Abstract
The behavior of rhabdoid meningiomas otherwise lacking malignant features remains unknown as most of the originally reported aggressive cases showed anaplastic histologic features independently of rhabdoid phenotype. We studied 44 patients with rhabdoid meningiomas lacking anaplastic features. Median age at diagnosis was 48.6 years (range 10–79). Location was supratentorial in 28 (63.6%), skull base in 15 (34.1%), and spinal in 1 (2.3%). Tumor grade was otherwise World Health Organization grade I (n = 22, 50%) or II (n = 22, 50%). Rhabdoid cells represented <20% of the tumor in 12 cases (27.3%), 20% to 50% in 18 (40.9%), and >50% in 14 (31.8%). Median clinical follow-up, available for 38 patients, was 5.0 years (range 0.17–14.2). Recurrence occurred in 9 patients (5-year recurrence-free survival, 73.7%) with a significantly higher risk in subtotally resected tumors (p = 0.043). Rhabdoid cell percentage was not associated with recurrence. Six patients died (4 of disease, 2 of unclear causes); 5-year overall survival was 86.7%, a mortality in excess of that expected in grade I–II meningiomas but much lower than originally reported. Review of 50 similar previously reported cases confirmed our findings. We suggest that rhabdoid meningiomas be graded analogously to nonrhabdoid tumors, with caution that some may still behave aggressively and close follow-up is recommended.
Keywords: Anaplastic meningioma, Meningioma, Rhabdoid meningioma, WHO grade
INTRODUCTION
Rhabdoid meningioma is a rare, aggressive meningioma subtype. Histologically, these tumors are characterized by sheets of loosely cohesive, plump cells with eccentric nuclei and glassy, eosinophilic inclusion-like cytoplasm. The tumor cells resemble rhabdoid cells as described in tumors at other sites, in particular the kidney, and in the atypical teratoid rhabdoid tumors in the brain. Rhabdoid meningioma was initially defined in 1998 in 2 case series by Kepes et al (1) and Perry et al (2), who described a total of 19 patients with rhabdoid meningiomas. In the series of 15 cases by Perry et al, 13 patients had tumor recurrence and 8 died, with a median time to death of 3.1 years after initial appearance of rhabdoid morphology. This led to the classification of meningiomas with rhabdoid features as anaplastic (World Health Organization [WHO] grade III), in the 2000 WHO Tumors of the Central Nervous System (3). However, independent of rhabdoid morphology, the majority of rhabdoid meningiomas described in the initial series could be classified as anaplastic/malignant (grade III), based on mitotic rate or frankly anaplastic histology. As such, the behavior of meningiomas showing rhabdoid features in the absence of other features of malignancy has not been well studied and remains largely unknown (3, 4).
In this study, we describe the clinicopathologic features of 44 patients with meningiomas that showed rhabdoid features and lacked other histologic features of malignancy. Additionally, we analyzed 50 previously reported cases with similar features among 160 previously published rhabdoid meningiomas.
MATERIALS AND METHODS
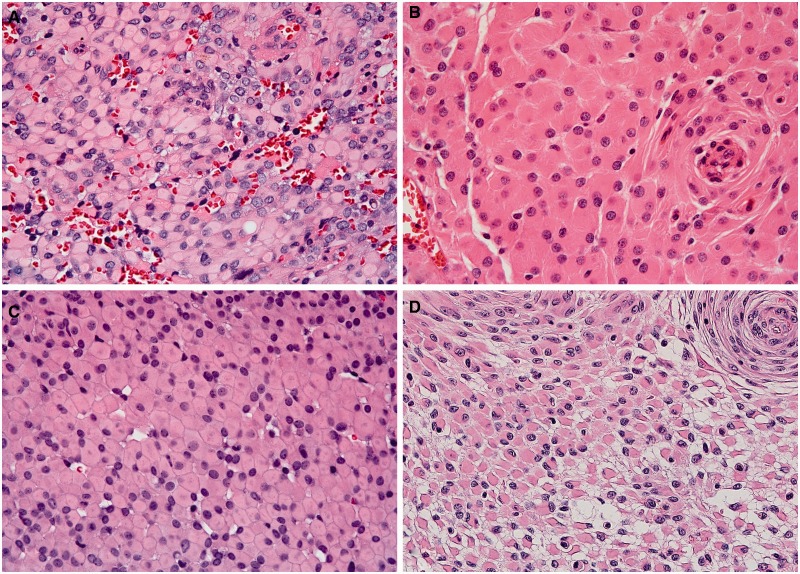
Forty-four cases of meningioma with rhabdoid features were identified in the surgical and consult archives of Mayo Clinic Rochester (15 cases), University of California San Francisco (10 cases), Washington University St. Louis (16 cases), and Johns Hopkins Hospital (3 cases) (Table 1; Supplementary Data Table 1). The hematoxylin and eosin-(H&E) stained slides were centrally reviewed by 2 neuropathologists (CG and RAV) and evaluated for the presence of rhabdoid features, characterized by plump cells with eccentric nuclei and abundant eosinophilic inclusion-like cytoplasm (Fig. 1). In 3 tumors, rhabdoid features were present only at tumor recurrence; the slides included in this study were from the first specimen displaying rhabdoid features. For each case, the percentage of the tumor with rhabdoid morphology was estimated semiquantitatively (<20%, 20%–50%, >50%). When other histological patterns were present, they were recorded.
TABLE 1.
Patient Demographics (N = 44)
| Agea (years), Median (range) | 48.6 (9.8–79.1) |
| Gender | |
| Male | 18 (40.9%) |
| Female | 26 (59.1%) |
| Tumor Site | |
| Supratentorial | 28 (63.6%) |
| Skull base | 15 (34.1%) |
| Spinal cord | 1 (2.3%) |
| Extent of Resection | |
| GTR or NTR | 26 (68.4%) |
| STR | 12 (31.6%) |
| Unknown | 6 |
| Adjuvant Therapy | |
| None | 23 (67.6%) |
| Radiation therapy | 9 (26.5%) |
| Chemotherapy (for concurrent neuroendocrine tumor) | 1 (2.9%) |
| Gamma knife | 1 (2.9%) |
| Unknown | 10 |
| Appearance of Rhabdoid Features | |
| Primary resection | 41 (93.2%) |
| Recurrence | 3 (6.8%) |
GTR, gross total resection; NTR, near total resection; STR, subtotal resection.
aAt first appearance of rhabdoid features.
FIGURE 1.
(A–D) Histologic features of rhabdoid meningioma, characterized by plump cells with eccentric nuclei and abundant eosinophilic cytoplasm. (A, case 5), (B, case 24), and (C, case 7), were predominantly rhabdoid. (D) Case 14 also had a prominent meningothelial growth pattern (hematoxylin and eosin [H&E], 400x).
The tumors were graded according to WHO criteria as grade I or II independent of the presence of rhabdoid features (3). WHO II tumors were defined by a mitotic rate of ≥ 4 mitoses per 10 high-power fields or the presence of 3 or more atypical histologic features (hypercellularity, small cell change, macronucleoli, sheet-like growth, and necrosis). Brain invasion, when present, was also noted. WHO III tumors (displaying frank anaplasia or mitotic rate ≥20 per 10 high-power fields) were excluded.
Clinical follow-up was obtained by chart review or correspondence with clinicians. Follow-up data were available in 38 of 44 cases. For the 3 patients whose tumors showed rhabdoid features at recurrence, follow-up was reported from the first appearance of rhabdoid features.
Electron microscopy studies were performed in 9 cases, 1 from tissue primarily fixed in glutaraldehyde for electron microscopy, 2 initially fixed in formalin and transferred to glutaraldehyde, and 6 from tissue that was primarily fixed in formalin and embedded in paraffin. This tissue was deparaffinized and reprocessed for electron microscopy.
Patient data were summarized with medians and ranges or frequencies and percentages, as appropriate. The percentage of the tumor with rhabdoid morphology was categorized into approximate tertiles. The risk of recurrence and death were each compared by rhabdoid percentage (<20% vs 20%–50% vs >50%), WHO grade (I vs II), tumor site (supratentorial vs skull base), and extent of resection (gross or near total vs subtotal) using log-rank tests. The recurrence-free and overall survival rates were each estimated at 2 and 5 years postdiagnosis, using the Kaplan-Meier method, along with 95% confidence intervals (CIs). The distribution of WHO grade was compared with the rhabdoid percentage categories with a Fisher exact test. P values less than 0.05 were considered significant. Analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC).
We identified 160 previously published cases of rhabdoid meningioma through an OvidMedline search followed by careful review of each paper’s bibliography, (Supplementary Data Table 2). We reviewed the cases and tabulated the histological characteristics of each tumor to the best of the information provided. However, many of the case reports lacked details regarding tumor histology, mitotic rate, or the presence of atypical features. In papers in which the WHO grade was not explicitly reported, we assigned the tumors a WHO grade based on the histologic descriptions provided. In some cases, the authors used descriptions of mitotic rate, such as “scattered” or “not conspicuous,” and we were unable to distinguish them reliably as WHO I or II. Descriptors of mitotic rate such as “frequent” or “many” were considered to be WHO II–III. Several clinical series did not provide patient-level data and were excluded from analysis (5–7). Recurrence-free and overall survival rates were estimated using the same methods as described above.
RESULTS
Histologic Features
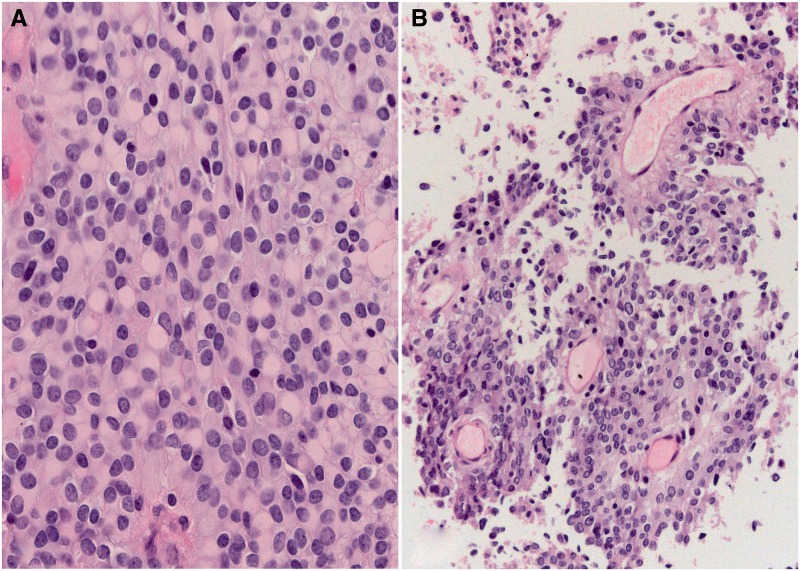
Rhabdoid features were identified in 41 initial resection specimens from the 44 cases reviewed; in the remaining 3 cases, rhabdoid morphology was present only at tumor recurrence. The extent of the rhabdoid features present within the tumor specimen was variable (Fig. 1). Rhabdoid features accounted for <20% of the examined area in 12 tumors (27.3%), 20% to 50% in 18 tumors (40.9%), and >50% in 14 tumors (31.8%) (Table 2). In addition to rhabdoid morphology, other histologic patterns were present in 36 cases. Additional growth patterns were predominantly meningothelial or transitional (in 33 cases); fibrous and metaplastic morphology were rare. In 2 tumors, papillary features were identified and comprised approximately 30% of the tumor in both (Fig. 2). Based on mitotic rate and/or the presence of atypical histology features, 22 tumors (50%) were graded as WHO I, and 22 tumors (50%) were graded as WHO II (Table 2). Brain invasion was identified in a single WHO II tumor. The extent of rhabdoid morphology did not correlate with WHO grade (p = 0.24).
TABLE 2.
Patient Outcomes by Percentage of Rhabdoid Component
| Percent Rhabdoid | All Tumors (N = 44) | <20% (N = 12, 27.3%) | 20%–50% (N = 18, 40.9%) | >50% (N = 14, 31.8%) |
|---|---|---|---|---|
| WHO Grade | ||||
| WHO I | 22 (50%) | 7 (58.3%) | 6 (33.3%) | 9 (64.3%) |
| WHO II | 22 (50%) | 5 (41.7%) | 12 (66.7%) | 5 (35.7%) |
| Tumor Recurrence | ||||
| None | 27 | 8 | 10 | 9 |
| 1 | 7 | 2 | 3 | 2 |
| ≥ 2 | 2 | 0 | 1 | 1 |
| Unknown or no follow-up | 8 | 2 | 4 | 2 |
| Death | ||||
| DOD | 4 | 1 | 2 | 1 |
| Other/unknown cause | 2 | 0 | 1 | 1 |
| Follow-up Available, N | 38 | 10 | 15 | 13 |
| Follow-up, Median (range) | 5.0 years (61 days–14.2 years) | 5.1 years (182 days–14.2 years) | 5.5 years (219 days–13.8 years) | 4.0 years (61 days–6.0 years) |
DOD, died of disease; WHO, World Health Organization.
FIGURE 2.
Composite rhabdoid-papillary meningioma. Both rhabdoid (A) and papillary features (B) were present in case 35. The tumor was otherwise a WHO grade II tumor, based on mitotic rate.
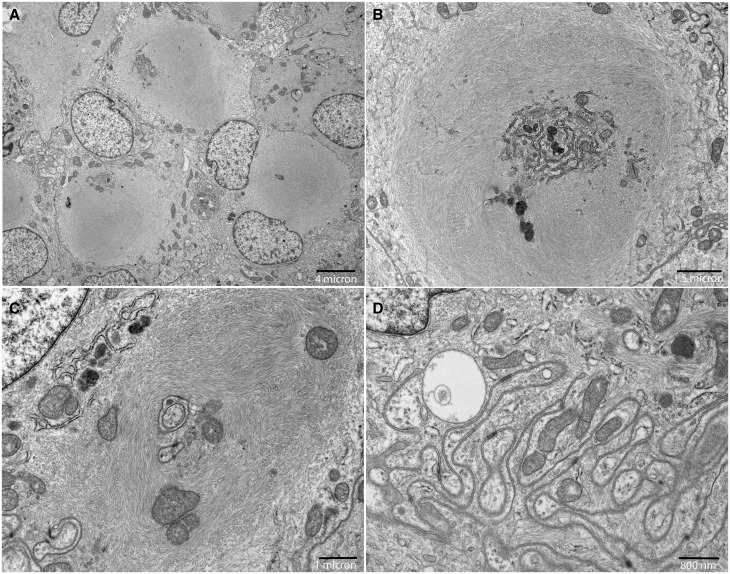
To confirm the presence of a true rhabdoid phenotype, electron microscopy was performed in a subset of 9 tumors. Seven cases demonstrated the presence of intracytoplasmic whorls of intermediate filaments, which define a true rhabdoid phenotype (Fig. 3). Electron microscopy from paraffin-embedded tissue was unsuccessful in 2 cases due to inadequate tissue preservation.
FIGURE 3.
Rhabdoid meningioma ultrastructure. (A–C) Electron microscopy was performed on glutaraldehyde-fixed tissue in case 5; the corresponding H&E section is shown in Figure 1A. Rhabdoid morphology is defined by intracytoplasmic whorls of intermediate filaments (A), which entrap cellular organelles, including endoplasmic reticulum (B) and mitochondria (C). (D) The characteristic features of meningothelial cells, including intercellular junctions formed by interdigitating cell processes, were also present.
Clinical/Therapeutic Data
The overall clinicopathologic features of the 44 cases are summarized in Table 1; individual case details are in Supplementary Data Table 1. There were 26 (59.1%) female patients and 18 (40.9%) male patients; the median age at diagnosis was 48.6 years (range 9.8–79.1). The majority of the tumors involved the supratentorial compartment (63.6%) and skull base (34.1%), with a single tumor occurring in the spinal cord (2.3%). The extent of resection was known in 38 cases; 26 (68.4%) patients underwent gross total or near total resection; subtotal resection was achieved in 12 (31.6%) cases. Nine patients (26.5%) received adjuvant radiotherapy, and 1 received chemotherapy for a concurrent neuroendocrine tumor in addition to radiotherapy.
Patient Outcome
Clinical follow-up information was available for 38 patients, with a median follow-up length of 5.0 years (range 61 days–14.2 years) after the first identification of rhabdoid features (Table 2). Overall, there were 9 tumor recurrences corresponding to a 5-year recurrence-free survival of 73.7% (95% CI: 57.8%, 89.6%). Of the 9 recurrences, 4 tumors were WHO I tumors and 5 were WHO II. Recurrences occurred in tumors that were <20% rhabdoid (n = 2), 20%–50% rhabdoid (n = 4), and >50% rhabdoid (n = 3). Recurrence-free survival was not significantly associated with the extent of rhabdoid morphology (p = 0.77) (Table 3). Similarly, recurrence-free survival was not significantly different between WHO I and WHO II tumors (5-year recurrence-free survival was 75.0% for WHO I and 71.6% for WHO II, p = 0.73). Only the extent of tumor resection correlated with recurrence-free survival, with subtotally resected tumors significantly more likely to recur (5-year recurrence-free survival 53.6% vs 84.6%, p = 0.043). Skull base tumors showed a trend toward lower recurrence-free survival when compared to supratentorial tumors but this was not significant (p = 0.08). Adjuvant radiation therapy was not associated with recurrence-free survival (p = 0.87).
TABLE 3.
Five-Year Recurrence-Free Survival
| Number of Cases | Number of Recurrences | Five-Year Recurrence-Free Survival (95% Confidence Interval) | P Valuea | |
|---|---|---|---|---|
| Overall | 44 | 9 | 73.7% (57.8%, 89.6%) | |
| Extent of Resection | ||||
| Gross or Near Total | 26 | 3 | 84.6% (68.5%, 100%) | 0.043 |
| Subtotal | 12 | 6 | 53.6% (23%, 84.2%) | |
| Tumor Siteb | ||||
| Supratentorial | 28 | 3 | 86.6% (68.4%, 100.0%) | 0.08 |
| Skull base | 15 | 5 | 61.5% (35.1%, 88.0%) | |
| Adjuvant Radiation | ||||
| Radiation | 25 | 5 | 86.7% (72.7%, 100.0%) | 0.87 |
| None | 9 | 2 | 87.5% (64.6%, 100.0%) | |
| % Rhabdoid | ||||
| < 20% | 12 | 2 | 78.8% (52.5%, 100%) | 0.77 |
| 20%–50% | 18 | 4 | 75.2% (50.6%, 99.8%) | |
| > 50% | 14 | 3 | 70.0% (41.6%, 98.4%) | |
| WHO Grade | ||||
| WHO I | 22 | 4 | 75.0% (53.7%, 96.4%) | 0.73 |
| WHO II | 22 | 5 | 71.6% (47.3%, 95.8%) |
WHO, World Health Organization.
Log-rank test.
The single spinal cord tumor was excluded from statistical analysis.
There were 6 patient deaths over the follow-up interval, corresponding to a 5-year overall survival of 86.7% (95% CI: 74.2%, 99.1%) (Table 2). Of these, 4 male patients, aged 18, 43, 53, and 56 years, died of disease. Additionally, 2 patients died of unknown causes. One patient was a 59-year-old man with no clinical follow-up; the other was a 78-year-old woman with a history of both renal cell carcinoma and insular thyroid carcinoma. There were too few deaths for formal statistical analysis; however, the deaths occurred in tumors across the range of focal to predominantly rhabdoid tumors. Disease-related deaths occurred in 3 patients with WHO II tumors and 1 with a WHO I tumor. Of note, 2 of the disease-related deaths occurred in the patients (aged 18 and 43 years) whose tumors also had papillary features.
When including only the patients in which the rhabdoid phenotype was present at first diagnosis (excluding the 3 patients whose tumors showed rhabdoid features only at recurrence), there were 35 patients with clinical follow-up. Five-year overall survival in this group was 88.5% (95% CI: 76.0%, 100.0%), and 5-year recurrence-free survival was 81.7% (95% CI: 67.0%, 96.5%). Each of the 3 patients with tumors that were rhabdoid at recurrence had additional tumor recurrences and 1 patient died.
Literature Review
Since the initial case series reports (1, 2), we identified 52 publications in the English language literature detailing 160 cases of rhabdoid meningioma (1, 2, 4–54) (Table 4; Supplementary Data Table 2). In 29 of these publications, describing a total of 50 patients, the authors either assigned the tumors a WHO grade independent of rhabdoid phenotype or reported histologic information that we considered sufficient to grade the tumors as WHO I or II. The majority of these patients (n = 35) had WHO II tumors; there were fewer WHO I lesions (n = 9). Six additional tumors were definitely in the WHO I–II range, but sufficiently detailed data were not available to distinguish between grades I or II (Table 5). Only a small number of cases contained information regarding the extent of rhabdoid features, precluding analysis of this variable. Intriguingly, a high proportion of the WHO grade I and II tumors (n = 11, 22%) was also reported to have papillary features in addition to rhabdoid morphology.
TABLE 4.
Patient Demographics of 50 Reported Cases of WHO Grade I–II Rhabdoid Meningiomas
| Agea (years), Median (range) | 36.0 (3–84) |
| Gender | |
| Male | 20 (40.0%) |
| Female | 30 (60.0%) |
| Tumor Site | |
| Supratentorial | 31 (62.0%) |
| Skull base, posterior fossa, or infratentorial | 16 (32.0%) |
| Spinal cord | 3 (6.0%) |
| Extent of Resection | |
| GTR or NTR | 32 (68.1%) |
| STR | 15 (31.9%) |
| Unknown | 3 |
| Adjuvant Therapy | |
| None | 32 (65.3%) |
| Radiation therapy | 12 (24.5%) |
| Chemotherapy | 1 (2.0%) |
| Radiation and chemotherapy | 4 (8.2%) |
| Unknown | 1 |
| Appearance of Rhabdoid Features | |
| Primary | 46 (92.0%) |
| Recurrence | 4 (8.0%) |
| Papillary Morphology | 11 (22.0%) |
GTR, gross total resection; NTR, near total resection; STR, subtotal resection.
At first appearance of rhabdoid features.
TABLE 5.
Patient Outcomes of Reported Cases of WHO Grade I–II Rhabdoid Meningiomas
| Overall (N = 50) | WHO I (N = 9) | WHO I-IIa (N = 6) | WHO II (N = 35) | |
|---|---|---|---|---|
| Tumor Recurrence | ||||
| None | 26 | 7 | 3 | 16 |
| 1 | 11 | 1 | 1 | 9 |
| ≥2 | 11 | 1 | 2 | 8 |
| Unknown | 3 | 0 | 0 | 3 |
| Death | ||||
| DOD | 9 | 2 | 1 | 6 |
| Other/unknown cause | 2 | 0 | 0 | 2 |
| Follow-up Available, N | 47 | 9 | 6 | 32 |
| Follow-up, Median (range) | 2 years (2 days–17 years) | 3.8 years (2 days–10.9 years) | 1.7 years (5 days–5.4 years) | 1.8 years (1 month–17 years) |
WHO, World Health Organization; DOD, died of disease.
aMitotic rate and/or tumor description were insufficient to differentiate WHO I vs WHO II tumors.
The overall patient characteristics in these 50 published cases of rhabdoid meningioma lacking anaplastic features were similar to our series, although the median age of 36 years (range 3–84 years) was slightly younger (Table 4). Patients received similar treatments, with a comparable proportion of gross-total resection and adjuvant therapies. Available follow-up was shorter than observed for our patients (47 patients with follow-up, median 2 years, range 2 days–17 years).
Of these 47 reported cases with follow-up, 22 patients had tumor recurrence, corresponding to a 2-year recurrence-free survival of 55.2% (95% CI: 37.4%, 73.0%) and a 5-year recurrence-free survival of 34.2% (95% CI: 14.4%, 54%). For this group, 2-year survival estimates are likely more stable as limited follow-up was available at 5 years. However, both 2- and 5-year estimates are reported for comparison to our series. Recurrence-free survival showed a trend towards association with WHO grade (p = 0.06) but no clear associations with extent of resection (p = 0.37), tumor site (p = 0.28), or presence of papillary features (p = 0.28). There were 11 total deaths in this cohort, with a 2-year overall survival of 86.8% (95% CI: 75.7%, 98.0%) and 5-year overall survival of 77.6% (95% CI: 62%, 93.3%). Nine patients died from disease, and 2 patients died of unknown or other causes (Table 5).
When we combined all reports of cases of rhabdoid meningioma that described individual patient outcomes (96 cases, including WHO III tumors), outcome was strongly correlated with WHO grade (Table 6). There were 17 patients with WHO III tumors defined by mitotic rate. Of these, 10 patients died, corresponding to a 2-year survival of 60.2% (95% CI: 34.8%, 85.5%) and 5-year survival of 34.4% (95% CI: 8.0%, 60.8%). There were 7 cases in which the histologic grade could not be reliably defined as WHO II or WHO III and 22 cases with insufficient information to reliably assign any histologic grade. These groups, respectively, had intermediate 2-year survivals of 60.3% and 53.0% and 5-year survivals of 53.3% and 53.0% (Table 6).
TABLE 6.
Five-Year Survival of All Published Cases
| WHO Grade | Number of Cases | Number of Deaths | Two-Year Survival (95% CI) | Five-Year Survival (95% CI) |
|---|---|---|---|---|
| Our series | 44 | 6 | 91.0% (81.3%, 100.0%) | 86.7% (74.2%, 99.1%) |
| I–II | 50 | 11 | 86.8% (75.7%, 98.0%) | 77.6% (62.0%, 93.3%) |
| II–IIIa | 7 | 5 | 80.0% (44.9%, 100.0%) | 53.3% (4.7%, 100.0%) |
| III | 17 | 10 | 60.2% (34.8%, 85.5%) | 34.4% (8.0%, 60.8%) |
| Unknownb | 22 | 9 | 53.0% (30.4%, 75.7%) | 53.0% (30.4%, 75.7%) |
CI, confidence interval; WHO, World Health Organization.
aMitotic rate and/or tumor description were insufficient to differentiate WHO II vs WHO III tumors.
bCases contained insufficient histologic information to reliably assign any WHO grade.
DISCUSSION
Since the initial definition of rhabdoid meningioma in 1998, the behavior of rhabdoid meningiomas lacking the histologic features of malignancy has remained an open question. According to WHO 2007 (4), “A minority of meningiomas with rhabdoid features shows this only focally and lacks other histologic features of malignancy; the behavior of these tumors remains to be determined.” A major challenge to answering this question is that rhabdoid meningioma is a rare entity. Across 4 large academic institutions, we were able to identify 44 cases of rhabdoid meningioma that lacked other features of malignancy treated over a period of 29 years. We also identified 50 additional reported cases of rhabdoid meningioma, lacking other features of malignancy published since 1998. The overall patient age in our study (median of 48.6 years) and in the published cases (median of 36.0 years) was somewhat younger than a typical meningioma case series (Tables 1 and 5). Similarly, there was a slightly higher proportion of male patients than expected in our series (40.9%) and in the published case series (40.0%). The overall patient presentations and treatment were otherwise typical of meningioma case series (Tables 1 and 5).
Histologically, the tumors in our series demonstrated a range of morphologies from tumors with focal (<20%) to predominant (>50%) rhabdoid features (Fig. 1). However, this percentage did not correlate with recurrence-free survival (p = 0.77, Table 3), suggesting that the extent of rhabdoid features is not an independent risk factor for tumor recurrence. Similarly, the extent of rhabdoid features was not associated with WHO grade (Table 2). In our series, only extent of tumor resection had a significant effect on recurrence-free survival (p = 0.043, Table 3).
Within our series of 44 patients, 9 patients had tumor recurrence, corresponding to a 5-year recurrence-free survival of 73.7% (95% CI: 57.8%, 89.6%). In contrast, within the 50 published WHO I–II cases (Table 5), there were 22 tumor recurrences, corresponding to a 5-year recurrence-free survival of 34.2% (95% CI: 14.4%, 54%). This lower recurrence-free survival may in part reflect a bias toward publication of more aggressive cases. Consistently, the previously published cohort of patients also contained a higher proportion of tumors that were WHO II (70% compared to 50% in our series), as well as a high proportion (22%) of tumors with papillary features, another aggressive variant classified as WHO III (3). However, the presence of papillary morphology in these cases was not associated with lower recurrence-free survival (p = 0.28). Within our series of 44 cases, there were 6 patient deaths (Table 2), corresponding to a 5-year overall survival of 86.7% (95% CI: 74.2%, 99.1%). This was comparable to, although slightly higher than, what was observed in our analysis of the published WHO I–II tumors with similar features. In the latter group there were 11 patient deaths (Table 5), corresponding to a 5-year overall survival of 77.6% (95% CI: 62%, 93.3%).
Among the original 15 cases reported by Perry et al (2), 13 patients had tumor recurrence and 8 died, with a median time to death of 3.1 years (range 10 months–10.7 years) after appearance of rhabdoid morphology. Of the 15 tumors, 8 were overtly malignant (WHO III), independent of rhabdoid morphology, 4 were WHO II, and 3 were WHO I at the initial identification of rhabdoid features. Irrespective of rhabdoid morphology, it is likely that the presence of high mitotic rate and other features of malignancy had a significant impact on tumor outcome in this initial series. In our review of the published literature of rhabdoid meningiomas, we identified 17 tumors that were WHO III based on mitotic rate and independent of rhabdoid morphology (Table 6). These tumors behaved extremely aggressively, with 5-year survival of 34.4%. An additional group of 22 similar tumors of unclear histologic grade had a 5-year survival of 53%, comparable to the original series (2).
Our case series and review of the literature suggest that most meningiomas that have rhabdoid features and lack other features of malignancy (WHO I or II) are not as aggressive as rhabdoid meningiomas with independent histological features consistent with WHO grade III. Given the small number of patient deaths in our series, we were unable to perform a formal statistical analysis. However, the 6 deaths in our case series and 11 deaths in the reviewed literature are more than expected in a meningioma series of comparable grade (55). Although the published literature may be biased toward publication of recurrent/more aggressive cases, even in our series, the overall mortality data are higher than expected for a similar cohort of patients with WHO I-–II tumors of typical meningothelial morphologies. This suggests that a subset of these tumors may still behave aggressively, even when lacking other features of malignancy. Although the median follow-up in our series (5.0 years) is longer than the median time to death in the initial series (1, 2), it is also possible that a longer follow-up period might have revealed additional tumors with aggressive behavior.
At present, there are no histologic features that can identify the subset of tumors with an aggressive clinical course. Therefore, we suggest that rhabdoid meningiomas be graded similarly to nonrhabdoid meningiomas but with an additional diagnosis comment that a subset of the otherwise grade I and II tumors will behave aggressively. We therefore recommend close clinical follow-up. While it may be the most exhaustive study to date, our case series still contains a relatively small number of cases that were examined retrospectively. Additional studies will be necessary to clarify the significance of rhabdoid morphology.
Supplementary Material
Acknowledgments
This study was supported by funds from the Mayo Clinic Department of Laboratory Medicine and Pathology, Rochester MN. Dr. Giannini was partially supported by 5P50CA108961 from the National Institutes of Heath. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NIH. The authors have no duality or conflicts of interest to declare. Supplementary Data can be found at http://www.jnen.oxfordjournals.org.
REFERENCES
- 1.Kepes JJ, Moral LA, Wilkinson SB, et al. Rhabdoid transformation of tumor cells in meningiomas: A histologic indication of increased proliferative activity: Report of four cases. Am J Surg Pathol 1998;22:231–8 [DOI] [PubMed] [Google Scholar]
- 2.Perry A, Scheithauer BW, Stafford SL, et al. “Rhabdoid” meningioma: An aggressive variant. Am J Surg Pathol 1998;22:1482–90 [DOI] [PubMed] [Google Scholar]
- 3.Louis DN, Scheithauer BW, Budka H, et al. Meningiomas In: Kleihues P, Cavenee WK, eds. WHO Pathology and Genetics of Tumours of the Nervous System. Lyon: IARC Press; 2000:176–83 [Google Scholar]
- 4.Perry A, Louis DN, Scheithauer BW, et al. Meningiomas In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. eds. WHO Classification of Tumours of the Central Nervous System. Lyon: IACR Press; 2007:163–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim EY, Weon YC, Kim ST, et al. Rhabdoid meningioma: Clinical features and MR imaging findings in 15 patients. AJNR Am J Neuroradiol 2007;28:1462–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ko KW, Nam DH, Kong DS, et al. Relationship between malignant subtypes of meningioma and clinical outcome. J Clin Neurosci 2007;14:747–53 [DOI] [PubMed] [Google Scholar]
- 7.Kwon MJ, Sung CO, Kang SY, et al. Differential expression of extracellular matrix-related genes in rare variants of meningioma. Hum Pathol 2013;44:260–8 [DOI] [PubMed] [Google Scholar]
- 8.Abolfotoh M, Tavanaiepour D, Hong C, et al. Primary calcified rhabdoid meningioma of the cranio-cervical junction: A case report and review of literature. J Craniovertebr Junction Spine 2012;3:32–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bleggi-Torres LF, Reis-Filho JS, Faoro LN, et al. April 2001: A 70-year-old woman with recurrent meningioma. Brain Pathol 2001;11:481–2;87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hojo H, Abe M. Rhabdoid papillary meningioma. Am J Surg Pathol 2001;25:964–9 [DOI] [PubMed] [Google Scholar]
- 11.Kesavan S. Meningioma with rhabdoid transformation. Singapore Med J 2000;41:464–7 [PubMed] [Google Scholar]
- 12.Saito A, Nakazato Y, Yoshii Y, et al. Anaplastic meningioma with papillary, rhabdoid, and epithelial features: A case report. Brain Tumor Pathol 2001;18:155–9 [DOI] [PubMed] [Google Scholar]
- 13.Bannykh SI, Perry A, Powell HC, et al. Malignant rhabdoid meningioma arising in the setting of preexisting ganglioglioma: A diagnosis supported by fluorescence in situ hybridization. Case report. J Neurosurg 2002;97:1450–5 [DOI] [PubMed] [Google Scholar]
- 14.Jansen JC, Turner J, Sheehy J, et al. Recurrent ehabdoid meningioma: Case report. Skull Base 2003;13:51–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirby PA. Rhabdoid meningioma: Intraoperative diagnosis using smear preparation. Diagn Cytopathol 2003;29:292–6 [DOI] [PubMed] [Google Scholar]
- 16.Cooper WA, Shingde M, Lee VK, et al. “Rhabdoid meningioma” lacking malignant features. Report of two cases. Clin Neuropathol 2004;23:16–20 [PubMed] [Google Scholar]
- 17.Endo K, Tanaka S, Komagata M, et al. Rhabdoid transformation of recurrent meningioma in the cervical cord: A case report. J Orthop Sci 2004;9:323–6 [DOI] [PubMed] [Google Scholar]
- 18.Parwani AV, Mikolaenko I, Eberhart CG, et al. Rhabdoid meningioma: Cytopathologic findings in cerebrospinal fluid. Diagn Cytopathol 2003;29:297–9 [DOI] [PubMed] [Google Scholar]
- 19.Koenig MA, Geocadin RG, Kulesza P, et al. Rhabdoid meningioma occurring in an unrelated resection cavity with leptomeningeal carcinomatosis. Case report. J Neurosurg 2005;102:371–5 [DOI] [PubMed] [Google Scholar]
- 20.Rittierodt M, Tschernig T, Samii M, et al. Evidence of recurrent atypical meningioma with rhabdoid transformation and expression of pyrogenic cytokines in a child presenting with a marked acute-phase response: Case report and review of the literature. J Neuroimmunol 2001;120:129–37 [DOI] [PubMed] [Google Scholar]
- 21.Xiao GQ, Burstein DE. Cytologic findings of rhabdoid meningioma in cerebrospinal fluid. Acta Cytol 2008;52:118–9 [DOI] [PubMed] [Google Scholar]
- 22.Al-Habib A, Lach B, Al Khani A. Intracerebral rhabdoid and papillary meningioma with leptomeningeal spread and rapid clinical progression. Clin Neuropathol 2005;24:1–7 [PubMed] [Google Scholar]
- 23.Batoroev YK, Nguyen GK. Rhabdoid meningioma diagnosed by imprint cytology. Acta Cytol 2005;49:464–5 [PubMed] [Google Scholar]
- 24.Mawrin C, Hahne R, Scherlach C, et al. June 2004: A male in his late 60s with recurrent extracerebral tumor. Brain Pathol 2004;14:457–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nozza P, Raso A, Rossi A, et al. Rhabdoid meningioma of the tentorium with expression of desmin in a 12-year-old Turner syndrome patient. Acta Neuropathol 2005;110:205–6 [DOI] [PubMed] [Google Scholar]
- 26.Wakabayashi K, Suzuki N, Mori F, et al. Rhabdoid cystic papillary meningioma with diffuse subarachnoid dissemination. Acta Neuropathol 2005;110:196–8 [DOI] [PubMed] [Google Scholar]
- 27.Chaturvedi S, Dua R, Singhal S, et al. Rhabdoid meningioma with cranial nerve involvement: Case report of a child. Clin Neuropathol 2008;27:248–51 [DOI] [PubMed] [Google Scholar]
- 28.Han JH, Seol HJ, Kim DG, et al. A Case of Rhabdoid Meningioma. J Korean Neurosurg Soc 2006;39:144–47 [Google Scholar]
- 29.Martinez-Lage JF, Ferri Niguez B, Sola J, et al. Rhabdoid meningioma: A new subtype of malignant meningioma also apt to occur in children. Childs Nerv Syst 2006;22:325–9 [DOI] [PubMed] [Google Scholar]
- 30.McMaster J, Ng T, Dexter M. Intraventricular rhabdoid meningioma. J Clin Neurosci 2007;14:672–5 [DOI] [PubMed] [Google Scholar]
- 31.Ahmad KE, Al-Jahdhami S, Ahmad O. Rhabdoid meningioma presenting with subependymal and diffuse meningeal involvement but no mass lesion. J Clin Neurosci 2010;17:1581–2 [DOI] [PubMed] [Google Scholar]
- 32.Cai C, Zhang Q, Shen C, et al. Rhabdoid meningioma in a child: Report of a case and literatures review. Chin J Clin Oncol 2008;5:67–71 [Google Scholar]
- 33.Dutta D, Lee HN, Munshi A, et al. Intracerebral cystic rhabdoid meningioma. J Clin Neurosci 2009;16:1073–4 [DOI] [PubMed] [Google Scholar]
- 34.Eom KS, Kim DW, Kim TY. Diffuse craniospinal metastases of intraventricular rhabdoid papillary meningioma with glial fibrillary acidic protein expression: A case report. Clin Neurol Neurosurg 2009;111:619–23 [DOI] [PubMed] [Google Scholar]
- 35.Morina A, Kelmendi F, Morina O, et al. Rhabdoid meningioma in an eight-year-old child. Med Arh 2010;64:123–4 [PubMed] [Google Scholar]
- 36.Santhosh K, Kesavadas C, Radhakrishnan VV, et al. Rhabdoid and papillary meningioma with leptomeningeal dissemination. J Neuroradiol 2008;35:236–9 [DOI] [PubMed] [Google Scholar]
- 37.Bansal M, Pathak VP, Kishore S, et al. Rhabdoid meningioma: Rapid intraoperative diagnosis on squash smears. Diagn Cytopathol 2010;38:594–6 [DOI] [PubMed] [Google Scholar]
- 38.Matyja E, Grajkowska W, Nauman P, et al. Necrotic rhabdoid meningiomas with aggressive clinical behavior. Clin Neuropathol 2010;29:307–16 [DOI] [PubMed] [Google Scholar]
- 39.Wu YT, Ho JT, Lin YJ, et al. Rhabdoid papillary meningioma: A clinicopathologic case series study. Neuropathology 2011;31:599–605 [DOI] [PubMed] [Google Scholar]
- 40.Wu YT, Lin JW, Wang HC, et al. Clinicopathologic analysis of rhabdoid meningioma. J Clin Neurosci 2010;17:1271–5 [DOI] [PubMed] [Google Scholar]
- 41.Buccoliero AM, Castiglione F, Rossi Degl'Innocenti D, et al. Pediatric rhabdoid meningioma: A morphological, immunohistochemical, ultrastructural and molecular case study. Neuropathology 2011;31:59–65 [DOI] [PubMed] [Google Scholar]
- 42.Lofrese G, Della Pepa GM, Sabatino G, et al. Intraparenchymal recurrence of a dural meningioma: Association with rhabdoid alterations with aggressive biological and clinical behaviour. Br J Neurosurg 2011;25:324–6 [DOI] [PubMed] [Google Scholar]
- 43.Martinez-Saez E, Malik I, Connor S, et al. Combined rhabdoid, papillary and adenocarcinomatous-like elements in a malignant meningioma—A potential diagnostic problem. Clin Neuropathol 2012;31:51–3 [DOI] [PubMed] [Google Scholar]
- 44.Motegi H, Kobayashi H, Terasaka S, et al. Hemorrhagic onset of rhabdoid meningioma after initiating treatment for infertility. Brain Tumor Pathol 2012;29:240–4 [DOI] [PubMed] [Google Scholar]
- 45.Rogerio F, de Araujo Zanardi V, Ribeiro de Menezes Netto J, et al. Meningioma with rhabdoid, papillary and clear cell features: Case report and review of association of rare meningioma variants. Clin Neuropathol 2011;30:291–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Z, Kong M, Li J, et al. Intraspinal rhabdoid meningioma metastasis to the liver. J Clin Neurosci 2011;18:714–6 [DOI] [PubMed] [Google Scholar]
- 47.Jeong J, Kim NR, Lee SG. Crush cytology of a primary intraspinal rhabdoid papillary meningioma: A case report. Acta Cytol 2013;57:528–33 [DOI] [PubMed] [Google Scholar]
- 48.Kashimura H, Mase T, Ogasawara K, et al. Unusual growth pattern of a meningioma. Surg Neurol Int 2012;3:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou Y, Xie Q, Gong Y, et al. Clinicopathological analysis of rhabdoid meningiomas: Report of 12 cases and a systematic review of the literature. World Neurosurg 2013;79:724–32 [DOI] [PubMed] [Google Scholar]
- 50.Karabagli P, Karabagli H, Yavas G. Aggressive rhabdoid meningioma with osseous, papillary and chordoma-like appearance. Neuropathology 2014;34:475–83 [DOI] [PubMed] [Google Scholar]
- 51.Mordechai O, Postovsky S, Vlodavsky E, et al. Metastatic rhabdoid meningioma with BRAF V600E mutation and good response to personalized therapy: Case report and review of the literature. Pediatric Hematol Oncol 2015;32:207–11 [DOI] [PubMed] [Google Scholar]
- 52.Parameshwaran Nair R, Vinod, Sarma Y, et al. Metastatic rhabdoid meningioma of the parotid—Mimicking primary salivary gland neoplasm. Int J Surg Case Rep 2015;6C:104–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reddy Ch K, Rao AD, Ballal CK, et al. Rhabdoid meningioma: Report of two cases. J Clin Diagn Res 2015;9:PD05–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tian Q, Zhang F, Bi L, et al. Rhabdoid meningioma: Analysis of one case. Childs Nerv Syst 2014;30:189–91 [DOI] [PubMed] [Google Scholar]
- 55.Rogers L, Barani I, Chamberlain M, et al. Meningiomas: Knowledge base, treatment outcomes, and uncertainties. A RANO review. J Neurosurg 2015;122:4–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.