Abstract
P21 activated kinase 2 (PAK2) is a member of Group I PAKs family and highly expressed in various cancers. Current studies have demonstrated that PAK2 played a pivotal role in tumor progression. However, the role of PAK2 in salivary adenoid cystic carcinoma is still unclear. This study aims to explore the expression and the function of PAK2 in AdCC. Human salivary gland tissue microarray, including 18 normal salivary glands (NSG), 12 pleomorphic adenoma (PMA) and 72 AdCC, and immunohistochemistry were used to evaluate the expression of PAK2. The result showed that PAK2 was significantly increased in AdCC compared with NSG and PMA. Then the Pearson correlation analysis using serial tissue sections showed a close correlation of PAK2 with Cyclin D1, Phospho-STAT3 at Tyrosine 705 (p-STAT3) and Ki-67. Further in vitro study utilizing PAK2 knockdown via siRNA transfection revealed significantly reduced migration and proliferation of AdCC cell lines compared with control group. Knockdown of PAK2 decreased the expression of Cyclin D1 in AdCC cell lines. In addition, the inhibition of STAT3 reduced the expression of PAK2 in AdCC cell lines. These findings suggested that PAK2 promotes AdCC cell migration and proliferation and may be a potential therapeutic target.
Keywords: P21 activated kinase 2, adenoid cystic carcinoma, migration, proliferation
Introduction
Adenoid cystic carcinoma (AdCC) is one of the most common salivary gland tumors, which arises in the salivary gland with predominant location of parotid, sublingual and minor salivary glands [1]. Based on the morphologically heterogeneous of AdCC tumor, three major histology types are distinguished including: tubular, cribriform and solid [2]. Currently, the treatment choices are limited to surgery with or without postoperative radiotherapy, consistent with the limited insight into the molecular mechanism and the inconspicuous clinical responses of chemotherapy [3]. Therefore, the study of molecular mechanism in AdCC may offer an enable treatment with novel targeted agents.
P21 activated kinases (PAKs), which are a family of serine/threonine-specific intracellular protein kinases comprised of six isoforms (PAK1-6), can be activated by Rac and Cdc42 [4]. Based on sequence and structure, the six isoforms of PAKs categorized into two subgroups: group I (PAK 1-3) and group II (PAK 4-6) [5]. PAKs isoforms frequently were overexpressed or aberrantly activated in various cancers and PAKs have been identified as drivers of tumor growth, proliferation and invasion [6].
P21 activated kinase 2 (PAK2) is one of isoforms which belongs to Group I (PAK1-3). Current studies have demonstrated that PAK2 is overexpressed in different types of human tumors, such as gastric cancer, ovarian cancer and head neck cancer [7-9]. PAK2 is activated not only by binding with Cdc42/Rac but also by caspase-3 and similar proteases [10]. Functional studies have also implicated PAK2 contribute to a number of processes involving tumor cell survival, mobility, proliferation and invasion [6]. PAK2 has both anti-apoptotic and pro-apoptotic functions based on the in vitro study [10,11]. In head neck cancer, the migration and invasion were reduced in PAK2 siRNA-treated cells [9]. However, the mechanism of PAK2 in AdCC remains mainly obscure. The aim of this study was to evaluate the expression of PAK2 and its function in AdCC.
In this study, we take advantage of AdCC tissue microarray to show the expression of PAK2 in AdCC. In addition, we analyzed the associated molecules with PAK2 using serial sections. Moreover, we applied PAK2 siRNA to identify the function of PAK2 in ACC cell lines.
Materials and methods
Cell culture and siRNA knockdown assay
SACC-LM and SACC-83 were cultured in RPMI 1640 medium (Hyclone) containing 10% FBS (fetal bovine serum) as previously described [12]. And the cell lines of human salivary AdCC (SACC-LM and SACC-83) were obtained from the China Center for Type Culture Collection. PAK2 siRNAs were purchased from GenePharma and transfected with a final concentration of 100 nM. The procedures were followed as previous described [13]. S3I-201 was purchased from Selleck Chemicals (Houston, TX, USA) and used at a final concentration of 100 μM.
Wound healing assay
SACC-LM and SACC-83 cells were plated in 6-well plates at a density of approximately 1.0×105 cells per well and grown to confluence. Then, the monolayer of cells was scratched with a sterile pipette tip to generate a constant gap and washed extensively to remove cellular debris. Next, the cells were incubated with medium with no FBS and documented by photograph at 12 hours.
Cell proliferation assay
Cell Counting Kit-8 (CCK-8, Dojindo, Japan) was used to test the cell proliferation. After 48 h of transfection, SACC-LM were seeded at density of 2×103 cells in 100 μl medium per well in a 96-well plate and cultured at 37°C in an atmosphere containing 5% CO2. 24 hours later and grew overnight. CCK-8 (10 μl per well in 100 ul medium) was added to each well at 0, 24, 48, 72 and 96 hours and incubated at 37°C for 2 hours. Then the absorbance at 450 nm was measured.
Clinical tissue samples and Ethics statement
Tissue samples were retrieved from the Department of Oral and Maxillofacial-Head and Neck Oncology, School of Hospital of Stomatology, Wuhan University. The patients have written informed consent and this study was approved by Medical Ethics Committee of Hospital of Stomatology, Wuhan University. The tissues were fixed with paraformaldehyde and embedded with paraffin. The tissue microarray was constructed in collaboration with Shanghai Biochip Company, Ltd, Shanghai, China and contained 72 adenoid cystic carcinoma (AdCC, 24 tubular pattern, 28 cribriform pattern, 20 solid pattern), 12 pleomorphic adenoma (PMAs) and 18 normal salivary gland (NSGs) as previous described [12].
Immunohistochemistry and immunofluorescence
A rabbit monoclonal antibody against PAK2 (Abcam) was used for immunohistochemistry in our study. The sections were rehydrated and antigen retrieved with sodium citrate in a pressure cooker. The endogenous peroxidase was blocked with 3% hydrogen peroxide. The non-specific binding was blocked with goat serum at 37°C for 1 hour. Antibody for PAK2 (Abcam), p-STAT3, Cyclin D1 and Ki-67 (Cell Signaling Technology) were diluted in Dako and the sections were incubated with them at 4°C overnight. Secondary biotin-labeled antibody and an avidin-biotin-peroxidase reagent were incubated consequently and DAB kit was applied to stain. The procedure of immunofluorescence was followed as previously described [12]. Antibody for PAK2 (Abcam, 1:200), p-STAT3 (Cell Signaling Technology, 1:200), Cyclin D1 (Cell Signaling Technology, 1:200) and Ki-67 (Cell Signaling Technology, 1:400) were used.
Western blot
The cells collected and lysed with RIPA lysis Buffer (Beyotime) and the concentrations were detected by BCA kit (Beyotime). The protein, denatured in loading buffer, was separated by 10% SDS-polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride (PVDF) membranes (Millipore) and probed with primary antibody against PAK2 (Abcam), Cyclin D1 (Cell Signaling Technology), p-STAT3 (Cell Signaling Technology). Proteins were detected by horseradish peroxidase conjugated secondary antibody and revealed using the ECL system (Advansta).
Scoring system and Hierarchical clustering
The immunohistochemical staining of whole slice was scanned using an Aperio ScanScope CS scanner (Vista, CA, USA) with background subtraction. The positive result was quantified using Aperio Quantification software (Version 9.1) for membrane, nuclear, or pixel quantification and histoscore were calculated using formula (3+)×3+(2+)×2+(1+)×1 as previous described [14]. The histoscore were converted into -3 to 3 and the cluster data were displayed with markers on the vertical axis and tissue samples on the horizontal axis, then hierarchical cluster 3.0 and Treeview 1.1.3 were performed as previously described [15].
Statistical analysis
GraphPad Prism software (6.0.1) was used to analyze the result. P value was considered statistically significant. Data were analyzed using One-way ANOVA followed post Turkey test. Pearson statistics were used to analyze the correlation of histioscore.
Results
The overexpression of PAK2 in AdCC tissues and its correlation with pathological features
To investigate the expression of PAK2 in human adenoid cystic carcinoma, immunohistochemistry was performed on a human AdCC tissue microarray containing 72 AdCC (24 tubular pattern, 28 cribriform pattern, 20 solid pattern), 12 common salivary glands benign tumor polymorphic adenoma (PMA) and 18 normal salivary gland (NSG). As shown in Figure 1A, the results of the immunohistochemistry using specific PAK2 antibody were displayed in NSG, PMA, cribriform pattern AdCC, tubular pattern AdCC and solid pattern AdCC. The positive expression of PAK2 mainly located in cytoplasm and highly expressed in AdCC, including cribriform pattern (patient #1), tubular pattern (patient #2) and solid pattern (patient #3, Figure 1A). In contrast, PAK2 is almost negative in NSG and PMA, which indicated that the overexpression of PAK2 may represent a malignant status in salivary tumor (Figure 1A). And the statistical analysis showed the expression of PAK2 significantly increased in AdCC compared with NSG and PMA (Figure 1B). Moreover, we analyzed the expression of PAK2 in cribriform, tubular and solid pattern of AdCC. And there is no significant correlation between PAK2 and histopathological pattern (Figure 1C).
Figure 1.
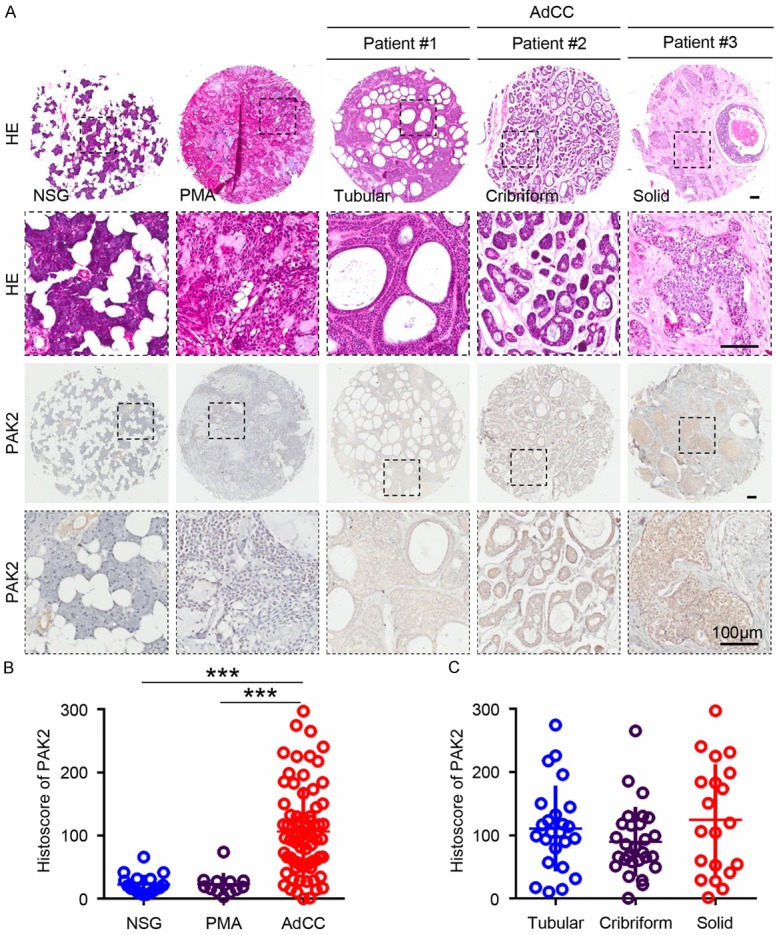
The expression of PAK2 is up-regulated in adenoid cystic carcinoma (AdCC). A. Serial sections of paraffin-embedded AdCC tissue microarray were stained with HE (upper panel) for orientation and immunohistochemistry for PAK2 (lower panel). Image from representative samples, including normal salivary glands (NSG), polymorphic adenoma (PMA), tubular AdCC (Patient #1), cribriform AdCC (Patient #2) and solid AdCC (Patient #3), were captured at the same low and high manification. Scale bar =100 µm. B. Quantification of histoscore of PAK2 in NSG (n=18), PMA (n=12) and AdCC (n=72). Data are shown as mean ± SEM. ***P<0.001. C. Quantification of histoscore of PAK2 in tubular AdCC (n=24), cribriform AdCC (n=28) and solid AdCC (n=20) show no significance.
PAK2, Ki-67, Cyclin D1 and p-STAT3 expression are positively correlated in human AdCC
To determine the potential associated molecule of PAK2 in AdCC, we analyzed serial sections of AdCC tissue microarray (Figure 2A) by the Spearman rank correlation coefficient test and linear tendency test. Elevated expression of Ki-67, Cyclin D1, Phospho-STAT3 at Tyrosine 705 (p-STAT3) have been reported in our previous study [12]. Of interest, the expression of PAK2 is positively associated with Ki-67, Cyclin D1 and p-STAT3 (Figure 2B). Precise P value and correlation coefficient are displayed in Figure 2C. Hierarchical clustering shows the expression of PAK2 is closer with Cyclin D1 and p-STAT3 compared with Ki-67 (Figure 2D). These results indicated that PAK2 may be correlated with Ki-67, Cyclin D1 and p-STAT3.
Figure 2.
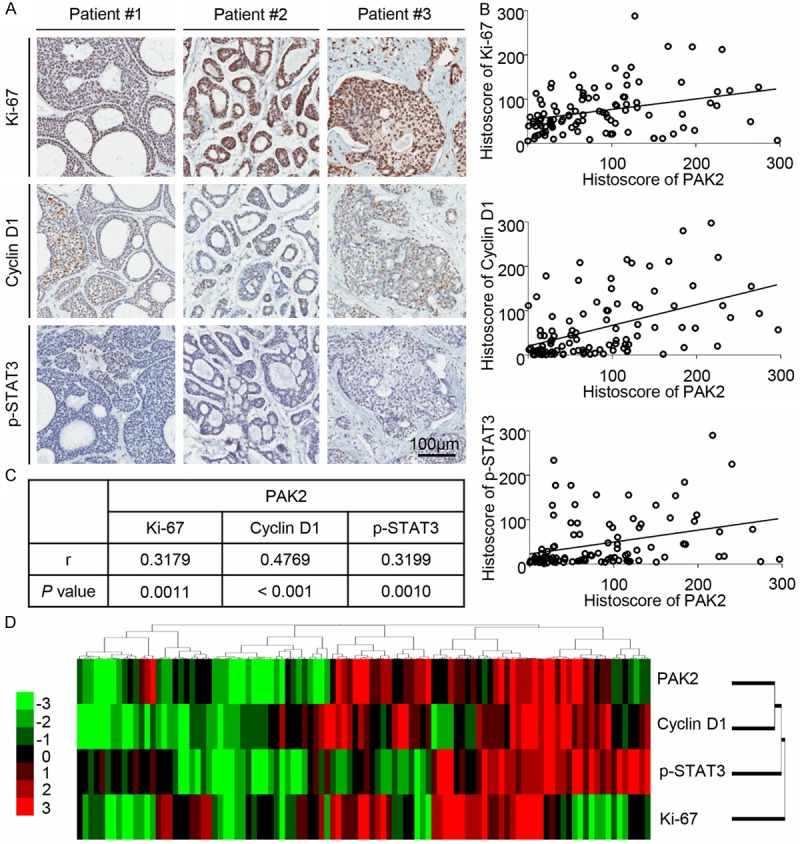
The expression of PAK2 is correlated with Ki-67, Cyclin D1 and p-STAT3. A. The typical immunohistochemical staining for Ki-67, Cyclin D1 and p-STAT3 in tubular AdCC (Patient #1), cribriform AdCC (Patient #2) and solid AdCC (Patient #3). Scale bar =100 µm. B. The PAK2 expression in NSG, PMA and AdCC correlated with Ki-67, Cyclin D1 and p-STAT3. A Pearson correlation was used to analyze the linear correlation between two variables. Data presented as dot plot of each specimen, statistic including NSG (n=18), PMA (n=12) and AdCC (n=72). C. The P value and correlation coefficient between PAK2 and Ki-67, Cyclin D1, p-STAT3 by the Pearson correlation analyses and linear tendency test. D. Hierarchical clustering of histoscore of PAK2, Ki-67, Cyclin D1 and p-STAT3 in NSG, PMA and AdCC (total n=102).
Knockdown of PAK2 inhibits cells migration and proliferation in SACC-LM and SACC-83 cells
To investigate the role of PAK2 in AdCC, PAK2 siRNAs (siRNA-1 and siRNA-2) were used to knockdown PAK2. The direct target effect of PAK2 siRNA was confirmed by Western blot in SACC-LM and SACC-83 (Figure 3A). The qualification of Western blot shows the protein level of PAK2 is significantly reduced in SACC-LM and SACC-83 cells transfected with PAK2 siRNA (Figure 3B). To identify whether knockdown of PAK2 could contribute to the inhibitory effect on tumor cells, wound healing assay and CCK8 cell proliferation assay were performed with SACC-LM and SACC-83 cells that was transfected with PAK2 siRNA. Based on the results shown in Figure 4A and 4B, when PAK2 was knocked down, the migration of AdCC cells was significantly reduced compared with untreated cells. In addition, the proliferation assay showed knockdown of PAK2 significantly inhibits the proliferation of tumor cells compared with control and negative control in SACC-LM and SACC-83 (Figure 4C). These data indicate that PAK2 affects the AdCC cells migration and proliferation.
Figure 3.
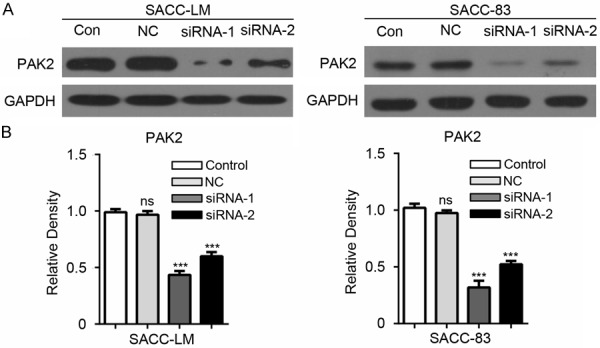
The direct target effect of PAK2 siRNA in SACC-LM and SACC-83 cells. A. Specific knockdown of PAK2 was monitored by Western blot with the PAK2 antibody in SACC-LM and SACC-83 cells. Control (Con), Negative Control (NC). B. The densitometric values were qualified with Image J software and the relative density (compared with control group) of the Western blot of PAK2 in SACC-LM and SACC-83 cells was calculated. The result was analyzed with One-way ANOVA by Graphpad 6.0 and the data are shown as mean ± SEM of three independent experiments. *P<0.05; ***P<0.001; ns, no significance.
Figure 4.
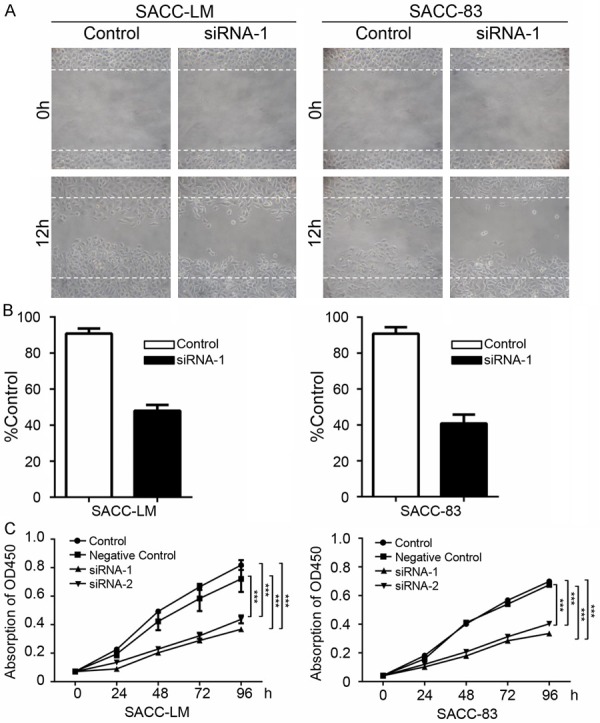
PAK2 knockdown inhibits SACC-LM and SACC-83 cells migration and proliferation. A. Wound healing assay in PAK2 siRNA-1 transfected SACC-LM and SACC-83 cells. B. Quantification of cell numbers indicate the statistical significance between PAK2 siRNA-1 and control. The cell number was calculated with Image J “Cell Counter” module. Mean ± SD. Unpaired t test with GraphPad 6.0. ***P<0.001. C. CCK8 proliferation assays with SACC-LM and SACC-83 cells transfected with PAK2 siRNA1, PAK2 siRNA2 and negative control compared with control. The absorption of OD450 was measured at 0, 24, 48, 72 and 96 hours. ***P<0.001.
Knockdown of PAK2 down-regulates Cyclin D1 in AdCC cell lines
To further define whether knockdown of PAK2 influence the expression of Cyclin D1 in AdCC cells, we test the protein level of Cyclin D1 and found the protein level of Cyclin D1 significantly downregulated in SACC-LM and SACC-83 (Figure 5A and 5B) transfected with PAK2 siRNA. And immunofluorescence also confirmed the decrease of Cyclin D1 after the knockdown of PAK2 in SACC-LM and SACC-83 (Figure 5C and 5D). Because of the pivotal role of Cyclin D1 in cells proliferation, these results suggest that PAK2 may promote the AdCC cells proliferation via Cyclin D1.
Figure 5.
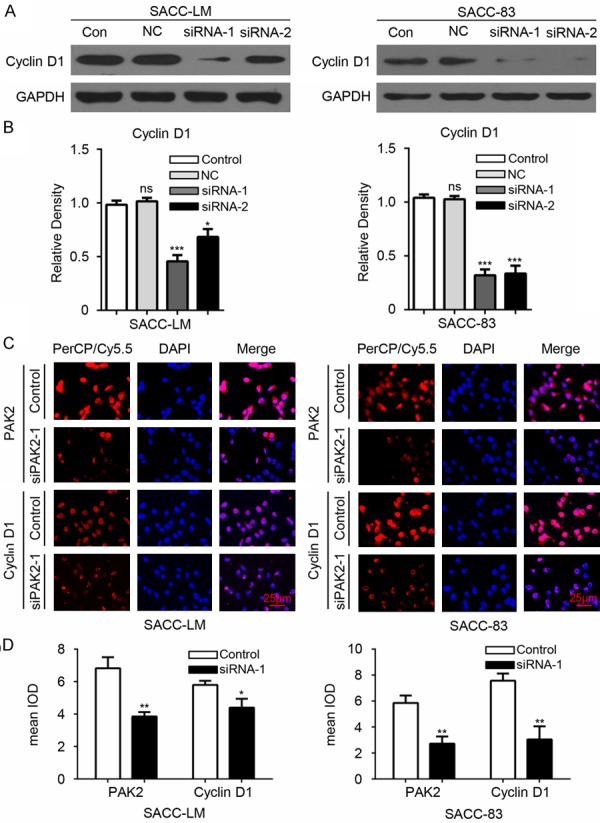
PAK2 knockdown reduces the expression of Cyclin D1 in SACC-LM and SACC-83 cells. A. Western blot shows the alternation of the protein level of Cyclin D1 in SACC-LM and SACC-83 cells. Control (Con), Negative Control (NC). B. The densitometric values were qualified with Image J software and the relative density (compared with control group) of the Western blot of Cyclin D1 in SACC-LM and SACC-83 cells was calculated. The result was analyzed with One-way ANOVA by Graphpad 6.0 and the data are shown as mean ± SEM of three independent experiments. *P<0.05; ***P<0.001; ns, no significance. C. Immunofluorescence shows knockdown of PAK2 with PAK2 siRNA1 decreases the expression of PAK2 and Cyclin D1 in SACC-LM and SACC-83 cell line. Scale bar =25 μm. D. The mean integrated optical density (IOD) of immunofluorescence of SACC-LM and SACC-83 cells was qualified with Image J software and analyzed with unpaired t test by Graphpad 6.0. *P<0.05; **P<0.01.
Inhibition of STAT3 suppresses the expression of PAK2 in AdCC cell lines
Our previous data have demonstrated that STAT3 inhibitor (S3I-201) could decrease the level of Cyclin D1 in SACC-LM and SACC-83 cell lines [12]. Based on previous study and the relationship of PAK2 with Cyclin D1 and p-STAT3, we tested the expression of PAK2 in AdCC cell lines after 100 µM S3I-201 treatment. As indicated by Western blot, the inhibition of STAT3 could downregulate the expression of PAK2 in SACC-LM and SACC-83 (Figure 6A and 6B). Similar results were observed by immunofluorescence in SACC-LM and SACC-83 (Figure 6C and 6D). These data suggested PAK2 may be the downstream of STAT3.
Figure 6.
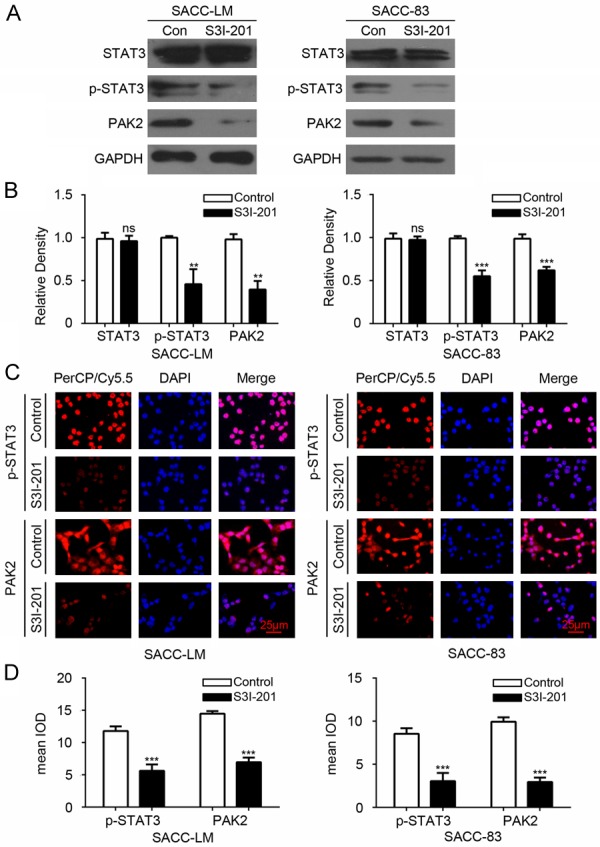
The inhibition of STAT3 reduced the protein level of PAK2 in SACC-LM and SACC-83 cells. A. Western blot shows that SACC-LM and SACC-83 cells treated with S3I-201 (100 μM) reduced the expression of p-STAT3 and PAK2. B. Qualification of the result of Western blot with Image J shows the protein level of p-STAT3 and PAK2 significantly decreased in SACC-LM and SACC-83 cells treated with S3I-201 compared with control group. The data were analyzed with One-way ANOVA by Graphpad 6.0 and shown as mean ± SEM of three independent experiments. **P<0.01. C. Representative immunofluorescence shows p-STAT3 and PAK2 were decreased in SACC-LM and SACC-83 cells after treatment with S3I-201. Scale bar =25 μm. D. Quantification of immunofluorescence with Image J software and analyzed with unpaired t test in SACC-LM and SACC-83 cells. Data are shown as mean ± SEM. ***P<0.001.
Discussion
In the study, we showed that the expression of PAK2 is significantly upregulated in AdCC compared with PMA and NSG. In addition, the expression of PAK2 is positively correlated with Ki-67 and Cyclin D1 by analyzing the AdCC tissue microarray, which suggested that PAK2 may promote tumor proliferation. And the result is supported by PAK2 siRNA knockdown study and proliferation assay in vitro. Meanwhile, the migration of AdCC cells is decreased after the PAK2 knockdown. Based on previous study and the correlation between PAK2 and p-STAT3, we speculate that PAK2 and p-STAT3 are potential associated molecules. And this relationship was verified in vitro study, which suggested PAK2 may be the downstream of p-STAT3. In conclusion, these results indicate that PAK2 is a potential therapeutic target in AdCC.
PAKs are overexpressed or amplified but not mutated in many types of cancer [16,17]. Based on the positive expression of PAK2 and its role in carcinogenesis in various cancers, we detected PAK2 in AdCC tissue microarray and firstly reported that PAK2 was highly expressed in AdCC. Because the effectiveness of current molecular targets for AdCC is not satisfactory, this study may offer possibility for new target and the molecular mechanisms of further research.
PAK2 appears to play a vital role in tumor cells migration, which is associated with generating new focal adhesions as well as limiting the sizes of focal adhesions [18]. PAK2 affected the ovarian cancer cells migration speed and PAK2 knockdown via siRNA transfection reduced migration of ovarian cancer cells affected by collagen type I, increasing the migratory ability of ovarian cancer cells [8,19]. In addition, suppression of PAK2 by siRNA repressed hepatoma cells migration in the presence of TGF-β, which could induce cell migration [20]. Our previous data reported that TGF-β1 is elevated in human AdCC and cell lines and involved in the tumor migration and invasion [21]. Here, in order to specifically assess the role of PAK2 in AdCC cells migration, knockdown of PAK2 with specific siRNA was performed and resulted in a significant decreasing of tumor cells migration.
PAK2 has been also reported to influence tumor cell proliferation. For example, knockdown of PAK2 inhibits tumor cell proliferation and decreases colony formation in melanoma [22,23]. And various microRNA, including miR-134, miR-137 and miR-224, could affect tumor cells proliferation by targeting or regulating PAK2 [22,24,25]. In addition, genetic alternation of Cyclin D1 is essential for tumor development and uncontrolled cell proliferation [26,27]. Meanwhile, the activity of Ki-67, a nuclear antigen, is identified as an effective molecular marker of tumor cell proliferation index and highly expressed in various cancers [28,29]. The overexpression of Cyclin D1 and Ki-67 has been observed in AdCC in our previous study [12]. In the present study, we found PAK2 is significantly associated with the expression of Ki-67 and Cyclin D1, which indicated that the function of PAK2 may be correlated with proliferation in AdCC. Additional study with PAK2 siRNA and CCK8 detection kit showed the knockdown of PAK2 significantly suppressed the cell proliferation of AdCC cells and downregulated the protein level of Cyclin D1, suggesting PAK2 may promote tumor cell proliferation by regulating Cyclin D1. This conclusion is consistent with the study, in which the expression of PAK2 abundantly elevated in meningioma cells [30]. And knockdown of PAK2 suppresses the activation of proliferation signals by inhibiting he activity of Cyclin D1, C-myc and β-catenin in schwannoma cells [30].
Recent study demonstrated that Group I PAKs could promote tumor cell survival and proliferation through the AKT1 and Raf/MAPK pathways [31]. MAPK Erk3 was identified as the substrate of PAK2 and could regulate cell growth in Kidney cancer [32]. STAT3 has been identified as a critical mediator of oncogenic signaling involving in encoding apoptosis inhibitor and cell cycle regulator [33]. Our previous study has indicated that p-STAT3 is essential for the proliferation of AdCC and STAT3 signaling blockade with STAT3 inhibitor SI-301 could suppress the expression of Cyclin D1 and induce apoptosis [12]. Otherwise, the expression of STAT3 is consistent with the deregulation of miR-134, which indicated it may be the target of miR-134 [34]. And in this study we found that PAK2 is significantly correlated with p-STAT3 in serial tissue sections and inhibiting STAT3 could downregulate the expression of PAK2. Although Cyclin D1 was directly activated by STAT3, we indicated that STAT3 may mediate the Cyclin D1 through PAK2 and proposed another possible mechanism in AdCC cell proliferation [35].
In conclusion, this study identified the expression of PAK2 was elevated in AdCC compared with PMA and NSG. And PAK2 was involved in the migration and proliferation of AdCC cells and the proliferation may be related with Cyclin D1 and Ki-67. Moreover, we revealed PAK2 may be the downstream of STAT3. The possibility of PAK2 as a therapeutic target need to be further investigated in future study for AdCC.
Acknowledgements
This work was supported by National Natural Science Foundation of China (81272963, 81472528) to Z.J. Sun and (81272964, 81472529) to W.F. Zhang. This work was also supported by program for new century excellent talents in university (NCET-13-0439), Ministry of Education of China to Z.J. Sun.
Disclosure of conflict of interest
None.
References
- 1.Sung MW, Kim KH, Kim JW, Min YG, Seong WJ, Roh JL, Lee SJ, Kwon TK, Park SW. Clinicopathologic predictors and impact of distant metastasis from adenoid cystic carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 2003;129:1193–1197. doi: 10.1001/archotol.129.11.1193. [DOI] [PubMed] [Google Scholar]
- 2.Gondivkar SM, Gadbail AR, Chole R, Parikh RV. Adenoid cystic carcinoma: a rare clinical entity and literature review. Oral Oncol. 2011;47:231–236. doi: 10.1016/j.oraloncology.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Chae YK, Chung SY, Davis AA, Carneiro BA, Chandra S, Kaplan J, Kalyan A, Giles FJ. Adenoid cystic carcinoma: current therapy and potential therapeutic advances based on genomic profiling. Oncotarget. 2015;6:37117–37134. doi: 10.18632/oncotarget.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bokoch GM. Biology of the p21-activated kinases. Annu Rev Biochem. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- 5.Arias-Romero LE, Chernoff J. A tale of two Paks. Biol Cell. 2008;100:97–108. doi: 10.1042/BC20070109. [DOI] [PubMed] [Google Scholar]
- 6.Ye DZ, Field J. PAK signaling in cancer. Cell Logist. 2012;2:105–116. doi: 10.4161/cl.21882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao C, Ma T, Pang L, Xie R. Activation of P21-activated protein kinase 2 is an independent prognostic predictor for patients with gastric cancer. Diagn Pathol. 2014;9:55. doi: 10.1186/1746-1596-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siu MK, Wong ES, Chan HY, Kong DS, Woo NW, Tam KF, Ngan HY, Chan QK, Chan DC, Chan KY, Cheung AN. Differential expression and phosphorylation of Pak1 and Pak2 in ovarian cancer: effects on prognosis and cell invasion. Int J Cancer. 2010;127:21–31. doi: 10.1002/ijc.25005. [DOI] [PubMed] [Google Scholar]
- 9.Park J, Kim JM, Park JK, Huang S, Kwak SY, Ryu KA, Kong G, Park J, Koo BS. Association of p21-activated kinase-1 activity with aggressive tumor behavior and poor prognosis of head and neck cancer. Head Neck. 2015;37:953–963. doi: 10.1002/hed.23695. [DOI] [PubMed] [Google Scholar]
- 10.Rudel T, Bokoch GM. Membrane and morphological changes in apoptotic cells regulated by caspase-mediated activation of PAK2. Science. 1997;276:1571–1574. doi: 10.1126/science.276.5318.1571. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Wen W, Liu K, Zhu F, Malakhova M, Peng C, Li T, Kim HG, Ma W, Cho YY, Bode AM, Dong Z, Dong Z. Phosphorylation of caspase-7 by p21-activated protein kinase (PAK) 2 inhibits chemotherapeutic drug-induced apoptosis of breast cancer cell lines. J Biol Chem. 2011;286:22291–22299. doi: 10.1074/jbc.M111.236596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bu LL, Deng WW, Huang CF, Liu B, Zhang WF, Sun ZJ. Inhibition of STAT3 reduces proliferation and invasion in salivary gland adenoid cystic carcinoma. Am J Cancer Res. 2015;5:1751–1761. [PMC free article] [PubMed] [Google Scholar]
- 13.Ma SR, Wang WM, Huang CF, Zhang WF, Sun ZJ. Anterior gradient protein 2 expression in high grade head and neck squamous cell carcinoma correlated with cancer stem cell and epithelial mesenchymal transition. Oncotarget. 2015;6:8807–8821. doi: 10.18632/oncotarget.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun ZJ, Zhang L, Hall B, Bian Y, Gutkind JS, Kulkarni AB. Chemopreventive and chemotherapeutic actions of mTOR inhibitor in genetically defined head and neck squamous cell carcinoma mouse model. Clin Cancer Res. 2012;18:5304–5313. doi: 10.1158/1078-0432.CCR-12-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang CF, Deng WW, Zhang L, Zhang WF, Sun ZJ. Expression of LC3, LAMP2, KEAP1 and NRF2 in Salivary Adenoid Cystic Carcinoma. Pathol Oncol Res. 2016;22:109–114. doi: 10.1007/s12253-015-9981-0. [DOI] [PubMed] [Google Scholar]
- 16.Ong CC, Jubb AM, Haverty PM, Zhou W, Tran V, Truong T, Turley H, O’Brien T, Vucic D, Harris AL, Belvin M, Friedman LS, Blackwood EM, Koeppen H, Hoeflich KP. Targeting p21-activated kinase 1 (PAK1) to induce apoptosis of tumor cells. Proc Natl Acad Sci U S A. 2011;108:7177–7182. doi: 10.1073/pnas.1103350108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen S, Auletta T, Dovirak O, Hutter C, Kuntz K, El-ftesi S, Kendall J, Han H, Von Hoff DD, Ashfaq R, Maitra A, Iacobuzio-Donahue CA, Hruban RH, Lucito R. Copy number alterations in pancreatic cancer identify recurrent PAK4 amplification. Cancer Biol Ther. 2008;7:1793–1802. doi: 10.4161/cbt.7.11.6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coniglio SJ, Zavarella S, Symons MH. Pak1 and Pak2 mediate tumor cell invasion through distinct signaling mechanisms. Mol Cell Biol. 2008;28:4162–4172. doi: 10.1128/MCB.01532-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flate E, Stalvey JR. Motility of select ovarian cancer cell lines: effect of extra-cellular matrix proteins and the involvement of PAK2. Int J Oncol. 2014;45:1401–1411. doi: 10.3892/ijo.2014.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sato M, Matsuda Y, Wakai T, Kubota M, Osawa M, Fujimaki S, Sanpei A, Takamura M, Yamagiwa S, Aoyagi Y. P21-activated kinase-2 is a critical mediator of transforming growth factor-beta-induced hepatoma cell migration. J Gastroenterol Hepatol. 2013;28:1047–1055. doi: 10.1111/jgh.12150. [DOI] [PubMed] [Google Scholar]
- 21.Zhao ZL, Ma SR, Wang WM, Huang CF, Yu GT, Wu TF, Bu LL, Wang YF, Zhao YF, Zhang WF, Sun ZJ. Notch signaling induces epithelial-mesenchymal transition to promote invasion and metastasis in adenoid cystic carcinoma. Am J Transl Res. 2015;7:162–174. [PMC free article] [PubMed] [Google Scholar]
- 22.Hao S, Luo C, Abukiwan A, Wang G, He J, Huang L, Weber CE, Lv N, Xiao X, Eichmuller SB, He D. miR-137 inhibits proliferation of melanoma cells by targeting PAK2. Exp Dermatol. 2015;24:947–952. doi: 10.1111/exd.12812. [DOI] [PubMed] [Google Scholar]
- 23.Li T, Zhang J, Zhu F, Wen W, Zykova T, Li X, Liu K, Peng C, Ma W, Shi G, Dong Z, Bode AM, Dong Z. P21-activated protein kinase (PAK2)-mediated c-Jun phosphorylation at 5 threonine sites promotes cell transformation. Carcinogenesis. 2011;32:659–666. doi: 10.1093/carcin/bgq271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shuang T, Wang M, Shi C, Zhou Y, Wang D. Down-regulated expression of miR-134 contributes to paclitaxel resistance in human ovarian cancer cells. FEBS Lett. 2015;589:3154–3164. doi: 10.1016/j.febslet.2015.08.047. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Takahashi S, Tasaka A, Yoshima T, Ochi H, Chayama K. Involvement of microRNA-224 in cell proliferation, migration, invasion, and anti-apoptosis in hepatocellular carcinoma. J Gastroenterol Hepatol. 2013;28:565–575. doi: 10.1111/j.1440-1746.2012.07271.x. [DOI] [PubMed] [Google Scholar]
- 26.Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 27.Li Z, Cui J, Yu Q, Wu X, Pan A, Li L. Evaluation of CCND1 amplification and CyclinD1 expression: diffuse and strong staining of CyclinD1 could have same predictive roles as CCND1 amplification in ER positive breast cancers. Am J Transl Res. 2016;8:142–153. [PMC free article] [PubMed] [Google Scholar]
- 28.Kim DK, Kim DW, Kim SW, Kim DY, Lee CH, Rhee CS. Ki67 antigen as a predictive factor for prognosis of sinonasal mucosal melanoma. Clin Exp Otorhinolaryngol. 2008;1:206–210. doi: 10.3342/ceo.2008.1.4.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaira K, Arakawa K, Shimizu K, Oriuchi N, Nagamori S, Kanai Y, Oyama T, Takeyoshi I. Relationship between CD147 and expression of amino acid transporters (LAT1 and ASCT2) in patients with pancreatic cancer. Am J Transl Res. 2015;7:356–363. [PMC free article] [PubMed] [Google Scholar]
- 30.Chow HY, Dong B, Duron SG, Campbell DA, Ong CC, Hoeflich KP, Chang LS, Welling DB, Yang ZJ, Chernoff J. Group I Paks as therapeutic targets in NF2-deficient meningioma. Oncotarget. 2015;6:1981–1994. doi: 10.18632/oncotarget.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menges CW, Sementino E, Talarchek J, Xu J, Chernoff J, Peterson JR, Testa JR. Group I p21-activated kinases (PAKs) promote tumor cell proliferation and survival through the AKT1 and Raf-MAPK pathways. Mol Cancer Res. 2012;10:1178–1188. doi: 10.1158/1541-7786.MCR-12-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De la Mota-Peynado A, Chernoff J, Beeser A. Identification of the atypical MAPK Erk3 as a novel substrate for p21-activated kinase (Pak) activity. J Biol Chem. 2011;286:13603–13611. doi: 10.1074/jbc.M110.181743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jing N, Tweardy DJ. Targeting Stat3 in cancer therapy. Anticancer Drugs. 2005;16:601–607. doi: 10.1097/00001813-200507000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Lages E, Guttin A, El Atifi M, Ramus C, Ipas H, Dupre I, Rolland D, Salon C, Godfraind C, deFraipont F, Dhobb M, Pelletier L, Wion D, Gay E, Berger F, Issartel JP. MicroRNA and target protein patterns reveal physiopathological features of glioma subtypes. PLoS One. 2011;6:e20600. doi: 10.1371/journal.pone.0020600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samsonov A, Zenser N, Zhang F, Zhang H, Fetter J, Malkov D. Tagging of genomic STAT3 and STAT1 with fluorescent proteins and insertion of a luciferase reporter in the cyclin D1 gene provides a modified A549 cell line to screen for selective STAT3 inhibitors. PLoS One. 2013;8:e68391. doi: 10.1371/journal.pone.0068391. [DOI] [PMC free article] [PubMed] [Google Scholar]


