Abstract
Objective. The aim of this study was to investigate omega-3 fatty acid (FA) supplement use and omega-3 FAs in erythrocyte membranes [omega-3 FA % in erythrocyte membranes (RBC)] and their association with anti-CCP autoantibodies in a population without RA, but who are at genetic risk for RA.
Methods. The multicentre Studies of the Etiology of RA (SERA) cohort includes RA-free subjects who are first-degree relatives of RA probands or are enriched with the HLA-DR4 allele. In a nested case-control study, 30 SERA cases were identified who were anti-CCP2 antibody positive. We further identified 47 autoantibody negative controls, frequency matched to cases on age at study visit, sex, race and study site. Anti-CCP2 status, self-reported omega-3 FA supplement use and omega-3 FA % in RBCs were obtained from a single visit.
Results. Anti-CCP2 positive cases were less likely than controls to report omega-3 FA supplement use (odds ratio: 0.14; 95% CI 0.03, 0.68). In addition, the likelihood of anti-CCP2 positivity was inversely associated with total omega-3 FA % in RBCs (odds ratio: 0.47; 95% CI 0.24, 0.92, for a s.d. increase).
Conclusion. The inverse association between anti-CCP2 positivity and self-reported omega-3 FA supplement use and omega-3 FA % in RBCs suggests that omega-3 FAs may protect against the development of RA-related autoimmunity in pre-clinical RA.
Keywords: rheumatoid arthritis, autoantibodies, epidemiology
Rheumatology key messages
Omega-3 fatty acid supplement use is inversely associated with presence of anti-CCP2.
Increasing levels of docosahexaenoic acid in erythrocyte membranes are inversely associated with the presence of anti-CCP2.
Introduction
Emerging evidence supports a pre-clinical period of elevated RA-related autoantibodies, in the absence of clinically apparent arthritis, which precedes the development of RA [1, 2]. In particular, autoantibodies to CCP antigen second generation (anti-CCP2) are highly specific (>95%) for established RA [3], and appear ∼3–5 years prior to the onset of RA [1, 2]. Furthermore, anti-CCP2 is strongly associated with a future diagnosis of classifiable RA [4–8]. This pre-clinical phase of RA suggests that genetic and environmental factors are acting prior to the development of classifiable RA to drive the initial development of autoantibodies. Understanding factors that underlie the development of autoimmunity in preclinical RA could ultimately lead to preventive interventions.
Heritable factors are estimated to contribute to ∼50% of RA risk [9, 10], suggesting environmental factors likely account for a significant portion of the remaining risk. A number of environmental factors have been investigated and been found to have varying associations with RA [11]. Omega-3 fatty acids (omega-3 FA) are an environmental factor of interest because of their anti-inflammatory properties [12]. The omega-3 FAs eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are of particular interest, as they serve as substrates for lipid mediators actively involved in the resolution of inflammation [13]. Additionally, there is evidence that omega-3 FAs reduced the risk of the development of pre-clinical islet autoimmunity in children at risk for type 1 diabetes [14, 15], suggesting a relationship between omega-3 FAs and autoimmunity.
Humans cannot endogenously synthesize the essential omega-3 FA α-linolenic acid (ALA), or the essential omega-6 FA linoleic acid, necessitating dietary intake [16]. The omega-6 FA linoleic acid can be converted into sequentially longer-chain FAs γ-linoleic acid and arachidonic acid, while the omega-3 FA ALA can be converted into sequentially longer-chain FAs EPA, docosapentaenoic acid (DPA), and finally DHA [17]. Both omega-3 and omega-6 FA conversions compete for the same rate-limiting delta-6 desaturation enzyme [17]. Given that the conversion of longer-chain FAs from the essential FAs is inefficient at best, dietary sources of longer-chain FAs are very important [17]. The most common type of omega-3 FAs in American diets is ALA, with the longer-chain omega-3 FAs EPA and DHA being less common [18]. Dietary sources of ALA include leafy green vegetables and the oils of certain plants and nuts, such as flaxseed oil. Dietary sources of EPA and DHA, and to a lesser extent DPA, include fatty fish (e.g. salmon and mackerel) and omega-3 FA supplements.
Epidemiological studies investigating omega-3 FAs (measured by fish consumption) in the aetiology of RA have found varying results, from no observed association [19–21], to non-significant trends towards an inverse association [22, 23], to a significant inverse association [24, 25]. Additionally, a recent study found that patients with RA had lower ALA and EPA levels in erythrocyte membranes when compared with controls [26]. Evolving evidence suggests that omega-3 FAs may be important in the aetiology of RA. However, the relationship between omega-3 FAs and the earlier phases of disease development, including the presence of otherwise asymptomatic pre-clinical RA-related autoimmunity, remains unknown.
This study evaluated omega-3 FA supplement use and omega-3 FAs in erythrocyte membranes (RBCs) in relation to anti-CCP2 positivity in participants without RA, but at increased genetic risk of developing clinically apparent RA. We hypothesized that omega-3 FAs would be inversely associated with anti-CCP2 positivity in RA-free participants.
Methods
Study subjects
Subjects were included from the Studies of the Etiology of Rheumatoid Arthritis (SERA), a multicentre, prospective cohort following RA-free subjects at risk of future RA located in the USA. The SERA at-risk population consists of 1763 first-degree relatives (FDRs) of probands with RA classified by the 1987 ACR criteria [27], and 634 parents of children who participated in the Diabetes Autoimmunity Study in the Young (DAISY). FDRs were recruited from study sites located in Denver, Los Angeles, Seattle, Nebraska, Chicago and New York City. Children in Denver were recruited into DAISY based on possession of type 1 diabetes risk alleles, which include HLA-DR4; therefore, the parent population of DAISY children have a higher proportion of the RA risk allele HLA-DR4 [28, 29], making them an at-risk population. This SERA at-risk population had no evidence of RA as determined by a 68-joint examination performed by SERA study trained personnel at their initial study visit. This study was an analysis of the existing SERA cohort; study participants from this nested case–control study and the entire SERA cohort study provided informed, written consent. The SERA study protocol was approved by the following institutional review boards (IRBs) at each SERA site: Colorado Multiple IRB, University of Nebraska Medical Center IRB, Benaroya Research Institute at Virginia Mason IRB, Cedars-Sinai Medical Center’s IRB, North Shore-LIJ IRB and the Chicago Biomedicine IRB. The SERA study ethical approval covers all analyses done using SERA data.
RA-related autoantibodies were measured in SERA participants as previously described [30]. RF was measured by nephelometry (Dade Behring, Newark, DE, USA) and RF isotypes, (IgG, IgM and IgA) were measured by ELISA (Quanta Lite) kits and reported in IU/ml, following manufacturer recommendations (Inova Diagnostics, San Diego, CA, USA). Cut-offs for positivity for RF in serum (for both nephelometry and isotype ELISAs) were defined using a threshold that was higher than that observed in >95% of 490 randomly selected healthy blood donor controls from the Denver area. Anti-CCP2 was measured in serum using ELISA kits (Diastat; Axis-Shield, Dundee, UK). Positivity for anti-CCP2 was defined as ≥5 units, based on manufacturer specifications.
SERA participants were genotyped for HLA shared epitope at the Benaroya Research Institute at Virginia Mason in Seattle, as described previously [30]. DNA was screened for HLA-DR4 and HLA-DR1 positivity using specific PCR primers. The HLA-DR4 subtypes considered as shared epitope positive included DRB1*0401, *0404, *0405, *0408, *0409, *0410, *0413, *0416, *0419 and *0421. The HLA-DR1 subtypes considered as shared epitope positive included DR1*0101, *0102, *0104, *0105, *0107, *0108 and *0111. For this study, a participant is deemed shared epitope positive if they are either DR4 or DR1 subtype positive.
Nested case–control study
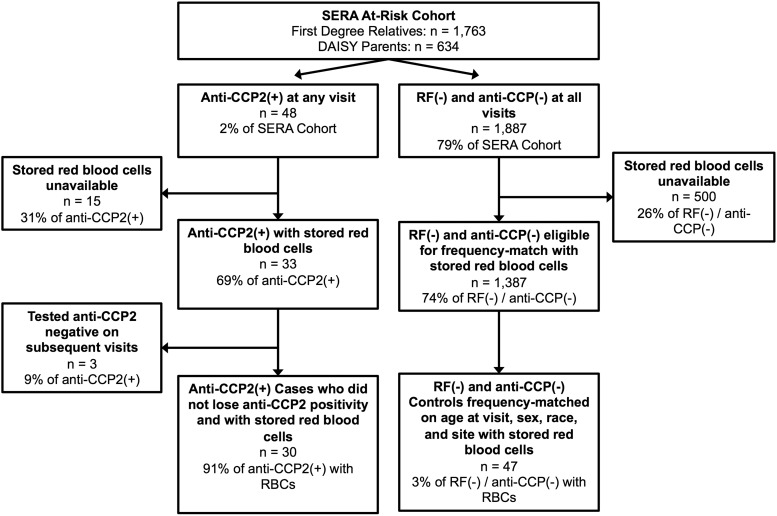
A nested case–control study design in the SERA at-risk cohort was utilized, as outlined in Fig. 1. Cases were defined as those testing anti-CCP2 positive on at least one visit, and never testing anti-CCP2 negative at subsequent visits (for participants with multiple visits), with stored RBCs available (n = 30). There were 18 anti-CCP2-positive participants who were not selected as cases because either they did not have stored RBCs available, or they lost anti-CCP2 positivity on subsequent visits. These 18 were generally similar to the 30 anti-CCP2-positive cases selected for this study with regard to mean age at visit (44 vs 46 years), sex (83% vs 70% female) and race (67% vs 60% non-Hispanic Whites). Controls were selected by identifying 1887 at-risk participants who were autoantibody negative for RF (all assays) and anti-CCP2 on all visits, of which 47 controls were identified that were always negative for anti-CCP2 and RF on all visits with stored RBCs. These 47 controls were frequency matched to cases so that the distributions of age at study visit, sex, race and site were not statistically different between the two groups, thereby controlling for these potential confounders and increasing efficiency via study design [31–33]. The selected cases and controls came from five SERA sites: Denver (n = 32), Los Angeles (n = 26), Nebraska (n = 9), Chicago (n = 9) and New York (n = 2). There was no familial relationship among and between anti-CCP2 cases and controls.
Fig. 1.
Flow diagram of the case–control study design nested within the SERA at-risk cohort
SERA: multicentre Studies of the Etiology of RA.
Omega-3 FA exposure measurement
Participant self-reported supplement use within the past year up until the date of the study visit was obtained by a standardized SERA questionnaire at the time of study visit. Supplement use variables are binary (yes or no) and are derived from the dietary supplement use question on the SERA epidemiological questionnaire that was routinely collected at each study visit. Participants were asked to report use of any supplement within the past year. If a participant reports yes to use of any supplement, they were then asked specific questions about use of fish oil, fish liver oil or omega-3 FA or use of multivitamins that contained omega-3 FAs in the past year. Additionally, another supplement variable was created that indicated use of any supplement (i.e. vitamin D, antioxidants, etc.) except omega-3 FA supplements. SERA did not collect information on dietary intake.
Percentages of omega-3 FAs in erythrocyte membranes (omega-3 FA % in RBCs) were measured in cases and controls as a biomarker of fatty acid status/exposure, as RBCs exhibit an in vivo lifespan of ∼120 days and allow one to capture the longer-term status of omega-3 FAs in the body, which corresponds to both dietary intake and physiological processes (i.e. conversion to longer-chain FAs) [34]. RBCs were separated within 30 min of blood draw, flash frozen in liquid nitrogen and stored at −70°C. In cases, the RBC sample collected at the first anti-CCP2-positive visit was assayed. In controls, the RBC sample collected from the frequency-matched age at visit sample was assayed. Selected samples were then sent to the University of Florida Analytical Toxicology Core Laboratory, where lipids were extracted for measurement of the FAs present. Samples were methylated and analysed by gas chromatography (Hewlett-Packard 6890; Agilent, Santa Clara, CA, USA) with mass spectral detection (Hewlett-Packard 5973; Agilent, Santa Clara, CA, USA), using an internal standard to monitor performance. Triplicate analysis of a composite control RBC sample yielded coefficients of variation <15% for all analytes. Fatty acids were measured as a percentage of the total lipid weight in the RBC sample [(µg FA/µg total lipid)*100]. The omega-3 FAs analysed included: 18:3 omega-3 (ALA), 20:5 omega-3 (EPA), 22:5 omega-3 (DPA) and 22:6 omega-3 (DHA), and a summed total of the aforementioned omega-3 FAs (total omega-3 FA % in RBCs). In addition, we created an omega-3 FA % in RBCs variable that summed EPA and DHA, which are the two most common types of omega-3 FA found in omega-3 FA supplements and fatty fish.
Statistical methods
The association between self-reported supplement use and anti-CCP2 case–control status was analysed using logistic regression analyses, adjusting for age at visit, sex, race, study site, current smoking status, presence of the shared epitope, reported education and reported income. Statistical adjustments of the frequency-matched variables were made as an analytical approach to capture any residual imbalance between cases and controls [31, 33]. Smoking and shared epitope were adjusted for to allow for their well-known associations with RA [35]. Adjustments for education and income were made on the basis of their potential confounding effects between omega-3 FAs and anti-CCP2.
The relationship between omega-3 FA supplement use and omega-3 FA % in RBCs, regardless of autoantibody status, was assessed using a t-test, allowing for unequal variances. If there was a significant mean difference in total omega-3 FA % in RBCs by omega-3 FA supplement use status, subsequent t-tests were performed evaluating the specific omega-3 FAs (ALA, EPA, DPA, DHA) by omega-3 FA supplement use status.
The relationship between omega-3 FA % in RBCs and anti-CCP2 case–control status was assessed using a t-test, allowing for unequal variances. If there was a significant mean difference in total omega-3 FA % in RBCs by anti-CCP2 case–control status, subsequent t-tests were performed evaluating the specific omega-3 FAs (ALA, EPA, DPA, DHA), comparing anti-CCP2 cases with controls.
Plots were created to demonstrate the mean omega-3 FA % in RBCs for ALA, EPA, DPA and DHA by omega-3 FA supplement use status, and then by anti-CCP2 case–control status. Similar plots were created for omega-6 FA % in RBCs (total omega-6 FA, LA, γ-linoleic acid and arachidonic acid) and included as supplementary Fig. S1, available at Rheumatology Online for comparison.
The distribution of omega-3 FA % in RBCs was assessed for normality. Due to skewness, both log-transformations and non-parametric tests were considered, but yielded essentially the same results as the t-tests.
Logistic regression was used to evaluate increasing levels of omega-3 FA % in RBCs and anti-CCP2 positivity, adjusting for age at visit, sex, race, site, current smoking status, shared epitope, education and income. Subsequent analyses were performed on ALA, EPA, DPA, DHA and EPA + DHA if a significant association between total omega-3 FA % in RBCs and anti-CCP2 positivity was observed. All omega-3 FA % in RBCs values were standardized (i.e. to calculate the odds ratio for a 1 s.d. increase in FA) to allow comparisons across FAs. Statistical adjustments were made based on the same rationale as outlined above.
All statistical analyses were performed using SAS software, Version 9.3 of the SAS System for Windows (Copyright, SAS Institute Inc. SAS, and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc., Cary, NC, USA). All plots were created using the ggplot2 package for R software, version 3.0.2 [36].
Results
Descriptive characteristics of the cases and controls are presented in Table 1. Anti-CCP2 cases were less likely to report using omega-3 FA supplements when compared with controls (odds ratio: 0.14, 95% CI 0.03, 0.69, P = 0.02), adjusting for age at visit, sex, race, current smoking status, shared epitope, study site, education and income (Table 2), which was not observed with use of other supplements in general (Table 2). Among the omega-3 FA supplement users, on average anti-CCP2 cases reported using omega-3 FA supplements for 4.5 months over the past year, compared with 10.25 months in controls.
Table 1.
Study population characteristics comparing anti-CCP2-positive cases with anti-CCP2-negative and RF-negative controls
| Descriptive variable | Anti-CCP2 (+) (n = 30) | Control (Ab−) (n = 47) | P-value |
|---|---|---|---|
| Age at study visit, mean (s.d.), years | 45.6 ± 16.5 | 48.6 ± 14.4 | 0.39 |
| Race, non-Hispanic Whites, n (%) | 18 (60.0) | 30 (63.8) | 0.74 |
| Gender, female, n (%) | 21 (70.0) | 35 (74.5) | 0.67 |
| Education, > High School, n (%) | 20 (66.7) | 37 (78.7) | 0.24 |
| Income, ≥$40 000 annually, n (%) | 21 (70.0) | 33 (70.2) | 0.98 |
| Study site, Denver, Colorado, n (%) | 12 (40.0) | 20 (42.6) | 0.82 |
| Shared epitope, positive, n (%) | 22 (73.3) | 31 (66.0) | 0.50 |
| Ever smoker, yes, n (%) | 8 (26.7) | 21 (44.7) | 0.11 |
| Current smoker, yes, n (%) | 2 (6.7) | 2 (4.6) | 0.64 |
Table 2.
Association between self-reported supplement use (yes vs no) and anti-CCP2 positivity
| Self-reported supplement | Anti-CCP2 (+) (n = 30) | Control (Ab−) (n = 47) | Odds ratioa (95% CI) | P-value |
|---|---|---|---|---|
| Other supplements, yes, n (%)b | 16 (53.3) | 21 (44.7) | 1.68 (0.60, 4.72) | 0.33 |
| Omega-3 FA supplement, yes, n (%) | 2 (6.7) | 16 (34.4) | 0.11 (0.02, 0.61) | 0.01 |
aAdjusted for age at visit, sex, race, site, current smoker, shared epitope, education and income. bIncludes all other dietary supplements except omega-3 FA supplements. FA: fatty acid.
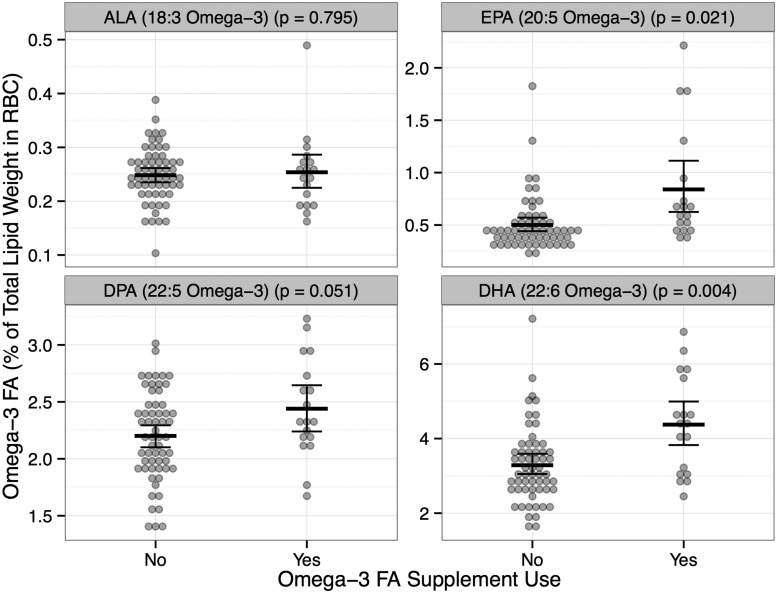
Participants who reported use of omega-3 FA supplements in the past year had significantly higher total omega-3 FA % in RBCs, compared with those who did not report use of omega-3 FA supplements (7.91% vs 6.24%, P < 0.01). Subsequent analyses of specific omega-3 FA % in RBCs found similar significant results for the longer-chain FAs EPA and DHA (Fig. 2). These results suggest that the questionnaire accurately represented whether participants were indeed using supplements containing omega-3 FAs.
Fig. 2.
Mean and 95% CIs of omega-3 FA % in RBCs for ALA, EPA, DPA and DHA by omega-3 FA supplement use status (Yes, n = 18; No, n = 59)
Dots represent individual participant omega-3 FA % in RBCs. A P < 0.05 indicates a significant difference in means between the two groups. RBC: erythrocyte membranes; ALA: omega-3 FA α-linolenic acid; EPA: omega-3 FAs eicosapentaenoic acid; DPA: docosapentaenoic acid; DHA: docosahexaenoic acid; FA: fatty acid.
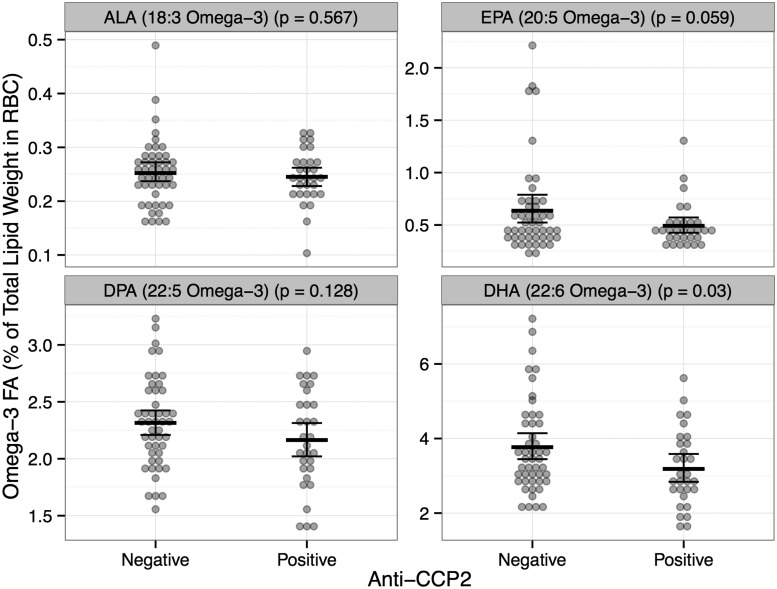
On average, total omega-3 FA % in RBCs values were significantly lower in anti-CCP2 cases compared with controls (6.09% vs 6.97% P = 0.02). Subsequent analyses of specific omega-3 FA % in RBCs found similar, but marginally significant results for the longer-chain FA EPA, and significant results for DHA (Fig. 3). While the focus of our analysis was to examine omega-3 FA % in RBCs as a biomarker of omega-3 FA supplement use, as a comparison we also present omega-6 FA % in RBCs in these subjects (supplementary Fig. S1, available at Rheumatology Online). Levels of omega-6 FA % in RBCs were not statistically different between anti-CCP2 cases and controls.
Fig. 3.
Mean and 95% CIs of omega-3 FA % in RBCs for ALA, EPA, DPA and DHA by anti-CCP2 status [anti-CCP2(+) n = 30, Control Ab(−) n = 47]
Dots represent individual participant omega-3 FA % in RBCs. P < 0.05 indicates a significant difference in means between the two groups. RBC: erythrocyte membranes; ALA: omega-3 FA α-linolenic acid; EPA: omega-3 FAs eicosapentaenoic acid; DPA: docosapentaenoic acid; DHA: docosahexaenoic acid; FA: fatty acid.
Adjusting for age at visit, sex, race, site, current smoker, shared epitope, education and income, anti-CCP2 cases were significantly more likely to have lower total omega-3 FA % in RBCs (by 1 s.d.) than controls (Table 3). Subsequent analyses of individual omega-3 FAs found that anti-CCP2 cases were significantly more likely to have lower levels of DHA and EPA + DHA, specifically, than controls.
Table 3.
Adjusted association between anti-CCP2 positivity and levels of erythrocyte membrane (RBC) omega-3 FAs, in anti-CCP2 cases (n = 30) vs controls (n = 47)
| Omega-3 FA % in RBCs | Adjusted odds ratioa (95% CI) | P-value |
|---|---|---|
| Total omega-3 FA | 0.44 (0.21, 0.92) | 0.03 |
| ALA (18:3 omega-3) | 0.87 (0.52, 1.45) | 0.59 |
| EPA (20:5 omega-3) | 0.55 (0.25, 1.21) | 0.14 |
| DPA (22:5 omega-3) | 0.65 (0.38, 1.13) | 0.13 |
| DHA (22:6 omega-3) | 0.50 (0.26, 0.97) | 0.04 |
| EPA + DHA | 0.47 (0.23, 0.97) | 0.03 |
aAdjusted for age at visit, sex, race, site, current smoker, shared epitope, education and income. The odds ratios reported are for a s.d. difference in omega-3 FA %. s.d. are 1.70 for total omega-3 FA, 0.06 for ALA, 0.58 for EPA, 2.26 for DPA, 3.54 for DHA and 4.12 for EPA + DHA. ALA: omega-3 FA α-linolenic acid; EPA: omega-3 FAs eicosapentaenoic acid; DPA: docosapentaenoic acid; DHA: docosahexaenoic acid; FA: fatty acid.
Discussion
Our results suggest an inverse association between omega-3 FAs and the RA-specific autoantibody anti-CCP2 in participants who are without RA but at an increased risk of future disease. These findings support those from other studies demonstrating that omega-3 FAs are inversely associated with RA [24–26]. Perhaps most intriguing is the demonstrated inverse association of omega-3 FAs and anti-CCP2 positivity in arthritis-free participants who are being followed prospectively through the unique SERA study, suggesting that omega-3 FAs may affect RA pathogenesis during the pre-clinical phase of disease.
Strengthening the validity of our findings are the observed associations between both self-reported omega-3 FA supplements, and a biomarker measurement of omega-3 FA % in RBCs with anti-CCP2 positivity. Furthermore, we did not observe associations between biomarkers of omega-6 FA % in RBCs and anti-CCP2 positivity, supporting our conclusions that these associations are specific to omega-3 FAs. Our variable assessing self-reported omega-3 FA supplement use within the past year is unlikely to be subject to differential recall between our cases and controls, as both groups were unaware of their anti-CCP2 status at the time they self-reported their FA supplement use. Furthermore, the omega-3 FA % in RBCs biomarker quantified a time period of ∼120 days prior to measurement. When considering both omega-3 FA supplement use and omega-3 FA % in RBCs in tandem, they provide convincing and supportive evidence that omega-3 FAs are inversely associated with anti-CCP2 positivity in arthritis-free participants who are at-risk of future RA.
Notably, we observed a strong, significant inverse association between omega-3 FA supplement use and anti-CCP2 positivity that was not observed with the use of other dietary supplements. This observation could suggest that the apparent protective effect is due to omega-3 FAs and not due to other beneficial factors associated with supplement use (i.e. a healthier lifestyle in general). It is also worth noting that we did not collect information on dietary sources of omega-3 FAs (i.e. fatty fish consumption). However, since intake of fatty fish in the USA is relatively low [37], and taking DHA and DHA/EPA supplements accounts for increases ranging from 44% to >100% in RBC FA content [38, 39], a large amount of the variability in FA status is due to supplement use. Moreover, participants who take fish oil supplements are more likely to consume more fish than those who do not take these supplements [40], where perhaps we may be underestimating the difference in FA status between our cases and controls. Therefore, accounting for dietary sources of EPA and DHA would likely not change the inverse association observed between omega-3 FA supplement use and anti-CCP2 positivity.
We took steps to guard against false discovery through the use of a priori hypotheses and a statistical approach that limits unwarranted comparisons. Based on our statistical approach, as well as the supporting evidence from two omega-3 FA measures (supplement use and omega-3 FA % in RBCs), we believe that our observed associations are not due to chance discoveries as a result of multiple comparisons, and warrant further investigation [41].
We adjusted for current smoking status rather than ever-smoking status in our models based on the hypothesis that current smoking would be a more relevant measure for the presence of anti-CCP2 in a RA-free population. Interestingly, no association was observed between smoking and anti-CCP2 positivity in our study. While smoking is a well-known risk factor for ACPA-positive RA, studies suggest that smoking may not be associated with ACPA positivity in individuals without RA [42–45]. We also note that we did not observe an association between shared epitope positivity and anti-CCP2 positivity, a result that is supported by other studies [42–46]. In aggregate, these findings suggest that smoking and shared epitope positivity may not act to initially trigger anti-CCP positivity, but will need to be explored carefully in future studies.
Anti-CCP2 was prevalent in ∼2% of the SERA at-risk RA-free cohort, which is not surprising, given that while the risk of development of RA in FDRs is increased over that of the general population [47], RA is relatively uncommon in the general population (prevalence of ∼0.5–1%) [48]. It is worth noting that this study consists of a relatively small sample of 30 anti-CCP2 cases and 47 controls. While our study had adequate power to detect the aforementioned novel associations between two different markers of omega-3 FA exposure and anti-CCP2 positivity, it is possible that due to limited power, associations of smaller magnitude may exist but were not detected. This is the first study to observe these associations in an at-risk population without RA. In order to dismiss the possibility of type 1 error and lend further evidence to this finding, it is important that this question be studied in other populations.
Furthermore, we were not able to assess other markers of inflammation, including cytokines (as we did not collect these data), nor the relationship between CRP, omega-3 FAs and anti-CCP2, as the sample size prohibited in-depth analysis of this relationship. In addition, we were unable to evaluate the relationship between omega-3 FAs and RF, as due to the case–control study design and our case definition, we had no RF-positive, anti-CCP2-negative individuals in this study. However, given our intriguing finding that omega-3 FAs appear to be protective against the presence of anti-CCP2, further exploration is needed to determine whether omega-3 FAs are associated with RF and other inflammatory markers, which could elucidate whether omega-3 FAs play a larger role in the pathogenesis of RA.
In terms of potential mechanisms explaining our findings, omega-3 FAs, and in particular DHA and EPA, are known to have important anti-inflammatory and inflammation-resolving properties [13]. In particular, DHA and EPA may suppress certain cytokines, such as IL-6, that participate in inflammatory responses that could ultimately result in activation and maturation of autoreactive B cells [49, 50]. In addition, there are also recently characterized omega-3 FA–related pro-resolution pathways, such as the generation of the EPA- and DHA-derived lipoxin, resolvin and protectin compounds [13], which could also, in part, explain these findings. These compounds are thought to actively play a role in the resolution of inflammation (e.g. inhibiting recruitment of neutrophils), and serve as bridges between the innate and adaptive immune systems (e.g. suppression of cytokines) [13]. These potential mechanisms warrant further evaluation in experimental and clinical studies that are better able to explore the specific role of omega-3 FAs in the generation of, as well as the persistence and evolution of, RA-related autoimmunity.
In summary, the results presented in this study could offer insight into a potential mechanism of action that would explain other observed relationships in which omega-3 FAs are protective against RA, perhaps through the early inhibition of RA-related autoimmunity. Future research should involve replication of our findings, and further exploration into the mechanisms that underlie the relationship between omega-3 FAs and RA in an at-risk population.
Supplementary Material
Acknowledgements
We would like to thank Kaylynn Aiona, Marie Feser and Jennifer Seifert for their help processing erythrocyte samples, and Peter DeWitt for his help plotting figures using R software. We would especially like to thank the SERA participants for their generous contributions.
Funding: This work is supported by the NIH Autoimmunity Prevention Center U19 AI050864 and U01 AI101981), the National Institutes of Health (grants R01 AR051394, M01 RR00069, M01 RR00425, K23 AR051461 and T32 AR007534), the General Clinical Research Centers Program, National Center for Research Resources, National Institutes of Health, National Center for Research Resources (grant UL1RR033176) and is now at the National Center for Advancing Translational Sciences (grant UL1TR000124), the Walter S. and Lucienne Driskill Foundation, the Research Support Fund grant from the Nebraska Medical Center and the University of Nebraska Medical Center.
Disclosure statement: The authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
- 1.Deane KD, Norris JM, Holers VM. Preclinical rheumatoid arthritis: identification, evaluation, and future directions for investigation. Rheum Dis Clin N Am 2010;36:213–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deane KD, El-Gabalawy H. Pathogenesis and prevention of rheumatic disease: focus on preclinical RA and SLE. Nat Rev Rheumatol 2014;10:212–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whiting PF. Systematic review: accuracy of anti-citrullinated peptide antibodies for diagnosing rheumatoid arthritis. Ann Intern Med 2010;152:456–64. [DOI] [PubMed] [Google Scholar]
- 4.Rantapää-Dahlqvist S, de Jong BAW, Berglin E, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum 2003;48:2741–9. [DOI] [PubMed] [Google Scholar]
- 5.Deane KD, O’Donnell CI, Hueber W, et al. The number of elevated cytokines and chemokines in preclinical seropositive rheumatoid arthritis predicts time to diagnosis in an age-dependent manner. Arthritis Rheum 2010;62:3161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nielen MMJ, van Schaardenburg D, Reesink HW, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum 2004;50:380–6. [DOI] [PubMed] [Google Scholar]
- 7.Vallbracht I, Rieber J, Oppermann M, et al. Diagnostic and clinical value of anti-cyclic citrullinated peptide antibodies compared with rheumatoid factor isotypes in rheumatoid arthritis. Ann Rheum Dis 2004;63:1079–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Venrooij WJ, van Beers JJBC, Pruijn GJM. Anti-CCP antibodies: the past, the present and the future. Nat Rev Rheumatol 2011;7:391–8. [DOI] [PubMed] [Google Scholar]
- 9.Viatte S, Plant D, Raychaudhuri S. Genetics and epigenetics of rheumatoid arthritis. Nat Rev Rheumatol 2013;9:141–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Vries R. Genetics of rheumatoid arthritis: time for a change! Curr Opin Rheumatol 2011;23:227–32. [DOI] [PubMed] [Google Scholar]
- 11.Miller FW, Alfredsson L, Costenbader KH, et al. Epidemiology of environmental exposures and human autoimmune diseases: findings from a National Institute of Environmental Health Sciences Expert Panel Workshop. J Autoimmun 2012;39:259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harbige LS. Fatty acids, the immune response, and autoimmunity: a question of n-6 essentiality and the balance between n-6 and n-3. Lipids 2003;38:323–41. [DOI] [PubMed] [Google Scholar]
- 13.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol 2008;8:349–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Norris JM, Yin X, Lamb MM, et al. Omega-3 polyunsaturated fatty acid intake and islet autoimmunity in children at increased risk for type 1 diabetes. JAMA 2007;298:1420–8. [DOI] [PubMed] [Google Scholar]
- 15.Norris JM, Kroehl M, Fingerlin TE, et al. Erythrocyte membrane docosapentaenoic acid levels are associated with islet autoimmunity: the Diabetes Autoimmunity Study in the Young. Diabetologia 2014;57:295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr 2006;83:1505S–19S. [DOI] [PubMed] [Google Scholar]
- 17.Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br J Clin Pharmacol 2013;75:645–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kris-Etherton PM, Taylor DS, Yu-Poth S, et al. Polyunsaturated fatty acids in the food chain in the United States. Am J Clin Nutr 2000;71:179S–88S. [DOI] [PubMed] [Google Scholar]
- 19.Linos A, Kaklamanis E, Kontomerkos A, et al. The effect of olive oil and fish consumption on rheumatoid arthritis—a case control study. Scand J Rheumatol 1991;20:419–26. [DOI] [PubMed] [Google Scholar]
- 20.Linos A, Kaklamani VG, Kaklamani E, et al. Dietary factors in relation to rheumatoid arthritis: a role for olive oil and cooked vegetables? Am J Clin Nutr 1999;70:1077–82. [DOI] [PubMed] [Google Scholar]
- 21.Benito-Garcia E, Feskanich D, Hu FB, et al. Protein, iron, and meat consumption and risk for rheumatoid arthritis: a prospective cohort study. Arthritis Res Ther 2007;9:R16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosell M, Wesley A-M, Rydin K, et al. Dietary fish and fish oil and the risk of rheumatoid arthritis. Epidemiology 2009;20:896–901. [DOI] [PubMed] [Google Scholar]
- 23.Pedersen M, Stripp C, Klarlund M, et al. Diet and risk of rheumatoid arthritis in a prospective cohort. J Rheumatol 2005;32:1249–52. [PubMed] [Google Scholar]
- 24.Shapiro JA, Koepsell TD, Voigt LF, et al. Diet and rheumatoid arthritis in women: a possible protective effect of fish consumption. Epidemiology 1996;7:256–63. [DOI] [PubMed] [Google Scholar]
- 25.Di Giuseppe D, Wallin A, Bottai M, et al. Long-term intake of dietary long-chain n-3 polyunsaturated fatty acids and risk of rheumatoid arthritis: a prospective cohort study of women. Ann Rheum Dis 2014;73:1949–53. [DOI] [PubMed] [Google Scholar]
- 26.Lee AL, Park Y. The association between n-3 polyunsaturated fatty acid levels in erythrocytes and the risk of rheumatoid arthritis in Korean women. Ann Nutr Metab 2013;63:88–95. [DOI] [PubMed] [Google Scholar]
- 27.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 28.Gregersen PK, Silver J, Winchester RJ. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum 1987;30:1205–13. [DOI] [PubMed] [Google Scholar]
- 29.Burton PR, Clayton DG, Cardon LR, et al. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 2007;447:661–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kolfenbach JR, Deane KD, Derber LA, et al. A prospective approach to investigating the natural history of preclinical rheumatoid arthritis (RA) using first-degree relatives of probands with RA. Arthritis Rheum 2009;61:1735–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins, 2008: 171–82.33.Stürmer T, Brenner H. Degree of matching and gain in power and efficiency in case-control studies. Epidemiology 2001;12:101–8. [DOI] [PubMed] [Google Scholar]
- 32.Friedlander Y, Merom DL, Kark JD. A comparison of different matching designs in case-control studies: an empirical example using continuous exposures, continuous confounders and incidence of myocardial infarction. Stat Med 1993;12:993–1004. [DOI] [PubMed] [Google Scholar]
- 33.Stürmer T, Brenner H. Degree of matching and gain in power and efficiency in case-control studies. Epidemiology 2001;12:101–8. [DOI] [PubMed] [Google Scholar]
- 34.Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res 2008;47:348–80. [DOI] [PubMed] [Google Scholar]
- 35.Karlson EW, Chang S-C, Cui J, et al. Gene–environment interaction between HLA-DRB1 shared epitope and heavy cigarette smoking in predicting incident rheumatoid arthritis. Ann Rheum Dis 2009;69:54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2013. http://www.R-project.org/ (11 July 2015, date last accessed) [Google Scholar]
- 37.Papanikolaou Y, Brooks J, Reider C, et al. U.S. adults are not meeting recommended levels for fish and omega-3 fatty acid intake: results of an analysis using observational data from NHANES 2003–2008. Nutr J 2014;13:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flock MR, Skulas-Ray AC, Harris WS, et al. Determinants of erythrocyte omega-3 fatty acid content in response to fish oil supplementation: a dose–response randomized controlled trial. J Am Heart Assoc 2013;2:e000513–e000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vidgren HM, Agren JJ, Schwab U, et al. Incorporation of n-3 fatty acids into plasma lipid fractions, and erythrocyte membranes and platelets during dietary supplementation with fish, fish oil, and docosahexaenoic acid-rich oil among healthy young men. Lipids 1997;32:697–705. [DOI] [PubMed] [Google Scholar]
- 40.Brustad M, Braaten T, Lund E. Predictors for cod-liver oil supplement use — the Norwegian Women and Cancer Study. Eur J Clin Nutr 2004;58:128–36. [DOI] [PubMed] [Google Scholar]
- 41.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990;1:43–6. [PubMed] [Google Scholar]
- 42.Arlestig L, Mullazehi M, Kokkonen H, et al. Antibodies against cyclic citrullinated peptides of IgG, IgA and IgM isotype and rheumatoid factor of IgM and IgA isotype are increased in unaffected members of multicase rheumatoid arthritis families from northern Sweden. Ann Rheum Dis 2012;71:825–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.El-Gabalawy HS, Robinson DB, Hart D, et al. Immunogenetic risks of anti-cyclical citrullinated peptide antibodies in a North American native population with rheumatoid arthritis and their first-degree relatives. J Rheumatol 2009;36:1130–35. [DOI] [PubMed] [Google Scholar]
- 44.Barra L, Scinocca M, Saunders S, et al. Anti-citrullinated protein antibodies in unaffected first-degree relatives of rheumatoid arthritis patients: ACPAs in first-degree relatives of RA patients. Arthritis Rheum 2013;65:1439–47. [DOI] [PubMed] [Google Scholar]
- 45.Terao C, Ohmura K, Ikari K, et al. Effects of smoking and shared epitope on the production of anti-citrullinated peptide antibody in a Japanese adult population. Arthritis Care Res 2014;66:1818–27. [DOI] [PubMed] [Google Scholar]
- 46.Svendsen AJ, Hjelmborg JV, Kyvik KO, et al. The impact of genes on the occurrence of autoantibodies in rheumatoid arthritis. A study on disease discordant twin pairs. J Autoimmun 2013;41:120–5. [DOI] [PubMed] [Google Scholar]
- 47.Hemminki K, Li X, Sundquist J, Sundquist K. Familial associations of rheumatoid arthritis with autoimmune disease and related conditions. Arthritis Rheum 2009;60:661–8. [DOI] [PubMed] [Google Scholar]
- 48.Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Arthritis Rheum 2008;58:12–25. [DOI] [PubMed] [Google Scholar]
- 49.Hughes-Austin JM, Deane KD, Derber LA, et al. Multiple cytokines and chemokines are associated with rheumatoid arthritis-related autoimmunity in first-degree relatives without rheumatoid arthritis: Studies of the Aetiology of Rheumatoid Arthritis (SERA). Ann Rheum Dis 2013;72:901–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sokolove J, Bromberg R, Deane KD, et al. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PLoS One 2012;7:e35296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.