic mice. Circ Res. 2009;105:1110–1117. doi: 10.1161/CIRCRESAHA.109.200741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Aluri SH, Chen Q, Hu Y, Toldo S, Abbate A, Budas RG, Lesnefsky EJ. Apoptosis signal regulating kinase-1 inhibitor decreases mitochondrial damage during ischemia-reperfusion. Circulation. 2012;126 abstratct 15526. [Google Scholar]
- 173.Szczepanek K, Lesnefsky EJ, Larner AC. Multi-tasking: Nuclear transcription factors with novel roles in the mitochondria. Trends Cell Biol. 2012;22:429–437. doi: 10.1016/j.tcb.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wegrzyn J, Potla R, Chwae YJ, Sepuri NB, Zhang Q, Koeck T, Derecka M, Szczepanek K, Szelag M, Gornicka A, Moh A, Moghaddas S, Chen Q, Bobbili S, Cichy J, Dulak J, Baker DP, Wolfman A, Stuehr D, Hassan MO, Fu XY, Avadhani N, Drake JI, Fawcett P, Lesnefsky EJ, Larner AC. Function of mitochondrial stat3 in cellular respiration. Science. 2009;323:793–797. doi: 10.1126/science.1164551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chen J, Huang X, Halicka D, Brodsky S, Avram A, Eskander J, Bloomgarden NA, Darzynkiewicz Z, Goligorsky MS. Contribution of p16ink4a and p21cip1 pathways to induction of premature senescence of human endothelial cells: Permissive role of p53. Am J Physiol Heart Circ Physiol. 2006;290:H1575–1586. doi: 10.1152/ajpheart.00364.2005. [DOI] [PubMed] [Google Scholar]
- 176.Levy DE, Lee CK. What does stat3 do? J Clin Invest. 2002;109:1143–1148. doi: 10.1172/JCI15650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Boengler K, Hilfiker-Kleiner D, Drexler H, Heusch G, Schulz R. The myocardial jak/stat pathway: From protection to failure. Pharmacol Ther. 2008;120:172–185. doi: 10.1016/j.pharmthera.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 178.Boengler K, Hilfiker-Kleiner D, Heusch G, Schulz R. Inhibition of permeability transition pore opening by mitochondrial stat3 and its role in myocardial ischemia/reperfusion. Basic Res Cardiol. 2010;105:771–785. doi: 10.1007/s00395-010-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Heusch G, Musiolik J, Gedik N, Skyschally A. Mitochondrial stat3 activation and cardioprotection by ischemic postconditioning in pigs with regional myocardial ischemia/reperfusion. Circ Res. 2011;109:1302–1308. doi: 10.1161/CIRCRESAHA.111.255604. [DOI] [PubMed] [Google Scholar]
- 180.Zhang J, Yang J, Roy SK, Tininini S, Hu J, Bromberg JF, Poli V, Stark GR, Kalvakolanu DV. The cell death regulator grim-19 is an inhibitor of signal transducer and activator of transcription 3. Proc Natl Acad Sci U S A. 2003;100:9342–9347. doi: 10.1073/pnas.1633516100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Phillips D, Reilley MJ, Aponte AM, Wang G, Boja E, Gucek M, Balaban RS. Stoichiometry of stat3 and mitochondrial proteins: Implications for the regulation of oxidative phosphorylation by protein-protein interactions. J Biol Chem. 2010;285:23532–23536. doi: 10.1074/jbc.C110.152652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Szczepanek K, Chen Q, Derecka M, Salloum FN, Zhang Q, Szelag M, Cichy J, Kukreja RC, Dulak J, Lesnefsky EJ, Larner AC. Mitochondrial-targeted signal transducer and activator of transcription 3 (stat3) protects against ischemia-induced changes in the electron transport chain and the generation of reactive oxygen species. J Biol Chem. 2011;286:29610–29620. doi: 10.1074/jbc.M111.226209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Marchenko ND, Wolff S, Erster S, Becker K, Moll UM. Monoubiquitylation promotes mitochondrial p53 translocation. Embo J. 2007;26:923–934. doi: 10.1038/sj.emboj.7601560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Marchenko ND, Zaika A, Moll UM. Death signal-induced localization of p53 protein to mitochondria. A potential role in apoptotic signaling. J Biol Chem. 2000;275:16202–16212. doi: 10.1074/jbc.275.21.16202. [DOI] [PubMed] [Google Scholar]
- 185.Zhao Y, Chaiswing L, Velez JM, Batinic-Haberle I, Colburn NH, Oberley TD, St Clair DK. P53 translocation to mitochondria precedes its nuclear translocation and targets mitochondrial oxidative defense protein-manganese superoxide dismutase. Cancer Res. 2005;65:3745–3750. doi: 10.1158/0008-5472.CAN-04-3835. [DOI] [PubMed] [Google Scholar]
- 186.Ong SB, Hall AR, Hausenloy DJ. Mitochondrial dynamics in cardiovascular health and disease. Antioxid Redox Signal. 2013;19:400–414. doi: 10.1089/ars.2012.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Carreira RS, Lee P, Gottlieb RA. Mitochondrial therapeutics for cardioprotection. Curr Pharm Des. 2011;17:2017–2035. doi: 10.2174/138161211796904777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Calo L, Dong Y, Kumar R, Przyklenk K, Sanderson TH. Mitochondrial dynamics: An emerging paradigm in ischemia-reperfusion injury. Curr Pharm Des. 2013;19:6848–6857. doi: 10.2174/138161281939131127110701. [DOI] [PubMed] [Google Scholar]
- 189.Dorn GW., 2nd Mitochondrial dynamism and heart disease: Changing shape and shaping change. EMBO Mol Med. 2015;7:865–877. doi: 10.15252/emmm.201404575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Gottlieb RA, Gustafsson AB. Mitochondrial turnover in the heart. Biochim Biophys Acta. 2011;1813:1295–1301. doi: 10.1016/j.bbamcr.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ikeda Y, Sciarretta S, Nagarajan N, Rubattu S, Volpe M, Frati G, Sadoshima J. New insights into the role of mitochondrial dynamics and autophagy during oxidative stress and aging in the heart. Oxid Med Cell Longev. 2014;2014:210934. doi: 10.1155/2014/210934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lee S, Jeong SY, Lim WC, Kim S, Park YY, Sun X, Youle RJ, Cho H. Mitochondrial fission and fusion mediators, hfis1 and opa1, modulate cellular senescence. J Biol Chem. 2007;282:22977–22983. doi: 10.1074/jbc.M700679200. [DOI] [PubMed] [Google Scholar]
- 193.Park YY, Lee S, Karbowski M, Neutzner A, Youle RJ, Cho H. Loss of march5 mitochondrial e3 ubiquitin ligase induces cellular senescence through dynamin-related protein 1 and mitofusin 1. J Cell Sci. 2010;123:619–626. doi: 10.1242/jcs.061481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Donato AJ, Morgan RG, Walker AE, Lesniewski LA. Cellular and molecular biology of aging endothelial cells. J Mol Cell Cardiol. 2015;89:122–135. doi: 10.1016/j.yjmcc.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Park YY, Nguyen OT, Kang H, Cho H. March5-mediated quality control on acetylated mfn1 facilitates mitochondrial homeostasis and cell survival. Cell Death Dis. 2014;5:e1172. doi: 10.1038/cddis.2014.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Joseph AM, Adhihetty PJ, Wawrzyniak NR, Wohlgemuth SE, Picca A, Kujoth GC, Prolla TA, Leeuwenburgh C. Dysregulation of mitochondrial quality control processes contribute to sarcopenia in a mouse model of premature aging. PLoS One. 2013;8:e69327. doi: 10.1371/journal.pone.0069327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sharp WW, Beiser DG, Fang YH, Han M, Piao L, Varughese J, Archer SL. Inhibition of the mitochondrial fission protein dynamin-related protein 1 improves survival in a murine cardiac arrest model. Crit Care Med. 2015;43:e38–47. doi: 10.1097/CCM.0000000000000817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sumida M, Doi K, Ogasawara E, Yamashita T, Hamasaki Y, Kariya T, Takimoto E, Yahagi N, Nangaku M, Noiri E. Regulation of mitochondrial dynamics by dynamin-related protein-1 in acute cardiorenal syndrome. J Am Soc Nephrol. 2015;26:2378–2387. doi: 10.1681/ASN.2014080750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Maejima Y, Adachi S, Ito H, Hirao K, Isobe M. Induction of premature senescence in cardiomyocytes by doxorubicin as a novel mechanism of myocardial damage. Aging Cell. 2008;7:125–136. doi: 10.1111/j.1474-9726.2007.00358.x. [DOI] [PubMed] [Google Scholar]
- 200.Russell LK, Mansfield CM, Lehman JJ, Kovacs A, Courtois M, Saffitz JE, Medeiros DM, Valencik ML, McDonald JA, Kelly DP. Cardiac-specific induction of the transcriptional coactivator peroxisome proliferator-activated receptor gamma coactivator-1alpha promotes mitochondrial biogenesis and reversible cardiomyopathy in a developmental stage-dependent manner. Circ Res. 2004;94:525–533. doi: 10.1161/01.RES.0000117088.36577.EB. [DOI] [PubMed] [Google Scholar]
- 201.Hammerling BC, Gustafsson AB. Mitochondrial quality control in the myocardium: Cooperation between protein degradation and mitophagy. J Mol Cell Cardiol. 2014;75:122–130. doi: 10.1016/j.yjmcc.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Huang C, Andres AM, Ratliff EP, Hernandez G, Lee P, Gottlieb RA. Preconditioning involves selective mitophagy mediated by parkin and p62/sqstm1. PLoS One. 2011;6:e20975. doi: 10.1371/journal.pone.0020975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gustafsson AB, Gottlieb RA. Autophagy in ischemic heart disease. Circ Res. 2009;104:150–158. doi: 10.1161/CIRCRESAHA.108.187427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Minkler PE, Stoll MS, Ingalls ST, Kerner J, Hoppel CL. Quantitative acylcarnitine determination by uhplc-ms/ms - going beyond tandem ms acylcarnitine “profiles”. Mol Genet Metab. 2015;116:231–241. doi: 10.1016/j.ymgme.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Muller AL, Freed D, Hryshko L, Dhalla NS. Implications of protease activation in cardiac dysfunction and development of genetic cardiomyopathy in hamsters. Can J Physiol Pharmacol. 2012;90:995–1004. doi: 10.1139/y2012-034. [DOI] [PubMed] [Google Scholar]
- 206.Chen Q, Lesnefsky EJ. Heart mitochondria and calpain 1: Location, function, and targets. Biochim Biophys Acta. 2015;1852:2372–2378. doi: 10.1016/j.bbadis.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 207.Wang K, Zhang J, Liu J, Tian J, Wu Y, Wang X, Quan L, Xu H, Wang W, Liu H. Variations in the protein level of omi/htra2 in the heart of aged rats may contribute to the increased susceptibility of cardiomyocytes to ischemia/reperfusion injury and cell death: Omi/htra2 and aged heart injury. Age (Dordr) 2013;35:733–746. doi: 10.1007/s11357-012-9406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kang S, Louboutin JP, Datta P, Landel CP, Martinez D, Zervos AS, Strayer DS, Fernandes-Alnemri T, Alnemri ES. Loss of htra2/omi activity in non-neuronal tissues of adult mice causes premature aging. Cell Death Differ. 2013;20:259–269. doi: 10.1038/cdd.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Delaval E, Perichon M, Friguet B. Age-related impairment of mitochondrial matrix aconitase and atp-stimulated protease in rat liver and heart. Eur J Biochem. 2004;271:4559–4564. doi: 10.1111/j.1432-1033.2004.04422.x. [DOI] [PubMed] [Google Scholar]
- 210.Kuo CY, Chiu YC, Lee AY, Hwang TL. Mitochondrial lon protease controls ros-dependent apoptosis in cardiomyocyte under hypoxia. Mitochondrion. 2015;23:7–16. doi: 10.1016/j.mito.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 211.Terman A, Brunk UT. Myocyte aging and mitochondrial turnover. Exp Gerontol. 2004;39:701–705. doi: 10.1016/j.exger.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 212.Boengler K, Konietzka I, Buechert A, Heinen Y, Garcia-Dorado D, Heusch G, Schulz R. Loss of ischemic preconditioning’s cardioprotection in aged mouse hearts is associated with reduced gap junctional and mitochondrial levels of connexin 43. Am J Physiol Heart Circ Physiol. 2007;292:H1764–1769. doi: 10.1152/ajpheart.01071.2006. [DOI] [PubMed] [Google Scholar]
- 213.Abete P, Testa G, Ferrara N, De Santis D, Capaccio P, Viati L, Calabrese C, Cacciatore F, Longobardi G, Condorelli M, Napoli C, Rengo F. Cardioprotective effect of ischemic preconditioning is preserved in food-restricted senescent rats. Am J Physiol Heart Circ Physiol. 2002;282:H1978–1987. doi: 10.1152/ajpheart.00929.2001. [DOI] [PubMed] [Google Scholar]
- 214.Tani M, Honma Y, Hasegawa H, Tamaki K. Direct activation of mitochondrial k(atp) channels mimics preconditioning but protein kinase c activation is less effective in middle-aged rat hearts. Cardiovasc Res. 2001;49:56–68. doi: 10.1016/s0008-6363(00)00240-6. [DOI] [PubMed] [Google Scholar]
- 215.Ishihara M, Sato H, Tateishi H, Kawagoe T, Shimatani Y, Ueda K, Noma K, Yumoto A, Nishioka K. Beneficial effect of prodromal angina pectoris is lost in elderly patients with acute myocardial infarction. Am Heart J. 2000;139:881–888. doi: 10.1016/s0002-8703(00)90021-8. [DOI] [PubMed] [Google Scholar]
- 216.Lee TM, Su SF, Chou TF, Lee YT, Tsai CH. Loss of preconditioning by attenuated activation of myocardial atp-sensitive potassium channels in elderly patients undergoing coronary angioplasty. Circulation. 2002;105:334–340. doi: 10.1161/hc0302.102572. [DOI] [PubMed] [Google Scholar]
- 217.Przyklenk K, Maynard M, Darling CE, Whittaker P. Aging mouse hearts are refractory to infarct size reduction with post-conditioning. J Am Coll Cardiol. 2008;51:1393–1398. doi: 10.1016/j.jacc.2007.11.070. [DOI] [PubMed] [Google Scholar]
- 218.Abete P, Testa G, Galizia G, Mazzella F, Della Morte D, de Santis D, Calabrese C, Cacciatore F, Gargiulo G, Ferrara N, Rengo G, Sica V, Napoli C, Rengo F. Tandem action of exercise training and food restriction completely preserves ischemic preconditioning in the aging heart. Exp Gerontol. 2005;40:43–50. doi: 10.1016/j.exger.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 219.Niemann B, Chen Y, Issa H, Silber RE, Rohrbach S. Caloric restriction delays cardiac ageing in rats: Role of mitochondria. Cardiovasc Res. 2010;88:267–276. doi: 10.1093/cvr/cvq273. [DOI] [PubMed] [Google Scholar]
- 220.Shinmura K. Effects of caloric restriction on cardiac oxidative stress and mitochondrial bioenergetics: Potential role of cardiac sirtuins. Oxid Med Cell Longev. 2013;2013:528935. doi: 10.1155/2013/528935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Cencioni C, Spallotta F, Mai A, Martelli F, Farsetti A, Zeiher AM, Gaetano C. Sirtuin function in aging heart and vessels. J Mol Cell Cardiol. 2015;83:55–61. doi: 10.1016/j.yjmcc.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 222.Winnik S, Auwerx J, Sinclair DA, Matter CM. Protective effects of sirtuins in cardiovascular diseases: From bench to bedside. Eur Heart J. 2015;36:3404–3412. doi: 10.1093/eurheartj/ehv290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Sack MN, Finkel T. Mitochondrial metabolism, sirtuins, and aging. Cold Spring Harb Perspect Biol. 2012:4. doi: 10.1101/cshperspect.a013102. [DOI] [PMC free article] [PubMed]
- 224.Ascensao A, Ferreira R, Magalhaes J. Exercise-induced cardioprotection--biochemical, morphological and functional evidence in whole tissue and isolated mitochondria. Int J Cardiol. 2007;117:16–30. doi: 10.1016/j.ijcard.2006.04.076. [DOI] [PubMed] [Google Scholar]
- 225.Jahangir A, Ozcan C, Holmuhamedov EL, Terzic A. Increased calcium vulnerability of senescent cardiac mitochondria: Protective role for a mitochondrial potassium channel opener. Mech Ageing Dev. 2001;122:1073–1086. doi: 10.1016/s0047-6374(01)00242-1. [DOI] [PubMed] [Google Scholar]
- 226.Paradies G, Ruggiero FM, Petrosillo G, Gadaleta MN, Quagliariello E. The effect of aging and acetyl-l-carnitine on the acitivity of the phophate carrier and on the phospholipid composition in rat heart mitochondria. Biochem Biophys Acta. 1992;406:136–138. doi: 10.1016/0005-2736(92)90103-s. [DOI] [PubMed] [Google Scholar]
- 227.Lesnefsky EJ, He D, Moghaddas S, Hoppel CL. Reversal of mitochondrial defects before ischemia protects the aged heart. Faseb J. 2006;20:1543–1545. doi: 10.1096/fj.05-4535fje. [DOI] [PubMed] [Google Scholar]
- 228.Gadaleta MN, Petruzzella V, Daddabbo L, Olivieri C, Fracasso F, Loguercio Polosa P, Cantatore P. Mitochondrial dna transcription and translation in aged rat. Effect of acetyl-l-carnitine. Ann N Y Acad Sci. 1994;717:150–160. doi: 10.1111/j.1749-6632.1994.tb12082.x. [DOI] [PubMed] [Google Scholar]
- 229.Rosca MG, Lemieux H, Hoppel CL. Mitochondria in the elderly: Is acetylcarnitine a rejuvenator? Adv Drug Deliv Rev. 2009;61:1332–1342. doi: 10.1016/j.addr.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T. A role for the mitochondrial deacetylase sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A. 2008;105:14447–14452. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A, Yang Y, Chen Y, Hirschey MD, Bronson RT, Haigis M, Guarente LP, Farese RV, Jr, Weissman S, Verdin E, Schwer B. Mammalian sir2 homolog sirt3 regulates global mitochondrial lysine acetylation. Mol Cell Biol. 2007;27:8807–8814. doi: 10.1128/MCB.01636-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Sack MN. The role of sirt3 in mitochondrial homeostasis and cardiac adaptation to hypertrophy and aging. J Mol Cell Cardiol. 2012;52:520–525. doi: 10.1016/j.yjmcc.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Braidy N, Guillemin GJ, Mansour H, Chan-Ling T, Poljak A, Grant R. Age related changes in nad+ metabolism oxidative stress and sirt1 activity in wistar rats. PLoS One. 2011;6:e19194. doi: 10.1371/journal.pone.0019194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ruggieri S, Orsomando G, Sorci L, Raffaelli N. Regulation of nad biosynthetic enzymes modulates nad-sensing processes to shape mammalian cell physiology under varying biological cues. Biochim Biophys Acta. 2015;1854:1138–1149. doi: 10.1016/j.bbapap.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 235.Mendelsohn AR, Larrick JW. Partial reversal of skeletal muscle aging by restoration of normal nad(+) levels. Rejuvenation Res. 2014;17:62–69. doi: 10.1089/rej.2014.1546. [DOI] [PubMed] [Google Scholar]
- 236.Gong B, Pan Y, Vempati P, Zhao W, Knable L, Ho L, Wang J, Sastre M, Ono K, Sauve AA, Pasinetti GM. Nicotinamide riboside restores cognition through an upregulation of proliferator-activated receptor-gamma coactivator 1alpha regulated beta-secretase 1 degradation and mitochondrial gene expression in alzheimer’s mouse models. Neurobiol Aging. 2013;34:1581–1588. doi: 10.1016/j.neurobiolaging.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ochoa JJ, Quiles JL, Huertas JR, Mataix J. Coenzyme q10 protects from aging-related oxidative stress and improves mitochondrial function in heart of rats fed a polyunsaturated fatty acid (pufa)-rich diet. J Gerontol A Biol Sci Med Sci. 2005;60:970–975. doi: 10.1093/gerona/60.8.970. [DOI] [PubMed] [Google Scholar]
- 238.Quiles JL, Pamplona R, Ramirez-Tortosa MC, Naudi A, Portero-Otin M, Araujo-Nepomuceno E, Lopez-Frias M, Battino M, Ochoa JJ. Coenzyme q addition to an n-6 pufa-rich diet resembles benefits on age-related mitochondrial dna deletion and oxidative stress of a mufa-rich diet in rat heart. Mech Ageing Dev. 2010;131:38–47. doi: 10.1016/j.mad.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 239.Sohal RS, Kamzalov S, Sumien N, Ferguson M, Rebrin I, Heinrich KR, Forster MJ. Effect of coenzyme q10 intake on endogenous coenzyme q content, mitochondrial electron transport chain, antioxidative defenses, and life span of mice. Free Radic Biol Med. 2006;40:480–487. doi: 10.1016/j.freeradbiomed.2005.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Wilkinson JE, Burmeister L, Brooks SV, Chan CC, Friedline S, Harrison DE, Hejtmancik JF, Nadon N, Strong R, Wood LK, Woodward MA, Miller RA. Rapamycin slows aging in mice. Aging Cell. 2012;11:675–682. doi: 10.1111/j.1474-9726.2012.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Zhang Y, Bokov A, Gelfond J, Soto V, Ikeno Y, Hubbard G, Diaz V, Sloane L, Maslin K, Treaster S, Rendon S, van Remmen H, Ward W, Javors M, Richardson A, Austad SN, Fischer K. Rapamycin extends life and health in c57bl/6 mice. J Gerontol A Biol Sci Med Sci. 2013;69:119–130. doi: 10.1093/gerona/glt056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Flynn JM, O’Leary MN, Zambataro CA, Academia EC, Presley MP, Garrett BJ, Zykovich A, Mooney SD, Strong R, Rosen CJ, Kapahi P, Nelson MD, Kennedy BK, Melov S. Late-life rapamycin treatment reverses age-related heart dysfunction. Aging Cell. 2013;12:851–862. doi: 10.1111/acel.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Dai DF, Karunadharma PP, Chiao YA, Basisty N, Crispin D, Hsieh EJ, Chen T, Gu H, Djukovic D, Raftery D, Beyer RP, MacCoss MJ, Rabinovitch PS. Altered proteome turnover and remodeling by short-term caloric restriction or rapamycin rejuvenate the aging heart. Aging Cell. 2014;13:529–539. doi: 10.1111/acel.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.