Abstract
Objective. To describe trajectories of functional decline over 84 months and study associated risk factors among adults initially without limitation who had or were at risk of knee OA.
Methods. We used annual measures of WOMAC physical function over 84 months from the OA Initiative. We included knees with no functional limitation (i.e. WOMAC = 0) at baseline. Knee-based trajectories of functional decline from WOMAC were identified from a group-based trajectory model (PROC TRAJ).
Results. We identified five trajectories from 2110 knees (1055 participants, age 61.0 ± 9.3, BMI 27.1 ± 4.4, 52% women). Half of the knees (54%) remained free of limitation over 84 months, 26% slowly declined to a WOMAC of 1.5, 9% were limitation free for the first 36 months and declined to a WOMAC of 11.3, 6% rapidly declined over the first 12 months and gradually recovered to a WOMAC of 3.3 and 5% steadily declined to a WOMAC of 13.2. Baseline radiographic disease, knee pain, obesity and depressive symptoms at baseline were associated with trajectories of worse functional decline.
Conclusion. Five per cent of our sample initially without limitation was on a trajectory of progressive functional decline over 84 months later. We found worse disease and health status at baseline to be associated with faster decline over time.
Keywords: knee osteoarthritis, physical function, trajectory
Rheumatology key messages
We observed 5 unique trajectories of functional decline among people with knee osteoarthritis.
A small minority of people with knee osteoarthritis had progressively worsening physical function over time.
Worse disease, pain and depression may be important predictors of functional decline in knee osteoarthritis.
Introduction
OA ranks as the 11th highest contributor to global disability [1] and is the leading cause of functional limitation in older adults, such as difficulty walking and climbing stairs [2, 3]. Furthermore, arthritis-related conditions are the second most common reason for medical visits related to chronic conditions, second only to hypertension [4]. As such, OA-related disability is a major public health problem impacting the lives of many older adults. Presently, there is no proven strategy to prevent or cure knee OA, but rather the focus of intervention is to address pain and functional limitation.
Understanding patterns of functional decline is necessary to effectively treat functional limitation in knee OA. Anecdotally, slow steady decline is thought to occur; however, it is not known whether this is universally true, or whether unique patterns of decline occur over time. To date, large epidemiological studies have provided rather crude characterizations of functional decline in knee OA, with few repeated measures and limited follow-up time [5–7]. This is a major limitation since functional ability is known to fluctuate over time [8].
Should different patterns of functional decline exist, it is important to identify which risk factors precipitate worsening of function. To date, little is known about the association of the severity of radiographic disease with functional decline, though structure–symptom discordance in knee OA has been reported [9, 10]. In order to evaluate the association of severity of radiographic disease with trajectories of physical function we examined data from a well-known OA cohort, the OA Initiative (OAI), which includes study participants with or at high risk of knee OA. As well, there is a paucity of evidence to show the extent to which potentially modifiable factors, such as obesity and depressive symptoms, are associated with the development of functional limitation in knee OA. Such an investigation will enable risk stratification efforts and help develop therapeutic targets for early intervention to potentially mitigate or prevent the development of functional limitation in people with or at high risk of knee OA. The purpose of this study was to describe trajectories of functional decline among initially well-functioning people with or at risk of knee OA and examine the association of demographic, disease severity and modifiable factors with trajectories.
Methods
Study sample
The OAI is an ongoing longitudinal cohort study of the risk factors and natural history of OA. Adults between 45 and 79 years of age at enrolment who had or were at high risk of knee OA were recruited from four clinical sites: Baltimore MD, Pittsburgh PA, Pawtucket RI and Columbus OH. Detail regarding the rationale and approach for the OAI can be found at http://www.oai.ucsf.edu/datarelease/About.asp. Briefly, OAI recruited two primary subcohorts. One had symptomatic knee OA at baseline and the other did not but was at high risk based on the presence of risk factors associated with the development of symptomatic knee OA. High risk was identified from age-specific criteria from established risk factors including knee symptoms in the past 12 months [11], being overweight from gender-specific cut-points [11–13], knee injury causing difficulty walking for at least 1 week [11, 14, 15], any knee surgery history [12, 16], family history of a total knee replacement [17, 18], Heberden’s nodes [11, 19] or repetitive knee bending at work or outside of work [20, 21]. People were excluded who had rheumatoid or inflammatory arthritis, end-stage disease defined as severe joint space narrowing in both knees at baseline, or bilateral total knee replacements, a positive pregnancy test or used ambulatory aids other than a cane. Participants included in the study were assessed annually. Institutional review board (IRB) approval for the OAI was obtained from all OAI sites. We did not obtain specific IRB approval for our study since the parent study already had approval. We used baseline and annual follow-up visit data to the 84-month visit among study subjects with no functional limitation (as defined below) at the baseline visit from the entire OAI cohort.
Study outcome
Functional limitation was assessed with the WOMAC physical function subscale [22]. The physical function subscale includes 17 items measuring self-reported difficulty with mobility, such as climbing stairs, rising from sitting and squatting, activities of daily living, such as putting on socks, bathing and getting on/off the toilet, and heavy and light domestic duties. Item responses range from 0: no difficulty to 4: extreme difficulty. Responses were summed (range: 0–68) with higher scores indicating worse function. OAI study participants rated each knee separately and both scores were used in the analyses.
Study exposures
Baseline age (<60, 60–70 and >70 years), sex (men/women) and race (white, non-white) were measured by self-report. Baseline radiographic knee OA was assessed from weight-bearing posteroanterior and lateral fixed flexion radiographic evaluations of both knees [23]. Radiographs were independently graded twice among three expert readers (two rheumatologists and a musculoskeletal radiologist) for joint space narrowing and osteophytes in the tibiofemoral joint according to Kellgren and Lawrence (KL) criteria (grades 0–4) [24]. Any disagreements were adjudicated among all three expert readers to reach consensus. Knee-specific KL grades were used for analyses.
Knee pain severity was rated on an ordinal scale ranging from 0 to 10 with 0 representing no pain and 10 being pain as bad as you can imagine [25, 26]. Participants were asked to rate the worst pain in each knee over the last 7 days.
Baseline modifiable factors included BMI and depressive symptoms. Subjects with a BMI between ⩾25 kg/m2 and <30 kg/m2 at baseline were classified as overweight and those with a BMI ⩾30 kg/m2 at baseline were classified as obese. Depressive symptoms were considered to be present with a score ⩾16 on the Center for Epidemiologic Studies Depression Scale [27].
Analysis
We included study participants who reported no difficulty on any of the WOMAC physical function items for both knees at baseline. In addition, participants needed a minimum of two follow-up time points in order to provide an adequate number of data points for trajectory analyses [28, 29]. We compared subjects meeting study criteria at baseline with those who did not have at least two follow-up visits, that is, were and were not included in the analytic dataset.
We identified trajectories of functional decline by first determining the number of trajectory groups using a group based trajectory model (PROC TRAJ) [30]. We used Bayesian Information Criterion to select the optimal number of trajectory groups, that is, the number of trajectory groups was varied until the best-fitting model was obtained as indicated by the Bayesian Information Criterion [31]. In addition, we required the smallest trajectory group to include ⩾5% of the subjects from the sample in order to provide a meaningful and pragmatic description of patterns of change from a clinical perspective. We modeled intercept only, linear, quadratic or cubic polynomial terms. We used the posterior probabilities of group membership from each individual to assess the fit of the model, which was provided by the PROC TRAJ macro. High probability of membership into a single group represents a good model fit.
Next, we examined the association of baseline risk factors with trajectories of functional decline using multinomial logistic regression. These risk factors included demographic (age, sex, race), disease severity (KL grades and knee pain severity) and modifiable (obesity and depressive symptoms) risk factors. Analyses were mutually adjusted for baseline risk factors in multivariable models [32]. We also described the number of total knee and hip replacements that occurred after the baseline visit. All new replacements were adjudicated by radiographs and/or medical records.
Each study subject contributed two knees to analyses and our statistical methodology is unable to account for the within-person correlations. Therefore, we randomly selected one knee from each participant and repeated all analyses.
Results
Of the 4796 participants at the baseline OAI visit, 1118 participants had no functional limitation in either knee at baseline. Of these, 1055 participants contributing 2110 knees had at least two follow-up visits and contributed to the analytic cohort. The average age (s.d.) was 61.0 (9.3) years, 53% were women, 91% were white, 72% had at least a college education and few had one or more comorbidities (17%). At baseline, over half had a KL of 0 and 19% a KL of 1. The average (s.d.) knee pain was 0.5 (1.2) and 85% rated their knee pain as 0 or 1 on a 10 point scale. The average BMI (s.d.) was 27.1 (4.4) with 35% having a healthy BMI (<25 kg/m2), 41% were overweight and the remainder were obese. Of the participants, <5% had depressive symptoms and <2% of knees or hips were replaced after the baseline visit (Table 1). The 63 participants with no functional limitation at baseline who did not have at least two follow-up visits were more likely to be older, black, have less than a college education, and have at least one comorbidity compared with the 1055 subjects included in the analytic cohort (supplementary Table S1, available at Rheumatology Online).
Table 1.
Baseline subject characteristics across trajectory groups
| Trajectory group |
||||||
|---|---|---|---|---|---|---|
| Characteristic | All knees (n = 2110) | High functioning (n = 1143) | Minimal limitation (n = 549) | Late worsening (n = 185) | Remitting (n = 129) | Progressive worsening (n = 104) |
| Age, mean (s.d.; min–max), years | 61.0 (9.3; 45–79) | 61.0 (9.5; 45–79) | 60.4 (8.9; 45–79) | 62.0 (9.5; 45–79) | 61.7 (10; 45–79) | 61.6 (8.7; 45–78) |
| <60, % | 47 | 48 | 49 | 43 | 43 | 41 |
| 60–70, % | 29 | 26 | 31 | 31 | 29 | 36 |
| ≥70, % | 24 | 25 | 20 | 25 | 27 | 23 |
| Women, % | 53 | 50 | 54 | 56 | 57 | 55 |
| White, % | 91 | 91 | 92 | 91 | 88 | 87 |
| College graduate, % | 72 | 75 | 73 | 66 | 65 | 54 |
| Depressive symptoms, % CES-D ≥16 | 4 | 3 | 4 | 7 | 6 | 14 |
| At least 1 comorbidity, % | 17 | 15 | 19 | 18 | 21 | 29 |
| BMI, mean (s.d.; min–max), kg/m2 | 27.1 (4.4; 16.9–42.5) | 26.5 (4.4; 16.9–41.6) | 27.3 (4.5; 18.4–40.7) | 28.4 (4.3; 19.5–39.1) | 27.9 (4.2; 19.5–42.5) | 28.9 (4.3; 19.8–42.5) |
| Healthy weight (<25.0), % | 35 | 41 | 33 | 22 | 26 | 13 |
| Overweight (25–30), % | 41 | 39 | 41 | 45 | 43 | 50 |
| Obese (≥30), % | 24 | 20 | 26 | 33 | 31 | 37 |
| Kellgren and Lawrence grade of knee OA (%) | ||||||
| 0 | 52 | 57 | 54 | 41 | 37 | 32 |
| 1 | 19 | 20 | 16 | 19 | 20 | 18 |
| 2 | 20 | 17 | 23 | 25 | 23 | 32 |
| 3 or 4 | 9 | 6 | 7 | 16 | 20 | 17 |
| Knee pain in the last 30 days (0–10), median (IQR; min–max) | 0 (0; 0–0) | 0 (0; 0–0) | 0 (1; 0–1) | 0 (1; 0–1) | 0 (2; 0–2) | 0 (2; 0–2) |
| No knee pain ≤1 out of 10, % | 85 | 90 | 83 | 77 | 73 | 68 |
| Mild knee pain 2 or 3 out of 10, % | 12 | 8 | 13 | 17 | 19 | 25 |
| Moderate knee pain ≥4 out of 10, % | 3 | 2 | 4 | 6 | 8 | 7 |
| New total knee replacement after baseline, n | 18 | 0 | 2 | 3 | 8 | 5 |
| New total hip replacement after baseline, n | 33 | 11 | 5 | 8 | 6 | 3 |
n: number.
Trajectories of functional decline
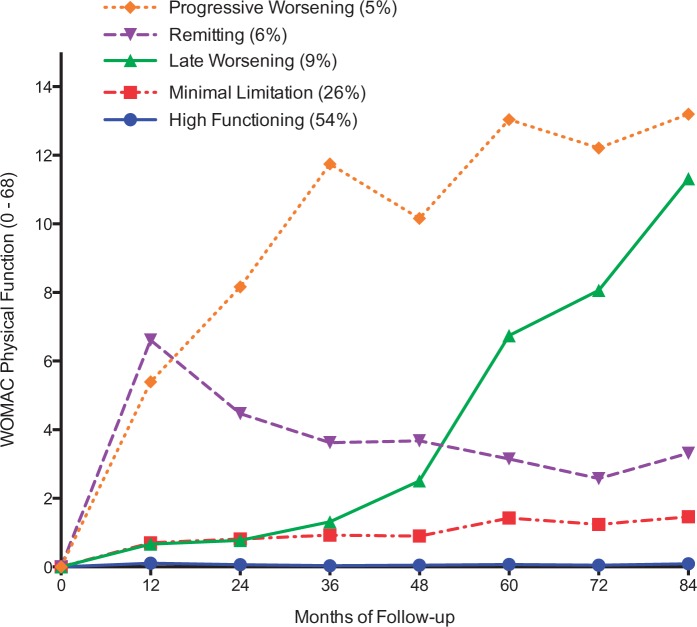
We identified five trajectories over 84 months (Fig. 1, Table 2 and Supplementary Table S2, available at Rheumatology Online). The high functioning trajectory included a majority (54%) of knees, was characterized by little to no decline throughout the 84 months of follow-up [mean (s.d., range), median (IQR) WOMAC = 0.1 (0.3, 0–2.3), 0 (0–0) at the 84-month study visit]. Twenty-six per cent of knees were on a minimal limitation trajectory that had mild difficulty on one to two items from the entire physical function scale on average by the end of follow-up [mean (s.d., range), median (IQR) WOMAC = 1.5 (2.2, 0–11.3), 0 (0–2)]. Nine per cent of knees were on a late worsening trajectory, initially experiencing little to no limitation until the 36-month follow-up visit, at which time minimal difficulty on at least half of the WOMAC physical function subscale items or moderate difficulty on at least 5 of the 17 items was reported [mean (s.d., range), median (IQR) WOMAC =11.3 (8.7, 0–48.0), 10.6 (5–17)]. The remainder of knees were on either a remitting (6%) or a progressive worsening (5%) trajectory. Both trajectories had mild difficulty on five or six items or moderate difficulty on two or three items on average at 12 months. Thereafter, knees in the remitting trajectory had improvement in physical function by the last visit [mean (s.d., range), median (IQR) WOMAC = 3.3 (4.3, 0–20), 2 (0–5)]. In contrast, knees in the progressive worsening trajectory continued to decline [mean (s.d., range), median (IQR) WOMAC = 13.2 (9.9, 0–34.0), 12.5 (5.3, 20.6)]. The posterior probability of allocating each knee into trajectories was ⩾ 0.92, indicating that there was a 92% probability on average of each individual knee trajectory fitting the respective group trajectory.
Fig. 1.
Trajectories of functional decline (number of knees = 2110)
Each point represents the average WOMAC physical function score for each trajectory group.
Table 2.
Number of knees at each follow-up visit by trajectory group
| Month of follow-up | ||||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 12 | 24 | 36 | 48 | 60 | 72 | 84 | |
| Progressive worsening | 104 | 104 | 102 | 100 | 103 | 97 | 95 | 94 |
| Remitting | 129 | 127 | 126 | 123 | 123 | 116 | 114 | 111 |
| Late worsening | 185 | 180 | 180 | 180 | 184 | 175 | 170 | 169 |
| Minimal limitation | 549 | 536 | 539 | 537 | 541 | 515 | 505 | 497 |
| High functioning | 1143 | 1117 | 1118 | 1114 | 1111 | 1031 | 1010 | 1007 |
Subject characteristics within trajectory groups
As shown in Table 1, sex, age and race were evenly distributed among trajectory groups. Study participants in the progressive worsening trajectory had a higher prevalence of comorbidity, depressive symptoms, obesity, higher KL grades and mild or moderate knee pain compared with those in the high functioning trajectory. Loss to follow-up was highest in the remitting trajectory (14%) followed by the high functioning trajectory (12%). The other trajectory groups had <10% loss to follow-up. Eleven or fewer new knee or hip replacements occurred within each trajectory group after the baseline visit.
Association of demographic, disease severity and modifiable factors with trajectory groups
The severity of radiographic knee OA and knee pain, and the presence of obesity and depressive symptoms at baseline were associated with decline (Table 3). For example, a KL grade of 3 or 4 was associated with 4.9, 5.1 and 3.5 times the odds of being in the progressive worsening, remitting and late worsening trajectories, respectively, compared with a KL grade of 0. The corresponding odds ratios for being in the progressive worsening, remitting and late worsening trajectories were 4.3, 4.8 and 3.6 for moderate knee pain compared with knees without pain, 5.0, 2.3 and 3.0 for obesity compared with those who were not obese, and 6.7, 2.8 and 3.0 for depressive symptoms compared with those without depressive symptoms, respectively. No apparent association was observed for age, sex and race with trajectory groups.
Table 3.
Association of demographic, OA and modifiable factors at baseline with trajectory groups
| Trajectory group | |||||
|---|---|---|---|---|---|
| Factor | High functioning, odds ratio (95% CI) (n = 1143) | Minimal limitation, odds ratio (95% CI) (n = 549) | Late worsening, odds ratio (95% CI) (n = 185) | Remitting, odds ratio (95% CI) (n = 129) | Progressive worsening, odds ratio (95% CI) (n = 104) |
| Baseline demographic factorsa | |||||
| Age 60–70 vs < 60 | 1.0 (Reference) | 1.1 (0.9, 1.4) | 1.3 (0.9, 1.9) | 1.2 (0.8, 1.9) | 1.6 (1.0, 2.5) |
| Age >70 vs < 60 | 1.0 (Reference) | 0.8 (0.6, 1.0) | 1.1 (0.7, 1.6) | 1.2 (0.8, 1.9) | 1.1 (0.6, 1.8) |
| Men vs women | 1.0 (Reference) | 0.9 (0.7, 1.1) | 0.8 (0.6, 1.1) | 0.8 (0.5, 1.1) | 0.9 (0.6, 1.3) |
| White vs non-white | 1.0 (Reference) | 1.2 (0.8, 1.7) | 1.0 (0.6, 1.8) | 0.8 (0.4, 1.4) | 0.7 (0.4, 1.2) |
| Baseline OA factorsb | |||||
| KL 1 vs KL 0 | 1.0 (Reference) | 0.8 (0.6, 1.1) | 1.3 (0.8, 2.0) | 1.5 (0.9, 2.5) | 1.6 (0.9, 3.0) |
| KL 2 vs KL 0 | 1.0 (Reference) | 1.5 (1.1, 2.0) | 2.1 (1.4, 3.1) | 2.2 (1.3, 3.6) | 3.5 (2.0, 6.1) |
| KL 3 or 4 vs KL 0 | 1.0 (Reference) | 1.3 (0.8, 2.0) | 3.5 (2.0, 5.9) | 5.1 (2.9, 9.2) | 4.9 (2.4, 9.7) |
| Mild vs no knee pain | 1.0 (Reference) | 1.9 (1.3, 2.6) | 2.8 (1.8, 4.5) | 3.1 (1.8, 5.2) | 6.0 (3.4, 10.4) |
| Moderate vs no knee pain | 1.0 (Reference) | 2.1 (1.1, 4.0) | 3.6 (1.7, 7.9) | 4.8 (2.1, 10.9) | 4.3 (1.6, 11.6) |
| Baseline modifiable factorsc | |||||
| Overweight vs healthy weight | 1.0 (Reference) | 1.3 (1.0, 1.7) | 2.2 (1.5, 3.4) | 1.7 (1.0, 2.7) | 4.2 (2.2, 8.3) |
| Obese vs healthy weight | 1.0 (Reference) | 1.6 (1.2, 2.2) | 3.0 (1.9, 4.8) | 2.3 (1.4, 4.0) | 5.0 (2.5, 10.) |
| Depressed vs not depressed | 1.0 (Reference) | 1.7 (1.0, 3.0) | 3.0 (1.5, 6.3) | 2.8 (1.3, 6.6) | 6.7 (3.1, 14.3) |
aMutually adjusted for age, sex and race.
bKL adjusted for age, sex, race, education and comorbidity. The model with pain was additionally adjusted for KL grade in addition to age, sex, race, education and comorbidity.
cAdjusted for age, sex, race, knee pain, KL grade, education, comorbidity and mutually adjusted for obesity and depressive symptoms.
KL: Kellgren and Lawrence.
Using one randomly selected knee from each study participant, we found the similar functional limitation trajectories as the primary analysis (supplementary Fig. S1, supplementary Tables S3 and S4, available at Rheumatology Online). We also found similar associations of demographic, disease severity, and modifiable factors with trajectory groups as with the primary analysis. One exception to this was the effect estimates for depressive symptoms with trajectory groups using one randomly selected knee from each study participant (supplementary Table S5, available at Rheumatology Online).
Discussion
We identified five distinct trajectories of functional decline over a period of 7 years among adults with or at high risk of knee OA who had no limitation at baseline. We also found radiographic disease severity, knee pain, obesity and depressive symptoms to be associated with decline. These findings provide a preliminary description of patterns of functional decline over 7 years and their associated risk factors in knee OA, which is the most common cause of functional limitation in older adults [2, 3].
The distinct patterns we observed in the OAI are contrary to the notion that functional decline is slow and gradual in people with or at high risk of knee OA. While 25% were on a trajectory of slow and steady decline, another 5% had fast and progressive decline. For the remainder, we observed non-linear patters of change over time. For instance, 9% had virtually no decline for 3 years followed by rapid decline for the remainder of follow-up, while 6% had rapid decline in the first year followed by gradual recovery thereafter. These findings indicate that an array of functional decline patterns likely accompany the natural history of knee OA. A similar heterogeneous pattern of decline has been described for older adults in the last year of life [33]. Thus, the medical needs for people with knee OA, for example, rehabilitation, may vary over time, with some requiring care sooner than others, and treatment intensity needs may change over time as well.
We found several baseline risk factors, including knee pain, obesity, depressive symptoms and the severity of radiographic disease, to be strong predictors for subsequent trajectories of functional decline. These findings are consistent with previous longitudinal studies investigating changes in existing physical function over time [5, 34–36]. Based on our findings, these risk factors may aid in identifying people on a trajectory of developing future limitation. At present, conservative approaches, such as strengthening exercise and aerobic walking, are prescribed at a late stage of disease, for example, just prior to total knee replacement, which have minimal efficacy for reducing functional limitation [37]. An early intervention approach leveraging the same conservative approaches is likely to be more effective at preventing or delaying the onset of functional limitation associated with knee OA than intervention at a late stage. Using a risk factor profile may be an appropriate strategy for targeting these conservative approaches to those at risk of developing such limitations.
We recognize that by the 84-month visit the absolute values of functional limitation were relatively minor. For instance, in the progressive worsening trajectory, which had the fastest rate of decline, the average WOMAC physical function value was 13 out of 68, which represents mild difficulty for most types of physical functioning or extreme difficulty on four items. Nevertheless, the differences between trajectory groups and subsequent severity of limitation will become greater over time; our trajectory analysis provides only a snapshot of these changes that are occurring over much longer time periods. As such, eventual limitation will be more prominent over time. Hence, people on a late worsening and progressive worsening trajectory will likely exceed a patient acceptable symptom state [38] sooner than those on a trajectory of little to no functional decline.
Limitations to the current study warrant comment. First, each study subject contributed two knees in analyses and we were unable to account for potential within-person correlations. However, our findings did not change materially in the sensitivity analyses where each subject contributed one randomly selected knee to analyses. Second, we did not directly address the impact of total joint replacement occurring over 7 years on trajectory groups. Since the number of total knee and hip replacements comprised 2% of the sample (51/2110), we believe trajectory groups were not dramatically influenced by total joint replacement after the baseline visit. Third, we did not evaluate how changes in demographic, OA and modifiable factors over time contributed to change within trajectories. We decided to focus on baseline factors rather than inter-current events with trajectories of functional decline in order to enable risk stratification efforts and identify potential potent therapeutic targets for early intervention. Fourth, we are unable to address whether people with and without radiographic knee OA at baseline have similar trajectory patterns since we included both at baseline in our analytic sample. Fifth, we did not track pharmacological and non-pharmacological interventions in our analyses, and these may have influenced trajectory patterns.
Despite these limitations, our study has several strengths and clinical implications. First, we employed a large prospective longitudinal dataset of adults with or at risk of knee OA to investigate trajectories of functional decline. The OAI is a well-established cohort with standardized annual measures of disease, impairment and physical function over 84 months. Second, to the best of our knowledge, this is the first study to describe trajectories of functional decline in initially well-functioning people with or at risk of knee OA. These findings are important for patients, clinicians and policy-makers to understand the natural history of functional decline in knee OA and develop methods to risk-stratify those at highest risk for functional limitation.
Conclusion
In summary, we found five distinct trajectories of functional decline among initially well-functioning adults with or at risk of knee OA. Those with worse radiographic disease and knee pain, and higher excess body weight and depressive symptoms were most at risk for being on a trajectory of decline. These findings are important to help identify adults with or at risk of knee OA who are at risk of developing functional limitation, and intervene upon potential risk factors that may limit or prevent decline.
Supplementary Material
Acknowledgements
The OAI is a public–private partnership comprising five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health (NIH), a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript was prepared using an OAI public use data set and does not necessarily reflect the opinions or views of the OAI investigators, the NIH, or the private funding partners.
Funding for the authors was provided by the Boston Rehabilitation Outcomes Center (Boston ROC) R24HD0065688, ACR/RRF Bridge Funding Award, NIAMS R01AR062506, P60AR047785, NIH U54 GM104941. None of the funders of this manuscript had any role in the design and conduct of this study; collection, management, analysis and interpretation of the data; or preparation of the manuscript.
Funding: No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: The authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
- 1.Cross M, Smith E, Hoy D. et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis 2014;73:1323–30 [DOI] [PubMed] [Google Scholar]
- 2.Guccione AA, Felson DT, Anderson JJ. et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health 1994; 84:351–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray CJ, Richards MA, Newton JN. et al. UK health performance: findings of the Global Burden of Disease Study 2010. Lancet 2013;381:997–1020 [DOI] [PubMed] [Google Scholar]
- 4.Cherry DK, Hing E, Woodwell DA, Rechtsteiner EA. National Ambulatory Medical Care Survey: 2006 summary. Natl Health Stat Report 2008;1–39 [PubMed] [Google Scholar]
- 5.Sharma L, Cahue S, Song J. et al. Physical functioning over three years in knee osteoarthritis: role of psychosocial, local mechanical, and neuromuscular factors. Arthritis Rheum 2003;48:3359–70 [DOI] [PubMed] [Google Scholar]
- 6.White DK, Keysor JJ, Lavalley MP. et al. Clinically important improvement in function is common in people with or at high risk of knee OA: the MOST study. J Rheumatol 2010;37:1244–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dieppe P, Cushnaghan J, Tucker M, Browning S, Shepstone L. The Bristol 'OA500 study': progression and impact of the disease after 8 years. Osteoarthritis Cartilage 2000;8:63–8 [DOI] [PubMed] [Google Scholar]
- 8.Gill TM, Hardy SE, Williams CS. Underestimation of disability in community-living older persons. J Am Geriatr Soc 2002;50:1492–7 [DOI] [PubMed] [Google Scholar]
- 9.Hannan MT, Felson DT, Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol 2000;27:1513–7 [PubMed] [Google Scholar]
- 10.McAlindon TE, Cooper C, Kirwan JR, Dieppe PA. Determinants of disability in osteoarthritis of the knee. Ann Rheum Dis 1993; 52:258–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper C, Snow S, McAlindon TE. et al. Risk factors for the incidence and progression of radiographic knee osteoarthritis. Arthritis Rheum 2000;43:995–1000 [DOI] [PubMed] [Google Scholar]
- 12.Felson DT, Zhang Y. An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthritis Rheum 1998;41:1343–55 [DOI] [PubMed] [Google Scholar]
- 13.Manninen P, Riihimaki H, Heliovaara M, Makela P. Overweight, gender and knee osteoarthritis. Int J Obes Relat Metab Disord 1996;20:595–7 [PubMed] [Google Scholar]
- 14.Felson DT, Zhang Y, Hannan MT. et al. Risk factors for incident radiographic knee osteoarthritis in the elderly: the Framingham Study. Arthritis Rheum 1997;40:728–33 [DOI] [PubMed] [Google Scholar]
- 15.Roos H, Adalberth T, Dahlberg L, Lohmander LS. Osteoarthritis of the knee after injury to the anterior cruciate ligament or meniscus: the influence of time and age. Osteoarthritis Cartilage 1995;3:261–7 [DOI] [PubMed] [Google Scholar]
- 16.Roos H, Lauren M, Adalberth T. et al. Knee osteoarthritis after meniscectomy: prevalence of radiographic changes after twenty-one years, compared with matched controls. Arthritis Rheum 1998;41:687–93 [DOI] [PubMed] [Google Scholar]
- 17.Spector TD, Cicuttini F, Baker J, Loughlin J, Hart D. Genetic influences on osteoarthritis in women: a twin study. BMJ 1996;312:940–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chitnavis J, Sinsheimer JS, Clipsham K. et al. Genetic influences in end-stage osteoarthritis. Sibling risks of hip and knee replacement for idiopathic osteoarthritis. J Bone Joint Surg Br 1997;79:660–4 [DOI] [PubMed] [Google Scholar]
- 19.Hart DJ, Doyle DV, Spector TD. Incidence and risk factors for radiographic knee osteoarthritis in middle-aged women: the Chingford Study. Arthritis Rheum 1999;42:17–24 [DOI] [PubMed] [Google Scholar]
- 20.Felson DT, Hannan MT, Naimark A. et al. Occupational physical demands, knee bending, and knee osteoarthritis: results from the Framingham Study. J Rheumatol 1991;18:1587–92 [PubMed] [Google Scholar]
- 21.Cooper C, McAlindon T, Coggon D, Egger P, Dieppe P. Occupational activity and osteoarthritis of the knee. Ann Rheum Dis 1994;53:90–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988;15:1833–40 [PubMed] [Google Scholar]
- 23.Peterfy C, Li J, Zaim S. et al. Comparison of fixed-flexion positioning with fluoroscopic semi-flexed positioning for quantifying radiographic joint-space width in the knee: test-retest reproducibility. Skeletal Radiol 2003;32:128–32 [DOI] [PubMed] [Google Scholar]
- 24.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 1957;16:494–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bellamy N. Musculoskeletal Clinical Metrology. London: Kluwer Academic Publishers, 1993 [Google Scholar]
- 26.Turk D, Melzack R. Handbook of Pain Assessment, 2nd edn New York: Guilford Press, 2001 [Google Scholar]
- 27.Radloff L. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401 [Google Scholar]
- 28.Nagin D. Group-Based Modeling of Development. Cambridge, MA: Harvard University Press, 2005 [Google Scholar]
- 29.Hejazi S, Dahinten VS, Marshall SK, Ratner PA. Developmental pathways leading to obesity in childhood. Health Rep 2009;20:63–9 [PubMed] [Google Scholar]
- 30.Jones B, Nagin D, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Social Methods Res 2001;29:374–93 [Google Scholar]
- 31.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol 2010;6:109–38 [DOI] [PubMed] [Google Scholar]
- 32.Westreich D, Greenland S. The table 2 fallacy: presenting and interpreting confounder and modifier coefficients. Am J Epidemiol 2013;177:292–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gill TM, Gahbauer EA, Han L, Allore HG. Trajectories of disability in the last year of life. N Engl J Med 2010;362:1173–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riddle DL, Stratford PW. Body weight changes and corresponding changes in pain and function in persons with symptomatic knee osteoarthritis: a cohort study. Arthritis Care Res 2013;65:15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wesseling J, Bierma-Zeinstra SM, Kloppenburg M, Meijer R, Bijlsma JW. Worsening of pain and function over 5 years in individuals with 'early' OA is related to structural damage: data from the Osteoarthritis Initiative and CHECK (Cohort Hip & Cohort Knee) study. Ann Rheum Dis 2013; 74:347–53 [DOI] [PubMed] [Google Scholar]
- 36.Holla JF, van der Leeden M, Heymans MW. et al. Three trajectories of activity limitations in early symptomatic knee osteoarthritis: a 5-year follow-up study. Ann Rheum Dis 2014;73:1369–75 [DOI] [PubMed] [Google Scholar]
- 37.Gill SD, McBurney H. Does exercise reduce pain and improve physical function before hip or knee replacement surgery? A systematic review and meta-analysis of randomized controlled trials. Arch Phys Med Rehabil 2013;94:164–76 [DOI] [PubMed] [Google Scholar]
- 38.Tubach F, Ravaud P, Baron G. et al. Evaluation of clinically relevant states in patient reported outcomes in knee and hip osteoarthritis: the patient acceptable symptom state. Ann Rheum Dis 2005;64:34–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.