Abstract
Background: Long noncoding RNAs (lncRNAs) played important roles in several biological processes through regulating the expression of protein. However, the function of lncRNA BCYRN1 in airway smooth muscle cells (ASMCs) has not been reported. Methods: Male Sprague-Dawley (SD) rats were divided into control and asthma groups and the ovalbumin (OVA) model was constructed. The expression of BCYRN1 and transient receptor potential 1 (TRPC1) were detected in the ASMCs separated from these rats. Then 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium (WST-1) assay, Roche real-time cell analyzer (RTCA) DP assay and Transwell cell migration assay were performed to detect the effect of BCYRN1 on the viability/proliferation and migration of ASMCs. RNA pull-down assays and RNA immunoprecipitation assay were used to identify and verify the binding between BCYRN1 and TRPC1. Inspiratory resistance and expiratory resistance were measured in OVA challenged rats with BCYRN1 knockdown. Results: We foundthe high expression of BCYRN1 and TRPC1 in asthma groups and ASMCs treated with PDGF-BB. Overexpression of BCYRN1 greatly promoted the proliferation and migration of ASMCs. In addition,TRPC1 overexpression reversed the function of si-BCYRN1 indecreasing the viability/proliferation and migration of ASMCs treated with PDGF-BB. BCYRN1 could up-regulate the protein level of TRPC1 through increasing the stability of TRPC1. Finally, we found that BCYRN1 knockdown reduced the inspiratory resistance and expiratory resistance in OVA challenged rats. Conclusion: Our study indicated that BCYRN1 promotedthe proliferation and migration of rat ASMCs in asthma via upregulating the expression of TRPC1.
Keywords: BCYRN1, transient receptor potential 1, airway smooth muscle cells, asthma, proliferation, migration
Introduction
Asthma is a chronic airway inflammatory disease that affects numerous people globally, with the immunopathological features of chronic airway inflammation and airway remodeling [1]. Airway smooth muscle (ASM) has been found to be a key player in the pathogenesis of airway remodeling. It has been known that the proliferation of airway smooth muscle cells (ASMCs) is involved in the remodeling and irreversible obstruction of airways during severe asthma [2]. However, the mechanisms underlying this disease process are not fully understood.
The role of ASM in asthma pathogenesis has beencontentious, yet emerging evidence suggests that the contractile activity of ASM largely contributes to the clinical symptoms and disease pathogenesis ofacute airway narrowing [3]. In addition, intracellular Ca2+ is a critical signal transduction element in regulating muscle contraction [4]. It has been reported that in-creases in cytosolic free Ca2+ concentration may trigger bronchial constriction and bronchial wall thickening in asthma [5]. In ASMCs, at least three kinds of Ca2+ channels are found in the plasma membrane: second messenger activated non-selective cation channels (NSCCs), voltage-dependent Ca2+ channels (VDCCs), and store-operated Ca2+ channels (SOCCs) [6]. Nowadays, members of the canonical or classic transient receptor potential (TRPC) family are identified as the molecular counterparts of SOCCs and NSCCs, which play important roles in the function of ASMCs [7]. To date, seven TRPC proteins (TRPC1-7) have been identified. Among them, TRPC1 is proposed to be important for contraction and proliferation of vascular SMC [8]. Evidence indicated that TRPC1 knockdown may inhibit the proliferation of pulmonary artery SMC in culture [9].
Accumulating evidence indicated that long noncoding RNAs (lncRNAs) can regulate multiplebiological responses through interacting with DNA, RNA and protein [10]. BCYRN1 (brain cytoplasmic RNA 1 or BC200) is a 200 bases long lncRNA that operates as a translational modulator [11]. BCYRN1 and its rodent counterpart BC1 RNA are primarily expressed in neurons and regulate the expression profile in somatic cells [12]. The aberrant expression of BCYRN1 is observed in Alzheimer’s disease and may contribute to the maintenance of long term synaptic plasticity [13]. BCYRN1 is also found highly expressed in some carcinomas of the breast, cervix, lung and ovary [14,15]. Recently, BCYRN1 was found to be dysregulated in primary ASMCs and might act as the miRNA ‘sponge’ [16]. However, the function of BCYRN1 in ASMCs has not been reported.
Here, this study was designed to investigate the effects of BCYRN1 on ASMC proliferation and migration, and the expression and activity of TRPC1 channel in asthma, with an objective to understand the relationship between BCYRN1 and TRPC1 channel in the ASMC over-proliferation in asthma.
Materials and methods
Establishment of rat chronic asthmatic model and grouping
All experimental procedures were carried out in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of People’s Hospital Affiliated to Zhengzhou University. The animals were cared for in accordance with the National Animal Welfare and Protection Law of China.Specific-pathogen-free juvenile female SD rats (60-80 g) obtained from the Experimental Animal Center of People’s Hospital Affiliated to Zhengzhou University were randomly divided into 2 groups: the control group (n=10) and the asthmatic group (n=10). The ovalbumin (OVA) model was constructed. Rats were injected with 1 mL antigen sensitization liquid containing 100 mg ovalbumin, 100 mg aluminum hydroxide and 5 × 109 inactivated Bordetella pertussis on the 1st and 8th day. In control group, rats injected with an equal volume of saline were used as controls. The total experimental time was eight weeks. Rats were excited by ultrasonic atomizing inhalation with 1% ovalbumin for 30 min 3 times per week with the next six weeks. The control group was treated with normal saline instead of ovalbumin for 2 weeks. After sensitized, the rats showed symptoms of asthmatic attack such as agitation, bucking, cyanosis, tachypnea, and so on. The rats were sacrificed within 18-24 hours after the final challenge in each group.
Isolation and culture of rat ASMCs
ASMCs were isolated from the airway of SD rat conforming to a protocol approved by the Institutional Animal Care and Use Committee of People’s Hospital Affiliated to Zhengzhou University. The rat tracheal was dissected under sterile conditions in normal saline solution. Airways without cartilages were selected and airway smooth muscle bundles were micro-dissected free from surrounding tissues. The epithelial layer was removed. The smooth muscle section was then seeded into sterile 25 mL culture flasks and 3 mL DMEM supplemented with 10% FBS, 100 U/mL penicillin, and 0.1 mg/mL streptomycin at 37°C in humidified air containing 5% CO2. Culture medium was replaced every 3 days until they formed a confluence and passaged with 0.25% trypsin-EDTA solution. All experiments were performed with ASMCs from passages 6 to 10.
Cell viability/proliferation assay
WST-1 assay and Roche real-time cell analyzer (RTCA) DP assay were performed to estimate ASMC viability/proliferation. ASMCs were seeded into 96 well plate with DMEM containing 10% FBS. The cells were arrested by removing serum for 24 h. Cells were then stimulated with PDGF-BB (25 ng/mL) for 24 h and assays were performed by adding WST-1 directly to the culture wells and incubating them for 120 minutes at 37°C. Plates were read by a scanning multiwell spectrophotometer by measuring the absorbance of the dye with a wavelength of 450 nm and a reference wavelength of 630 nm. Percentage of viability was obtained by comparing OD values to that of ASMCs in a control group. The cell proliferation was determined using the Roche RTCA DP assay according to the manufacturer’s instructions. ASMCs proliferation variation was obtained by calculating the changes of ASMCs to baseline. Data were generated from ASMCs with three replicates.
Boyden chamber migration assay
The migration assay was performed using the Transwell system. The lower compartment was filled with 0.6 mL of DMEM containing 1% FBS with Ad-BCYRN1 or Ad-control or PDGF-BB alone or together with si-BCYRN1, or pcDNA-TPRC1. ASMCs (1 × 105) were re-suspended in 0.1 mL of DMEM and placed in the upper part of the Transwell plate. ASMCs were fixed with 4% paraformaldehyde and stained with 0.5% crystal violet for 10 min. The migrated cells were determined by counting the cells that migrated to the lower side of the filter using a microscope.
Western blot
Fifteen micrograms of protein was separated on a 15% SDS-PAGE gel and electrophoretically transferred to a polyvinylidene difluoride membrane. The membrane was then probed with TPRC1 antibody or β-actin antibody for 1 hour at room temperature. After secondary incubation in peroxidase-conjugated IgG (1:10000 diluted), the membranes were subsequently washed and the blots were visualized using a Bio-Rad Gel Doc™ XR + Imaging system. The band densities were quantified using Quantity One software (Bio-Rad Laboratories, Inc.).
Quantitative real-time PCR
Cells were lysed and RNA was purified using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. Total RNA was used to synthesize cDNA with an Omniscript reverse transcriptase kit (Qiagen). Amplified PCR products were analyzed by agarose gel electrophoresis (2%) in the presence of ethidium bromide. An ABI Prism 7900HT sequence detection system (Applied Biosystems) was used for reverse-transcription of total RNA and the subsequent real-time PCR. The β-actin gene was used as an internal control to normalize the difference in the amount of total RNA in each sample. The comparative method 2-ΔΔCt was used to calculate the relative expression of the target gene.
RNA pull-down assays
Biotin-labelled BCYRN1 were in vitro transcribed with the Biotin RNA labelling mix (Roche) and T7 or SP6 RNA polymerase (Roche) and purified with RNeasy Mini Kit (Qiagen, Valencia, CA). Then one milligram of protein from ASMCs extracts was mixed with 50 pmol of biotinylated RNA biotin-labeled RNAs, incubated with streptavidin agarose beads (Invi-trogen, Carlsbad, CA), and washed. The proteins binding to the streptavidin-coupled dynabeads were detected by the standard western blotting technique.
RNA immunoprecipitation assay
RIP was performed using a Magna RIP RNA-Binding Protein Immunoprecipitation Kit (Millipore, Bedford, MA) according to the manufacturer’s instructions. Briefly, ASMCs were lysed in completeRNA lysis buffer, and then were incubated with RIP buffer containing magneticbeads conjugated with anti-TRPC1 antibody and negative control normal mouse IgG (Millipore, Billerica, MA, USA). The co-precipitated RNAs were detected by reverse-transcription polymerase chain reaction (RT-PCR).
Cell transfection
The full-length cDNA of human TRPC1 was inserted into the Not I and Apa I sites of expression vector pcDNA3.0(+) (Invitrogen) with the cytomegalovirus promoter (pcDNA-TRPC1). Control and BCYRN1-specific siRNAs were synthesized by Shanghai GenePharma Co., Ltd (Shanghai, China). ASMCs were isolated and cultured for 24 hours and then transiently transfected with pcDNA-TRPC1 or siRNA specific for BCYRN1 or the control for 10 to 12 hours with RNAiMax Lipofectamine (Invitrogen), according to the manufacturer’s instructions.
Airway resistance measurement
Airway reactivity to methacholine was assessed to calculate the inspiratory and expiratory resistances of the respiratory system as described in a previous study [17]. Briefly, rats were intravenously injected with methacholine (dissolved in 0.9% sodium chloride) at an initial dose of 0.0625 mg/kg. To obtain a response curve of lung resistance, the dose was increased 2-fold with each injection up to 1 mg/kg. The spacing intervals of injections were 5 min. Prior to the next methacholine injection, fifty microliter of methacholine was administered over 3-4 sec according to the return of resistance curves to the pre-methacholine level. Following the methacholine administration, response was measured immediately as the peak increase above the baseline.
Statistical analysis
Data are expressed as the mean ± standard deviation and the differences between groups were analyzed using analysis of variance or non-paired Student’s t-test if the continuous variables were not normally distributed. All statistical analyses were performed using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
The expression of BCYRN1 andTRPC1 in ASMCs from sensitized rats
To investigate the role of BCYRN1 and TRPC1 in asthma, we detected the expression of BCYRN1 and TRPC1 in ASMCs from sensitized rats and control. Chronic asthmatic model in rats was established as described in method. As shown in Figure 1, the mRNA level of BCYRN1 and protein level of TRPC1 were both higher in ASMCs from sensitized rats than that from normal rats.
Figure 1.
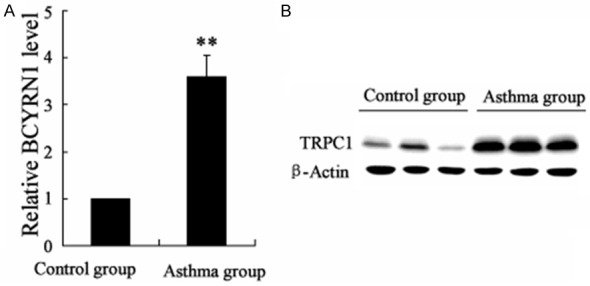
The expression of BCYRN1 and TRPC1 in ASMCs ofrats in asthma group and control group. A. The mRNA level of BCYRN1 in ASMCs of rats in asthma group and control group. B. The protein level of TRPC1 in ASMCs of rats in asthma group and control group. Data are expressed as mean ± SD for each group. **VS control group, P<0.01.
The effects of BCYRN1 overexpression on the ASMCs viability/proliferation and migration
To detect the effect of BCYRN1 on the viability/proliferation and migration of ASMCs, ASMCs were separated from rats and were transfected with Ad-BCYRN1 to overexpress BCYRN1. It has been shown that Ad-BCYRN1 significantly up-regulated the expression of BCYRN1 in ASMCs (Figure 2A). Then WST-1 assay and Roche RTCA DP assay were performed to examine the ASMCs viability and proliferation. As shown in Figure 2B and 2C, BCYRN1 overexpression highly enhanced the viability and proliferation of ASMCs over the transfection time. In addition, we also examined the effect of BCYRN1 on ASMCs migration with Transwell system. The result showed that BCYRN1 overexpression greatly increased the migration ability of ASMCs. These data indicated that BCYRN1 might be involved in the viability/proliferation and migration of ASMCs.
Figure 2.
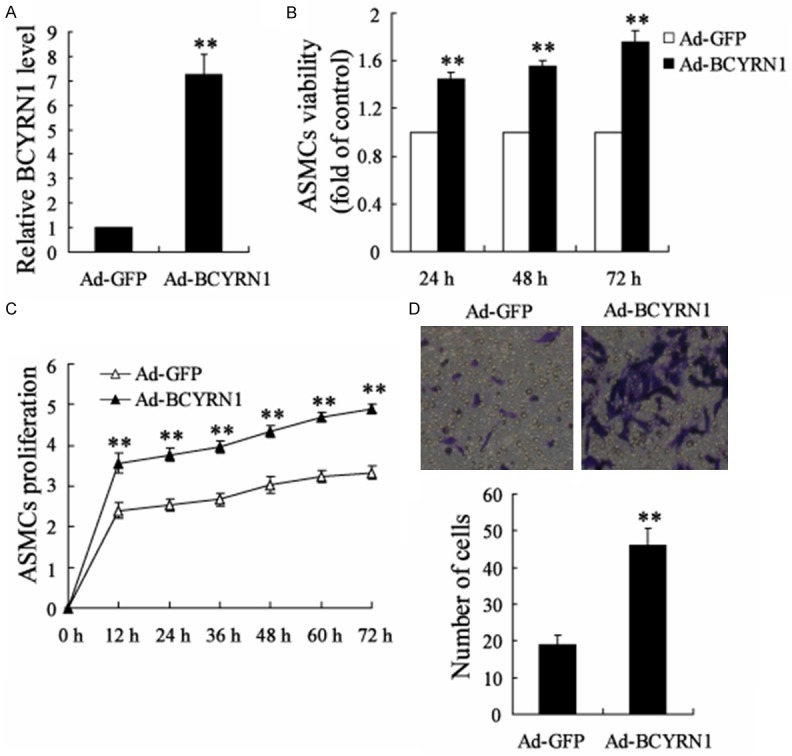
Effects of BCYRN1 overexpression on the viability/proliferation and migration of ASMCs separated from rats. A. The effect of Ad-BCYRN1 on the expression of BCYRN1 in ASMCs. B. The effect of Ad-BCYRN1 on ASMCs viability to that of HC by WST-1 assay. C. The effect of Ad-BCYRN1 on ASMCs proliferation with that of HC by Roche RTCA DP assay. D. The effects of Ad-BCYRN1 on ASMCs migration. Ad-GFP: adenovirus vector as the control; Ad-BCYRN1: adenovirus vector overexpressing BCYRN1. Data are expressed as mean ± SD for each group. **VS Ad-GFP group, P<0.01.
The expression of BCYRN1 and TRPC1 in PDGF-BB-induced ASMCs
To further verify the dysregulation of BCYRN1 and TRPC1 in ASMCs, platelet-derived growth factor (PDGF)-BB was used to induce the proliferation and migration of ASMCs. It has been shown that the mRNA level of BCYRN1 was up-regulated in PDGF-BB treated ASMCs (Figure 3A). The protein level of TRPC1 was also enhanced in PDGF-BB treated ASMCs (Figure 3B). These data confirmed the important roles of BCYRN1 and TRPC1 in ASMCs.
Figure 3.
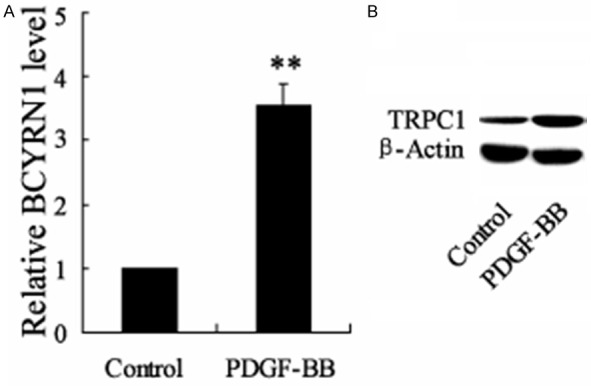
Theexpression of BCYRN1 and TRPC1 in PDGF-BB-induced ASMCs. PDGF-BB was used to induce ASMCs proliferation and migration. A. The mRNA level of BCYRN1 in PDGF-BB-induced ASMCs and control ASMCs. B. The protein level of TRPC1 in PDGF-BB-induced ASMCs and control ASMCs. Data are expressed as mean ± SD for each group. **VS control group, P<0.01.
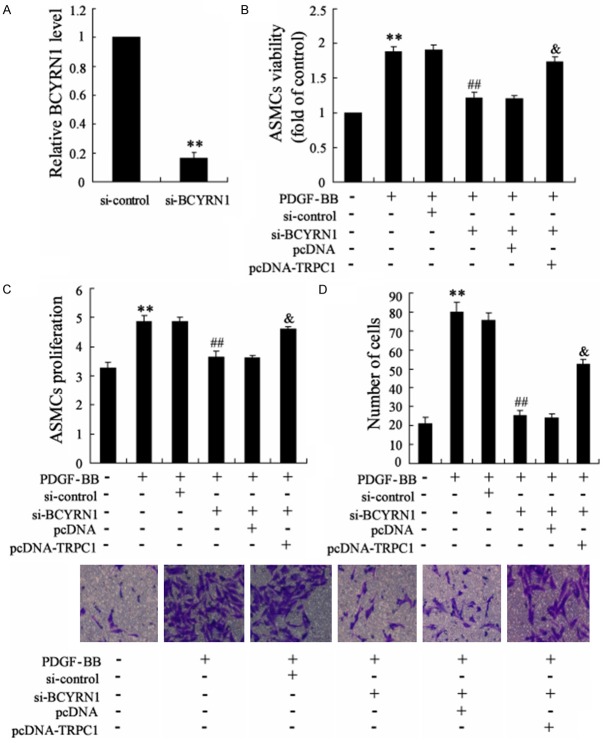
The effect of BCYRN1 knockdown and TRPC1 overexpression on PDGF-BB-induced ASMCs proliferation and migration
Next, we examined the effect of BCYRN1 knockdown and TRPC1 overexpression on PDGF-BB-induced ASMCs proliferation and migration. The expression of BCYRN1 was significantly reduced by si-BCYRN1, as shown in Figure 4A. Then ASMCs were divided into six groups: control group, PDGF-BB group, PDGF-BB + si-control group, PDGF-BB + si-BCYRN1 group, PDGF-BB + si-BCYRN1 + pcDNA group, PDGF-BB + si-BCYRN1 + pcDNA-TRPC1 group. PDGF-BB indeed increased the viability/proliferation and migration of ASMCs. We also found that si-BCYRN1 attenuated the effect of PDGF-BB on the viability/proliferation and migration of ASMCs. Moreover, TRPC1 overexpression reversed the function of si-BCYRN1 in decreasing the viability/proliferation and migration of ASMCs (Figure 4B-D). These dataindicated that BCYRN1 and TRPC1 played critical roles in PDGF-BB-induced ASMCs viability/proliferation and migration.
Figure 4.
The effect of BCYRN1 knockdown and TRPC1 overexpressing on PDGF-BB-induced ASMCs proliferation and migration. (A) The effect of si-BCYRN1 on the expression of BCYRN1 in ASMCs. (B-D) ASMCswere divided into six groups: control group, PDGF-BB group, PDGF-BB + si-control group, PDGF-BB + si-BCYRN1 group, PDGF-BB + si-BCYRN1 + pcDNA group, PDGF-BB + si-BCYRN1 + pcDNA-TRPC1 group. The ASMCs viability, proliferation and migration of these group were showed in (B-D), respectively. Data are expressed as mean ± SD for each group. **VS control group, P<0.01; ##VS PDGF-BB + si-control, P<0.01; & VS PDGF-BB + si-BCYRN1 + pcDNA, P<0.01.
The effect of BCYRN1 on the expression of TRPC1 in ASMCs
LncRNAs have been reported to regulate the activity of proteins through binding to the protein. To investigate whether BCYRN1 functions through this mechanism, we performed an RNA pull-down assay to identify the binding between TRPC1 and BCYRN1. The result showed that protein TRPC1 but not TRPC6 was specifically associated with BCYRN1 (Figure 5A). To further validate the association between BCYRN1 and TRPC1, we next performed an RIP assay with an antibody against TRPC1 on ASMCs cellular extracts. Consistently, we observed a significantly higher enrichment level of BCYRN1 with the TRPC1 antibody compared with the nonspecific IgG control antibody (Figure 5B). In addition, the protein level of TRPC1 could be up-regulated or down-regulated by pcDNA-BCYRN1 or si-BCYRN1 (Figure 5C). But the mRNA level of TRPC1 has no change (data not shown). Cycloheximide (CHX) was known to block protein synthesis in vivo and in vitro. MG132 was used to inhibit the proteasome-mediated protein degradation. Here, we found that the protein level of TRPC1 was reduced in ASMCs treated with CHX and increased by MG132 (Figure 5D). In addition, BCYRN1 overexpression remarkably increased the protein level of TRPC1 in the presence of translational inhibitor CHX. And it has no effect on the expression of TRPC1 in the presence of MG132 (Figure 5E). These data indicated that BCYRN1 could up-regulate the protein level of TRPC1 through increasing the stability of TRPC1.
Figure 5.
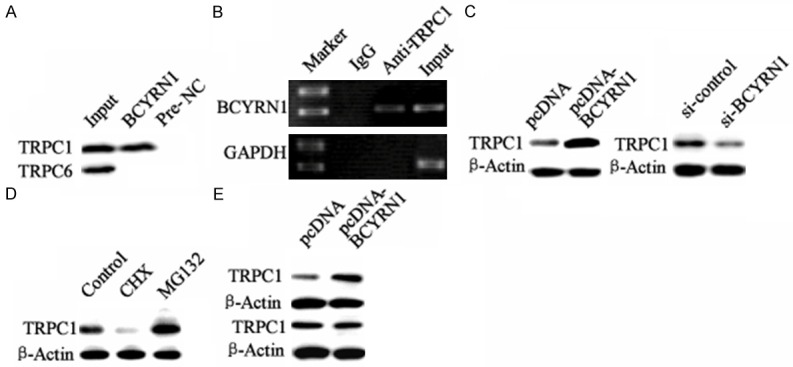
The effect of BCYRN1 on the expression of TRPC1 in ASMCs. A. DNA binding analysis between BCYRN1 and TRPC1 or TRPC6 by RNA pull-down assays in ASMCs. “Input”: chromosome. B. DNA binding analysis about TRPC1 and BCYRN1 promoter by RIP assay in ASMCs. “IgG”: with anti-IgG as negative control; “anti-TRPC1”: with anti-TRPC1; “Input”: chromosome. C. The regulation of BCYRN1 on the protein level of TRPC1 in ASMCs. D. The effect of cycloheximide (CHX) and MG132 on the protein level of TRPC1 in ASMCs. E. The effect of BCYRN1 overexpression on the protein level of TRPC1 in ASMCs treat with CHX or MG132.
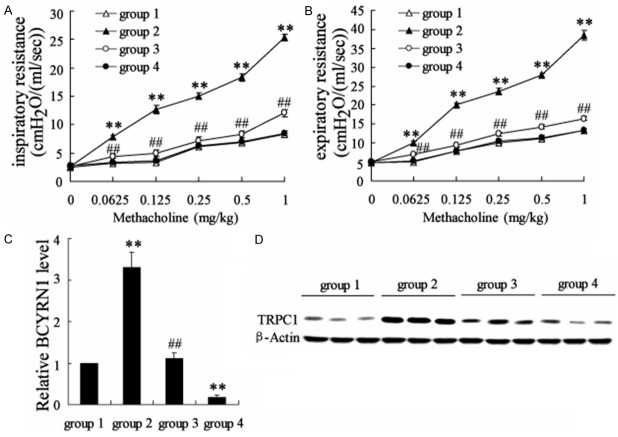
The effect of BCYRN1 knockdown on the airway resistance in sensitized rats
To investigate the effect of BCYRN1 knockdown on the airway resistance in sensitized rats, rats were divided into four groups: control (group 1), asthma + Ad-GFP (group 2), asthma + Ad-SM22α-siBCYRN1 (group 3), control + Ad-SM22α-siBCYRN1 (group 4). Ad-SM22α-siBCYRN1 was specifically expressed in ASMCs and sent to lung through nasal spray. Inspiratory resistance and expiratory resistance were both increased in sensitized rats compared to the control. In addition, BCYRN1 knockdown reduced the inspiratory resistance and expiratory resistance in sensitized rats (Figure 6A and 6B). Through separating the ASMCs from these rats, qRT-PCR revealed that Ad-SM22α-siBCYRN1 significantly decreased the expression of BCYRN1 (Figure 6C). The results of western blot indicated that the protein level of TRPC1 was decreased by Ad-SM22α-siBCYRN1 (Figure 6D).
Figure 6.
The effect of BCYRN1 knockdown on the airway resistance in sensitized rats. Rats were divided into four groups: Control (group 1), Asthma + Ad-GFP (group 2), Asthma + Ad-SM22α-siBCYRN1 (group 3), Control + Ad-SM22α-siBCYRN1 (group 4). Ad-SM22α-siBCYRN1 was specifically expressed in ASMCs and sent to lung through nasal spray. A. The inspiratory resistance of rats in these groups. B. The expiratory resistance of rats in these groups. C. The mRAN level of BCYRN1 in rats of these groups. D. The protein level of TRPC1 in rats of these groups. Data are expressed as mean ± SD for each group. **VS group 1, P<0.01; ##VS group 2, P<0.01.
Discussion
To date, considerable evidence have showed that ASMCs played critical roles in the development and pathology of asthma [18]. One of the significant characteristics of asthma is known to be the airway remodeling, which contributes to structural changes, including increase in basal membrane thickness, bronchial fibrosis, smooth muscle hyperplasia and hypertrophy and so on [19]. In particular, an increase of ASM mass resulting from proliferation and hypertrophy of ASMCs plays an important role in the pathophysiology of airway remodeling in asthma. It has been shown that increased ASM mass is associated with severe asthma and likely contributes to enhanced airway contractility and a narrowed lumen [20]. ASM is also identified as a source of extracellular matrix proteins and as a producer of pro- and anti-inflammatory mediators that affect cell proliferation, migration, and apoptosis [21]. Abnormal proliferation and migration of ASMCs in the airway cause airway wall thickening, which is involved in the development of airway remodeling in asthma. Therefore, studying the molecular mechanism of ASMC proliferation is a necessary in asthma research.
Some studies have reported that the concentration of free cytoplasmic calcium ([Ca2+]cyt) in ASM participates in many cellular processes, such as proliferation, contraction, gene transcription and secretion of signaling mediators [22]. Canonical transient receptor potential (TRPC) channels are originally identified in Drosophila and composed of proteins that are highly related to photoreceptor signal transduction pathway. Expression of TRPC genes in mammalian cells causes the formation of Ca2+-permeable channels that are activated by SR Ca2+ depletion [23]. Among the seven homologous TRPC proteins (TRPC1-7), TRPC1 was found to be important for contraction and proliferation of vascular SMC. Using TRPC1 siRNA have demonstrated unequivocally that inhibition of TRPC1 expression or function selectively blocks store-operated Ca2+ entry in pulmonary arterial smooth muscle cells [24]. Golovina et al. suggested that TRPC1 may be involved in the proliferation of ASMC [25]. TRPC1 is also increased in the hyperplasia that follows arterial injury [26]. In this study, we found the higher level of TRPC1 in rats model of asthma and PDGF-BB treated ASMCs, which suggested the important role of TRPC1 in ASMCs.
To further investigate the molecular mechanism involving in the TRPC1-midiated proliferation, we detected the expression ofone lncRNA, named BCYRN1.LncRNAs are longer than 200 nt and participate in diverse biological processes, such as proliferation, differentiation, and development through various modes of action [27,28]. InASMCs, there are currently only a few studies examining the roles of lncRNAs. For example, Perry et al.recently reported that PVT1 regulated the TGF-β-induced proliferative response in ASMCs from severe asthmatics [29]. Another lncRNA GAS5 has been demonstrated to be correlated with the corticosteroid insensitivity observed in primary human ASM cells from severe asthmatics [30]. In 2014, Perry et al. adopted a transcriptomic approach to investigate the differential expression of miRNAs and lncRNAs in primary ASM cells, and found the dysregulation of BCYRN1 acting as miRNA ‘sponges’ for 4 miRNAs [16]. Here, we found the up-regulation of BCYRN1 in rats model of asthma and PDGF-BB treatedASMCs. In addition, BCYRN1 overexpression resulted in the proliferation and migration of ASMCs separated from rats model of asthma. Through RNA pull-down assay and RIP assay, we found that BCYRN1 could up-regulate the protein level of TRPC1 through increasing the stability of TRPC1. Moreover, TRPC1 involved in the pro-proliferative and pro-migratory function of BCYRN1 in ASMCs.
In summary, these findings suggest that BCYRN1 may promote the proliferation and migration of ASMC in asthma remodeling. This pro-proliferative effect of BCYRN1 was probably mediated partially by up-regulation of TRPC1 channel. Although clinical applications require further investigation, this study has provided the scientific experimental basis for the important role of BCYRN1 in abnormal proliferation and migration of ASMCs in the asthma.
Acknowledgements
This work was supported by a grant from the National Natural Science Foundation of China (No.U1304801).
Disclosure of conflict of interest
None.
References
- 1.Elias JA, Zhu Z, Chupp G, Homer RJ. Airway remodeling in asthma. J Clin Invest. 1999;104:1001–1006. doi: 10.1172/JCI8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeganeh B, Xia C, Movassagh H, Koziol-White C, Chang Y, Al-Alwan L, Bourke JE, Oliver BG. Emerging mediators of airway smooth muscle dysfunction in asthma. Pulm Pharmacol Ther. 2013;26:105–111. doi: 10.1016/j.pupt.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 3.Zhao L, Sullivan MN, Chase M, Gonzales AL, Earley S. Calcineurin/Nuclear Factor of Activated T Cells-Coupled Vanilliod Transient Receptor Potential Channel 4 Ca2+ Sparklets Stimulate Airway Smooth Muscle Cell Proliferation. Am J Respir Cell Mol Biol. 2014;50:1064–1075. doi: 10.1165/rcmb.2013-0416OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen YF, Cao J, Zhong JN, Chen X, Cheng M, Yang J, Gao YD. Plasma membrane Ca2+-ATPase regulates Ca2+ signaling and the proliferation of airway smooth muscle cells. Eur J Pharmacol. 2014;740:733–741. doi: 10.1016/j.ejphar.2014.05.055. [DOI] [PubMed] [Google Scholar]
- 5.Sweeney M, McDaniel SS, Platoshyn O, Zhang S, Yu Y, Lapp BR, Zhao Y, Thistlethwaite PA, Yuan JX. Role of capacitative Ca2+ entry in bronchial contraction and remodeling. J Appl Physiol (1985) 2002;92:1594–1602. doi: 10.1152/japplphysiol.00722.2001. [DOI] [PubMed] [Google Scholar]
- 6.Karaki H, Ozaki H, Hori M, Mitsui-Saito M, Amano K, Harada K, Miyamoto S, Nakazawa H, Won KJ, Sato K. Calcium movements, distribution, and functions in smooth muscle. Pharmacol Rev. 1997;49:157–230. [PubMed] [Google Scholar]
- 7.Ong HL, Brereton HM, Harland ML, Barritt GJ. Evidence for the expression of transient receptor potential proteins in guinea pig airway smooth muscle cells. Respirology. 2003;8:23–32. doi: 10.1046/j.1440-1843.2003.00424.x. [DOI] [PubMed] [Google Scholar]
- 8.Dietrich A, Chubanov V, Kalwa H, Rost BR, Gudermann T. Cation channels of the transient receptor potential superfamily: their role in physiological and pathophysiological processes of smooth muscle cells. Pharmacol Ther. 2007;112:744–760. doi: 10.1016/j.pharmthera.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Sweeney M, Yu Y, Platoshyn O, Zhang S, McDaniel SS, Yuan JX. Inhibition of endogenous TRP1 decreases capacitative Ca2+ entry and attenuates pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol. 2002;283:L144–L155. doi: 10.1152/ajplung.00412.2001. [DOI] [PubMed] [Google Scholar]
- 10.Turner M, Galloway A, Vigorito E. Noncoding RNA and its associated proteins as regulatory elements of the immune system. Nat Immunol. 2014;15:484–491. doi: 10.1038/ni.2887. [DOI] [PubMed] [Google Scholar]
- 11.Wu P, Zuo X, Deng H, Liu X, Liu L, Ji A. Roles of long noncoding RNAs in brain development, functional diversification and neurodegenerative diseases. Brain Res Bull. 2013;97:69–80. doi: 10.1016/j.brainresbull.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Chen W, Böcker W, Brosius J, Tiedge H. Expression of neural BC200 RNA in human tumours. J Pathol. 1997;183:345–351. doi: 10.1002/(SICI)1096-9896(199711)183:3<345::AID-PATH930>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 13.Mus E, Hof PR, Tiedge H. Dendritic BC200 RNA in aging and in Alzheimer’s disease. Proc Natl Acad Sci U S A. 2007;104:10679–10684. doi: 10.1073/pnas.0701532104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vikram R, Ramachandran R, Abdul KS. Functional significance of long non-coding RNAs in breast cancer. Breast Cancer. 2014;21:515–521. doi: 10.1007/s12282-014-0554-y. [DOI] [PubMed] [Google Scholar]
- 15.Hu T, Lu YR. BCYRN1, a c-MYC-activated long non-coding RNA, regulates cell metastasis of non-small-cell lung cancer. Cancer Cell Int. 2015;15:36–43. doi: 10.1186/s12935-015-0183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perry MM, Tsitsiou E, Austin PJ, Lindsay MA, Gibeon DS, Adcock IM, Chung KF. Role of non-coding RNAs in maintaining primary airway smooth muscle cells. Respir Res. 2014;15:58–69. doi: 10.1186/1465-9921-15-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang YG, Tian WM, Zhang H, Li M, Shang YX. Nerve growth factor exacerbates allergic lung inflammation and airway remodeling in a rat model of chronic asthma. Exp Ther Med. 2013;6:1251–1258. doi: 10.3892/etm.2013.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kudo M, Ishigatsubo Y, Aoki I. Pathology of asthma. Front Microbiol. 2013;4:263. doi: 10.3389/fmicb.2013.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prakash YS. Airway smooth muscle in airway reactivity and remodeling: what have we learned? Am J Physiol Lung Cell Mol Physiol. 2013;305:L912–933. doi: 10.1152/ajplung.00259.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ammit AJ, Panettieri RA Jr. Airway smooth muscle cell hyperplasia: a therapeutic target in airway remodeling in asthma? Prog Cell Cycle Res. 2002;5:49–57. [PubMed] [Google Scholar]
- 21.Koopmans T, Anaparti V, Castro-Piedras I, Yarova P, Irechukwu N, Nelson C, Perez-Zoghbi J, Tan X, Ward JP, Wright DB. Ca2+ handling and sensitivity in airway smooth muscle: Emerging concepts for mechanistic understanding and therapeutic targeting. Pulm Pharmacol Ther. 2014;29:108–120. doi: 10.1016/j.pupt.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Mahn K, Ojo OO, Chadwick G, Aaronson PI, Ward JP, Lee TH. Ca2+ homeostasis and structural and functional remodelling of airway smooth muscle in asthma. Thorax. 2010;65:547–552. doi: 10.1136/thx.2009.129296. [DOI] [PubMed] [Google Scholar]
- 23.Parekh AB, Penner R. Store depletion and calcium influx. Physiol Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- 24.Lin MJ, Leung GP, Zhang WM, Yang XR, Yip KP, Tse CM, Sham JS. Chronic Hypoxia-Induced Upregulation of Store-Operated and Receptor-Operated Ca2+ Channels in Pulmonary Arterial Smooth Muscle Cells A Novel Mechanism of Hypoxic Pulmonary Hypertension. Circ Res. 2004;95:496–505. doi: 10.1161/01.RES.0000138952.16382.ad. [DOI] [PubMed] [Google Scholar]
- 25.Golovina VA, Platoshyn O, Bailey CL, Wang J, Limsuwan A, Sweeney M, Rubin LJ, Yuan JX. Upregulated TRP and enhanced capacitative Ca2+ entry in human pulmonary artery myocytes during proliferation. Am J Physiol Lung Cell Mol Physiol. 2001;280:H746–H755. doi: 10.1152/ajpheart.2001.280.2.H746. [DOI] [PubMed] [Google Scholar]
- 26.Kumar B, Dreja K, Shah S, Cheong A, Xu S-Z, Sukumar P, Naylor J, Forte A, Cipollaro M, McHugh D. Upregulated TRPC1 channel in vascular injury in vivo and its role in human neointimal hyperplasia. Circ Res. 2006;98:557–563. doi: 10.1161/01.RES.0000204724.29685.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Wilusz JE, Sunwoo H, Spector DL. Long noncoding RNAs: functional surprises from the RNA world. Genes Dev. 2009;23:1494–1504. doi: 10.1101/gad.1800909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perry M, Austin P, Adcock I, Chung KF. The long intergenic non-coding RNA, PVT1, regulates the TGF-β-induced proliferative response in airway smooth muscle cells from severe asthmatics. European Respiratory Journal. 2013;42:1520. [Google Scholar]
- 30.Tu A, Austin P, Adcock I, Chung K. Expression Of The Long Intergenic Non-Coding Rna, Gas5, Is Correlated With The Corticosteroid Insensitivity Observed In Airway Smooth Muscle Cells From Severe Asthmatics. Am J Respir Crit Care Med. 2014;189:A3373. [Google Scholar]