Abstract
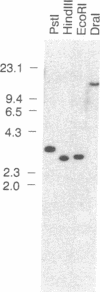
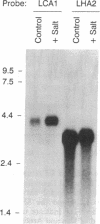
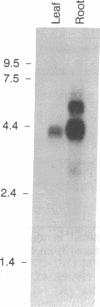
Calcium-dependent regulatory mechanisms participate in diverse developmentally, hormonally, and environmentally regulated processes, with the precise control of cytosolic Ca2+ concentration being critical to such mechanisms. In plant cells, P-type Ca(2+)-ATPases localized in the plasma membrane and the endoplasmic reticulum are thought to play a central role in regulating cytoplasmic Ca2+ concentrations. Ca(2+)-ATPase activity has been identified in isolated plant cell membranes, but the protein has not been characterized at the molecular level. We have isolated a partial-length cDNA (LCA1) and a complete genomic clone (gLCA13) encoding a putative endoplasmic reticulum-localized Ca(2+)-ATPase in tomato. The deduced amino acid sequence specifies a protein (Lycopersicon Ca(2+)-ATPase) of 1048 amino acids with a molecular mass of 116 kDa, eight probable transmembrane domains, and all of the highly conserved functional domains common to P-type cation-translocating ATPases. In addition, the protein shares approximately 50% amino acid sequence identify with animal sarcoplasmic/endoplasmic reticulum Ca(2+)-ATPases but less than 30% identity with other P-type ATPases. Genomic DNA blot hybridization analysis indicates that the Lycopersicon Ca(2+)-ATPase is encoded by a single gene. RNA blot hybridization analysis indicates the presence of three transcript sizes in root tissue and a single, much less abundant, transcript in leaves. Lycopersicon Ca(2+)-ATPase mRNA levels increase dramatically upon a 1-day exposure to 50 mM NaCl. Thus this report describes the primary structure of a higher-plant Ca(2+)-ATPase and the regulation of its mRNA abundance by salt stress.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen J. P., Vilsen B. Primary ion pumps. Curr Opin Cell Biol. 1990 Aug;2(4):722–730. doi: 10.1016/0955-0674(90)90116-v. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter-Lowe L. A., Guo J. Z., Bergstrom E. E., Hokin L. E. Molecular cloning of the Na,K-ATPase alpha-subunit in developing brine shrimp and sequence comparison with higher organisms. FEBS Lett. 1989 Oct 23;257(1):181–187. doi: 10.1016/0014-5793(89)81816-2. [DOI] [PubMed] [Google Scholar]
- Boutry M., Michelet B., Goffeau A. Molecular cloning of a family of plant genes encoding a protein homologous to plasma membrane H+-translocating ATPases. Biochem Biophys Res Commun. 1989 Jul 31;162(2):567–574. doi: 10.1016/0006-291x(89)92348-6. [DOI] [PubMed] [Google Scholar]
- Brandl C. J., Green N. M., Korczak B., MacLennan D. H. Two Ca2+ ATPase genes: homologies and mechanistic implications of deduced amino acid sequences. Cell. 1986 Feb 28;44(4):597–607. doi: 10.1016/0092-8674(86)90269-2. [DOI] [PubMed] [Google Scholar]
- Briskin D. P. Ca-translocating ATPase of the plant plasma membrane. Plant Physiol. 1990 Oct;94(2):397–400. doi: 10.1104/pp.94.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk S. E., Lytton J., MacLennan D. H., Shull G. E. cDNA cloning, functional expression, and mRNA tissue distribution of a third organellar Ca2+ pump. J Biol Chem. 1989 Nov 5;264(31):18561–18568. [PubMed] [Google Scholar]
- Carafoli E. Calcium pump of the plasma membrane. Physiol Rev. 1991 Jan;71(1):129–153. doi: 10.1152/physrev.1991.71.1.129. [DOI] [PubMed] [Google Scholar]
- Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Deikman J., Fischer R. L. Interaction of a DNA binding factor with the 5'-flanking region of an ethylene-responsive fruit ripening gene from tomato. EMBO J. 1988 Nov;7(11):3315–3320. doi: 10.1002/j.1460-2075.1988.tb03202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermont J. A., Wuytack F., De Jaegere S., Nelles L., Casteels R. Evidence for two isoforms of the endoplasmic-reticulum Ca2+ pump in pig smooth muscle. Biochem J. 1989 Jun 15;260(3):757–761. doi: 10.1042/bj2600757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. L., Moore R., Hasenstein K. H. How roots respond to gravity. Sci Am. 1986 Dec;255(6):112–119. doi: 10.1038/scientificamerican1286-112. [DOI] [PubMed] [Google Scholar]
- Ewing N. N., Wimmers L. E., Meyer D. J., Chetelat R. T., Bennett A. B. Molecular Cloning of Tomato Plasma Membrane H-ATPase. Plant Physiol. 1990 Dec;94(4):1874–1881. doi: 10.1104/pp.94.4.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Ghislain M., Goffeau A., Halachmi D., Eilam Y. Calcium homeostasis and transport are affected by disruption of cta3, a novel gene encoding Ca2(+)-ATPase in Schizosaccharomyces pombe. J Biol Chem. 1990 Oct 25;265(30):18400–18407. [PubMed] [Google Scholar]
- Giannini J. L., Ruiz-Cristin J., Briskin D. P. Calcium Transport in Sealed Vesicles from Red Beet (Beta vulgaris L.) Storage Tissue : II. Characterization of Ca Uptake into Plasma Membrane Vesicles. Plant Physiol. 1987 Dec;85(4):1137–1142. doi: 10.1104/pp.85.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy S., Fricker M. D., Read N. D., Trewavas A. J. Role of Calcium in Signal Transduction of Commelina Guard Cells. Plant Cell. 1991 Apr;3(4):333–344. doi: 10.1105/tpc.3.4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunteski-Hamblin A. M., Greeb J., Shull G. E. A novel Ca2+ pump expressed in brain, kidney, and stomach is encoded by an alternative transcript of the slow-twitch muscle sarcoplasmic reticulum Ca-ATPase gene. Identification of cDNAs encoding Ca2+ and other cation-transporting ATPases using an oligonucleotide probe derived from the ATP-binding site. J Biol Chem. 1988 Oct 15;263(29):15032–15040. [PubMed] [Google Scholar]
- Haro R., Garciadeblas B., Rodríguez-Navarro A. A novel P-type ATPase from yeast involved in sodium transport. FEBS Lett. 1991 Oct 21;291(2):189–191. doi: 10.1016/0014-5793(91)81280-l. [DOI] [PubMed] [Google Scholar]
- Harper J. F., Manney L., DeWitt N. D., Yoo M. H., Sussman M. R. The Arabidopsis thaliana plasma membrane H(+)-ATPase multigene family. Genomic sequence and expression of a third isoform. J Biol Chem. 1990 Aug 15;265(23):13601–13608. [PubMed] [Google Scholar]
- Harper J. F., Surowy T. K., Sussman M. R. Molecular cloning and sequence of cDNA encoding the plasma membrane proton pump (H+-ATPase) of Arabidopsis thaliana. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1234–1238. doi: 10.1073/pnas.86.4.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh W. L., Pierce W. S., Sze H. Calcium-pumping ATPases in vesicles from carrot cells : stimulation by calmodulin or phosphatidylserine, and formation of a 120 kilodalton phosphoenzyme. Plant Physiol. 1991 Dec;97(4):1535–1544. doi: 10.1104/pp.97.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano I., Nagai F., Satoh K., Ushiyama K., Nakao T., Kano K. Structure of the alpha 1 subunit of horse Na,K-ATPase gene. FEBS Lett. 1989 Jun 19;250(1):91–98. doi: 10.1016/0014-5793(89)80691-x. [DOI] [PubMed] [Google Scholar]
- Karin N. J., Kaprielian Z., Fambrough D. M. Expression of avian Ca2+-ATPase in cultured mouse myogenic cells. Mol Cell Biol. 1989 May;9(5):1978–1986. doi: 10.1128/mcb.9.5.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami K., Ohta T., Nojima H., Nagano K. Primary structure of the alpha-subunit of human Na,K-ATPase deduced from cDNA sequence. J Biochem. 1986 Aug;100(2):389–397. doi: 10.1093/oxfordjournals.jbchem.a121726. [DOI] [PubMed] [Google Scholar]
- Knight M. R., Campbell A. K., Smith S. M., Trewavas A. J. Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature. 1991 Aug 8;352(6335):524–526. doi: 10.1038/352524a0. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lytton J., MacLennan D. H. Molecular cloning of cDNAs from human kidney coding for two alternatively spliced products of the cardiac Ca2+-ATPase gene. J Biol Chem. 1988 Oct 15;263(29):15024–15031. [PubMed] [Google Scholar]
- Lytton J., Zarain-Herzberg A., Periasamy M., MacLennan D. H. Molecular cloning of the mammalian smooth muscle sarco(endo)plasmic reticulum Ca2+-ATPase. J Biol Chem. 1989 Apr 25;264(12):7059–7065. [PubMed] [Google Scholar]
- Maeda M., Ishizaki J., Futai M. cDNA cloning and sequence determination of pig gastric (H+ + K+)-ATPase. Biochem Biophys Res Commun. 1988 Nov 30;157(1):203–209. doi: 10.1016/s0006-291x(88)80033-0. [DOI] [PubMed] [Google Scholar]
- Maeda M., Oshiman K., Tamura S., Futai M. Human gastric (H+ + K+)-ATPase gene. Similarity to (Na+ + K+)-ATPase genes in exon/intron organization but difference in control region. J Biol Chem. 1990 Jun 5;265(16):9027–9032. [PubMed] [Google Scholar]
- Magyar A., Váradi A. Molecular cloning and chromosomal localization of a sarco/endoplasmic reticulum-type Ca2(+)-ATPase of Drosophila melanogaster. Biochem Biophys Res Commun. 1990 Dec 31;173(3):872–877. doi: 10.1016/s0006-291x(05)80867-8. [DOI] [PubMed] [Google Scholar]
- Mohana Rao J. K., Argos P. A conformational preference parameter to predict helices in integral membrane proteins. Biochim Biophys Acta. 1986 Jan 30;869(2):197–214. doi: 10.1016/0167-4838(86)90295-5. [DOI] [PubMed] [Google Scholar]
- Myers E. W., Miller W. Optimal alignments in linear space. Comput Appl Biosci. 1988 Mar;4(1):11–17. doi: 10.1093/bioinformatics/4.1.11. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov YuA, Modyanov N. N., Broude N. E., Petrukhin K. E., Grishin A. V., Arzamazova N. M., Aldanova N. A., Monastyrskaya G. S., Sverdlov E. D. Pig kidney Na+,K+-ATPase. Primary structure and spatial organization. FEBS Lett. 1986 Jun 9;201(2):237–245. doi: 10.1016/0014-5793(86)80616-0. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov YuA, Monastyrskaya G. S., Broude N. E., Ushkaryov YuA, Melkov A. M., Smirnov YuV, Malyshev I. V., Allikmets R. L., Kostina M. B., Dulubova I. E. Family of human Na+, K+-ATPase genes. Structure of the gene for the catalytic subunit (alpha III-form) and its relationship with structural features of the protein. FEBS Lett. 1988 Jun 6;233(1):87–94. doi: 10.1016/0014-5793(88)81361-9. [DOI] [PubMed] [Google Scholar]
- Pardo J. M., Serrano R. Structure of a plasma membrane H+-ATPase gene from the plant Arabidopsis thaliana. J Biol Chem. 1989 May 25;264(15):8557–8562. [PubMed] [Google Scholar]
- Rudolph H. K., Antebi A., Fink G. R., Buckley C. M., Dorman T. E., LeVitre J., Davidow L. S., Mao J. I., Moir D. T. The yeast secretory pathway is perturbed by mutations in PMR1, a member of a Ca2+ ATPase family. Cell. 1989 Jul 14;58(1):133–145. doi: 10.1016/0092-8674(89)90410-8. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesser A., Ulaszewski S., Ghislain M., Goffeau A. A second transport ATPase gene in Saccharomyces cerevisiae. J Biol Chem. 1988 Dec 25;263(36):19480–19487. [PubMed] [Google Scholar]
- Serrano R., Kielland-Brandt M. C., Fink G. R. Yeast plasma membrane ATPase is essential for growth and has homology with (Na+ + K+), K+- and Ca2+-ATPases. Nature. 1986 Feb 20;319(6055):689–693. doi: 10.1038/319689a0. [DOI] [PubMed] [Google Scholar]
- Shull G. E., Greeb J., Lingrel J. B. Molecular cloning of three distinct forms of the Na+,K+-ATPase alpha-subunit from rat brain. Biochemistry. 1986 Dec 16;25(25):8125–8132. doi: 10.1021/bi00373a001. [DOI] [PubMed] [Google Scholar]
- Shull G. E., Greeb J. Molecular cloning of two isoforms of the plasma membrane Ca2+-transporting ATPase from rat brain. Structural and functional domains exhibit similarity to Na+,K+- and other cation transport ATPases. J Biol Chem. 1988 Jun 25;263(18):8646–8657. [PubMed] [Google Scholar]
- Shull G. E., Lingrel J. B. Molecular cloning of the rat stomach (H+ + K+)-ATPase. J Biol Chem. 1986 Dec 25;261(36):16788–16791. [PubMed] [Google Scholar]
- Shull G. E., Schwartz A., Lingrel J. B. Amino-acid sequence of the catalytic subunit of the (Na+ + K+)ATPase deduced from a complementary DNA. Nature. 1985 Aug 22;316(6030):691–695. doi: 10.1038/316691a0. [DOI] [PubMed] [Google Scholar]
- Takeyasu K., Tamkun M. M., Renaud K. J., Fambrough D. M. Ouabain-sensitive (Na+ + K+)-ATPase activity expressed in mouse L cells by transfection with DNA encoding the alpha-subunit of an avian sodium pump. J Biol Chem. 1988 Mar 25;263(9):4347–4354. [PubMed] [Google Scholar]
- Verma A. K., Filoteo A. G., Stanford D. R., Wieben E. D., Penniston J. T., Strehler E. E., Fischer R., Heim R., Vogel G., Mathews S. Complete primary structure of a human plasma membrane Ca2+ pump. J Biol Chem. 1988 Oct 5;263(28):14152–14159. [PubMed] [Google Scholar]
- Váradi A., Gilmore-Heber M., Benz E. J., Jr Amplification of the phosphorylation site-ATP-binding site cDNA fragment of the Na+,K(+)-ATPase and the Ca2(+)-ATPase of Drosophila melanogaster by polymerase chain reaction. FEBS Lett. 1989 Dec 4;258(2):203–207. doi: 10.1016/0014-5793(89)81653-9. [DOI] [PubMed] [Google Scholar]