Abstract
Previous high-throughput screens to identify mitochondrial toxicants used immortalized cell lines and focused on changes in mitochondrial membrane potential, which may not be sufficient and do not identify different types of mitochondrial dysfunction. Primary cultures of renal proximal tubule cells (RPTC) were examined with the Seahorse Extracellular Flux Analyzer to screen 676 compounds (5 μM; 1 h) from the ToxCast Phase II library for mitochondrial toxicants. Of the 676 compounds, 19 were classified as cytotoxicants, 376 were electron transport chain (ETC) inhibitors, and 5 were uncouplers. The remaining 276 compounds were examined after a 5-h exposure to identify slower acting mitochondrial toxicants. This experiment identified 3 cytotoxicants, 110 ETC inhibitors, and 163 compounds with no effect. A subset of the ToxCast Phase II library was also examined in immortalized human renal cells (HK2) to determine differences in susceptibility to mitochondrial toxicity. Of the 131 RPTC ETC inhibitors tested, only 14 were ETC inhibitors in HK2 cells. Of the 5 RPTC uncouplers, 1 compound was an uncoupler in HK2 cells. These results demonstrate that 73% (491/676) of the compounds in the ToxCast Phase II library compounds exhibit RPTC mitochondrial toxicity, overwhelmingly ETC inhibition. In contrast, renal HK2 cells are markedly less sensitive and only identified 6% of the compounds as mitochondrial toxicants. We suggest caution is needed when studying mitochondrial toxicity in immortalized cell lines. This information will provide mechanisms and chemical-based criteria for assessing and predicting mitochondrial liabilities of new drugs, consumer products, and environmental agents.
Keywords: cell culture; in vitro and alternatives, kidney; systems toxicology; safety evaluation
The National Research Council’s report Toxicity Testing in the Twenty-first Century states the need to improve toxicity evaluation by focusing on molecular changes, developing rapid screening methods, and reducing the use of experimental animals (Council, 2007). In response, the Environmental Protection Agency (EPA) established a National Center for Computational Toxicology to develop new methods for predictive toxicology, and the ToxCast program to prioritize chemical research. The purpose of the ToxCast program is to develop and utilize high-throughput screening, toxicogenomics, and computational chemistry to enable more informative predictive toxicology (Dix et al., 2007). To this end the EPA has compiled 2 libraries of environmental contaminants: ToxCast Phase I and ToxCast Phase II. ToxCast Phase I (310 compounds) is primarily comprised of food-use pesticides. The ToxCast Phase II library (676 compounds) is more diverse including phthalates, pesticides, food additives, and 111 failed pharmaceutical compounds (Sipes et al., 2013). The toxicity assays within the ToxCast program primarily focus on the effects of toxicants at the cellular level, including receptor binding, inhibition of enzymatic activity, and genotoxicity (Judson et al., 2009).
Many chemicals in the environment have deleterious effects on mitochondrial function including pesticides, insecticides, antibiotics, and multiple pharmaceutical compounds (Chan et al., 2005; Dykens and Will, 2007; Sherer et al., 2007). Mitochondria have a significant role in maintaining cellular homeostasis through the regulation of energy production, oxidation, cellular proliferation, calcium homeostasis, and cell death (Birch-Machin and Turnbull, 2001; Hirsch et al., 1998). Toxicants that disrupt mitochondrial function can result in damage at the cellular level and ultimately result in organ toxicities including hepatotoxicity, cardiotoxicity, and nephrotoxicity (Wallace and Starkov, 2000). Therefore, there is a need to develop high-throughput toxicity assays to evaluate chemicals for their potential to disrupt mitochondrial function (Beeson et al., 2010; Rogers et al., 2011).
Immortalized and tumor-derived cell lines have been the primary tool for conducting in vitro toxicity studies due to the low cost and technical ease. Many tumor-derived cells lines are adapted for survival and growth in hypoxic environments, and therefore acquire energy through glycolysis rather that mitochondrial oxidative phosphorylation (Marroquin et al., 2007; Rodríguez-Enríquez et al., 2001). Therefore many mitochondrial toxicants may have little to no effect on cell growth or viability in cultured cell lines. Combined with the use of cell lines, mitochondrial membrane potential has been used as a marker of mitochondrial function (Lemasters and Ramshesh, 2007). Although mitochondrial membrane potential is easily measured, it provides little information on the mechanism of mitochondrial dysfunction.
We developed a multi-well plate respirometric assay utilizing the Seahorse Biosciences 96-well Extracellular Flux Analyzer (XF-96) (Beeson et al., 2010; Wills et al., 2013, 2012) and primary cultures of renal proximal tubule cells (RPTC) to identify mitochondrial toxicants. This platform can be utilized to measure basal oxygen consumption rates (OCR), and allows for the injection of multiple probes to identify and quantify the sources of functional impairment. Injection of the proton ionophore carbonylcyanide p-trifluoromethoxyphenylhydrazone (FCCP) uncouples the production of ATP from consumption of oxygen. Exposure of the cells to FCCP serves as a stress test to quantify the maximal activity of the electron transport chain (ETC) (Beeson et al., 2010; Schnellmann et al., 1987). Previous research in our laboratory confirmed the mitochondrial toxicity of cisplatin, gentamicin, and HgCl2 in this model using the FCCP stress test (Beeson et al., 2010).
As stated earlier, immortalized and cancer cell lines have limitations for toxicity testing, particularly their dependence on glycolysis for energy metabolism. Primary cultures of RPTC are a valuable model for evaluating mitochondrial toxicity because they acquire their energy from oxidative phosphorylation and maintain differentiated functions and membrane polarization (Nowak and Schnellmann, 1995, 1996). Under these improved culture conditions RPTC exhibit respiration and gluconeogenesis rates comparable to that found in vivo (Beeson et al., 2010; Nowak and Schnellmann, 1996).
The goal of this study was to utilize the integrated respiratory assay to screen the ToxCast Phase II library for mitochondrial toxicants in primary cultures of RPTC. These results were then compared with cultured human kidney epithelial cells (HK2) to determine differences in sensitivity to mitochondrial toxicity.
MATERIALS AND METHODS
Ethics statement
The following research study was conducted in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocols used were approved by the Institutional Animal Care and Use Committee at the Medical University of South Carolina.
Unless otherwise noted all of the chemicals were purchased from Sigma-Aldrich (St. Louis, Missouri). ToxCast Phase II was provided by the U.S. EPA.
Isolation and culture of RPTC
Female New Zealand white rabbits (1.5–2.0 kg) were purchased from Charles River (Wilmington, Massachusetts). Rabbit renal tubules were isolated using the iron oxide perfusion method as previously described (Nowak and Schnellmann, 1995). The resulting proximal tubules were plated on 100-mm tissue culture-grade plastic Petri dishes constantly swirled on an orbital shaker at 80 rpm. The culture medium was a 50:50 mixture of Dulbecco’s modified Eagle’s essential medium and Ham’s F12 (lacking glucose, phenol red, and sodium pyruvate; Gibco, Grand Island, New York) and supplemented with HEPES (15 mM), glutamine (2.5 mM), pyridoxine HCl (1 μM), sodium bicarbonate (15 mM), and lactate (6 mM). Hydrocortisone (50 nM), selenium (5 ng/ml), human transferrin (5 μg/ml), bovine insulin (10 nM), and l-ascorbic acid-2-phosphate (50 μM) were added to fresh culture medium (Nowak and Schnellmann, 1996). After 3 days the cells were trypsinized and re-plated onto XF-96 multi-well plates at a concentration of 18 000 cells/well and maintained in a 37°C incubator for 2 days prior to experimentation (Wills et al., 2013).
Culture of HK-2 cells
HK-2 are derived from a primary proximal tubule cell culture from normal adult human renal cortex that was immortalized by transduction with HPV 16 E6/E7 genes (Ryan et al., 1994) HK-2 (human kidney epithelial cells) were purchased from ATCC (Manassas, Virginia; CRL-2190). HK-2 were cultured in RPMI + GlutaMAX (Gibco, Grand Island, New York) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Flowery Branch, Georgia). The Roswell Park Memorial Institute medium (RPMI) contains 10 mM glucose. Cells were plated onto XF-96 multi-well plates at a concentration of 15 000 cells/well and maintained in a 37°C incubator for 2 days prior to experimentation.
Respirometric assay
The OCR measurements were performed using a Seahorse Bioscience XF-96 instrument as previously described (Beeson et al., 2010; Wills et al., 2013). Experimental plates were treated with vehicle control (0.5% DMSO), blank controls, and 5 µM of the compound of interest. The respirometric and the cell viability analyses are expressed as percent vehicle control. Exposure of the RPTC and the HK-2 to 0.5% DMSO had no deleterious effects on mitochondrial respiration or cellular viability (data not shown). The XF96 protocol consisted of basal OCR (1 measurement/1.5 min), injection of FCCP (0.5 µM), and three measurements of uncoupled OCR (1 measurement/1.5 min). The OCR was calculated from the continuous average slope of the O2 partitioning among plastic, atmosphere, and cellular uptake (Ferrick et al., 2008; Gerencser et al., 2009). Quality control evaluations included the basal and uncoupled rates of the vehicle control, positive control, and variances between duplicate treatment wells. Based on preliminary studies the toxicity threshold value was < 0.85 for the mean ratio of (chemical treatment FCCP-OCR/vehicle control FCCP-OCR). This threshold is ≥ 1 SD below the historic mean for the vehicle control.
An alternate XF96 protocol was developed to examine compounds for the ability to act as mitochondrial uncouplers. Basal OCR (1 measurement/1.5 min) were determined and each well was injected with DMSO, FCCP (0.5 µM), or the compound of interest (5 µM). The wells were mixed for 3 min followed by measurements of OCR. Finally the wells were injected with oligomycin (0.5 µM) followed by OCR measurement.
Assessment of cellular viability
After assessment of respirometric activity, RPTC or HK-2 were washed and fixed with 4% neutral buffer formalin solution. Each well was stained with 0.25 µM Hoechst (AnaSpec Inc.). Samples were analyzed by fluorescence microscopy using 350-nm excitation and 486-nm emission wavelengths in the IN Cell Analyzer 2000 (GE Healthcare). Analysis was performed using InCell Analyzer 3.7 software. The parameters for normal nuclear morphology and size were set between 100 and 200 µm2 (Wills et al., 2013).
Statistical analysis
Data are presented as mean ± SEM and were tested for normality. The cellular and mitochondrial respiration data failed a normality test, therefore a Kruskal-Wallis 1-way ANOVA on ranks was conducted. Multiple means were compared utilizing Dunn’s post hoc test and were considered statistically different when p < .05. RPTC and mitochondria isolated from a single animal represented an individual experiment (N = 1) and were repeated until an N ≥ 4 was obtained.
RESULTS
ETC Inhibitors and Uncouplers in ToxCast Phase II Chemical Library After 1 - and 5-h Exposures in RPTC
A respirometric analysis was conducted in RPTC to assess the mitochondrial effects of the 676 compounds in ToxCast Phase II. In the first experiment, RPTC plated on XF 96-well plates were treated with the vehicle (DMSO, 0.5%, control) or 5 µM of the test compounds for 1 h and evaluated on the XF-96 for basal and FCCP-uncoupled OCR (FCCP-OCR). Control basal and FCCP-OCR were monitored for cell quality. Additionally evaluation of the variances between duplicate wells (maximum accepted variance 15%) was determined. Subsequently, cell viability was determined as described earlier.
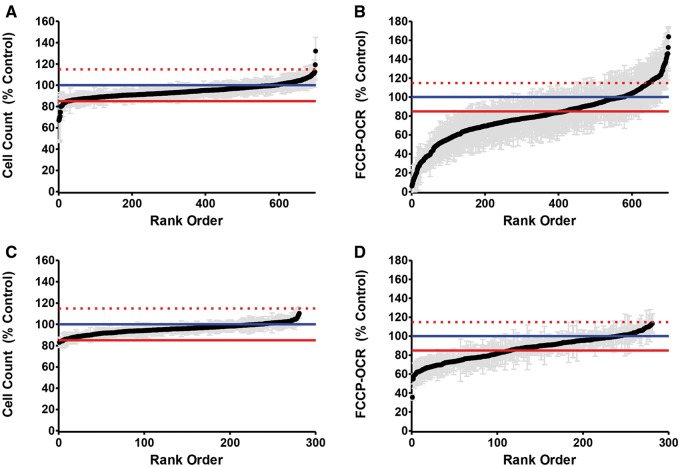
Previous research by Beeson et al. (2010) and Wills et al. (2013) demonstrated that a decrease in FCCP-OCR to a level that is ≤ 85% of the vehicle control without a decrease in cell viability is an ETC inhibitor. A compound was classified as a cytotoxicant if exposure resulted in a decrease in nuclear count ≤ 85% of the vehicle control. A compound was classified as having high basal activity if exposure resulted in an increase in basal-OCR ≥115% of the vehicle control. The results of the assay identified 19 cytoxicants (3%), 379 ETC inhibitors (56%), 26 high basal compounds (4%), and 252 no effect compounds (37%) (Fig. 1 and B; Table 1; Supplementary Fig. S1).
FIG. 1.
Cell count and respiration in RPTC 1 and 5 h after exposure to 5 µM of ToxCast Phase II compounds. A, Average cell count relative to the vehicle control 1 h after exposure. B, Average FCCP-OCR relative to the vehicle control 1 h after exposure. C, Average cell count relative to the vehicle control 5 h after exposure. D, Average FCCP-OCR relative to the vehicle control 5 h after exposure. Data represented as mean ± SEM of 4 biological replicates. The blue line indicates the mean for vehicle control wells. The solid red line indicates results <85% of the mean. The dashed red line indicates results >115% above the mean.
TABLE 1.
Classification of ToxCast Phase II Compounds After 1-h Exposure to 5 µM in RPTC
| Category | Number | % |
|---|---|---|
| Cytotoxicants | 19 | 3 |
| ETC Inhibitors | 379 | 56 |
| High Basal | 26 | 4 |
| No effect | 252 | 37 |
| Total | 676 |
In the second experiment, RPTC were dosed for 5 h with 5 µM of all the compounds that had no effect after a 1 h, to identify slower acting mitochondrial toxicants. The 5-h exposure was selected based on a previous study by Attene-Ramos et al. (2013), used 1 and 5 h to assess the mitochondrial toxicity of 1408 compounds of interest to the National Toxicity Program. These compounds were evaluated for mitochondrial toxicity using both alterations in mitochondrial membrane potential and oxygen consumption in HepG2 cells. Utilizing these time points the researchers were able to identify multiple mitochondrial toxicants and cluster compounds based on their ability to act at either/ and or both time points.
This subset was comprised of the 252 compounds classified as no effect after 1-h exposure and 24 compounds classified as high basal after 1-h exposure. The 5-h exposure identified 3 cytotoxicants (1%), 110 ETC inhibitors (40%), and 163 no effect compounds (59%) (Fig. 1C and D; Table 2; Supplementary Fig. S2). In total, after 1 - or 5-h exposures there were 22 cytotoxicants (3%), 489 ETC inhibitors (72%), 26 high basal compounds (4%), and 163 no effect compounds (24%).
TABLE 2.
Classification of ToxCast Phase II Compounds After 5-h Exposure to 5 µM in RPTC
| Category | Number | % |
|---|---|---|
| Cytotoxicants | 3 | 1 |
| ETC Inhibitors | 110 | 40 |
| No effect | 163 | 59 |
| Total | 276 |
Mitochondrial Uncouplers From ToxCast Phase II in RPTC
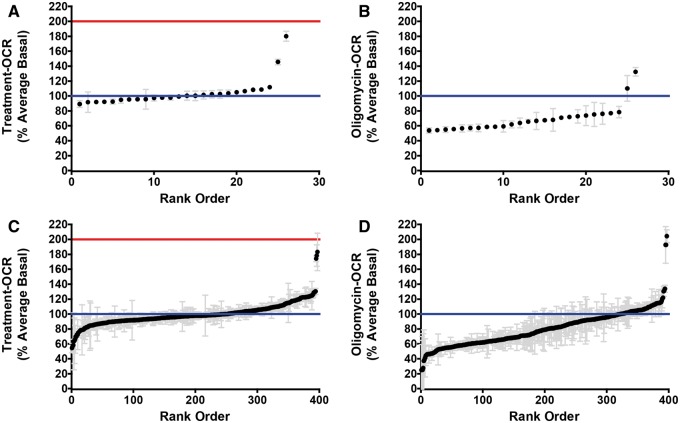
All 26 compounds classified as having high basal activity and the 379 compounds classified as ETC inhibitors after the 1-h exposure were further tested as potential mitochondrial uncouplers. RPTC were exposed to the high basal compounds for 3 min and evaluated for respiratory capacity. Injection of 0.5 µM of the proton ionophore FCCP was used as a positive control. Exposure to FCCP resulted in a 2-fold increase in OCR compared with vehicle control (Fig. 2A and C). A compound was classified as an uncoupler based on a treatment-OCR ≥1.4-fold of the vehicle control. Subsequently RPTC were exposed to 0.5 µM of the ATP synthase inhibitor oligomycin. The purpose of the oligomycin injection was to confirm the increase in OCR was due mitochondrial uncoupling.
FIG. 2.
Treatment and oligomycin OCR in RPTC. A, Average treatment-OCR relative to the vehicle control 3 min after exposure to 5 µM of high basal compounds. B, Average oligomycin-OCR relative to the vehicle control after exposure to 5 µM of high basal compounds. C, Average treatment-OCR relative to the vehicle control 3 min after exposure to 5 µM of ETC inhibitors. D, Average oligomycin-OCR relative to the vehicle control after exposure to 5 µM of ETC inhibitors. The blue line indicates the mean for vehicle control wells. The solid red line indicates the mean for the positive control FCCP response in RPTC.
Five compounds: 2-methyl-4,6-dinitrophenol (4,6-dinitro-o-cresol, abbreviated DNOC), pentachlorophenol, perfluorooctanesulfonamide, tributyltin, and didecyldimethylammonium chloride, were confirmed as uncouplers after a 3-min exposure (Fig. 2A and C). DNOC and pentachlorophenol were originally classified as having high basal activity after a 1-h exposure. Perfluorooctanesulfonamide, tributyltin, and didecyldimethylammonium chloride were originally classified as ETC inhibitors after a 1-h exposure. The OCR of all 5 compounds remained elevated after exposure to oligomycin confirming that the increased OCR was not coupled to ATP synthesis (Fig. 2B and D). In total 5 of the 676 compounds in ToxCast Phase II (0.7%) were identified as mitochondrial uncouplers (Fig. 3).
FIG. 3.
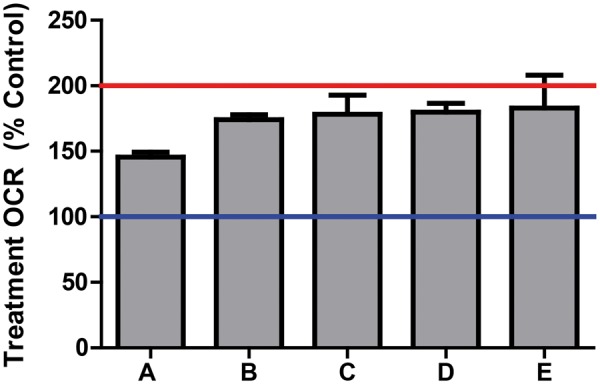
Treatment-OCR in RPTC 3 min after exposure to 5 µM of mitochondrial uncouplers from ToxCast Phase II. A, 2-methyl-4,6-dinitrophenol; B, perfluorooctanesulfonamide; C, tributyltin; D, pentachlorophenol, E, didecyldimethylammonium chloride. Data represented as mean ± SEM of 4 biological replicates. The blue line indicates the mean for vehicle control wells. The red line indicates the mean for the positive control FCCP response in RPTC.
Cytotoxicants and ETC Inhibitors From ToxCast Phase II in HK-2 cells After 1-h Exposure
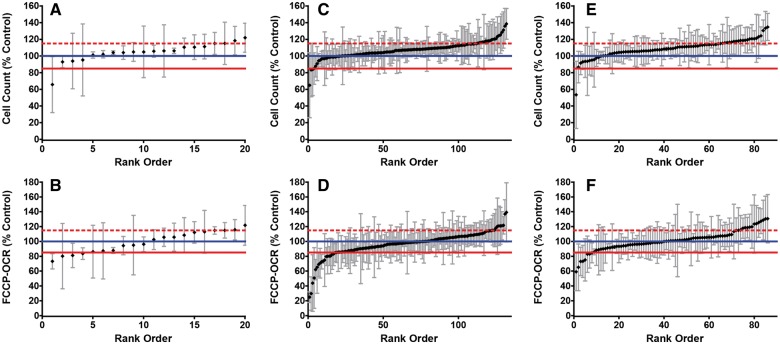
To test the differences in toxicant susceptibility between cells in primary culture and cultured cell lines, HK-2 (human kidney-2) cells were exposed to a subset of ToxCast Phase II and the results were compared with those of the RPTC. HK-2 cells were exposed for 1 h to 5 µM of the 19 cytotoxicants identified in RPTC after 1-h exposure. Only 1 (5%) of the tested cytotoxicants was confirmed to be cytotoxic in the HK-2 cells. Of the remaining 18 compounds: 3 (16%) were ETC inhibitors, and 15 (79%) had no effect (Fig.4A and B; Table 3).
FIG. 4.
Cell count and respiration in HK2 1 h after exposure to 5 µM of select compounds from ToxCast Phase II. A, Average cell count and B, average FCCP-OCR of cytotoxicants relative to the vehicle control. C, Average cell count and D, average FCCP-OCR of ETC inhibitors relative to the vehicle control. E, Average cell count and F, average FCCP-OCR of no effect compounds relative to the vehicle control. The blue line indicates the mean for vehicle control wells. The solid red line indicates results <85% of the mean. The dashed red line indicates results >115% above the mean.
TABLE 3.
Classification of RPTC Identified Cytotoxicants in HK2 After 1-h Exposure to 5 µM
| Category | Number | % |
|---|---|---|
| Cytotoxicants | 1 | 5 |
| ETC inhibitors | 3 | 16 |
| No effect | 15 | 79 |
| Total | 19 |
HK-2 cells were exposed for 1 h to 5 µM of 1/3 (131) of the compounds identified in RPTC as ETC inhibitors. Only 14 (11%) of the ETC inhibitors were confirmed to have a similar effect in HK-2 (Table 4). Of the remaining 117 compounds 3 (2%) were cytotoxicants, and 114 (87%) had no effect (Fig.4C and D; Table 5). Exposure of HK-2 cells for 1 h to 5 µM of 1/3 (85) of the compounds identified in RPTC as having no effect confirmed that 78 (92%) of the compounds had no effect in HK-2 (Fig. 4E and F; Table 6). One of the compounds (1%) was identified as being cytotoxic, and 6 (7%) were identified as ETC inhibitors.
TABLE 4.
ETC Inhibitors in RPTC and HK2 After 1-h Exposure to 5 µM
| Chemical Name | CAS Numbers | Average RPTC FCCP-OCR Relative to Vehicle Control | Average HK-2 FCCP-OCR Relative to Vehicle Control |
|---|---|---|---|
| 2,4-Bis(1-methyl-1-phenylethyl)phenol | 2772-45-4 | 44.0 ± 19.0 | 70.7 ± 9.9 |
| 2,4-Bis(2-methylbutan-2-yl)phenol | 120-95-6 | 17.9 ± 5.1 | 24.9 ± 3.6 |
| 3-chloro-2-[(3 R)-5-chloro-1 -(2,4-dimethoxybenzyl)-3-methyl-2-oxo-2,3-dihydro-1H-indol-3-yl]-N-ethyl-N-(pyridin-3-ylmethyl)benzamide hydrochloride | NOCAS_47379 | 60.8 ± 19.1 | 83.2 ± 7.9 |
| 3-Methylaniline | 108-44-1 | 84.4 ± 19.4 | 74.9 ± 17.8 |
| 4,4'-Methylenebis(N,N-dimethylaniline) | 101-61-1 | 71.0 ± 25.9 | 82.8 ± 10.2 |
| 5-fluoro-1 -(3-fluorobenzyl)-N-(1H-indol-5-yl)-1H-indole-2-carboxamide | NOCAS_47366 | 40.6 ± 13.9 | 72.7 ± 4.9 |
| Carbosulfan | 55285-14-8 | 46.0 ± 17.1 | 79.6 ± 6.1 |
| Clotrimazole | 23593-75-1 | 58.8 ± 26.9 | 65.3 ± 17.5 |
| Gentian violet | 548-62-9 | 15.9 ± 3.9 | 43.8 ± 19.1 |
| Heptachlor | 76-44-8 | 65.0 ± 12.5 | 80.0 ± 11.8 |
| Mercuric chloride | 7487-94-7 | 32.0 ± 11.5 | 29.7 ± 11.3 |
| Octrizole | 3147-75-9 | 48.2 ± 6.4 | 80.2 ± 6.3 |
| Oryzalin | 19044-88-3 | 67.7 ± 26.7 | 80.4 ± 11.9 |
| Phenylmercuric acetate | 62-38-4 | 11.4 ± 2.6 | 50.7 ± 20.4 |
TABLE 5.
Classification of a Subset of RPTC Identified ETC Inhibitors in HK2 After 1-h Exposure to 5 µM
| Category | Number | % |
|---|---|---|
| Cytotoxicants | 3 | 2 |
| ETC Inhibitors | 14 | 11 |
| No Effect | 114 | 87 |
| Total | 131 |
TABLE 6.
Classification of a Subset of RPTC Identified No Effect Compounds in HK2 After 1-h Exposure to 5 µM
| Category | Number | % |
|---|---|---|
| Cytotoxicants | 1 | 1 |
| ETC inhibitors | 6 | 7 |
| No effect | 78 | 92 |
| Total | 85 |
Mitochondrial Uncouplers From ToxCast Phase II Identified in HK-2 Cells
Compounds classified as mitochondrial uncouplers in RPTC were tested for their effects in HK-2 cells. HK-2 cells were exposed to the compounds for 3 min and evaluated for respiratory capacity. Injection of 0.5 µM of the proton ionophore FCCP was used as a positive control. Exposure to FCCP resulted in an increase in OCR to 1.2-fold of the vehicle control compared with the 2-fold increase in FCCP OCR observed in RPTC (Fig. 5A). A compound was classified as an uncoupler based on a treatment-OCR ≥ 115% of the vehicle control. Subsequently HK-2 cells were exposed to 0.5 µM of the ATP synthase inhibitor oligomycin. Only perfluorooctanesulfonamide was confirmed as mitochondrial uncoupler after a 3-min exposure (Figs. 5A and 6). The OCR of perfluorooctanesulfonamide remained elevated after exposure to oligomycin, confirming its functionality as a mitochondrial uncoupler (Fig. 5B).
FIG. 5.
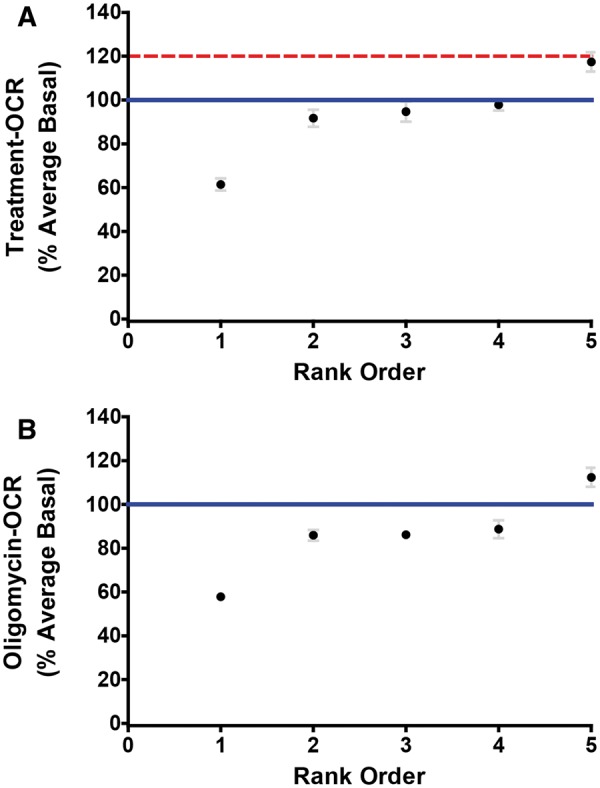
Treatment and oligomycin OCR in HK-2. A, Average treatment-OCR relative to the vehicle control 3 min after exposure to 5 µM of uncouplers identified in HK-2. B, Average oligomycin-OCR relative to the vehicle control after exposure to 5 µM of uncouplers identified in HK-2. The blue line indicates the mean for vehicle control wells. The dashed red line indicates the mean for the positive control FCCP response in HK-2.
DISCUSSION
The EPA and the NIH have developed a long-term goal of using more in vitro toxicity testing to streamline decision processes with more mechanistic guidance. These efforts, highlighted in the ToxCast project, focus on integrating molecular, chemical, and computation toxicology to evaluate thousands of environmental contaminants that have yet to be tested. One of the goals of the ToxCast program is to develop in vitro cell models to examine specific cellular targets of toxicity as a method for determining the mechanism of toxicity as well as provide a framework for creating predictive toxicological models in the future.
Previously, our laboratory developed and utilized a phenotypic respirometric assay combined with cellular microscopy to screen chemical libraries for mitochondrial toxicity in primary RPTC (Wills et al., 2013). This assay was used to acquire toxicity data from 2 chemically diverse libraries, the Sigma LOPAC (1280 compounds) and a 480 compound subset of the ChemBridge DIVERSet. Although these libraries are not enriched for toxicants, 31 compounds were identified that cause mitochondrial dysfunction. These mitochondrial toxicants were further analyzed for chemical similarity resulting in the identification of a predictive toxicophore of mitochondrial injury. The respirometric assay highlighted in this research provides further mechanistic insights into the types of dysfunction caused when compared with mitochondrial membrane potential analysis (Attene-Ramos et al., 2013). The combined measurement of cytotoxicity and OCR allows for the examination of the mechanism of action and efficient distinction between cytotoxicants, mitochondrial uncouplers, and ETC inhibitors. For these reasons, we utilized this method to evaluate ToxCast Phase II for the abundance of mitochondrial toxicants.
RPTC were exposed to the 676 compounds in ToxCast Phase II for 1 h to identify compounds that cause mitochondrial toxicity quickly after exposure. Exposure of RPTC to 5 µM of the 676 compounds for 1 h identified 19 cytoxicants, decreased in cell count irrespective of the resulting effects on mitochondrial function (Fig. 1A; Table 1). The assay also identified 26 compounds that exhibited increased basal respiration (Table 1) as potential mitochondrial uncouplers. The assay identified 379 compounds that were ETC inhibitors, decreased FCCP-OCR, in RPTC after a 1-h exposure at 5 µM concentration (Fig. 1B; Table 1). We chose a concentration of 5 µM for the toxicity assays because it is a common concentration used to screen pharmacologically active compounds. It is possible that other identified compounds could be toxic if examined under alternative experimental conditions, including higher concentrations. Although multiple concentrations of the toxicants were not tested, an evaluation of potency may be the focus of a future studies.
A secondary screen was conducted in RPTC after a 5-h exposure to identify any slower acting mitochondrial toxicants. The 252 compounds from ToxCast Phase II that had no effect on mitochondrial function and the 24 that caused high basal respiration were examined after a 5-h exposure to 5 µM. Three compounds were identified as cytotoxicants (Fig. 1C; Table 2) and 110 compounds were identified as ETC inhibitors after the 5-h exposure (Fig. 1D; Table 2). These compounds were not classified as mitochondrial toxicants after 1 h, indicating that biotransformation or disruption of signaling pathways may be required to elicit their detrimental effects on mitochondrial function. A number of P450 isoforms are expressed in the kidney of rodents, including members of the CYP1A, 2B, 2C, 2E, 2J, 3A, 4A, and 4F subfamilies (Cummings et al., 1999; Ma et al., 1999; Stec et al., 2003). Within the kidney, the renal proximal tubule has the highest concentrations of P450s and cytochrome P450 reductase (Cummings et al., 1999). In total, after the 1- and 5-h exposure in RPTC with 5 µM of the 676 compounds within ToxCast Phase II we classified 22 (3%) cytotoxicants, 486(72%) ETC inhibitors, 5 (1%) uncouplers, and 163 (24%) compounds with no measurable effect on cellular viability or mitochondrial function (Table 7). Previous research in our laboratory indicated that a longer exposure time (up to 24 h) would not yield a large increase in the number of mitochondrial toxicants identified (Wills et al., 2013). For any compounds identified as mitochondrial toxicants after an exposure time >5 h mitochondrial dysfunction may be a secondary effect of an alteration in protein translation or cellular signaling.
TABLE 7.
Classification of ToxCast Phase II Compounds After 1- and 5-h Exposure to 5 µM in RPTC
| Category | Number | % |
|---|---|---|
| Cytotoxicants | 22 | 3 |
| ETC inhibitors | 486 | 72 |
| Uncouplers | 5 | 1 |
| No effect | 163 | 24 |
| Total | 676 |
Only 5 compounds were identified and confirmed as uncouplers: DNOC, pentochlorophenol, perfluorootanesulfonamide, tributyltin and didecyldimethylammonium chloride (Figs. 2 and 3). DNOC is an herbicide that has been identified as an uncoupler in rat liver mitochondria, causing a decrease transmembrane potential and an increase in mitochondrial respiration in both the presence and absence of ADP (Castilho et al., 1997). Pentochlorophenol is used as both a pesticide and a disinfectant, and has been established as a protonophoric uncoupler (Bakker et al., 1974; Valmas et al., 2008). Perfluorootanesulfonamide was used as an ingredient in Scotchgard, and it was added to food packaging to repel water and grease (Fromme et al., 2009). Schnellmann and Manning (1990) established that perfluorootanesulfonamide is a protonophore that uncouples mitochondrial oxidative phosphorylation.
Tributyltin is a biocide that was used for decades as an anti-fouling agent for ships. Although not previously identified as a mitochondrial uncoupler, it has been shown to be mitochondrially toxic by inducing the release of cytochrome c eventually resulting in apoptosis of the affected cells (Nishikimi et al., 2001). Didecyldimethylammonium chloride is used as a fungicide and a microbiocide (Lim and Chung, 2014; van Slooten et al., 2015). Currently there is no research classifiying didecyldimethylammonium chloride as a mitochondrial toxicant. Although these findings do not contribute to the toxicological knowledge of these compounds, these data do provide proof of principle in the ability of the assay to identify mitochondrial toxicants.
To compare the differences between primary and cultured cells for their ability to detect mitochondrial toxicity, HK-2 cells were exposed to 5 µM of a subset of the ToxCast library for 1 h. Of 19 cytotoxicants identified in RPTC, 1 (5%) compound was confirmed to be cytotoxic, 3 (16%) were ETC inhibitors, and 15 (79%) had no effect (Fig. 4A and B; Table 3). HK-2 cells were also exposed to a third (86) of the compounds that had no effect on the RPTC after 1-h exposure. Seventy-nine (92%) of these compounds also had no effect on mitochondrial respiration or cellular count in the HK-2, 1 (1%) was cytotoxic, and 6 (7%) were ETC inhibitors (Fig. 4E and F; Table 6). HK-2 cells were exposed to a third (131) of the compounds classified as ETC inhibitors in RPTC. Three (2%) of the compounds were cytotoxic, and 114 (87%) had no effect (Fig. 4C and D; Table 4). Only 14 (11%) of the RPTC ETC inhibitors were confirmed to be ETC inhibitors in the HK-2 (Table 5). Finally, only perfluorootanesulfonamide was confirmed to be a mitochondrial uncoupler in HK-2 cells (Figs. 5 and 6). These data demonstrate a marked decrease in the sensitivity of HK-2 cells to cytotoxicity and in the identification of ETC inhibitors and mitochondrial uncouplers compared with RPTC. However, of those compounds that had no effect on RPTC after 1-h exposure, 92% of them also had no effect in HK-2 cells, demonstrating concordance in the identification of nontoxic chemicals. However, HK-2 cells may lead to false negatives with respect cytotoxic and mitochondrial toxic chemicals.
FIG. 6.
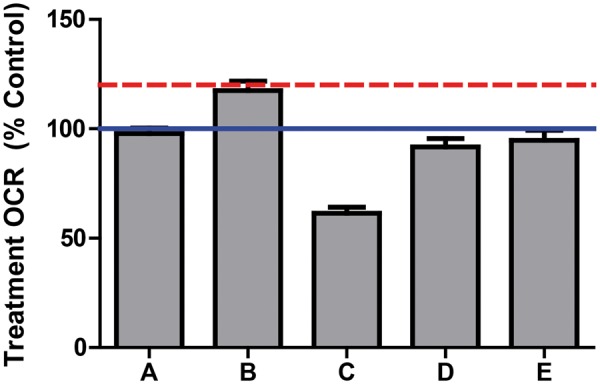
Treatment-OCR in HK-2 3 min after exposure to 5 µM of mitochondrial uncouplers from ToxCast Phase II. A, 2-methyl-4,6-dinitrophenol; B, perfluorooctanesulfonamide; C, tributyltin, D, pentachlorophenol; E, didecyldimethylammonium chloride. Data represented as mean ± SEM of 4 biological replicates. The solid line indicated the mean for the vehicle control wells. The dashed line indicates the mean for the positive control FCCP response in HK-2.
The 14 identified common mitochondrial toxicants were further evaluated for their chemical and toxicological profiles. The purpose of this analysis was to determine if these compounds shared chemical similarity accounting for the comparable effects in both primary and cultured cells. We also wanted to determine if there was available research previously identifying any of these compounds as mitochondrial toxicants. Within the 14 common ETC inhibitors there is a high level of chemical diversity and only 3 have known mitochondrial liability. Clotrimazole is an anti-fungal agent and potential cancer therapeutic that reduces mitochondrial activity in breast cancer cells (Furtado et al., 2012). Gentian violet is both an anti-bacterial and anti-fungal agent that causes mitochondrial swelling and disrupts the production of ATP (Docampo et al., 1988). Mercuric chloride exposure causes the mitochondrial release of cytochrome c and induction of the mitochondrial permeability transition (Araragi et al., 2003). Of the remaining 11 compounds 4 have no known toxicity and 7 have toxicity profiles that do not include mitochondrial dysfunction.
HK-2 cells are a proximal tubule epithelial cell line immortalized from adult human kidney using E6/E7 genes from human papilloma virus (Ryan et al., 1994). Although this method of transformation does enable the cells to maintain some specific features of differentiation, it is unclear whether these cells are reliant enough on mitochondrial oxidative phosphorylation to serve as an ideal model for characterizing mitochondrial toxicity (Marroquin et al., 2007). In contrast the primary culture of RPTC utilized in this study have been grown under improved culture conditions which have been shown to promote cell growth, maintain normal cellular transport, and stimulate in vivo-like respiration (Nowak and Schnellmann, 1996). As a result of these differences the use of the respirometric assay in RPTC was able to identify mitochondrial toxicity within 73% of the ToxCast Phase II library, while the HK-2 were only able to identify 10%. These differences in toxic susceptibility have implications when endeavoring to construct an informative database that will be used for predictive toxicology in the future. Attempts have been made to culture HK-2 in a low glucose environment in order to reduce the reliance on glycolysis and increase the reliance on mitochondrial ATP. These efforts have mixed results, and in our laboratory have not resulted in a cell line with respiration as robust as the primary RPTC. Although they are more costly in time and effort, the use of primary cells in mitochondrial assays will provide the more informative data of mitochondrial toxicity.
Mitochondria are a key component of the cellular response to environmental contaminants and stress. They are essential for maintaining energy through ATP production, as well as regulating cellular homeostasis. Compounds that deregulate mitochondrial processes have the potential to cause severe cellular, tissue and organ damage. Furthermore, chronic disruption of mitochondrial processes may sensitize a tissue to a toxic exposure. In the process of regulating current and emerging chemicals in the environment, it is essential that the potential for mitochondrial toxicity is assessed. The goal of this research is to improve current approaches for determining mitochondrial toxicity. The results of this assay are more informative of the potential effects on human health than the previous methods that use cell lines dependent on glycolysis rather than mitochondrial respiration. Ultimately these data can be used to develop mitochondrially specific toxicophores to predict the mitochondrial risks of novel chemicals.
FUNDING
Funding for this project was provided through grant 2R44-ES019378-02.
Supplementary Material
ACKNOWLEDGMENTS
We would like to acknowledge the Environmental Protection Agency for access to ToxCast Phase II and the MUSC/Seahorse Biosciences Academic Core Facility.
REFERENCES
- Araragi S., Kondoh M., Kawase M., Saito S., Higashimoto M., Sato M. (2003). Mercuric chloride induces apoptosis via a mitochondrial-dependent pathway in human leukemia cells. Toxicology 184, 1–9. [DOI] [PubMed] [Google Scholar]
- Attene-Ramos M. S., Huang R., Sakamuru S., Witt K. L., Beeson G. C., Shou L., Schnellmann R. G., Beeson C. C., Tice R. R., Austin C. P., Xia M. (2013). Systematic study of mitochondrial toxicity of environmental chemicals using quantitative high throughput screening. Chem. Res. Toxicol. 26, 1323–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker E. P., Van Den Heuvel E. J., Van Dam K. (1974). The binding of uncouplers of oxidative phosphorylation to rat-liver mitochondria. Biochim. Biophys. Acta 333, 12–21. [DOI] [PubMed] [Google Scholar]
- Beeson C. C., Beeson G. C., Schnellmann R. G. (2010). A high-throughput respirometric assay for mitochondrial biogenesis and toxicity. Anal. Biochem. 404, 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch-Machin M. A., Turnbull D. M. (2001). Assaying mitochondrial respiratory complex activity in mitochondria isolated from human cells and tissues. In Methods in Cell Biology (Liza E. A. S., Pon A., Eds.), Vol. 65, pp. 97–117. Academic Press, New York. [DOI] [PubMed] [Google Scholar]
- Castilho R. F., Vicente J. A. F., Kowaltowski A. J., Vercesi A. E. (1997). 4,6-Dinitro-o-cresol uncouples oxidative phosphorylation and induces membrane permeability transition in rat liver mitochondria. Int. J. Biochem. Cell Biol. 29, 1005–1011. [DOI] [PubMed] [Google Scholar]
- Chan K., Truong D., Shangari N., O’Brien P. J. (2005). Drug-induced mitochondrial toxicity. Exp. Opin. Drug Metab. Toxicol. 1, 655–669. [DOI] [PubMed] [Google Scholar]
- Council N. R. (2007). Committee on Toxicity Testing and Assessment of Environmental Agents, National Research Council. [Google Scholar]
- Cummings B. S., Zangar R. C., Novak R. F., Lash L. H. (1999). Cellular distribution of cytochromes P-450 in the rat kidney. Drug Metab. Dispos. 27, 542–548. [PubMed] [Google Scholar]
- Dix D. J., Houck K. A., Martin M. T., Richard A. M., Setzer R. W., Kavlock R. J. (2007). The toxcast program for prioritizing toxicity testing of environmental chemicals. Toxicol. Sci. 95, 5–12. [DOI] [PubMed] [Google Scholar]
- Docampo R., Moreno S. N., Gadelha F. R., de Souza W., Cruz F. S. (1988). Prevention of Chagas’ disease resulting from blood transfusion by treatment of blood: Toxicity and mode of action of gentian violet. Biomed. Environ. Sci. 1, 406–413. [PubMed] [Google Scholar]
- Dykens J. A., Will Y. (2007). 91 Drug-induced mitochondrial toxicity: Drug safety considerations. Mitochondrion 7, 430–431. [Google Scholar]
- Ferrick D. A., Neilson A., Beeson C. (2008). Advances in measuring cellular bioenergetics using extracellular flux. Drug Discov. Today 13, 268–274. [DOI] [PubMed] [Google Scholar]
- Fromme H., Tittlemier S. A., Völkel W., Wilhelm M., Twardella D. (2009). Perfluorinated compounds—Exposure assessment for the general population in western countries. Int. J. Hyg. Environ. Health 212, 239–270. [DOI] [PubMed] [Google Scholar]
- Furtado C. M., Marcondes M. C., Sola-Penna M., de Souza M. L. S., Zancan P. (2012). Clotrimazole preferentially inhibits human breast cancer cell proliferation, viability and glycolysis. PLoS One 7, e30462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerencser A. A., Neilson A., Choi S. W., Edman U., Yadava N., Oh R. J., Ferrick D. A., Nicholls D. G., Brand M. D. (2009). Quantitative microplate-based respirometry with correction for oxygen diffusion. Anal. Chem. 81, 6868–6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch T., Susin S. A., Marzo I., Marchetti P., Zamzami N., Kroemer G. (1998). Mitochondrial permeability transition in apoptosis and necrosis. Cell Biol. Toxicol. 14, 141–145. [DOI] [PubMed] [Google Scholar]
- Judson R. S., Houck K. A., Kavlock R. J., Knudsen T. B., Martin M. T., Mortensen H. M., Reif D. M., Rotroff D. M., Shah I., Richard A. M., Dix D. J. (2009). In Vitro Screening of Environmental chemicals for targeted testing prioritization: The ToxCast project. Environ. Health Perspect. 118, 485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemasters J. J., Ramshesh V. K. (2007). Imaging of mitochondrial polarization and depolarization with cationic fluorophores. In Methods in Cell Biology (Liza A. P., Eric A. S., Eds.), Vol. 80, pp. 283–295. Academic Press, New York. [DOI] [PubMed] [Google Scholar]
- Lim C.-H., Chung Y.-H. (2014). Effects of Didecyldimethylammonium chloride on sprague-dawley rats after two weeks of inhalation exposure. Toxicol. Res. 30, 205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Qu W., Scarborough P. E., Tomer K. B., Moomaw C. R., Maronpot R., Davis L. S., Breyer M. D., Zeldin D. C. (1999). Molecular cloning, enzymatic characterization, developmental expression, and cellular localization of a mouse cytochrome P450 highly expressed in kidney. J. Biol. Chem. 274, 17777–17788. [DOI] [PubMed] [Google Scholar]
- Marroquin L. D., Hynes J., Dykens J. A., Jamieson J. D., Will Y. (2007). Circumventing the crabtree effect: Replacing media glucose with galactose increases susceptibility of HepG2 cells to mitochondrial toxicants. Toxicol. Sci. 97, 539–547. [DOI] [PubMed] [Google Scholar]
- Nishikimi A., Kira Y., Kasahara E., Sato E. F., Kanno T., Utsumi K., Inoue M. (2001). Tributyltin interacts with mitochondria and induces cytochrome c release. Biochem. J. 356, 621–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak G., Schnellmann R. G. (1995). Improved culture conditions stimulate gluconeogenesis in primary cultures of renal proximal tubule cells. Am. J. Physiol. Cell. Physiol. 268, C1053–C1061. [DOI] [PubMed] [Google Scholar]
- Nowak G., Schnellmann R. G. (1996). L-ascorbic acid regulates growth and metabolism of renal cells: Improvements in cell culture. Am. J. Physiol. Cell Physiol. 271, C2072–C2080. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Enríquez S., Juárez O., Rodríguez-Zavala J. S., Moreno-Sánchez R. (2001). Multisite control of the Crabtree effect in ascites hepatoma cells. Eur. J. Biochem. 268, 2512–2519. [DOI] [PubMed] [Google Scholar]
- Rogers G. W., Brand M. D., Petrosyan S., Ashok D., Elorza A. A., Ferrick D. A., Murphy A. N. (2011). High throughput microplate respiratory measurements using minimal quantities of isolated mitochondria. PLoS One 6, e21746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan M. J., Johnson G., Kirk J., Fuerstenberg S. M., Zager R. A., Torok-Storb B. (1994). HK-2: An immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int. 45, 48–57. [DOI] [PubMed] [Google Scholar]
- Schnellmann R. G., Ewell F. P., Sgambati M., Mandel L. J. (1987). Mitochondrial toxicity of 2-bromohydroquinone in rabbit renal proximal tubules. Toxicol. Appl. Pharmacol. 90, 420–426. [DOI] [PubMed] [Google Scholar]
- Schnellmann R. G., Manning R. O. (1990). Perfluorooctane sulfonamide: A structurally novel uncoupler of oxidative phosphorylation. Biochim. Biophys. Acta 1016, 344–348. [DOI] [PubMed] [Google Scholar]
- Sherer T. B., Richardson J. R., Testa C. M., Seo B. B., Panov A. V., Yagi T., Matsuno-Yagi A., Miller G. W., Greenamyre J. T. (2007). Mechanism of toxicity of pesticides acting at complex I: relevance to environmental etiologies of Parkinson’s disease. J. Neurochem. 100, 1469–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipes N. S., Martin M. T., Kothiya P., Reif D. M., Judson R. S., Richard A. M., Houck K. A., Dix D. J., Kavlock R. J., Knudsen T. B. (2013). Profiling 976 toxcast chemicals across 331 enzymatic and receptor signaling assays. Chem. Res. Toxicol. 26, 878–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stec D. E., Flasch A., Roman R. J., White J. A. (2003). Distribution of cytochrome P-450 4A and 4F isoforms along the nephron in mice. Am. J. Physiol. Renal Physiol. 284, F95–F102. [DOI] [PubMed] [Google Scholar]
- Valmas N., Zuryn S., Ebert P. R. (2008). Mitochondrial uncouplers act synergistically with the fumigant phosphine to disrupt mitochondrial membrane potential and cause cell death. Toxicology 252, 33–39. [DOI] [PubMed] [Google Scholar]
- van Slooten C., Peperzak L., Buma A. G. J. (2015). Assessment of didecyldimethylammonium chloride as a ballast water treatment method. Environ. Technol. 36, 435–449. [DOI] [PubMed] [Google Scholar]
- Wallace K. B., Starkov A. A. (2000). Mitochondrial targets of drug toxicity. Annu. Rev. Pharmacol. Toxicol. 40, 353–388. [DOI] [PubMed] [Google Scholar]
- Wills L. P., Beeson G. C., Trager R. E., Lindsey C. C., Beeson C. C., Peterson Y. K., Schnellmann R. G. (2013). High-throughput respirometric assay identifies predictive toxicophore of mitochondrial injury. Toxicol. Appl. Pharmacol. 272, 490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills L. P., Trager R. E., Beeson G. C., Lindsey C. C., Peterson Y. K., Beeson C. C., Schnellmann R. G. (2012). The β2-adrenoceptor agonist formoterol stimulates mitochondrial biogenesis. J. Pharmacol. Exp. Therap. 342, 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.