Abstract
Gastric cancer is a prevalent disease causing a high annual death rate worldwide. Recent studies suggest the pivotal regulatory role of microRNAs in gastric cancer and the aberrant expression of microRNA-141 (miR-141) in gastric cancer cells. This study aims to explore the role and possible mechanism of miR-141 in gastric cancer prognosis and cell proliferation. A total of 30 gastric cancer patients were recruited for miR-141 level detection and a follow up of 115 weeks. Human adenocarcinoma cell line AGS was transfected with miR-141 mimic or inhibitor for cell viability, colony formation and cell cycle assays. A gastric cancer mouse model was constructed by implantation of transfected AGS cells. Insulin-like growth factor 1 receptor (IGF1R) was overexpressed in AGS cells to investigate miR-141 mechanism. Results showed that miR-141 was significantly down-regulated in gastric cancer tissue (P < 0.001). The patients with lower miR-141 levels exhibited poorer prognosis. miR-141 inhibited AGS cell viability (P < 0.01), colony formation (P < 0.01) and cell cycle (P < 0.05), and the mice implanted with miR-141 mimic cells showed an obvious smaller tumor size (P < 0.01), suggesting the anti-proliferative role of miR-141. Both the phosphorylated and total IGF1R protein levels were inhibited by miR-141, while IGF1R overexpression reversed the effects of miR-141 in AGS cell proliferation. These results indicate the potential roles of miR-141 as a prognostic factor and as a therapeutic alternative for gastric cancer. Its mechanism may be associated with IGF1R, and further research is necessary for more detail information.
Keywords: Gastric cancer, miR-141, insulin-like growth factor 1 receptor, cell proliferation, prognosis
Introduction
Gastric cancer is a prevalent public health problem with high incidence and mortality. It is the second cause of cancer death following lung cancer and leads to approximate 10% of annual cancer deaths [1,2]. Gastric cancer may be attributed to obesity, tobacco and other unhealthy life habits [3]; besides, Epstein-Barr virus and Helicobacter pylori are causative microorganisms of gastric cancer, which accounts for a considerable proportion of newly detected cases every year [4,5]. Surgery remains the only curative option for gastric cancer in the short term, and adjuvant chemotherapy can improve the outcome [6], but the development of new therapies seems to come to a halt. Despite the descending trend in gastric cancer mortality [7], more intensive efforts are still required to understand the biomechanism of this disease.
Unconstrained proliferation and invasion ability is the pivotal characteristic of cancer cells, which contributes to the initiation and progression of gastric cancer as well. According to recent findings, the cell proliferation ability of gastric cancer cells can be promoted by several pro-growth factors [8] including insulin-like growth factor 1 receptor (IGF1R) whose up-regulation has also been reported in other cancer cells [9,10]. Both mRNA and protein levels including the phosphorylated forms of IGF1R are up-regulated in gastric cancer tissues [11,12], and IGF1R has become a potential target for controlling gastric cancer [13].
microRNAs are small non-coding RNAs that play fundamental roles in biological and pathological processes of various disease via their post-transcriptional regulation on key genes involved. The diagnostic and prognostic implications of several microRNAs have been revealed in gastric cancer [14,15]. microRNA-141 (miR-141), a member of the miR-200 family, is down-regulated in primary gastric cancer tissues and several human gastric cancer cell lines, and its overexpression with its precursors inhibits MGC-803 cell growth [16]. However, more research is needed to elucidate detailed roles and mechanism of miR-141 in gastric cancer.
This study aims to explore the role of miR-141 in gastric cancer prognosis and cell proliferation. A follow up of 115 weeks in 30 gastric cancer patients was performed to analyze the prognostic implications of higher and lower miR-141 level. Human gastric adenocarcinoma cell line AGS and implanted gastric cancer mouse model were employed to assess cell proliferation and tumor growth in vitro and in vivo. We also tried to analyze the possible mechanism of miR-141 regarding IGF1R in gastric cancer cell proliferation. These findings may facilitate a better understanding of microRNAs in gastric cancer.
Materials and methods
Patients and tissue samples
Altogether 30 gastric cancer patients (15 males and 15 females) hospitalized from March 2012 to March 2013 were enrolled in this study. The stage of these patients was evaluated according to the TNM Classification of Malignant Tumors (7th edition) proposed in 2010: 5 patients in stage I, 2 patients in stage II, 6 patients in stage III, and 17 patients in stage IV. The gastric cancer tissue and the adjacent normal tissue of each patient were collected during gastroscopy and stored immediately at -80°C for RNA extraction. The informed consent was obtained from all the patients before the sampling procedure, and the protocol conformed to the guidelines of the ethics committee. A follow up of 115 weeks was carried out by looking up the records or telephone inquiring. The 30 patients were divided according to the higher or lower miR-141 level in gastric cancer tissue, with no significant differences in the gender, age (60.73 ± 3.78 and 65.13 ± 2.59) or stage between groups (P > 0.05). Then the survival curve was drawn to analyze miR-141 in the prognosis of gastric cancer.
Cells
Poorly differentiated human gastric adenocarcinoma cell line AGS (ATCC, Manassas, VA) was cultured in Roswell Park Memorial Institute (RPMI)-1640 medium supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin G and 100 μg/mL streptomycin (Gibco, Carlsbad, CA) and incubated in humidified atmosphere with 5% CO2 at 37°C. Cells were passaged every 4 days.
Cell transfection
The specific mimic, inhibitor and the corresponding controls (miR controls) for hsa-miR-141 were synthesized by RiboBio (Guangzhou, China). For IGF1R overexpression, the complete coding sequence of human IGF1R (GenBank accession No. AY332722) was cloned into pcDNA3.1 vector (Thermo Scientific, Carlsbad, CA) and sequenced for verification. Blank vector were used as a control. Transfection was performed in antibiotic-free medium in 24-well plates (2 × 105 cells in each well) with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s instruction. The final concentration of miR-141 mimic, miR-141 inhibitor, miR controls and vectors was 50 nM, 200 nM, 100 nM and 2 μg/mL, respectively. At 48 h after transfection, the cells were used in the following experiments.
Cell viability assay
Cell viability was detected by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method using MTT Cell Proliferation Assay (ATCC) according to the manufacturer’s instructions. The assay was performed in 96-well plates with 5 × 103 in each well. The absorbance at 570 nm was detected by a microplate reader iMark (Bio-Rad, Hercules, CA).
Cell proliferation assay
Cell proliferation was assessed by the colony formation experiment. Briefly, cells in the logarithmic phase were digested by 0.25% Trypsin to single-cell suspension. A total of 200 cells were seeded in the pre-warmed culture medium in each dish, which were then incubated at 37°C for 2 to 3 weeks. When microscopic colonies were observed, the culture medium was discarded and the cells were washed in phosphate-buffered saline (PBS) for 2 times, fixed in 4% paraformaldehyde for 15 min and stained in Giemsa (Sigma-Aldrich, Shanghai, China) for 20 min. Then the colony number was counted.
Cell cycle assay
Cell cycle analysis was performed using the Cell Cycle Analysis Kit (GenScipt, Nanjing, China) according to the manufacturer’s instruction. Briefly, the cells were adjusted density to 1 × 106/mL and fixed in 70% ethanol at 4°C overnight. After incubated in RNase A at 37°C for 30 min, the cells were stained with propidium iodide (PI) at 4°C in dark for 30 min. Then the cells were observed at 488 nm of excitation wavelength by a flow cytometry BD FACSCanto II (BD Biosciences, San Jose, CA).
Tumor implantation in mice
BALB/c nu/nu mice (specific pathogen-free grade, 6 weeks old, Vital River Laboratories, Beijing, China) were kept with controlled temperature at 22 ± 1°C, humidity of 65% and standard diet ad libitum. The experiments on mice were approved by the ethics committee. The implantation was performed based on existed studies [17,18]. The transfected AGS cells in the logarithmic phase were digested in 0.25% Trypsin (Gibco) and adjusted to a density of 1 × 109 cells/mL, after which 0.2 mL was intraperitoneally injected to 2 mouse individuals. When the mice showed obvious abdominal distention, about 0.2 mL of the ascites was collected from each mouse and the ascites cells were cultured in RPMI-1640 medium supplemented with 10% FBS. The ascites cells post passage in the logarithmic phase were adjusted to a density of 5 × 108 cells/mL, 0.2 mL of which was then subcutaneously implanted to the back of 5 mouse individuals. The same procedures were performed with the AGS cells transfected by miR-141 mimic or miR-141 inhibitor and the corresponding control. At 1 week after the implantation, the tumor size of each group was measured.
Western blot
Protein samples of cells were extracted by M-PER Mammalian Protein Extraction Reagent (Thermo Scientific, Carlsbad, CA) and quantified by BCA Protein Assay Kit (Beyotime, Shanghai, China). The protein samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane. The membrane was first blocked in 5% skim milk at room temperature for 2 h, and then incubated in the specific primary antibodies for total IGF1R (ab39675, Abcam, Cambridge, UK), phosphate-IGF1R at Tyr-1161 (ab39398) and GAPDH (ab181602), which was used here as an internal control, overnight at 4°C. After washed in PBS, the membrane was incubated in the horse radish peroxidase-conjugated secondary antibodies at room temperature for 1 h. ECL Plus Western Blotting Substrate (Pierce, Carlsbad, CA) was used to develop positive bands on the membrane, and the software ImageJ 1.49 (National Institutes of Health, Bethesda, MD) was used to analyze the relative grey level of sample bands compared to that of GAPDH bands.
qRT-PCR
The miRNAs in gastric cancer tissue and the adjacent normal tissue were extracted by miRNeasy Mini Kit (Qiagen, Shenzhen, China). Reverse transcription was performed with the specific reverse transcription primer for hsa-miR-141-3p (5’-CTC AAC TGG TGT CGT GGA GTC GGC AAT TCA GTT GAG CCA TCT TT-3’) under the catalysis of PrimeScript Reverse Transcriptase (Takara, Dalian, China). qRT-PCR was performed on the QuantStudio 6 Flex Realtime PCR system (Applied Biosystems, Carlsbad, CA) with the specific primer for hsa-miR-141-3p (Fw: 5’-ACA CTC CAG CTG GGT AAC ACT GTC TGG TAA-3’ and Rv: 5’-TGG TGT CGT GGA GTC G-3’). Human U6 (Fw: 5’-CTC GCT TCG GCA GCA CA-3’ and Rv: 5’-AAC GCT TCA CGA ATT TGC GT-3’) was used as an internal control. Data were analyzed with the 2-ΔΔCt method.
Statistical analysis
All experiments were repeated for 3 times and results were represented as the mean ± standard deviation. Data were analyzed by SPSS 20 (IBM, New York, US). Specifically, the composition difference in the stage or gender between the two groups of patients was analyzed by Fisher’s exact test. The survival curve was assessed by the Kaplan-Meier method and the two groups were compared by the log-rank test. The statistical difference between groups in the other experiments was analyzed by F test for homogeneity of variance and then t test. P < 0.05 indicates significant differences.
Results
miR-141 is down-regulated in gastric cancer tissue
In gastric cancer patients, the miR-141 level was significantly down-regulated in cancer tissue compared to the adjacent normal tissue (P < 0.001, Figure 1A), which might imply the involvement of miR-141 in gastric cancer. The 30 patients were divided into two groups, namely, high and low, based on the miR-141 level in gastric cancer tissue, and survival curves were drawn after the 115-week follow up (Figure 1B). A total of 7 censored data were recorded, 6 in the high group and 1 in the low group. Log-rank test indicated the significant difference between the two survival curves (χ 2 = 5.844, P < 0.05). The median survival time of the high and low groups was 81 and 59 weeks, respectively. These results suggested that miR-141 was aberrantly down-regulated in gastric cancer tissue and that the lower miR-141 level might be associated with poorer prognosis in gastric cancer patients.
Figure 1.
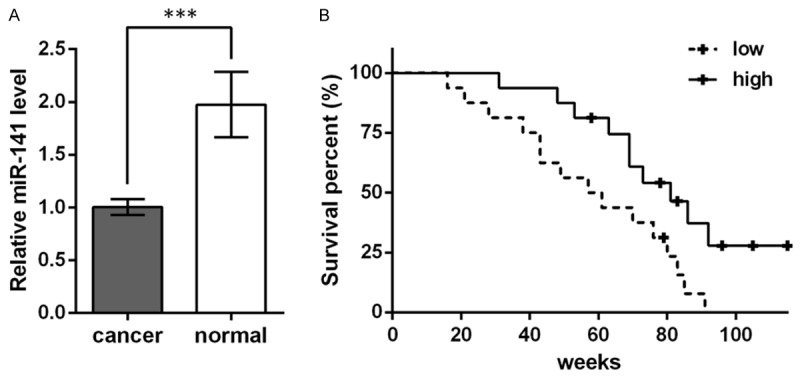
miR-141 is down-regulated in gastric cancer tissue and is associated with the prognosis of gastric cancer patients. A. Relative miR-141 level in gastric cancer tissue (cancer) and the adjacent normal tissue (normal) of 30 gastric cancer patients. U6 is used as an internal control for qRT-PCR. B. Survival curves showing the survival percent of the 30 patients. The patients are divided into two groups (high and low) based on the higher or lower miR-141 level in their gastric cancer tissue. The follow up lasts for 115 weeks. +, a censored data represents a patient loss to follow-up.
miR-141 inhibits cell proliferation in AGS cells and tumor growth in implanted mice
miR-141 level was altered by transfection of its specific mimic and inhibitor in human gastric adenocarcinoma cell line AGS, and qRT-PCR showed the successful transfection, with the mimic promoting miR-141 level and the inhibitor inhibiting miR-141 level (P < 0.01, Figure 2A). At 48 h post-transfection, MTT assay showed the decreased cell viability by the mimic (P < 0.01) and increased viability by the inhibitor (P < 0.001, Figure 2B). miR-141 mimic also decreased the colony number significantly (P < 0.01), and the inhibitor had the opposite effects (P < 0.01), as shown by colony formation assay (Figure 2C and 2D). Cell cycle changes were then detected by flow cytometry and results showed that miR-141 mimic handicapped AGS cell cycle, significantly decreasing the S-phase fraction (SPF, P < 0.01, Figure 2E and 2F) that reflects cell proliferation activity [19,20], and that the inhibitor possessed the opposite effects (P < 0.05). Taken together, miR-141 overexpression could inhibit AGS cell proliferation.
Figure 2.
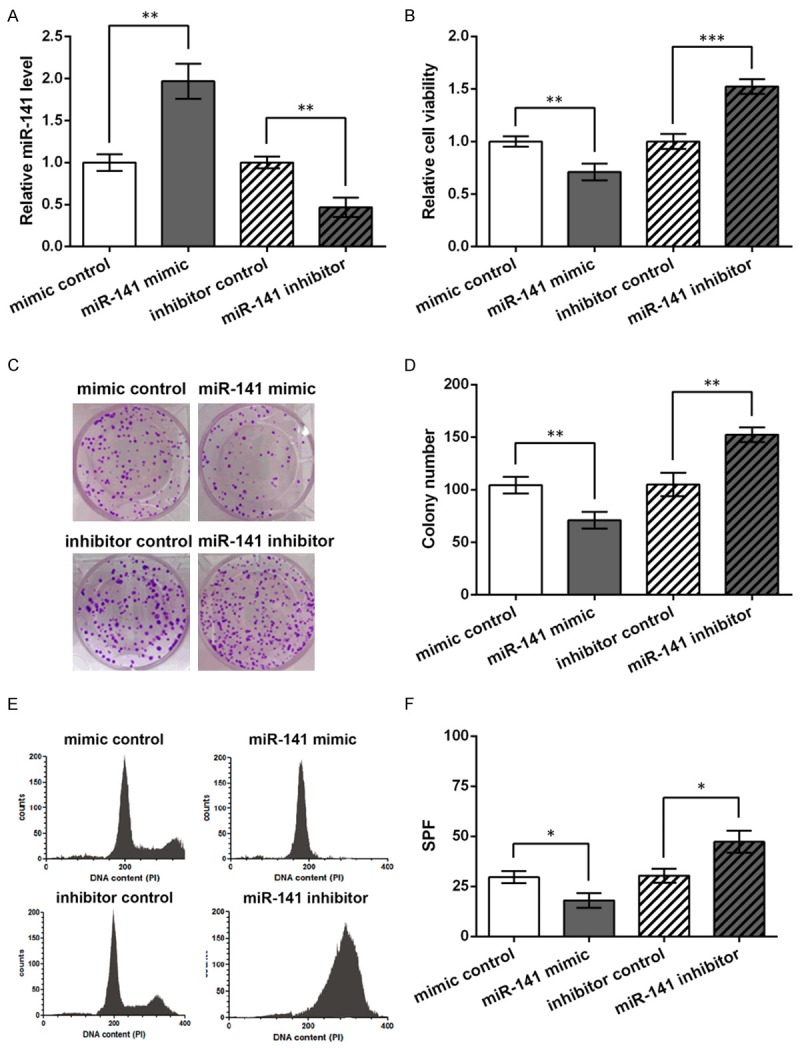
miR-141 inhibits cell proliferation in human gastric adenocarcinoma cell line AGS. AGS cells are transfected with miR-141 mimic, inhibitor or the corresponding control (mimic/inhibitor control). MTT assay, colony formation assay and flow cytometry are performed at 48 h post-transfection. A. qRT-PCT showing relative miR-141 levels in the transfected AGS cells, with U6 as an internal control. B. Relative cell viability in the transfected cells. C. Pictures of colony formation assay representing the four cell groups with 200 cells seeded in each dish. D. Colony number comparison based on the colony formation results. E. Cell cycle analyzed by flow cytometry. PI, propidium iodide. F. S-phase fraction (SPF) based on the flow cytometry results. *P < 0.05. **P < 0.01. ***P < 0.001.
Then the transfected AGS cells were implanted subcutaneously to the back of nude mice to generate tumor. At 1 week post-implantation, the tumor was excised and the tumors with miR-141 mimic were obviously smaller than the control, while the inhibitor generated much larger tumors (Figure 3A). The measurement of tumor size showed significantly differences between groups (P < 0.01, Figure 3B). These results indicated that miR-141 had the potential of inhibiting tumor growth in the implanted gastric cancer mouse model.
Figure 3.
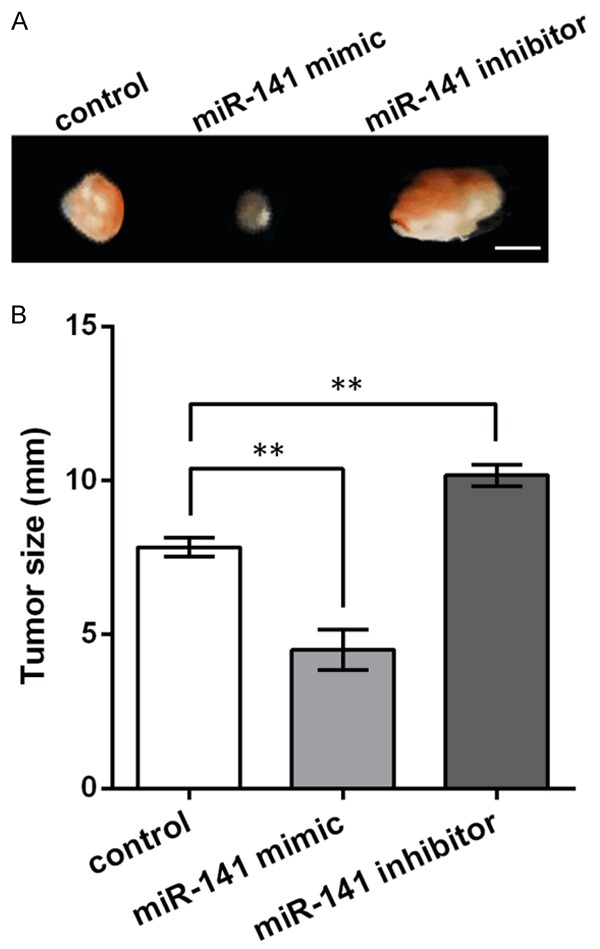
miR-141 inhibits tumor growth in the gastric cancer mouse model. A. Picture of tumors generated from the implanted mice. Bar = 5 mm. B. Histogram showing the tumor size of the implanted mice. **P < 0.01.
Effects of miR-141 in AGS cells can be abrogated by IGF1R
Existed studies have reported the promotive role of IGF1R in gastric cancer, as aforementioned. In this study, we tried to determine whether the role of miR-141 in gastric cancer cells was related to this crucial tumor promoter. IGF1R overexpression vector was transfected in the AGS cells with miR-141 mimic, and then both p-IGF1R and total IGF1R protein levels were detected by Western blot. Results showed that miR-141 mimic could inhibit both phosphorylated and total levels of IGF1R protein, and the IGF1R overexpression vector promoted the suppressed IGF1R levels in miR-141 mimic-transfected cells (Figure 4A). So next we examined whether IGF1R overexpression in the miR-141 mimic-transfected cells could reverse the effect of miR-141 on AGS cell proliferation. Colony formation assay indicated that IGF1R overexpression could again abrogate the suppressive effects of miR-141 on AGS cell proliferation, inducing a significant increase in the number of cell colonies (Figure 4B and 4C). These results implied that IGF1R might be involved in the anti-proliferative role of miR-141 in gastric cancer cells.
Figure 4.
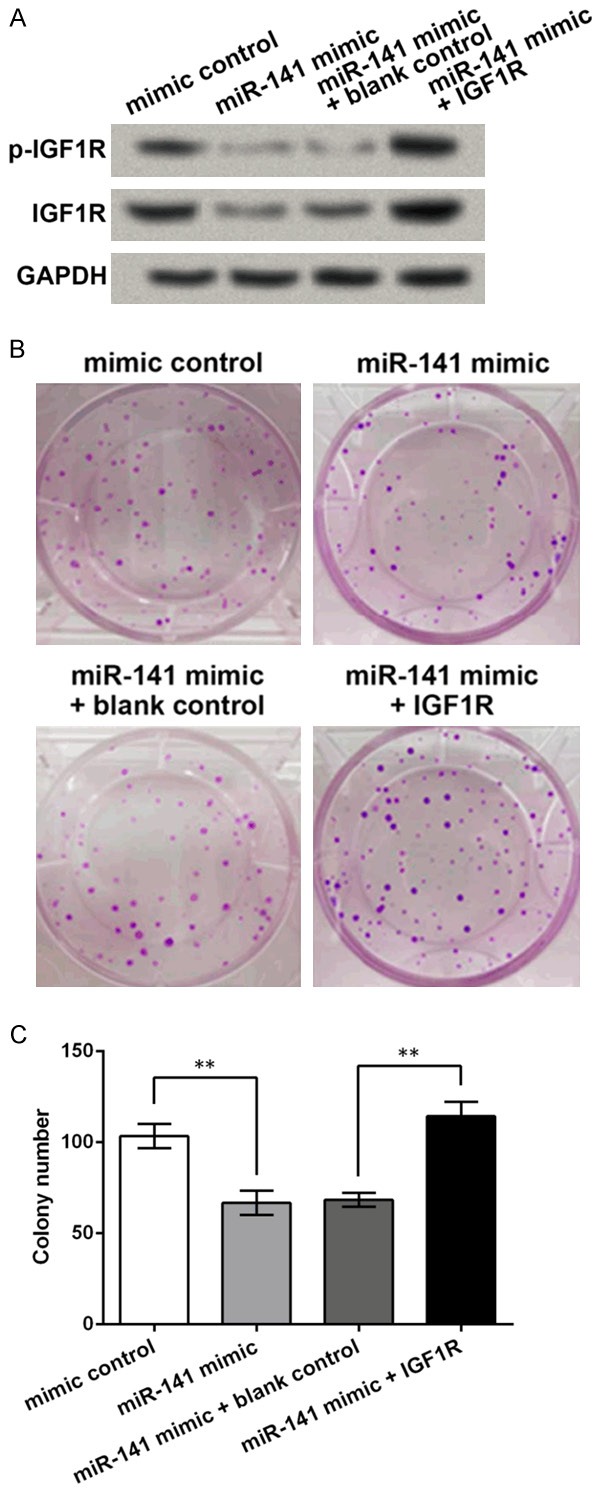
IGF1R is influenced by miR-141 and is involved in the anti-proliferative role of miR-141 in AGS cells. IGF1R overexpression vector is transfected to the AGS cells with miR-141 mimic. A. Western blot showing the levels of phosphorylated IGF1R (p-IGF1R) and total IGF1R. GAPDH is used as an internal control. B. Pictures of colony formation assay. C. Colony number comparison. **P < 0.01. IGF1R, insulin-like growth factor 1 receptor.
Discussion
In this study, miR-141 is found down-regulated in gastric cancer tissue and its lower level is associated with poorer diagnosis of gastric cancer patients. Its overexpression inhibits cell proliferation in gastric adenocarcinoma cell line AGS and represses tumor growth in gastric cancer mouse model induced by AGS implantation. IGF1R expression is influenced by miR-141 and IGF1R overexpression recovers the cell proliferative ability inhibited by miR-141.
This study followed up a total of 30 gastric cancer patients, 15 of which with relatively lower miR-141 levels showed poorer prognosis during the 115-week follow up, which implies that miR-141 may be associated with the prognosis of gastric cancer. In existed studies, circulating miR-141 is shown to be a reliable prognostic factor in colon cancer, breast cancer and bone lesions of prostate cancer [21-23]. Besides, its sibling miR-200c is elevated in the blood of gastric cancer patients and is a potential predictor of gastric cancer progression [24], while reports on miR-141 only refer to its negative correlation with different TNM stages [25]. In this study, we discovered the underlying association of miR-141 and the prognosis of gastric cancer patients, suggesting that miR-141 is a promising prognostic factor for this disease.
The down-regulated miR-141 has been reported in gastric cancer before, which is related to the regulation on cancer cell abilities. When overexpressing miR-141 in gastric cancer cell lines, researchers observed consistent results that the in vitro gastric cancer cell proliferation, migration and invasion are suppressed, and the underlying mechanisms involve diverse growth-related factors and non-coding RNAs [26-28]. Similar results are also found in renal neoplasm [29], hepatocellular carcinoma [30], among others. Together with the anti-proliferative role of miR-141 in this study, it is deduced that the roles of miR-141 in cancers are generally conserved, implying the potential of miR-141 in gastric cancer treatment.
The miR-141 regulatory network has been depicted by several studies regarding its methylation and target mRNAs, among which, however, IGF1R has not been mentioned [31,32]. Being a predictor of poor outcomes in gastric cancer patients, IGF1R promotes gastric cancer cell growth and invasion [33,34] and can be regulated by miR-7 and miR-143 in gastric cancer [35,36]. Its full activation is achieved when three tyrosines (Tyr-1135, 1131 and 1136) are in turn phosphorylated [37]. This study detected the inhibited p-IGF1R and total IGF1R protein levels when miR-141 was over-expressed, suggesting miR-141 may regulate IGF1R in AGS cells. Besides, IGF1R overexpression could reverse the effects of miR-141 on AGS cell proliferation, indicating the involvement of IGF1R in miR-141 mechanisms. Through the online database TargetScanHuman (v7.0, targetscan.org) [38], IGF1R mRNA was predicted to be a target for miR-141, which is worth the effort to be verified in our future studies.
In summary, miR-141 is a potential prognostic factor for gastric cancer. miR-141 inhibits gastric cancer cell proliferation and tumor growth, and the regulatory mechanism may be associated with IGF1R. So miR-141 provides a therapeutic strategy for gastric cancer treatment. Further investigation is necessary for the understanding of its mechanism.
Disclosure of conflict of interest
None.
References
- 1.Correa P. Gastric cancer: overview. Gastroenterol Clin North Am. 2013;42:211–217. doi: 10.1016/j.gtc.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guggenheim DE, Shah MA. Gastric cancer epidemiology and risk factors. J Surg Oncol. 2013;107:230–236. doi: 10.1002/jso.23262. [DOI] [PubMed] [Google Scholar]
- 3.Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354–362. doi: 10.3748/wjg.v12.i3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yau TO, Tang CM, Yu J. Epigenetic dysregulation in Epstein-Barr virus-associated gastric carcinoma: disease and treatments. World J Gastroenterol. 2014;20:6448–6456. doi: 10.3748/wjg.v20.i21.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamamoto K, Kato M, Takahashi M, Haneda M, Shinada K, Nishida U, Yoshida T, Sonoda N, Ono S, Nakagawa M, Mori Y, Nakagawa S, Mabe K, Shimizu Y, Moriya J, Kubota K, Matsuno Y, Shimoda T, Watanabe H, Asaka M. Clinicopathological analysis of early-stage gastric cancers detected after successful eradication of Helicobacter pylori. Helicobacter. 2011;16:210–216. doi: 10.1111/j.1523-5378.2011.00833.x. [DOI] [PubMed] [Google Scholar]
- 6.Orditura M, Galizia G, Sforza V, Gambardella V, Fabozzi A, Laterza MM, Andreozzi F, Ventriglia J, Savastano B, Mabilia A, Lieto E, Ciardiello F, De Vita F. Treatment of gastric cancer. World J Gastroenterol. 2014;20:1635–1649. doi: 10.3748/wjg.v20.i7.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferro A, Peleteiro B, Malvezzi M, Bosetti C, Bertuccio P, Levi F, Negri E, La Vecchia C, Lunet N. Worldwide trends in gastric cancer mortality (1980-2011), with predictions to 2015, and incidence by subtype. Eur J Cancer. 2014;50:1330–1344. doi: 10.1016/j.ejca.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 8.Jang BG, Kim WH. Molecular pathology of gastric carcinoma. Pathobiology. 2011;78:302–310. doi: 10.1159/000321703. [DOI] [PubMed] [Google Scholar]
- 9.Hellawell GO, Turner GD, Davies DR, Poulsom R, Brewster SF, Macaulay VM. Expression of the type 1 insulin-like growth factor receptor is up-regulated in primary prostate cancer and commonly persists in metastatic disease. Cancer Res. 2002;62:2942–2950. [PubMed] [Google Scholar]
- 10.Krueckl SL, Sikes RA, Edlund NM, Bell RH, Hurtado-Coll A, Fazli L, Gleave ME, Cox ME. Increased insulin-like growth factor I receptor expression and signaling are components of androgen-independent progression in a lineage-derived prostate cancer progression model. Cancer Res. 2004;64:8620–8629. doi: 10.1158/0008-5472.CAN-04-2446. [DOI] [PubMed] [Google Scholar]
- 11.Numata K, Oshima T, Sakamaki K, Yoshihara K, Aoyama T, Hayashi T, Yamada T, Sato T, Cho H, Shiozawa M, Yoshikawa T, Rino Y, Kunisaki C, Akaike M, Imada T, Masuda M. Clinical significance of IGF1R gene expression in patients with Stage II/III gastric cancer who receive curative surgery and adjuvant chemotherapy with S-1. J Cancer Res Clin Oncol. 2016;142:415–422. doi: 10.1007/s00432-015-2039-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng WY, Li N, Wan XB, Luo SX, Zhang YW. Phosphorylated insulin-like growth factor-1 receptor expression predicts poor prognosis of Chinese patients with gastric cancer. Med Oncol. 2014;31:141. doi: 10.1007/s12032-014-0141-2. [DOI] [PubMed] [Google Scholar]
- 13.Gong Y, Ren J, Liu K, Tang LM. Tumor suppressor role of miR-133a in gastric cancer by repressing IGF1R. World J Gastroenterol. 2015;21:2949–2958. doi: 10.3748/wjg.v21.i10.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan HW, Li SC, Tsai KW. MicroRNA Dysregulation in Gastric Cancer. Current Pharmaceutical Design. 2013;19:1273–1284. doi: 10.2174/138161213804805621. [DOI] [PubMed] [Google Scholar]
- 15.Kim BH, Hong SW, Kim A, Choi SH, Yoon SO. Prognostic implications for high expression of oncogenic microRNAs in advanced gastric carcinoma. J Surg Oncol. 2013;107:505–510. doi: 10.1002/jso.23271. [DOI] [PubMed] [Google Scholar]
- 16.Du Y, Xu Y, Ding L, Yao H, Yu H, Zhou T, Si J. Down-regulation of miR-141 in gastric cancer and its involvement in cell growth. J Gastroenterol. 2009;44:556–561. doi: 10.1007/s00535-009-0037-7. [DOI] [PubMed] [Google Scholar]
- 17.Shin VY, Wu WK, Ye YN, So WH, Koo MW, Liu ES, Luo JC, Cho CH. Nicotine promotes gastric tumor growth and neovascularization by activating extracellular signal-regulated kinase and cyclooxygenase-2. Carcinogenesis. 2004;25:2487–2495. doi: 10.1093/carcin/bgh266. [DOI] [PubMed] [Google Scholar]
- 18.Lin HL, Chiou SH, Wu CW, Lin WB, Chen LH, Yang YP, Tsai ML, Uen YH, Liou JP, Chi CW. Combretastatin A4-induced differential cytotoxicity and reduced metastatic ability by inhibition of AKT function in human gastric cancer cells. J Pharmacol Exp Ther. 2007;323:365–373. doi: 10.1124/jpet.107.124966. [DOI] [PubMed] [Google Scholar]
- 19.Holte H, Suo Z, Smeland EB, Kvaloy S, Langholm R, Stokke T. Prognostic Value of Lymphoma-specific S-Phase Fraction Compared with that of Other Cell Proliferation Markers. Acta Oncologica. 2009;38:495–503. doi: 10.1080/028418699432040. [DOI] [PubMed] [Google Scholar]
- 20.Sakamoto Y, Kato S, Takahashi M, Okada Y, Yasuda K, Watanabe G, Imai H, Sato A, Ishioka C. Contribution of autophagic cell death to p53-dependent cell death in human glioblastoma cell line SF126. Cancer Sci. 2011;102:799–807. doi: 10.1111/j.1349-7006.2011.01857.x. [DOI] [PubMed] [Google Scholar]
- 21.Navarro A, Cheng H, Zhang L, Cogdell DE, Zheng H, Schetter AJ, Nykter M, Harris CC, Chen K, Hamilton SR, Zhang W. Circulating Plasma MiR-141 Is a Novel Biomarker for Metastatic Colon Cancer and Predicts Poor Prognosis. PLoS One. 2011;6:e17745. doi: 10.1371/journal.pone.0017745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antolin S, Calvo L, Blanco-Calvo M, Santiago MP, Lorenzo-Patino MJ, Haz-Conde M, Santamarina I, Figueroa A, Anton-Aparicio LM, Valladares-Ayerbes M. Circulating miR-200c and miR-141 and outcomes in patients with breast cancer. BMC Cancer. 2015;15:297. doi: 10.1186/s12885-015-1238-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang HL, Qin XJ, Cao DL, Zhu Y, Yao XD, Zhang SL, Dai B, Ye DW. An elevated serum miR-141 level in patients with bone-metastatic prostate cancer is correlated with more bone lesions. Asian J Androl. 2013;15:231–235. doi: 10.1038/aja.2012.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valladares-Ayerbes M, Reboredo M, Medina-Villaamil V, Iglesias-Díaz P, Lorenzo-Patiño MJ, Haz M, Santamarina I, Blanco M, Fernández-Tajes J, Quindós M, Carral A, Figueroa A, Antón-Aparicio LM, Calvo L. Circulating miR-200c as a diagnostic and prognostic biomarker for gastric cancer. J Transl Med. 2012;10:186. doi: 10.1186/1479-5876-10-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou X, Wang Y, Shan B, Han J, Zhu H, Lv Y, Fan X, Sang M, Liu XD, Liu W. The downregulation of miR-200c/141 promotes ZEB1/2 expression and gastric cancer progression. Med Oncol. 2015;32:428. doi: 10.1007/s12032-014-0428-3. [DOI] [PubMed] [Google Scholar]
- 26.Chen B, Huang T, Jiang J, Lv L, Li H, Xia S. miR-141 suppresses proliferation and motility of gastric cancer cells by targeting HDGF. Mol Cell Biochem. 2014;388:211–218. doi: 10.1007/s11010-013-1912-3. [DOI] [PubMed] [Google Scholar]
- 27.Zhou X, Ji G, Ke X, Gu H, Jin W, Zhang G. MiR-141 Inhibits Gastric Cancer Proliferation by Interacting with Long Noncoding RNA MEG3 and Down-Regulating E2F3 Expression. Dig Dis Sci. 2015;60:3271–3282. doi: 10.1007/s10620-015-3782-x. [DOI] [PubMed] [Google Scholar]
- 28.Zuo QF, Zhang R, Li BS, Zhao YL, Zhuang Y, Yu T, Gong L, Li S, Xiao B, Zou QM. MicroRNA-141 inhibits tumor growth and metastasis in gastric cancer by directly targeting transcriptional co-activator with PDZ-binding motif, TAZ. Cell Death and Disease. 2015;6:e1623. doi: 10.1038/cddis.2014.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Senanayake U, Das S, Vesely P, Alzoughbi W, Fröhlich LF, Chowdhury P, Leuschner I, Hoefler G, Guertl B. miR-192, miR-194, miR-215, miR-200c and miR-141 are downregulated and their common target ACVR2B is strongly expressed in renal childhood neoplasms. Carcinogenesis. 2012;33:1014–1021. doi: 10.1093/carcin/bgs126. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Ding Y, Huang J, Wang S, Ni W, Guan J, Li Q, Zhang Y, Ding Y, Chen B, Chen L. MiR-141 Suppresses the Migration and Invasion of HCC Cells by Targeting Tiam1. PLoS One. 2014;9:e88393. doi: 10.1371/journal.pone.0088393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vrba L, Jensen TJ, Garbe JC, Heimark RL, Cress AE, Dickinson S, Stampfer MR, Futscher BW. Role for DNA methylation in the regulation of miR-200c and miR-141 expression in normal and cancer cells. PLoS One. 2010;5:e8697. doi: 10.1371/journal.pone.0008697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saha S, Choudhury J, Ain R. MicroRNA-141-3p and miR-200a-3p regulate insulin-like growth factor 2 during mouse placental development. Mol Cell Endocrinol. 2015;414:186–193. doi: 10.1016/j.mce.2015.07.030. [DOI] [PubMed] [Google Scholar]
- 33.Matsubara J, Yamada Y, Hirashima Y, Takahari D, Okita NT, Kato K, Hamaguchi T, Shirao K, Shimada Y, Shimoda T. Impact of insulin-like growth factor type 1 receptor, epidermal growth factor receptor, and HER2 expressions on outcomes of patients with gastric cancer. Clin Cancer Res. 2008;14:3022–3029. doi: 10.1158/1078-0432.CCR-07-1898. [DOI] [PubMed] [Google Scholar]
- 34.Ge J, Chen Z, Huang J, Yuan W, Den Z, Chen Z. Silencing insulin-like growth factor-1 receptor expression inhibits gastric cancer cell proliferation and invasion. Mol Med Rep. 2015;11:633–638. doi: 10.3892/mmr.2014.2746. [DOI] [PubMed] [Google Scholar]
- 35.Zhao X, Dou W, He L, Liang S, Tie J, Liu C, Li T, Lu Y, Mo P, Shi Y, Wu K, Nie Y, Fan D. MicroRNA-7 functions as an anti-metastatic microRNA in gastric cancer by targeting insulin-like growth factor-1 receptor. Oncogene. 2013;32:1363–1372. doi: 10.1038/onc.2012.156. [DOI] [PubMed] [Google Scholar]
- 36.Zhuang M, Shi Q, Zhang X, Ding Y, Shan L, Shan X, Qian J, Zhou X, Huang Z, Zhu W, Ding Y, Cheng W, Liu P, Shu Y. Involvement of miR-143 in cisplatin resistance of gastric cancer cells via targeting IGF1R and BCL2. Tumour Biol. 2015;36:2737–2745. doi: 10.1007/s13277-014-2898-5. [DOI] [PubMed] [Google Scholar]
- 37.Li W, Favelyukis S, Yang J, Zeng Y, Yu J, Gangjee A, Miller WT. Inhibition of insulin-like growth factor I receptor autophosphorylation by novel 6-5 ring-fused compounds. Biochem Pharmacol. 2004;68:145–154. doi: 10.1016/j.bcp.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 38.Agarwal V, Bell GW, Nam JW, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. Elife. 2015:4. doi: 10.7554/eLife.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]


