Abstract
Poly r(C) binding protein (PCBP) 1 or heterogeneous ribonucleoprotein (hnRNP) E1 is a RNA binding protein functional in multiple biological processes. PCBP1 has been shown to function as a tumor suppressor by negatively regulating translation of EMT inducer proteins in different cancers. Loss of PCBP1 expression or its Akt2-mediated phosphorylation at serine residue 43 has both been indicated to de-repress its regulation of EMT inducer proteins. However, its role in thyroid carcinoma has not been elucidated. Here we report that PCBP1 expression is significantly downregulated in thyroid carcinoma patients. In vitro kinase assay revealed that immunoprecipitated PCBP1 from transient or stably transfected thyroid carcinoma cells can be phosphorylated by recombinant Akt2 kinase. In situ analysis revealed that PCBP1 is a putative target of miR-490-3p, which was further confirmed by PCBP1 3’UTR-based reporter assays using the wild-type or a miR-490 seed mutant 3’UTR. The endogenous regulation of the PCBP1 3’UTR reporter by miR-490-3p could be rescued by transfection of miR-490 antagomir in WRO and BCPAP cells. Stably overexpressing PCBP1 BCPAP cells attenuated tumor formation completely as compared to empty vector overexpressing cells in xenograft assay. Cumulatively, our results indicate that PCBP1 functions as a tumor suppressor in thyroid carcinoma and that its expression is down regulated by high expression of the miR-490-3p observed in thyroid carcinoma patients.
Keywords: Poly r(C) binding protein, PCBP1, BCPAP, miR-490
Introduction
Among all endocrine malignancies, thyroid cancer is the most common, accounting for almost 90-95% of all endocrine tumors [1,2]. It is also one of the cancers with a steady increase in the incidence rate, especially in men [1]. Importantly, nearly 30% of palpable nodules observed in thyroid disorders are ultimately diagnosed as malignant [3]. Thyroid cancer is classified as papillary, follicular, medullary, or anaplastic [4]. Papillary thyroid cancer is the most common and accounts for 70% of all thyroid cancers, whereas follicular thyroid cancer accounts for 10-15% of cases [5,6]. Papillary thyroid cancers spread to lymph nodes in the neck in 50% of cases [7,8]. However, the precise mechanism(s) involved in metastatic dissemination is not well understood [9].
It has been recently shown that the RNA binding protein, heterogeneous nuclear ribonucleoprotein (hnRNP) E1 or poly r(C) binding protein (PCBP) 1 is central to maintenance of stemness in prostate cancer cells [10]. In LNCaP and DU145 prostate cancer cells, it was shown that after TGF-β treatment there is a progressive loss of PCBP1 protein expression, which in turn contributes to the enrichment and maintenance of CD44+CD24-CD133+ prostate stem cells [10]. In addition, it was shown that either loss of PCBP1 expression or phosphorylation-mediated post translational modification has a pro-tumorigenic and pro-metastatic effect in pancreatic, ovarian, breast, lung, gastric, and Burkitt lymphoma [11-17]. However, it is not clear if PCBP1 expression or its post translational modification participated in thyroid cancer pathogenesis.
In the current study, we show that loss of PCBP1 expression is commonly associated with papillary thyroid cancer and that this loss is perhaps the regulation of gene expression by miR-490-3p at the post-transcriptional level. Modulation of PCBP1 levels can affect in vivo tumorigenesis in thyroid cancer cell lines.
Materials and methods
Patient samples
Fresh-frozen and paraffin-embedded tissue specimens of papillary thyroid carcinoma and corresponding adjacent non-tumorous thyroid tissue samples were obtained from 10 Chinese patients at Tianjin Medical University General Hospital between 2014 and 2015. Only samples from those patients that did not undergo pre-operative local or systemic treatment were included in the current study. The study protocol was approved by the Institutional Review Board of Tianjin Medical University General Hospital, China. For freshly harvested samples, they were suspended in RNAlater (Life Technologies, Shanghai, China) before being snap frozen and stored in liquid nitrogen until use.
Immunohistochemistry
This part of the study was approved by the Institutional Review Board of Tianjin Medical University General Hospital. The tumor and adjacent normal tissue specimens obtained from 10 patients with papillary thyroid carcinoma were stained for PCBP1 expression (#ab-133421, Abcam, Waltham, MA, USA). The stained slides were scored as positive and negative by a pathologist blinded to tissue identity.
Cell and tissue lysis and western blot
Tissue specimens were homogenized in liquid nitrogen. Homogenized tissue specimens and cells were lysed in IP lysis buffer (Life Technologies, Shanghai, China) supplemented with Mini protease inhibitor cocktail (Roche Diagnostics, Indianapolis, USA). Whole cell lysates were resolved on a 4-20% NuPAGE gel (Life Technologies, Shanghai, China) and probed with PCBP1 antibody (#ab-133421, Abcam, Waltham, MA, USA). The blots were also probed with anti-GAPDH antibody (#ab-9485, Abcam, Waltham, MA, USA) to ensure equal loading across lanes.
Cell culture and treatment
WRO and BCAP cells were obtained from the cell and tissue bank at our center and cultured in RPMI1640 medium, containing glutamine, 5% FBS (Lonza, Germany), and penicillin/streptomycin. Cells were maintained at 37°C under a humidified atmosphere of 5% carbon dioxide.
Gene construction
The pENTR-PCBP1 plasmid was obtained from Addgene and cloned into the p3XFLAG-CMV10 vector (Sigma-Aldrich, St. Louis, MO, USA). The PCBP1 3’ UTR clone in pMirTarget was obtained from Origene. The PCBP1 3’ UTR reporter was constructed by amplifying the endogenous PCBP1 3’ UTR from the Origene. XhoI and ApaI sites were added to the 5’ and 3’ ends of the fragment during the preceding PCR reaction and cloned into the XhoI and ApaI site on the Rr-luc-6XCXCR4 Renilla luciferase vector (Addgene, Cambridge, MA, USA). To make the PCBP1 3’UTR mutant construct, site-directed mutagenesis was used to delete 77-83 region, corresponding to the hsa-miR-490-3p binding site. A firefly luciferase vector was used as transfection and normalization control in all luciferase assays. Constructs were sequence verified before being used for experiments.
Transfection
WRO and BCAP cells (4 × 104) were transiently transfected with FLAG-PCBP1 using Lipofectamine 3000 (Life Technologies, Shanghai, China). Stable clones were generated using G418 selection. For transient transfection, WRO cells were harvested 72 hours post-transfection. Stable BCAP clones overexpressing GLB1 and PCBP1 were also generated in cells that were initially transduced with firefly luciferase. For luciferase assay, BCAP and WRO cells (4 × 104) were transiently transfected with the indicated constructs using Lipofectamine 3000 (Life Technologies, Shanghai, China). Where indicated, cells were transfected with miR-490-3p antagomir (Life Technologies, Shanghai, China) along with the PCBP1 3’UTR constructs.
Luciferase assays
The dual-luciferase reporter assay system (Promega, Madison, Wisconsin, USA) was used to quantify renilla and firefly luciferase activities 96 hours post transfection. Each reporter plasmid was transfected at least twice in triplicate. Post-normalized data was represented as relative fluorescence units (RFU) ± standard deviation (SD).
In vitro kinase assay
In vitro kinase assays were performed using purified, active Akt2 enzyme (SignalChem, Richmond, Canada). Whole cell lysates for immunoprecipitation (IP) from WRO cells were prepared in IP lysis buffer (Life Technologies, Shanghai, China) containing protease inhibitors and 1 mM Na3V04. Lysates were sonicated and clarified by centrifugation at 4°C for 10 minutes in a Beckman tabletop microcentrifuge at maximum speed. Cell lysates (1000 μg) were incubated overnight at 4°C with 10 μg of either mouse anti-FLAG antibody or mouse IgG (Sigma-Aldrich, St. Louis, MO, USA) crosslinked to protein A/G beads (Pierce Crosslink IP Kit, Life Technologies, Grand Island, NY, USA), made up to a total volume of 300 μl with IP lysis buffer. The immune complexes were collected by centrifugation, washed five times with IP lysis buffer, and eluted with 10 μl of 3 × FLAG peptide. The eluant was diluted to 30 μl with 1 × kinase buffer (25 mM Tris, pH 7.5, 5 mM β-glycerolphosphate, 10 μM ATP, 2 mM dithiothreitol, 0.1 mM Na3VO4, 10 mM MgCl2). The kinase reactions were initiated by adding 200 ng of Akt2 and 0.5 μCi of [γ-32P]ATP (3000 Ci/mmol, PerkinElmer Life Sciences) in 20 μl of kinase buffer. The reactions were stopped after 15-minute incubation at 30°C by adding SDS-PAGE loading buffer and heating to 95°C for 10 minutes. The samples were resolved on a 4-12% SDS-PAGE gel and analyzed by autoradiography.
Xenograft assay
The experiments were approved by the Institutional Animal Care and Use Committee of Tianjin Medical University General Hospital. 106 BCAP cells stably overexpressing PCBP1 or GLB1 were injected subcutaneously in the hind flank of six-week-old female nude mice (n=5 per group). Mice were assessed for tumor formation weekly for 35 days using in vivo bioluminescence imaging with an IVIS Imaging System (IVIS imaging system 200, Xenogen Corporation, PerkinElmer, Waltham, USA) fitted with an ultrasensitive CCD camera. Images at day 35 were obtained along with X-ray to confirm the presence or absence of tumor.
Statistical analyses
SPSS version 20.0 (IBM Corporation, NY, USA) was used for all statistical analysis. Results are expressed as means ± SD. Statistical significance was based on two-tailed paired Student’s t-test; P<0.05 was considered to be significant.
Results
In order to evaluate PCBP1 expression level in papillary thyroid cancer, we performed immunohistochemistry and western blot by a previously validated PCBP1 antibody in 10 tumor and tumor-adjacent normal tissues. As shown in Figures 1A and 2A, robust expression of PCBP1 was detected in adjacent normal thyroid tissue. However, each tissue specimen of the 10 papillary thyroid cancers was scored as negative in the PCBP1 staining (Figure 1B), suggesting that loss of PCBP1 expression might be associated with thyroid cancer. In addition, none (2 out of 3 tested) or suppressed (1 out of 3 tested) PCBP1 expression was observed by western blot in comparison with the corresponding adjacent normal tissue specimens (Figure 2A). Cumulatively this suggested that loss of PCBP1 expression might be an underlying feature of thyroid cancer.
Figure 1.
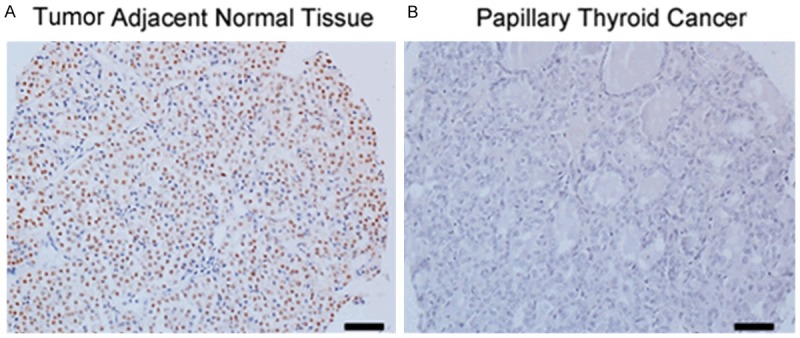
PCBP1 expression is suppressed in patients with papillary thyroid cancer. Representative immunohistochemistry images showing PCBP1 expression in papillary thyroid cancer and tumor adjacent normal tissues. Scale bar - 40 µm.
Figure 2.
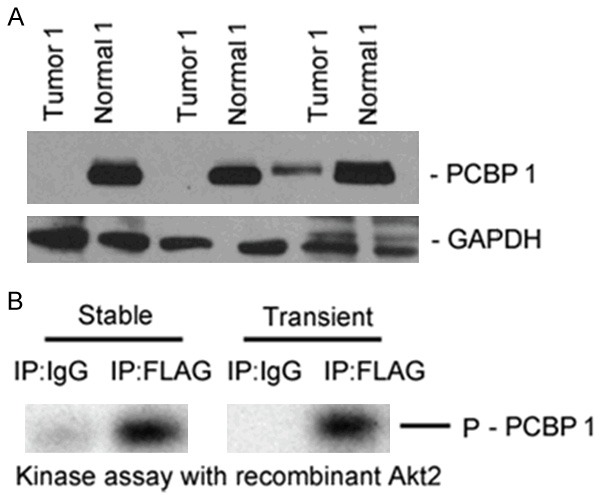
Downregulation of PCBP1 expression in papillary thyroid cancer patients seem to be widespread and its effect in thyroid cancer is perhaps independent of its post translational modification. A. Steady state expression of PCBP1 in papillary thyroid cancer and adjacent normal tissues from three different patients. The blots are representative of three independent experiments. B. In vitro kinase assay using immunoprecipitated PCBP1 from either transiently transfected or stably transfected WRO cells using recombinant Akt2 kinase showed robust PCBP1 phosphorylation.
Given that it was shown that Akt2-kinase-mediated phosphorylation of PCBP1 at serine residue 43 can induce translation of genes required for metastatic progression in the context of breast and lung cancer [12,13,15], we next tested if PCBP1 was getting phosphorylated in the thyroid cancer cell line WRO. A FLAG-PCBP1 plasmid was ectopically overexpressed in a transient or stable manner and FLAG immunoprecipitates from corresponding cell lysates were used as substrates in an in vitro kinase reaction using recombinant Akt2 as the kinase. PCBP1 showed robust in vitro Akt2-mediated phosphorylation in both transiently and stably transfected WRO cells (Figure 2B), thus indicating that post-translational modifications of PCBP1 might not be responsible for its loss in thyroid cancer tissue specimens.
To explore if the loss of PCBP1 expression was due to being targeted by miRNA, we used in situ platforms, TargetScan and microCosm, to predict putative miRNAs targeting PCBP1. One of the miRNAs that came up in both platforms was miR-490-3p (Figure 3A and data not shown). The miR-490-3p seed region in the 3’UTR of PCBP1 was conserved across different species (data not shown).
Figure 3.
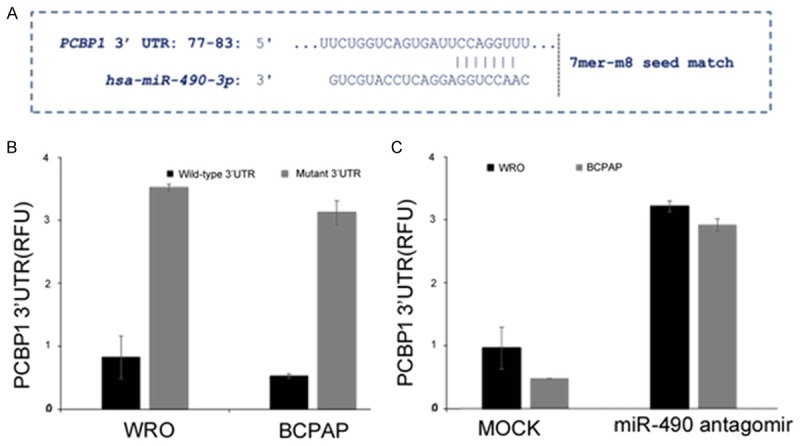
PCBP1 is a bona-fide target of miR-490-3p in the thyroid cancer cell lines, WRO and BCPAP. A. In situ prediction of complementary 7mer-m8 seed match between miR-490-3p and the 3’ UTR of PCBP1 as predicted by TargetScan software. B. Relative luciferase activity of transiently transfected luciferase reporter constructs containing full-length or mutated (miR-490 binding site deleted) PCBP1 3’ UTR in WRO and BCPAP cells. C. Relative luciferase activity of transiently transfected luciferase reporter constructs containing full-length PCBP1 3’ UTR in WRO and BCPAP cells, either mock transfected or transfected with miR-490 antagomir.
We next determined if PCBP1 is a bona fide target of miR-490-3p. To test this putative interaction, luciferase reporter constructs containing the wild-type PCBP1 3’UTR or a miR-490-3p binding mutant of the PCBP1 3’UTR reporter were transfected in WRO and BCAP cells (Figure 3B). Wild-type PCBP1 3’UTR containing reporter were inhibited 3.8 ± 0.02 folds (P=0.004) and 3.2 ± 0.05 folds (P=0.003) in WRO and BCPAP cells, respectively, compared to the miR-490-3p binding mutant reporter, which did not show any difference in the relative luciferase activity in these two cell lines (Figure 3B), confirming that PCBP1 mRNA was being targeted by the miR-490-3p in these thyroid cancer cell lines. Co-transfection of miR-490-3p antagomir also rescued the inhibition in both WRO and BCPAP cells further confirming PCBP1 as a target of miRNA-490-3p (Figure 3C).
In order to understand the in vivo role of PCBP1 in thyroid cancer formation, we finally did xenograft assays with BCPAP cells stably expressing firefly luciferase, and stably expressing either GLB1 or PCBP1; whereas, BCPAP/GLB1 cells formed steadily growing tumors by Day 35 (Figure 4A, 4B), the BCPAP/PCBP1 cells did not formed slowly regressing tumors (P<0.05) (Figure 4A, 4C). Cumulatively, this indicated that loss of PCBP1 expression function as a tumor suppressor in thyroid cancer, as revealed by lack of potent tumorigenic activity post-overexpression of PCBP1.
Figure 4.
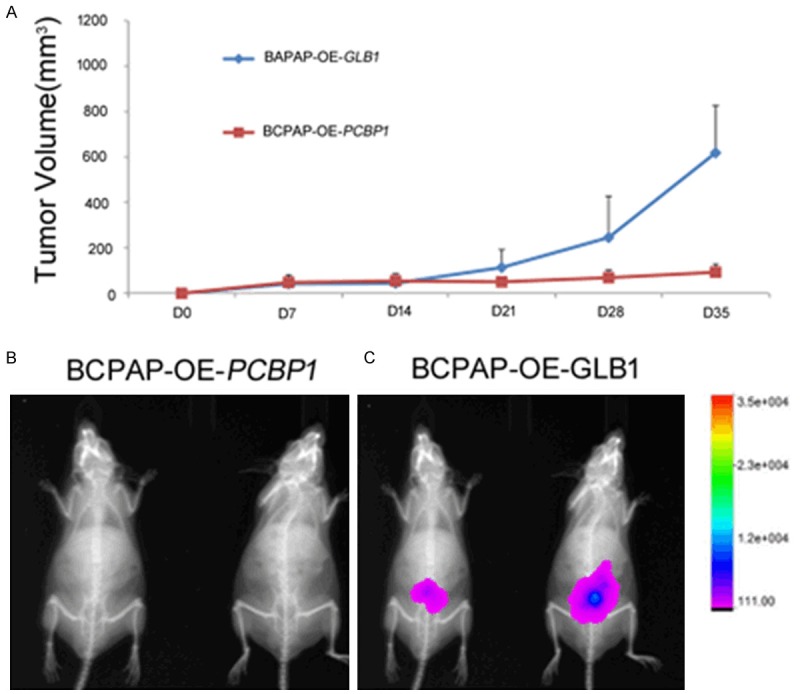
PCBP1 functions as a tumor suppressor in thyroid cancer. Luciferase expressing BCPAP stable transfectants, overexpressing either GLB1 (encoding beta galactosidase) or PCBP1, were injected sub cutanelously into nude mice. Tumor formation in the BCPAP-PCBP1 overexpressing cells-injected mice was largely attenuated as quantified by (A) tumor volume over 35 days post-injection, and (B) overlay of X-ray and in vivo luciferase imaging in representative animals in the two experimental groups.
Discussion
Our results cumulatively show that miR-490-3p targets PCBP1 in thyroid cancer resulting in its loss of expression. It remains to be determined if miR-490-3p expression is upregulated in thyroid cancer and if so by what mechanism. In addition, it also needs to be determined if loss of PCBP1 expression is an underlying feature of other types of thyroid cancers, too. Our experiments suggest a causative role of loss of PCBP1 expression in thyroid tumor formation and it will be important to determine how exactly loss of PCBP1 contributes to thyroid cancer pathogenesis.
PCBP1 has been previously shown to regulate the stability of the pro-oncogenic p63 transcript in pancreatic, ovarian and breast cancer cell line [11]. In addition, repression of PCBP1 has been shown to upregulate translation of genes and long non-coding RNA required for epithelial to mesenchymal transition and metastasis in different cancers, including breast, lung, and gastric cancer [12-16]. In addition, inactivating mutations in PCBP1 has been identified in Burkitt lymphoma [17]. Thus, it might be possible that downregulation of PCBP1 represents a central point, inhibition of which is a common mechanism to increase stemness and mesenchymal cell formation in a context dependent fashion, as observed by us in the current study and the others [11-17].
MiRNAs are evolutionarily conserved 21-23 nucleotides RNAs that regulate post-transcriptional gene expression either by blocking translation or degrading target messenger RNAs (mRNAs) and have been increasingly shown to function as tumor suppressors or oncogenes [18,19]. MiRNAs can function in both normal and transformed cells and have even been shown to play a role in metastasis [20-23].
Our findings are in line with previous experiments showing a positive role of miR-490-3p in hepatocellular carcinoma [24]. However, miR-490-3p expression has been shown to be downregulated in Ewing’s sarcoma and colon cancer [25,26]. Hence, it would appear that whether miR-490-3p functions as a tumor suppressor or oncomir is context dependent.
Acknowledgements
This study was supported by the project Mechanism and Effect of Hypothyroidism Induced by Chronic Intermittent Hypoxia (No. 2013KZ138) from the Science and Technology Foundation of Tianjin Municipal Health Bureau.
Disclosure conflict of interest
None.
References
- 1.Hodgson NC, Button J, Solorzano CC. Thyroid cancer: is the incidence still increasing? Ann Surg Oncol. 2004;11:1093–1097. doi: 10.1245/ASO.2004.03.066. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Mackenzie EJ, Mortimer RH. Thyroid nodules and thyroid cancer. Med J Aust. 2004;180:242–247. doi: 10.5694/j.1326-5377.2004.tb05894.x. [DOI] [PubMed] [Google Scholar]
- 4.Kondo T, Ezzat S, Asa SL. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer. 2006;6:292–306. doi: 10.1038/nrc1836. [DOI] [PubMed] [Google Scholar]
- 5.Fitzgibbons SC, Brams DM, Wei JP. The treatment of thyroid cancer. Am Surg. 2008;74:389–399. [PubMed] [Google Scholar]
- 6.Kitamura Y, Shimizu K, Ito K, Tanaka S, Emi M. Allelotyping of follicular thyroid carcinoma: frequent allelic losses in chromosome arms 7q, 11p, and 22q. J Clin Endocrinol Metab. 2001;86:4268–4272. doi: 10.1210/jcem.86.9.7853. [DOI] [PubMed] [Google Scholar]
- 7.Nikiforov YE. Thyroid carcinoma: molecular pathways and therapeutic targets. Mod Pathol. 2008;21(Suppl 2):S37–S43. doi: 10.1038/modpathol.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suster S. Thyroid tumors with a follicular growth pattern: problems in differential diagnosis. Arch Pathol Lab Med. 2006;130:984–988. doi: 10.5858/2006-130-984-TTWAFG. [DOI] [PubMed] [Google Scholar]
- 9.Vasko VV, Saji M. Molecular mechanisms involved in differentiated thyroid cancer invasion and metastasis. Curr Opin Oncol. 2007;19:11–17. doi: 10.1097/CCO.0b013e328011ab86. [DOI] [PubMed] [Google Scholar]
- 10.Chen Q, Cai ZK, Chen YB, Gu M, Zheng DC, Zhou J, Wang Z. Poly r(C) binding protein-1 is central to maintenance of cancer stem cells in prostate cancer cells. Cell Physiol Biochem. 2015;35:1052–1061. doi: 10.1159/000373931. [DOI] [PubMed] [Google Scholar]
- 11.Cho SJ, Jung YS, Chen X. Poly (C)-Binding Protein 1 Regulates p63 Expression through mRNA Stability. PLoS One. 2013;8:e71724. doi: 10.1371/journal.pone.0071724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaudhury A, Hussey GS, Ray PS, Jin G, Fox PL, Howe PH. TGF-beta-mediated phosphorylation of hnRNP E1 induces EMT via transcript-selective translational induction of Dab2 and ILEI. Nat Cell Biol. 2010;12:286–293. doi: 10.1038/ncb2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hussey GS, Chaudhury A, Dawson AE, Lindner DJ, Knudsen CR, Wilce MC, Merrick WC, Howe PH. Identification of an mRNP complex regulating tumorigenesis at the translational elongation step. Mol Cell. 2011;41:419–431. doi: 10.1016/j.molcel.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H, Vardy LA, Tan CP, Loo JM, Guo K, Li J, Lim SG, Zhou J, Chng WJ, Ng SB, Li HX, Zeng Q. PCBP1 suppresses the translation of metastasis-associated PRL-3 phosphatase. Cancer Cell. 2010;18:52–62. doi: 10.1016/j.ccr.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Gai L, Liu J, Cui Y, Zhang Y, Feng J. Expression of poly(C)-binding protein 1 (PCBP1) in NSCLC as a negative regulator of EMT and its clinical value. Int J Clin Exp Pathol. 2015;8:7165–7172. [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang ZZ, Shen ZY, Shen YY, Zhao EH, Wang M, Wang CJ, Cao H, Xu J. HOTAIR Long Noncoding RNA Promotes Gastric Cancer Metastasis through Suppression of Poly r(C)-Binding Protein (PCBP) 1. Mol Cancer Ther. 2015;14:1162–70. doi: 10.1158/1535-7163.MCT-14-0695. [DOI] [PubMed] [Google Scholar]
- 17.Wagener R, Aukema SM, Schlesner M, Haake A, Burkhardt B, Claviez A, Drexler HG, Hummel M, Kreuz M, Loeffler M, Rosolowski M, López C, Möller P, Richter J, Rohde M, Betts MJ, Russell RB, Bernhart SH, Hoffmann S, Rosenstiel P, Schilhabel M, Szczepanowski M, Trümper L, Klapper W, Siebert R ICGC MMML-Seq-Project; “Molecular Mechanisms in Malignant Lymphomas” Network Project of the Deutsche Krebshilfe. The PCBP1 gene encoding poly(rC) binding protein I is recurrently mutated in Burkitt lymphoma. Genes Chromosomes Cancer. 2015;54:555–564. doi: 10.1002/gcc.22268. [DOI] [PubMed] [Google Scholar]
- 18.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 20.Aleckovic M, Kang Y. Regulation of cancer metastasis by cell-free miRNAs. Biochim Biophys Acta. 2015;1855:24–42. doi: 10.1016/j.bbcan.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaur A, Jewell DA, Liang Y, Ridzon D, Moore JH, Chen C, Ambros VR, Israel MA. Characterization of microRNA expression levels and their biological correlates in human cancer cell lines. Cancer Res. 2007;67:2456–468. doi: 10.1158/0008-5472.CAN-06-2698. [DOI] [PubMed] [Google Scholar]
- 22.Kumar MS, Lu J, Mercer KL, Golub TR, Jacks T. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat Genet. 2007;39:673–677. doi: 10.1038/ng2003. [DOI] [PubMed] [Google Scholar]
- 23.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 24.Zhang LY, Liu M, Li X, Tang H. miR-490-3p modulates cell growth and epithelial to mesenchymal transition of hepatocellular carcinoma cells by targeting endoplasmic reticulum-Golgi intermediate compartment protein 3 (ERGIC3) J Biol Chem. 2013;288:4035–47. doi: 10.1074/jbc.M112.410506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamfjord J, Stangeland AM, Hughes T, Skrede ML, Tveit KM, Ikdahl T, Kure EH. Differential expression of miRNAs in colorectal cancer: comparison of paired tumor tissue and adjacent normal mucosa using high-throughput sequencing. PLoS One. 2012;7:e34150. doi: 10.1371/journal.pone.0034150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakatani F, Ferracin M, Manara MC, Ventura S, Del Monaco V, Ferrari S, Alberghini M, Grilli A, Knuutila S, Schaefer KL, Mattia G, Negrini M, Picci P, Serra M, Scotlandi K. miR-34a predicts survival of Ewing’s sarcoma patients and directly influences cell chemo-sensitivity and malignancy. J Pathol. 2012;226:796–805. doi: 10.1002/path.3007. [DOI] [PubMed] [Google Scholar]


