Abstract
Developing of personalized therapies for Triple Negative Breast Cancer (TNBC) requires a more detailed knowledge of its biology and a correct stratification of molecular subtypes. Androgen Receptor (AR) is expressed in a large part of TNBCs but its prognostic role in this Breast Cancer (BC) subtype is highly debated. In this study, we analyzed AR expression in a series of 238 TNBCs and correlated its expression with clinical-pathological features, survival, and metabolic profile. We showed a consistent association between AR expression and a better prognosis of TNBC patients, while its downregulation appeared strongly associated with diabetic disease. Since a recent prospective study reported a lower BC risk in diabetic women treated with drugs able to reduce circulating levels of glucose compared with non-diabetic woman, and in vitro studies showed that AR level are regulated directly by hyperglycemia, we speculate on the perspective of new integrated therapies for TNBC.
Keywords: Triple negative breast cancers, androgen receptor, diabetes
Introduction
Triple-negative breast cancer (TNBC), characterized by tumors that do not express estrogen receptor (ER), progesterone receptor (PR), and HER-2 genes account for 10%-24% of invasive breast cancers, and they are typically high-grade tumors with different histological types. Usually, patients with TNBC tend to have a higher recurrence rate after diagnosis, a short disease-free interval, reduced overall survival, especially for the lack of targeted therapies [1]. This has intensified the interest for pathogenesis of TNBC and the research of new molecular signatures tailored to this specific BC subtype.
Although TNBC lacks hormone receptors traditionally associated with BC, both molecular and immunohistochemical analyses demonstrate that a subset of TNBC expresses the androgen receptor (AR). Recently, Laehmann et al, by gene expression profiles studies, has stratified the TNBCs into 6 molecular subgroups, including a specific subtype with AR expression defined LAR (Luminal Androgen receptor) tumor [2].
Emerging data suggest that AR significantly influences breast cancer gene expression profiles and affects tumorigenic properties of TNBC. At present, divergent ideas on the prognostic value of AR are documented. In fact, several studies described that lack of AR expression is a poor prognostic marker, since low AR expression correlated with a more aggressive tumor behavior and a poorer OS [3,4]. On the contrary, other studies highlighted that LAR subtype, with overexpression of AR, was associated with a poor patient prognosis suggesting that this tumor may be less responsive to chemotherapy [5].
In any case, the use of anti-androgens drugs for the treatment of prostate cancer has increased the interest in hormonal therapy targeting AR also in the subset LAR of TNBC suggesting a novel therapeutic option that could improve prognosis.
Convincing evidence indicates that diabetes is associated with an excess risk of incidenceand mortality for overall and a number of site-specific cancers [6]. Type 2 diabetes is associated with increased risk of postmenopausal breast, colorectal, endometrial, liver, and pancreatic cancers, and non-Hodgkin lymphoma. Diabetes is also associated with premature death from cancers of the breast, liver, pancreas, ovary, colon-rectum, lung and bladder [7]. For instance, meta-analysis have showed that women with (versus without) diabetes have a statistically significant 20% increased risk of breast cancer [8]. Additionally, the patients with breast cancer and preexisting diabetes had an increased risk for distant metastasis and for all-cause mortality compared with their nondiabetic counterparts [9].
Recently, Barbosa-Desongles et al. showed that the increasing glucose concentrations was able to downregulate androgen receptor mRNA and protein levels through NF-κB activation in in vitro and animal model of prostate cancer [10].
To better define the prognostic role of AR in TNBCs and the relation with other clinic-pathologic features, including metabolic profile, we selected a large case series of TNBCs to analyze, by immunohistochemistry, AR expression, and identify LAR subtype.
Our data highlighted that high AR levels are strongly associated with a better survival in TNBCs patients and that its downregulation can be associated with diabetic disease.
Materials and methods
Patients and specimens
From 2003 to 2013, 238 patients who underwent a mastectomy, quadrantectomy or metastectomy at the National Cancer Institute “Giovanni Pascale Foundation” of Naples, Italy, were enrolled into this study.
In our institution, the percentage of tumors classified as Triple Negative is approximately 15-19% of the total number of breast cancer surgical samples. All cases of Triple Negative and non-Triple Negative breast samples were reviewed according to WHO classification criteria, using standard tissue sections and appropriate immunohistochemical slides.
Medical records for all cases of Triple Negative breast samples were reviewed for clinical information, including histologic parameters that were determined from the H&E slides. The following clinical and pathological parameters were evaluated for each tumor included in the study: patient age at initial diagnosis, tumor size, histologic subtype, histologic grade, nuclear grade, nodal status, number of positive lymph nodes, tumor stage, tumor recurrence or distant metastasis and type of surgery (for tumor removal).
In addition, all specimens were characterized for all routinely diagnostic immunophenotypic parameters.
TMA building
Tissue Micro-Array (TMA) was built using the most representative areas from each single case with one replicate. All tumours and controls were reviewed by two experienced pathologists (MDB/GB). Discrepancies between two pathologists from the same case were resolved in a joint analysis of the cases. Tissue cylinders with a diameter of 1 mm were punched from morphologically representative tissue areas of each ‘donor’ tissue block and brought into one recipient paraffin block (3 × 2.5 cm) using a semi-automated tissue arrayer (Galileo TMA).
Immunohistochemistry analysis
Immunohistochemical staining was done on slides from formalin-fixed, paraffin embedded tissues to evaluate the expression of ER PgR, HER2, Ki67 and AR markers. Paraffin slides were de-paraffinized in xylene and rehydrated through graded alcohols. Antigen retrieval was performed with slides heated in 0.01 M citrate buffer (pH 6.0) in a bath for 20 min at 97°C. After antigen retrieval, the slides allow to cool. The slides were rinsed with TBS and the endogenous peroxidase was inactivated with 3% hydrogen peroxide. After protein block (BSA 5% in PBS 1x), the slides were incubated with primary antibody to human to human ERα (Monoclonal Mouse Anti-Human Erα, Clone ID5, dilution 1:35, Dako North America, Inc., Carpinteria, CA, USA), PR (Monoclonal Mouse Anti-Human PR, Clone 636, dilution 1:50, Dako North America, Inc., Carpinteria, CA, USA), c-Erb B2 (Polyclonal Rabbit Anti-Human Oncoprotein, dilution 1:300, Dako North America, Inc., Carpinteria, CA, USA), Ki67 (Monoclonal Mouse Anti-Human Ki67 Ag Clone MIB-1, dilution 1:75, Dako North America, Inc., Carpinteria, CA, USA) for 30 minutes and AR (monoclonal mouse anti-human AR antibody clone AR441, dilution 1:75, #M3562; Dako North America, Inc., Carpinteria, CA, USA). The sections were rinsed in TBS and incubated for 20 min with Biotinylated Secondary Antibody (Novocastra RE7103), a biotin-conjugated secondary antibody formulation that recognized mouse and rabbit immunoglobulins. Then the sections were rinsed in TBS and incubated for 20 min with Streptavidin-HRP (Novocastra RE7104) and then peroxidase reactivity was visualized using a 3,3’-diaminobenzidine (DAB). Finally, the sections were counterstained with hematoxylin and mounted. Results are interpreted using a light microscope.
Evaluation of immunohistochemistry
Antigen expression was evaluated independently by a pathologist using a light microscopy. Observer was unaware of the clinical outcome. For each sample, at least five fields (inside the tumor and in the area exhibiting tumor invasion) and >500 cells were analysed. Using a semi-quantitative scoring system microscopically and referring to each antigen scoring method in other studies, an observer evaluated the intensity, extent and subcellular distribution.
The cutoff used to distinguish “positive” from “negative” cases was ≥1% ER/PR positive tumor cells. Immunohistochemical analyses of c-ErbB-2 expression describe the intensity and staining pattern of tumor cells. Only membrane staining intensity and pattern were evaluated using the 0 to 3+ score as illustrated in the HercepTest kit scoring guidelines. The ASCO/CAP 2013 describes a new HER2 Testing Algorithms identifying 4 categories: no staining, or incomplete and faint/barely perceptible membrane staining within ≤10% of tumor cells (0 negative); incomplete and faint/barely perceptible membrane staining within >10% of tumor (1+ negative); incomplete and circumferential weak/moderate membrane staining within >10% of tumor cells or complete and circumferential intense membrane staining within ≤10% of tumor cells (2+ equivocal); and complete and circumferential intense membrane staining within >10% of tumor cells (3+ positive). Cases with score 2+ underwent fluorescence in situ hybridization analysis. For the proliferative index Ki67 was defined as the percentage of immunoreactive tumour cells out of the total number of cells. The percentage of positive cells per case was scored according to 2 different groups: group 1: <30% (low proliferative activity); group 2: >30% (high proliferative activity).
For nuclear AR expression the cutoff used to distinguish “positive” from “negative” cases was ≥1% AR positive tumor cells.
Statistical analysis
The association between AR expression with clinical-pathological parameters and was conducted using the χ2 and T Student test.
The Pearson χ2 test was used to determine whether a relationship exists between the variables included in the study. The level of significance was defined as P<0.05. Overall survival (OS) and disease-free survival (DFS) curves were calculated using the Kaplan-Meier method. All the statistical analyses were carried out using the Statistical Package for Social Science v. 20 software (SPSS Inc., Chicago, IL, USA).
OS was defined as the time from diagnosis (first biopsy) to death by any cause or until the most recent follow-up. DFS was measured as the time from diagnosis to the occurrence of progression, relapse after complete remission, or death from any cause. DFS had a value of zero for patients who did not achieve complete remission. The follow-up duration was five years.
Results
Clinical-pathological characteristics and follow up data of TNBC patients
In our cohort, we have included 238 TNBC samples of breast cancers.
The age of patients ranged from 24-93 years, with an average age of 57 years. The percentages of tumor grading were: 82.8% grade III and 11.8% grade I-II. 13 cases were not able to retrieve that information. Tumor sizes were: lower than 2 cm in 42.9 % of the samples, between 2 and 5 cm in 43.3% (69/155) and larger than 5 cm in 8.4%. 13 cases were not able to retrieve that information. Metastatic lymph nodes (LNM) were found in 34.0% of patients and were not found in 46.6% of patients at surgery (this information for 46 patients was lost). Distant metastases were found in 17.6% and were not present in 55% of cases. 65 cases were unable to recover this information.
The expression of proliferation factor Ki67 was high (>20%) in 78.2%, and low (≤20%) in 17.6% of cases. This information for 10 patients was lost.
Regarding metabolic parameters, BMI evaluation was <30 in 30.3% of TNBCs patients and ≥30 in 15.1% of cases. This information was lost for 130 patients. 7.1% of TNBC patients were diabetic but this information was lost for 123 patients. The information about follow up was lost for 135 (56.7%) of patients. No evidence of disease was documented in 26.5% of patients, 10.1% were alive with disease and 6.7% were died of tumor. All information are schematized in Supplementary Table 1.
AR expression and LAR subtype identification in TNBCs cohort
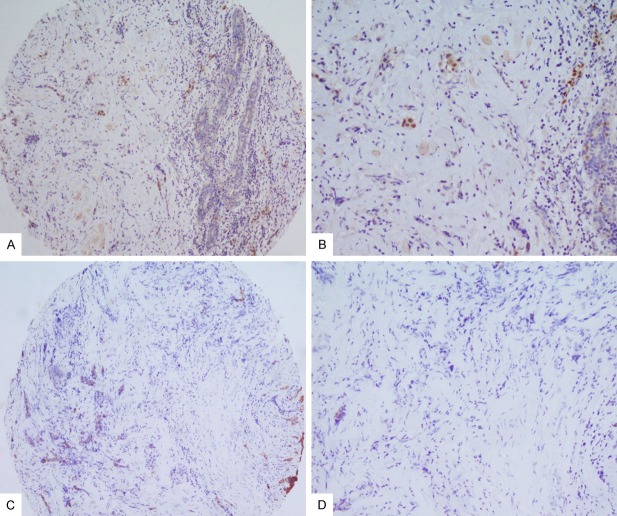
In order to immunohistochemically characterize the LAR subtype in our series of TNBCs, we analyzed the expression of luminal CK8/18 cytokeratin and AR receptor on TMAs (Figures 1 and 2).
Figure 1.
AR immunostaining in TNBC: A. Low expression of AR (10X); B. Detail of low expression of AR (20X); C. CK8/18 negative staining with normal tissue as positive internal control (10X); D. Detail of CK8/18 negative staining (20X).
Figure 2.
LAR TNBC immunephenotype: A. High expression of AR (10X); B. Detail of high expression of AR (20X); C. CK8/18 positive staining (10X); D. Detail of CK8/18 positive staining (20X).
Excluding the samples that could not be assessed, IHC expression was considered evaluable in 207/238 TMA cores. In detail, the expression of cytokeratin 8/18 was present in 40/207 (19%) TNBC samples, while nuclear AR protein expression was detected in 49/207 (23%) specimens (Table 1).
Table 1.
Statistical association of AR+ TNBC and LAR TNBC with clinic pathological features
| AR | LAR | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Negative | Positive | P value | R pearson | Negative | Positive | P value | R pearson | |
| Age (n=207) | ||||||||
| <40 | 23 (88%) | 3 (12%) | 24 (92%) | 2 (8%) | ||||
| ≥40≤60 | 69 (75%) | 23 (25%) | 0.295 | 0.085 | 72 (82%) | 16 (18%) | 0.141 | 0.137 |
| >60 | 66 (74%) | 23 (26%) | 67 (75%) | 22 (25%) | ||||
| Menopause (n=207) | ||||||||
| Pre | 58 (74.4%) | 20 (25.6%) | 0.604 | -0.036 | 62 (82.6%) | 13 (17.4%) | 0.516 | 0.046 |
| Post | 100 (77.5%) | 29 (22.5%) | 101 (78.9%) | 27 (21.1%) | ||||
| Size (n=199) | ||||||||
| ≤2 cm | 68 (78%) | 22 (30%) | 68 (80%) | 17 (20%) | ||||
| >2≤5 | 69 (73%) | 26 (27%) | 0.330 | -0.009 | 74 (79%) | 20 (21%) | 0.664 | -0.030 |
| >5 | 15 (88%) | 2 (12%) | 15 (88%) | 2 (12%) | ||||
| LNM (n=169) | ||||||||
| Negative | 82 (62%) | 50 (38%) | 0.078 | 0.136 | 84 (86%) | 14 (14%) | 0.056 | 0.148 |
| Positive | 17 (46%) | 20 (54%) | 51 (74%) | 18 (26%) | ||||
| Metastasis (n=151) | ||||||||
| Negative | 91 (77.1%) | 27 (22.9%) | 0.839 | -0.017 | 94 (80%) | 24 (20%) | 0.842 | -0.16 |
| Positive | 26 (78.7%) | 7 (21.3%) | 26 (81%) | 6 (19%) | ||||
| Grade (n=199) | ||||||||
| G1-G2 | 11 (58%) | 8 (42%) | 0.054 | -0.137 | 13 (68%) | 6 (32%) | ||
| G3 | 140 (78%) | 40 (22%) | 143 (81%) | 34 (19%) | 0.204 | -0.091 | ||
| Ki67 (n=200) | ||||||||
| ≤20% | 23 (74%) | 8 (26%) | 0.686 | -0,.029 | 24 (77%) | 7 (23%) | 0.624 | -0.035 |
| >20% | 131 (77.5%) | 38 (22.5%) | 134 (81%) | 31 (19%) | ||||
| Status (n=88) | ||||||||
| Alive | 45 (80%) | 11 (20%) | 48 (86%) | 8 (14%) | ||||
| Alive with disease | 11 (55%) | 9 (45%) | 0.009 | -0.038 | 12 (60%) | 8 (40%) | 0.008 | 0.000 |
| Dead | 12 (100%) | 0 (0%) | 12 (100%) | 0 (0%) | ||||
| Relapse (n=117) | ||||||||
| No | 59 (81%) | 14 (19%) | 0.308 | 0,.094 | 62 (85%) | 11 (15%) | 0.163 | 0.130 |
| Yes | 32 (73%) | 12 (27%) | 32 (74%) | 11 (26%) | ||||
IDC=Invasive Ductal Carcinoma; ILC=Invasive Lobular Carcinoma; MC=Medullary Carcinoma; MDLC=Mixed Ductal and Lobular Carcinoma; LNM=Lymph Node Metastasis.
All cases with luminal cytokeratin positivity also expressed AR. Thus the contribution of LAR subtype in our case series was of 19%.
In addition, our data showed that when the luminal cytokeratins are not expressed, the positivity of AR is always very low (Figure 1). On the contrary in the LAR, AR expression is always very consistent (Figure 2).
AR expression in premenopausal and postmenopausal TNBC patients
In order to evaluate the correlation of AR expression with menopausal status of TNBC patients we identify, on the basis of information present in medical file, premenopausal and postmenopausal subgroups.
Our results showed that both AR+ and LAR TNBCs do not show a statistical association with menopausal status. All data are schematized in Table 1.
AR expression association with clinical-pathological features and survival of TNBC patients
Based on statistical elaboration of AR protein expression analysis with the other clinical-pathological parameters in TNBC, we showed a trend of association between positivity and LNM metastases (p=0.078) and a stronger inverse trend of association with histological grade (p=0.054). The more significant inverse statistical association was present between AR expression and survival status (p=0.009). Regarding LAR subtype the inverse trend of association with histological grade is loss, while those with LNM metastases is reinforced (p=0.056). Also in this case the stronger inverse statistical association exists with the survival status (p=0.008).
All data are schematized in Table 1.
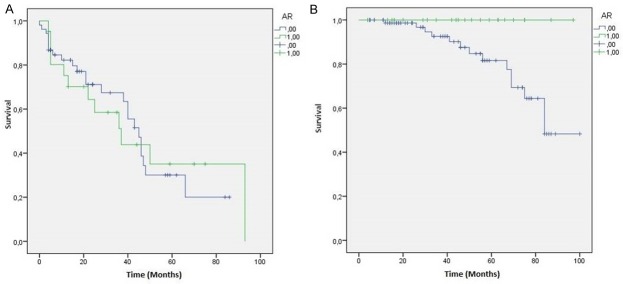
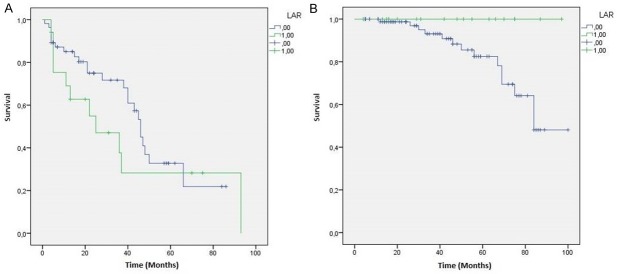
Regarding the relation with patients survival we showed a strong statistical association between AR+ TNBC and OS (p=0.035), while LAR subtype showed only a stronger trend with OS (p=0.054). The statistical association with DFS are not significant in both AR+ (p=0.998) that in LAR subtype (p=0.277) (Figures 3 and 4).
Figure 3.
AR+ TNBC patients Kaplan-Meier curves: A. Disease Free Survival (DFS) (p=0.998); B. Overall Survival (OS) (p=0.035).
Figure 4.
LAR TNBC patients Kaplan-Meier curves: A. Disease Free Survival (DFS) (p=0.277); B. Overall Survival (OS) (p=0.054).
Androgen receptor relation with metabolic features of TNBC patients
Based on statistical elaboration of AR protein expression with metabolic parameters in TNBC patients, we showed that a significant inverse association of AR expression with diabetic disease (p=0.028) was present. This association is lost, becoming a trend, in LAR subtype (p=0.054). All data are schematized in Table 2.
Table 2.
Statistical association of AR+ TNBC and LAR TNBC with metabolic features
| AR | LAR | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Negative | Positive | P value | R pearson | Negative | Positive | P value | R pearson | |
| BMI (n=92) | ||||||||
| <30 | 52 (81.2%) | 12 (18.8%) | 0.496 | 0.071 | 56 (87%) | 8 (13%) | 0.135 | 0.156 |
| ≥30 | 21 (75%) | 7 (25%) | 21 (75%) | 7 (25%) | ||||
| Diabetes (n=98) | ||||||||
| No | 62 (74.6%) | 21 (25.4%) | 0.028 | -0.222 | 66 (80%) | 17 (20%) | 0.054 | -0.195 |
| Yes | 15 (100%) | 0 (0%) | 15 (100%) | 0 (0%) | ||||
| Glycaemia (n=63) | ||||||||
| <110 | 36 (76.5%) | 11 (23.5%) | 0.699 | -0.049 | 38 (81%) | 9 (19%) | 0.972 | -0.004 |
| >110 | 13 (81.2%) | 3 (18.8%) | 13 (81%) | 3 (19%) | ||||
IDC=Invasive Ductal Carcinoma; ILC=Invasive Lobular Carcinoma; MC=Medullary Carcinoma; MDLC=Mixed Ductal and Lobular Carcinoma; LNM=Lymph Node Metastasis.
Discussion
Triple Negative breast cancer constitutes 15-20% of breast carcinoma with a lowest 5-years survival rate compared to other breast cancer types mostly for the lack of specific targeted therapies [11].
Several studies in the last years showed that nuclear IHC expression of AR can be detected in about 12-55% of TNBCs depending in particular by the criteria used in its IHC evaluation (positivity in >1% or >10% of cells) [12].
However the real prognostic value of AR protein expression in TNBC is highly controversial. In fact, Barton et al. described that AR+ TNBC is characterized by a lower Ki67 index than AR- TNBC [13]. For this reason probably they were less responsive to chemotherapy, identifying a more aggressive phenotype. On the contrary, Vera-Badillo et al. showed that AR expression was associated with a better OS and DFS in breast cancer [14].
We analyzed the AR expression in our TNBC cohort of 238 patients to verify the prognostic value of the receptor. We revealed that AR IHC expression was statistically associated with better survival, even if it has a trend of statistically association with LNM metastases. Therefore our data would seem in accordance with reports in the literature indicating a protective role of AR also in the TNBC subtype.
New clinical trials using anti AR drugs have shown an increase in survival of TNBC patients [15] suggesting new therapeutic chances at least in the portion of TNBC patients AR+. However, preclinical studies showed the efficacies of anti-androgens drugs in LAR cells models but also in several cell lines with low level of AR expression [2]. For this reason it becomes increasingly necessary to realize in vivo studies to identify AR+ TNBCs who might better respond to anti AR therapies, for properly stratifying TNBC patients.
In this study we have identified, within AR+ TNBC group, the LAR subtype, analyzing the expression of luminal cytokeratin CK8/18. We showed that the contribution of LAR pure in our cohort was of 19%.
However, correlating this subgroup with clinical-pathological features we have not detected significant changes in the statistical correlations. The more consistent observation was that, while a strong statistical association between AR+ TNBC and OS was present, this is lost, becoming only a stronger trend, in LAR patients. Therefore, our data support the idea that the only expression of AR would have a real prognostic value in TNBC patients.
It is known that after menopause, circulating androgens derive mainly from the adrenal gland and its production decreases with age [16]. However, in breast cancer several studies described that AR aberrant expression was more frequent in older women [17]. Moreover, Asseryanis E et al showed that postmenopausal status in BC women was correlated with BMI and androgen serum levels [18].
In this study we also evaluated the correlation of AR expression with menopausal condition of TNBC patients, but our results showed no statistical correlation in both AR+ that LAR TNBC patients.
Finally we associated AR expression with metabolic profile of TNBC patients, in particular with BMI and hyperglycemia. In fact, it is known that metabolic syndrome is consistently associated with an increased risk of several cancers in adults. In particular, the associations were stronger in women for some cancers (pancreas and rectal), and the magnitude of the associations was highest for sex-specific cancers (endometrial and breast postmenopausal) as showed by a recent meta-analysis study [19].
Our results showed no correlation of AR with BMI in TNBC patients but a strongly association between AR downregulation and the presence of diabetic disease.
It is known that in prostate cancer hyperglycemia plays an important role in protecting against cancer progression by reducing androgen receptor levels via NF-κB in vivo cell model [10].
Our results showed the same trend but this condition results not protective in our cohort of TNBC patients, rather diabetic disease appears correlated with reduction of androgen levels and consequently with the survival.
Therefore although AR level seems regulated directly by hyperglycemia, this condition would identify a subset of patients with poor prognosis.
In conclusion our data highlight again the favorable prognostic role of AR in TNBC cancers and that its expression may be under the control of hyperglycemia. A recent large prospective study reported a lower breast Cancer risk in diabetic women treated with metformin compared with non-diabetic woman [20].
This condition could open the perspective of new integrated therapies, that contemplate the use of AR antagonists and drugs able to reduce circulating levels of glucose by modulating the expression of the androgen receptor.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Elsamany S, Abdullah S. Triple-negative breast cancer: future prospects in diagnosis and management. Med Oncol. 2014;31:834. doi: 10.1007/s12032-013-0834-y. [DOI] [PubMed] [Google Scholar]
- 2.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim Y, Jae E, Yoon M. Influence of Androgen Receptor Expression on the Survival Outcomes in Breast Cancer: A Meta-Analysis. J Breast Cancer. 2015;18:134–142. doi: 10.4048/jbc.2015.18.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ricciardi GR, Adamo B, Ieni A, Licata L, Cardia R, Ferraro G, Franchina T, Tuccari G, Adamo V. Androgen Receptor (AR), E-Cadherin, and Ki-67 as Emerging Targets and Novel Prognostic Markers in Triple-Negative Breast Cancer (TNBC) Patients. PLoS One. 2015;10:e0128368. doi: 10.1371/journal.pone.0128368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi JE, Kang SH, Lee SJ, Bae YK. Androgen receptor expression predicts decreased survival in early stage triple-negative breast cancer. Ann Surg Oncol. 2015;22:82–89. doi: 10.1245/s10434-014-3984-z. [DOI] [PubMed] [Google Scholar]
- 6.Harding JL, Shaw JE, Peeters A, Cartensen B, Magliano DJ. Cancer risk among people with type 1 and type 2 diabetes: disentangling true associations, detection bias, and reverse causation. Diabetes Care. 2015;38:264–270. doi: 10.2337/dc14-1996. [DOI] [PubMed] [Google Scholar]
- 7.Shi Y, Hu FB. The global implications of diabetes and cancer. Lancet. 2014;383:1947–1948. doi: 10.1016/S0140-6736(14)60886-2. [DOI] [PubMed] [Google Scholar]
- 8.Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ. 2015;350:7607. doi: 10.1136/bmj.g7607. [DOI] [PubMed] [Google Scholar]
- 9.Cohen DH, LeRoith D. Obesity, type 2 diabetes, and cancer: the insulin and IGF connection. Endocr Relat Cancer. 2012;19:27–45. doi: 10.1530/ERC-11-0374. [DOI] [PubMed] [Google Scholar]
- 10.Barbosa-Desongles A, Hernández C, De Torres I, Munell F, Poupon MF, Simó R, Selva DM. Diabetes protects from prostate cancer by downregulating androgen receptor: new insights from LNCaP cells and PAC120 mouse model. PLoS One. 2013;8:74179. doi: 10.1371/journal.pone.0074179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyle P. Triple-negative breast cancer: epidemiological considerations and recommendations. Ann Oncol. 2012;23:7–12. doi: 10.1093/annonc/mds187. [DOI] [PubMed] [Google Scholar]
- 12.Safarpour D, Pakneshan S, Tavassoli FA. Androgen receptor (AR) expression in 400 breast carcinomas: is routine AR assessment justified? Am J Cancer Res. 2014;4:353–68. [PMC free article] [PubMed] [Google Scholar]
- 13.Barton VN, D’Amato NC, Gordon MA, Lind HT, Spoelstra NS, Babbs BL, Heinz RE, Elias A, Jedlicka P, Jacobsen BM, Richer JK. Multiple molecular subtypes of triple-negative breast cancer critically rely on androgen receptor and respond to enzalutamide in vivo. Mol Cancer Ther. 2015;14:769–778. doi: 10.1158/1535-7163.MCT-14-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vera-Badillo FE, Templeton AJ, de Gouveia P, Diaz-Padilla I, Bedard PL, Al-Mubarak M, Seruga B, Tannock IF, Ocana A, Amir E. Androgen receptor expression and outcomes in early breast cancer: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:319. doi: 10.1093/jnci/djt319. [DOI] [PubMed] [Google Scholar]
- 15.Gucalp A, Tolaney S, Isakoff SJ, Ingle JN, Liu MC, Carey LA, Blackwell K, Rugo H, Nabell L, Forero A, Stearns V, Doane AS, Danso M, Moynahan ME, Momen LF, Gonzalez JM, Akhtar A, Giri DD, Patil S, Feigin KN, Hudis CA, Traina TA. Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic Breast Cancer. Clin Cancer Res. 2013;19:5505–5512. doi: 10.1158/1078-0432.CCR-12-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McConnell DS, Stanczyk FZ, Sowers MR, Randolph JF Jr, Lasley BL. Menopausal transition stage-specific changes in circulating adrenal androgens. Menopause. 2012;19:658–663. doi: 10.1097/gme.0b013e31823fe274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Honma N, Sakamoto G, Akiyama F, Esaki Y, Sawabe M, Arai T, Hosoi T, Harada N, Younes M, Takubo K. Breast carcinoma in women over the age of 85: distinct histological pattern and androgen, oestrogen, and progesterone receptor status. Histopathology. 2003;42:120–127. doi: 10.1046/j.1365-2559.2003.01542.x. [DOI] [PubMed] [Google Scholar]
- 18.Asseryanis E, Ruecklinger E, Hellan M, Kubista E, Singer CF. Breast cancer size in postmenopausal women is correlated with body mass index and androgen serum levels. Gynecol Endocrinol. 2004;18:29–36. doi: 10.1080/09513590310001651759. [DOI] [PubMed] [Google Scholar]
- 19.Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care. 2012;35:2402–2411. doi: 10.2337/dc12-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chlebowski RT, McTiernan A, Wactawski-Wende J, Manson JE, Aragaki AK, Rohan T, Ipp E, Kaklamani VG, Vitolins M, Wallace R, Gunter M, Phillips LS, Strickler H, Margolis K, Euhus DM. Diabetes, metformin, and breast cancer in postmenopausal women. J. Clin. Oncol. 2012;30:2844–2852. doi: 10.1200/JCO.2011.39.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.