Abstract
Objectives. There is conflicting evidence regarding prognosis in patients with primary SS (pSS). The aim of this study was to estimate the rate, risk factors and causes of mortality in patients with pSS through a systematic review and meta-analysis.
Methods. Through a systematic review of multiple databases through October 2014, we identified cohort studies reporting relative risk (compared with standardized population), risk factors and causes of mortality in patients with pSS. We estimated summary risk ratios (RRs) with 95% CIs using random effects model.
Results. We identified 10 studies with 7888 patients (91% females) with pSS, of whom 682 patients died over a median average follow-up of 9 years. The pooled standardized mortality ratio in patients with pSS was 1.38 (95% CI 0.94, 2.01). Leading causes of mortality were cardiovascular diseases, solid-organ and lymphoid malignancies and infections; however, it is unclear whether these observed causes were overrepresented in patients with pSS as compared with the general population. Risk factors associated with increased mortality were advanced age at diagnosis [RR 1.09 (95% CI 1.07, 1.12)], male sex [RR 2.18 (95% CI 1.45, 3.27)], parotid enlargement [RR 1.81 (95% CI 1.02, 3.21)], abnormal parotid scintigraphy [RR 2.96 (95% CI 1.36, 6.45)], extraglandular involvement [RR 1.77 (95% CI 1.06, 2.95)], vasculitis [RR 7.27 (95% CI 2.70, 19.57)], anti-SSB positivity [RR 1.45 (95% CI 1.03, 2.04)], low C3 [RR 2.14 (95% CI 1.38, 3.32)] and C4 [RR 3.08 (95% CI 2.14, 4.42)] and cryoglobulinaemia [RR 2.62 (95% CI 1.77, 3.90)].
Conclusion. pSS is not associated with an increase in all-cause mortality as compared with the general population. However, a subset of patients with extraglandular involvement, vasculitis, hypocomplementaemia and cryoglobulinaemia may be at increased risk of mortality and require close follow-up.
Keywords: Sjögren’s syndrome, mortality, lymphoma, risk factors, vasculitis
Rheumatology key messages
There is no significant increase in all-cause mortality risk in patients with primary SS (pSS).
Older pSS patients with parotid enlargement, extraglandular involvement, low complement and cryoglobulinaemia may be at increased mortality risk.
Cardiovascular diseases, infections and solid-organ and haematological malignancies are leading causes of mortality in pSS.
Introduction
SS is one of the most prevalent autoimmune diseases, predominantly affecting females in the fourth–sixth decade of life, with inflammatory damage to lacrimal and salivary glands resulting in sicca symptoms [1, 2]. In a recent systematic review of 21 studies, the pooled incidence and prevalence of primary SS (pSS) was estimated at 6.92/100 000 person-years and 60.82/100 000 persons, respectively [3]. In addition to exocrine glands, patients with pSS may have systemic involvement and frequently demonstrate serologic findings such as ANA, anti-Ro/SSA, anti-La/SSB antibodies and RF [2]. pSS is also associated with an increased risk of malignancies [relative risk (RR) 1.53], in particular non-Hodgkin’s lymphoma (RR 13.76) [4].
The long-term prognosis of patients with pSS is unclear. The estimated 5 and 10 year survival after pSS diagnosis is 95% and 90%, respectively [5]. Population-based studies have shown conflicting evidence regarding relative mortality in pSS. In a recent study of 105 patients with pSS in Olmsted County, MN, USA, we observed no increase in mortality risk compared with age- and sex-matched populations [standardized mortality ratio (SMR) 0.85] [6]. On the other hand, in a Scottish study of 834 adults hospitalized with SS between 1981 and 2000, the overall mortality rate over 7 years was ∼2-fold higher as compared with the general population [7].
Risk factors associated with increased mortality in patients with pSS are poorly understood. Studies have variably suggested male sex, parotid gland enlargement, extraglandular involvement and certain serological markers such as low complement levels and cryoglobulinaemia as poor prognostic markers, but these are not consistent across studies [5, 8, 9].
Finally, causes of mortality in patients with pSS are unclear. While lymphoma may increase mortality, low-grade marginal zone lymphoma related to mucosa-associated lymphoid tissue, the most common lymphoid neoplasia in patients with SS, generally carries a very good prognosis [10]. In a recent multicentre referral cohort study from Spain, cardiovascular diseases and infections were the leading causes of mortality in patients with pSS [5]. Hence, to better understand the prognosis of pSS, we performed a systematic review and meta-analysis of observational studies reporting the rate, risk factors and causes of mortality in patients with pSS.
Methods
We conducted and reported this systematic review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and followed a priori established protocol [11].
Selection criteria
In this systematic review, we included cohort studies of patients with pSS that met the following criteria: population-based or representative of the community rheumatology practice or multicentre studies, reported mortality rate in pSS patients (with or without risk factors and causes of mortality) and compared the mortality rate with the general population or matched control population, reported as SMR or RR, respectively.
We excluded the following studies from the meta-analysis: single-centre studies with high attrition rate (<80% follow-up); lack of comparison of mortality rate to a control, non-SS population; and studies with insufficient information to allow estimation of SMR with 95% CI. Although these studies were excluded from quantitative synthesis of mortality risk in patients with pSS, key findings on risk factors for mortality, if reported in these studies, were included in the systematic review. In case of multiple studies from the same cohort, we included data from the most recent comprehensive report; if there was minimal overlap of time period, then both studies were included.
Search strategy
We conducted a comprehensive search of multiple electronic databases from each databases’ inception to 27 October 2014, with no language restrictions. The databases included Ovid Medline In-Process & Other Non-Indexed Citations, Ovid MEDLINE, Ovid EMBASE, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, Web of Science and Scopus. The search strategy was designed and conducted by an experienced medical librarian with input from the study’s investigators, using controlled vocabulary supplemented with keywords to search for cohort studies reporting mortality in SS. The details of the search strategy are included in the supplementary data, available at Rheumatology Online. Two authors, independently reviewed the title and abstract of studies identified in the search to exclude studies that did not address the research question of interest, based on pre-specified inclusion and exclusion criteria (see above). The full texts of the remaining articles were examined to determine whether they contained relevant information; discrepancy in article selection was resolved by consensus. Next, a recursive search of reference lists of all relevant articles was conducted to search for additional studies. Third, a manual search of conference proceedings from major rheumatology conferences (annual meetings of the ACR and EULAR) between 2010 and 2014 was conducted to identify additional studies published only in abstract form.
Data extraction and quality assessment
Data on the following study- and patient-related characteristics were independently abstracted onto a standardized form by two investigators (and discrepancies were resolved by consensus): study characteristics [primary author, time period of study/year of publication, follow-up, geographic location of the population studied, study design and setting (prospective vs retrospective, population-based vs referral cohort), case ascertainment method], patient characteristics [total number of patients with SS (including pSS and secondary SS), diagnostic criteria used, primary symptoms, serological characteristics]; risk factors for mortality [demographic, clinical and serological factors associated with mortality (both unadjusted and adjusted)] and outcomes (observed mortality rate, causes of mortality, expected mortality in age- and sex-matched population or comparable control population).
The methodological quality of observational studies was assessed independently by two study investigators using the Newcastle–Ottawa scale. In this scale, studies are scored across three categories: selection (four questions) and comparability (two questions) of study groups and ascertainment of the outcome of interest (three questions), with all questions with a score of 1, except for comparability of study groups, where separate points were awarded for controlling age and/or sex (maximum 2 points) [12].
Outcomes assessed
Primary outcome
The primary outcome of interest was the relative mortality in patients with pSS compared with the general population (expressed as SMR).
A priori hypotheses to explain potential heterogeneity in the direction and magnitude of effect among different observational studies included location of the study (North America vs Europe vs Asia) and study setting (population-based vs referral cohort). The following sensitivity analyses were conducted: after exclusion of prevalent cases, after excluding patients with secondary SS and based on the quality of included studies.
Secondary outcomes
In order to identify risk factors associated with mortality, we performed a meta-analysis of reported demographic, clinical and serological factors associated with mortality if reported in two or more studies. We preferentially used adjusted estimates for the pooled analysis. We reported main causes of mortality in patients with pSS as observed in individual studies.
Statistical analysis
We estimated the SMR of pSS with 95% CI using the random effects model described by DerSimonian and Laird [13]. We pooled maximally adjusted hazard ratios of risk factors associated with mortality reported in the respective studies. We assessed heterogeneity between study-specific estimates using the inconsistency index (I2 statistic) [14]. This estimates what proportion of total variances across studies was due to heterogeneity rather than chance; a value >50% is considered suggestive of substantial heterogeneity. Once heterogeneity was noted, we investigated between-study sources of heterogeneity using subgroup analyses by stratifying original estimates according to study characteristics as described above. We ascertained publication bias by visual inspection of funnel plots [15]. For all tests, a probability level <0.05 was considered statistically significant. All analyses were performed using Review Manager (RevMan) v5.3 (Nordic Cochrane Centre, Cochrane Collaboration, Copenhagen, Denmark, 2012).
Results
From a total of 611 studies identified using our search strategy, we included 10 studies in our meta-analysis [5–9, 16–20]. Five studies, from overlapping cohorts, and seven studies that reported overall mortality without a comparative estimate with the general population or control group were excluded. Fig. 1 shows the schematic diagram of study selection.
Fig. 1.
Study identification and selection flowchart
Characteristics and quality of included studies
Table 1 summarizes the studies included in the quantitative synthesis. Overall, these 10 studies included 7888 patients with pSS, of whom 682 patients died during a median average follow-up of 9 years across studies. The first study included patients diagnosed in 1975, and the most recent patients were recruited from 2010. Eight studies were conducted in Europe [5, 7–9, 16–19], one in the USA [6] and one in Asia [20]. Four studies were population-based, with the intention of capturing all patients diagnosed with SS in a defined geographic area [6–8, 20], and four studies were single-centre retrospective cohorts with high-rates of follow-up [9, 16, 18, 19]. One study was a multicentre prospective cohort study by the Spanish Group of Autoimmune Diseases–Sjögren’s Syndrome Study Group of 20 Spanish referral hospitals [5]. Five studies included only incident cases of pSS [6, 8, 16, 18, 20]. Nine studies were limited to patients with pSS; one study based on an administrative claims database of hospitalized patients with SS did not differentiate patients with primary or secondary SS [7].
Table 1.
Baseline characteristics of studies on mortality risk in patients with SS
| Reference | Location | Study design and setting | Time period; follow-up | Case finding; case ascertainment | No. of patients with pSS | Mortality (M/F) | Causes of death | Risk factors for mortality |
|---|---|---|---|---|---|---|---|---|
| Alamanos et al. [8] | Greece | Retrospective cohort; population-based; only pSS | 1982–2003; median 11 years; 16 patients lost to follow-up | All pSS patients referred to rheumatologists (systematic recording system for autoimmune rheumatic diseases with multisource retrieval) |
|
|
|
At diagnosis, older age and low C4 (not reported in Alamanos et al., but obtained from Skoupouli 2000) |
| Brito-Zeron et al. [16] | Spain | Retrospective cohort, single-centre, only pSS | 1984–2002; mean 9.1 years | Prospective enrolment of consecutive pSS patients seen at referral centre |
|
|
|
At diagnosis, systemic involvement, vasculitis, low C4, cryoglobulins |
| Brito-Zeron et al. [5] | Spain | Prospective cohort, multicentre, only pSS | NR; mean 10 years | Prospective enrolment of consecutive pSS patients seen at 20 referral centres in Spain |
|
|
|
At diagnosis, male, altered parotid scintigraphy, lymphopenia, SSA, monoclonal gammopathy, cryoglobulins, low C3, low C4 |
| Horvath et al. [9] | Hungary | Retrospective cohort, single-centre, only pSS | 1975–2010; mean 11 years | Systematic sampling of consecutive pSS patients seen at a referral centre |
|
|
|
At diagnosis or developing during follow-up, old age at diagnosis, polyarthritis, vasculitis, cryoglobulinaemia |
| Ioannidis et al.[17] | Greece | Prospective cohort, two centres, only pSS | 1981–99; mean 6 years; 34 patients lost to follow-up | Prospective enrolment of consecutive pSS patients seen at two referral centres |
|
|
|
At diagnosis, low C4 |
| Nannini et al. [6] | Minnesota, USA | Retrospective cohort, population-based, only pSS | 1976–2005; median 9 years | Administrative database with confirmation through record linkage of all pSS patients |
|
25 | NR | NR |
| Pertovaara et al.[18] | Finland | Retrospective cohort, single-centre, only pSS | 1977–92; median 9 years | Retrospective medical records review of all patients with sicca symptoms to identify pSS cohort |
|
17 |
|
NR |
| Theander et al.[19] | Sweden | Prospective cohort, single-centre, only pSS | 1984–2001; median 7 years | Prospective enrolment of consecutive pSS patients seen at one referral centre, with records linkage to national death register |
|
|
|
At diagnosis, older age at diagnosis, focal sialadenitis on biopsy, low C3, low C4 |
| Thomas et al. [7] | Scotland | Retrospective cohort, population-based, pSS and secondary SS | 1981–2000; mean 7 years | Administrative database of hospitalized patients, with records linkage to national death index |
|
|
NR | NR |
| Weng et al. [20] | Taiwan | Retrospective cohort, population-based, only pSS | 2005–07; NR | Administrative health claims database for pSS with records linkage to national death index |
|
|
NR | NR |
F: female; M: male; NR: not reported; pSS: primary SS.
Table 2 reports the baseline characteristics of patients included in the studies. The median average age of patients was 56 years (range 48–62). Overall, 91% of participants were female. Most studies with patients recruited after 2002 used the American-European consensus diagnostic criteria for pSS [21]. The serological profile was reported in seven studies; ANA (65–94%), RF (30–56%) and SSA (31–77%) and SSB (27–55%) were the most commonly observed positive serologies. In three studies that reported complement [5, 8, 9] and cryoglobulin levels [5, 8, 17], 3–22% and 6–28% of SS patients had hypocomplementaemia and cryoglobulinaemia, respectively.
Table 2.
Baseline clinical and serological characteristics of patients in included studies
| Reference | Age at diagnosis, mean (s.d.) or median (range) | Sex, n | Diagnostic criteria and testing | Serological profile |
|---|---|---|---|---|
| Alamanos et al. [8] | 55 (13) |
|
|
|
| Brito-Zeron et al. [16] | 57 (NR) |
|
|
NR |
| Brito-Zeron et al. [5] | 54 (15) |
|
|
|
| Horvath et al. [9] |
|
|
|
|
| Ioannidis et al. [17] | 53 (IQR 44–68) |
|
|
|
| Nannini et al. [6] | 59 (range 23–95) |
|
|
|
| Pertovaara et al. [18] | 62 (at last follow-up) |
|
|
|
| Theander et al. [19] | 57 (range 11–84) |
|
|
|
| Thomas et al. [7] |
|
|
ICD-9 diagnostic codes | NR |
| Weng et al. [20] |
|
|
ICD-9 diagnostic codes, based on American-European consensus criteria for pSS (2002) | NR |
F: female; ICD-9: International Classification of Diseases, 9th revision; IQR: interquartile range; M: male; NR: not reported; pSS: primary SS.
Supplementary Table S1, available at Rheumatology Online, shows the quality of included studies. Overall, the studies were at moderate risk of bias, particularly selection bias. In addition, studies did not adequately adjust for additional factors that may influence mortality.
Mortality in patients with SS
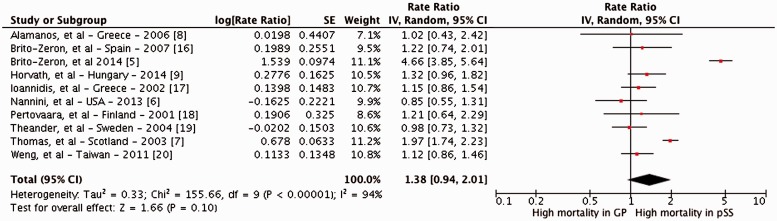
Meta-analysis of these studies revealed no statistically significant increase in the risk of mortality in patients with SS, as compared with the general population [SMR 1.38 (95% CI 0.94, 2.01)], with considerable heterogeneity between studies (I2 = 94%) (Fig. 2). Eight studies did not report a significant increase in mortality risk. The median cumulative 5 and 10 year survival after diagnosis of pSS was 96.4% and 90.5%, respectively.
Fig. 2.
Forest plot of the standardized mortality ratio in patients with primary Sjögren’s syndrome
df: degrees of freedom; GP: general population; I2: inconsistency index; IV: inverse variance; SE: standard error.
Subgroup analysis
The results were stable across study location (Europe vs North America vs Asia) and study setting (population-based vs referral cohorts) (Table 3).
Table 3.
Subgroup and sensitivity analysis on mortality risk in patients with SS
| Subgroups | Categories | No. of studies | RR (95% CI) | Heterogeneity, using I2 (%) | P-value for difference between subgroups |
|---|---|---|---|---|---|
| Subgroup analyses | |||||
| Location | Europe | 8 | 1.51 (0.98, 2.31) | 94 | 0.19 |
| North America | 1 | 0.85 (0.55, 1.31) | — | ||
| Asia | 1 | 1.12 (0.86, 1.46) | — | ||
| Setting | Population-based | 4 | 1.23 (0.77, 0.96) | 88 | 0.64 |
| Referral cohorts | 6 | 1.49 (0.78, 2.85) | 96 | ||
| Sensitivity analyses | |||||
| Only patients with pSS | 9 | 1.31 (0.80, 2.05) | 95 | — | |
| Only incident cases of pSS | 5 | 1.08 (0.89, 1.30) | 0 | — | |
| Excluding two studies with high selection bias towards severe disease | 8 | 1.11 (0.97, 1.26) | 0 | — | |
I2: inconsistency index; pSS: primary SS; RR: relative risk.
Sensitivity analysis
Results were stable on sensitivity analyses including only patients with pSS and only incident cases of pSS (Table 3). Two studies observed a statistically significant increase in mortality with SS—one was a multicentre referral cohort study [5] and another included only hospitalized patients with SS [7]—and both were biased towards patients with severe disease. After exclusion of these two studies, the summary SMR was 1.11 (95% CI 0.97, 1.26), with no observed heterogeneity (I2 = 0%).
Risk factors associated with mortality
Demographic and clinical factors
Meta-analysis revealed that increasing age [three studies; RR per 1 year increase in age at diagnosis 1.09 (95% CI 1.07, 1.12)] and male sex [six studies; RR 2.18 (95% CI 1.45, 3.27)] were associated with increased mortality. Among clinical factors, presence of vasculitis, systemic/extraglandular involvement, parotid enlargement and abnormal parotid scintigraphy were associated with increased mortality risk, with summary risk estimates ranging from 1.8 to 7.3, with low heterogeneity (Table 4). However, most of these variables were inconsistently studied.
Table 4.
Meta-analysis of clinical and serological factors associated with increased mortality in patients with SS
| Risk factor | No. of studies | Relative risk (95% CI) | I2 |
|---|---|---|---|
| Demographic characteristics | |||
| Age (per 1 year) | 3 | 1.09 (1.07, 1.12) | 0 |
| Male (vs females) | 6 | 2.18 (1.45, 3.27) | 40 |
| Clinical characteristics | |||
| Parotid enlargement | 2 | 1.81 (1.02, 3.21) | 0 |
| Abnormal parotid scintigraphy | 2 | 2.96 (1.36, 6.45) | 0 |
| Abnormal salivary gland biopsy | 3 | 1.45 (0.77, 2.71) | 22 |
| Systemic involvement | 3 | 1.77 (1.06, 2.95) | 44 |
| Vasculitis | 2 | 7.27 (2.70, 19.57) | 0 |
| Immunological characteristics | |||
| ANA positivity | 4 | 1.22 (0.81, 1.83) | 0 |
| RF positivity | 3 | 1.39 (0.72, 2.68) | 69 |
| Anti-SSA positivity | 2 | 1.30 (0.92, 1.85) | 0 |
| Anti-SSB positivity | 2 | 1.45 (1.03, 2.04) | 0 |
| Either Anti-SSA or anti-SSB positivity | 2 | 1.32 (0.82, 2.12) | 0 |
| Low C3 | 4 | 2.14 (1.38, 3.32) | 0 |
| Low C4 | 5 | 3.08 (2.14, 4.42) | 3 |
| Cryoglobulinaemia | 3 | 2.62 (1.77, 3.90) | 0 |
Values in bold represent statistically significant results. I2: inconsistency index.
Serological factors
Low C3 [four studies; RR 2.14 (95% CI 1.38, 3.32)] and C4 levels [five studies; RR 3.08 (95% CI 2.14, 4.42)] and cryoglobulinaemia [three studies; RR 2.62 (95% CI 1.77, 3.90)] were consistently associated with higher risk of mortality; the presence of anti-SSB but not anti-SSA was also associated with increased mortality (Table 4). The presence of ANA and RF did not significantly modify mortality risk. In all studies except one, risk factors present at diagnosis were recorded as potential predictors of mortality; Horvath et al. [9] recorded risk factors as either present at diagnosis or developing during the course of the disease.
Causes of mortality
Causes of mortality were reported in seven studies (Table 1) [5, 8, 9, 16–19]. Cardiovascular diseases, solid-organ and lymphoid malignancies and infections were the leading causes of mortality in most studies. However, in most studies there was no information on whether these observed causes were overrepresented in patients with pSS as compared with the general population. In one study, the risk of mortality due to cardiovascular causes or haematological malignancies was comparable in patients with pSS and the general population [19].
Publication bias
There was no evidence of publication bias on examination of the funnel plot (supplementary Fig. S1, available at Rheumatology Online).
Discussion
In this systematic review of 10 cohort studies of 7888 patients with pSS, of whom 682 died over a median follow-up of 9 years, we made several key observations. First, pSS is not associated with a significant increase in all-cause mortality as compared with the general population. In a subset of patients with severe disease, however, pSS may be associated with increased mortality. Second, certain clinical and serological factors may help identify this subset of patients at high risk of mortality and requiring close monitoring and aggressive treatment. These include older males and those with extraglandular and/or vasculitic involvement and parotid enlargement. Serologically, low complement levels and cryoglobulinaemia also increase mortality risk. While these are predictors of the development of lymphoid malignancies [22], these were independently associated with increased mortality. Third, cardiovascular diseases, solid-organ and lymphoid malignancies and infections are leading causes of mortality.
We confirmed previous observations that pSS is a chronic, slow-evolving, non-life-threatening illness in the vast majority of patients, with a 10 year cumulative survival >90%; there was no significant increase in mortality in patients with pSS on multiple subgroup and sensitivity analyses. However, two studies observed an increased mortality risk in patients with pSS, and both studies were potentially at risk of selecting patients with severe disease. In their multicentre prospective cohort study, Brito-Zeron et al. [5] included 1074 patients with pSS referred to 20 centres of excellence in Spain; >50% of patients had extraglandular involvement, a known adverse prognostic marker. Likewise, in their population-based Scottish study, Thomas et al. [7] followed a cohort of patients hospitalized for multiple rheumatologic diseases including SS over two decades and compared mortality with age- and sex-matched controls; conceivably these patients were sicker than usual pSS patients who are managed as outpatients.
Through pooled analysis, we identified several consistent clinical and serological factors associated with increased mortality. The identification of these high-risk baseline factors may be useful in identifying patients who require closer follow-up. Several of these factors, including parotid enlargement, anti-SSA and SSB antibodies and low C3 and C4, have also been associated with increased risk of lymphoma [22]. In a single-centre retrospective cohort study, Ramos-Casals et al. [23] observed that a low C4 level was associated with a high prevalence of systemic pSS involvement, including vasculitis (low vs normal C4; 31% vs 8%, respectively) and peripheral neuropathy (20% vs 5%), presence of cryoglobulins (35% vs 4%), as well as a higher risk of lymphoma (10% vs 2%). Hypocomplementaemia was associated with lower 10 year cumulative survival (vs normal complement levels; 73.6% vs 92.1%, respectively).
More recently, Brito-Zeron et al. [5] observed an association between baseline pSS disease activity and mortality. Based on this, they derived predictive models for death in patients with pSS that demonstrated higher mortality with high baseline EULAR pSS disease activity in at least one domain, EULAR pSS disease activity score ≥14, and more than one predictive laboratory variable (lymphopenia, anti-SSB, monoclonal gammopathy, low C3, low C4 and/or cryoglobulinaemia). While these variables were independently associated with a 1.8- to 2.8-fold increase in mortality, restricting their application to patients with systemic pSS involvement resulted in a 3- to 10-fold higher risk estimate of mortality.
We also observed that, in contrast to previous observations, lymphoid malignancies are not the leading cause of mortality in these patients. Cardiovascular diseases, infections and solid-organ malignancies were leading causes of mortality. However, it is unclear whether pSS confers an increased risk of these or whether these were representative of usual causes of death in an ageing population (particularly cardiovascular diseases and malignancies), regardless of pSS disease status. It is well known that patients with pSS have a higher risk of non-Hodgkin’s lymphoma as compared with the general population; this risk may be up to 13.8-fold higher [4]. While the SMR of pSS patients with non-Hodgkin’s lymphoma is increased at 3.25 (95% CI 1.32, 6.76), compared with 1.08 (95% CI 0.79, 1.45) in pSS patients without lymphoma, the prognosis of mucosa-associated lymphoid tissue lymphomas is usually good, with 5 year survival in excess of 90% [24].
Strengths and limitations
The strengths of this systematic review include a comprehensive and systematic literature search with well-defined inclusion criteria; quantitatively and qualitatively studying all aspects of mortality in pSS, including rate, risk factors and causes of death; and subgroup and sensitivity analyses to evaluate the stability of findings and identify potential factors responsible for inconsistencies.
Our study has several limitations, both at the meta-analysis level and individual study level. At a meta-analysis level, significant heterogeneity was observed in the pooled estimate of risk of mortality. This was explained by two studies with a high risk of selection bias [5, 7]. Second, in our attempt to quantify risk factors associated with mortality in pSS, we used available adjusted and unadjusted data from individual studies for pooling. Unfortunately, multivariate analysis was inconsistently reported with adjustment for different confounding variables across studies, and this somewhat limits the inferences that can be drawn from these observations.
We acknowledge that pooling unadjusted estimates is unable to account for confounding factors. As well, identified risk factors in isolation may not necessarily be the cause of increased mortality, but rather are associated with increased mortality because of their link to other disease features of pSS, such as extraglandular and/or vasculitic involvement.
Third, there were insufficient data to pool causes of mortality in patients with pSS, due to inconsistent reporting. It is unclear whether pSS itself increases the risk of cardiovascular and infection-related mortality, based on our analysis.
Fourth, we are unable to exclude publication bias even though there was no funnel plot asymmetry, given the small number of published studies. It is possible that small negative studies are not published. However, this would only bias results towards null and is unlikely to change observations in our analysis. At an individual study level, single referral-centre studies were at higher risk of selection bias, although rates of attrition were low. Finally, there was limited information on the treatment of pSS, and it is unclear how treatment may modify disease course.
In conclusion, patients with pSS are not at increased mortality risk as compared with the general population. However, as others have also observed, a subset of patients with high-risk clinical (older males, parotid enlargement, extraglandular involvement or vasculitis) and serological markers (hypocomplementaemia, cryoglobulinaemia) warrant close follow-up [25]. In addition, cardiovascular diseases, infections and solid-organ and lymphoid malignancies are leading causes of mortality. Although we were unable to examine this issue, it is likely that high inflammatory disease burden and poor disease control may be associated with increased risk of some of these outcomes. Future studies should focus on clinical and serological scoring indices to identify high-risk patients for risk stratification and inclusion in clinical trials of potential disease-modifying therapy.
Supplementary Material
Acknowledgements
We wish to thank Larry Prokop, Medical Librarian at the Mayo Clinic Library, for helping in the literature search for this systematic review and meta-analysis. Study concept and design: A.G.S., S.S.; acquisition of data: A.G.S., S.S.; analysis and interpretation of data: A.G.S., S.S., E.L.M.; drafting of the manuscript: A.G.S., S.S.; critical revision of the manuscript for important intellectual content: E.L.M.; approval of the final manuscript: A.G.S., S.S., E.L.M.; study supervision: E.L.M.
A.G.S. was supported by CTSA Grant UL1 TR000135 from the National Centre for Advancing Translational Sciences, a component of the National Institutes of Health. This study’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Funding: No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Disclosure statement: The authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
- 1.Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum 2008;58:25–35. [DOI] [PubMed] [Google Scholar]
- 2.Kassan SS, Moutsopoulos HM. Clinical manifestations and early diagnosis of Sjogren syndrome. Arch Intern Med 2004;164:1275–84. [DOI] [PubMed] [Google Scholar]
- 3.Qin B, Wang J, Yang Z, et al. Epidemiology of primary Sjogren’s syndrome: a systematic review and meta-analysis. Ann Rheum Dis 2014 Jun 17. pii: annrheumdis-2014-205375. doi: 10.1136/annrheumdis-2014-205375 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 4.Liang Y, Yang Z, Qin B, Zhong R. Primary Sjogren’s syndrome and malignancy risk: a systematic review and meta-analysis. Ann Rheum Dis 2014;73:1151–6. [DOI] [PubMed] [Google Scholar]
- 5.Brito-Zeron P, Kostov B, Solans R, et al. Systemic activity and mortality in primary Sjogren syndrome: predicting survival using the EULAR-SS Disease Activity Index (ESSDAI) in 1045 patients. Ann Rheum Dis 2014 Nov 28. pii: annrheumdis-2014-206418. doi: 10.1136/annrheumdis-2014-206418 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 6.Nannini C, Jebakumar AJ, Crowson CS, Ryu JH, Matteson EL. Primary Sjogren’s syndrome 1976–2005 and associated interstitial lung disease: a population-based study of incidence and mortality. BMJ Open 2013;3:e003569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas E, Symmons DPM, Brewster DH, Black RJ, Macfarlane GJ. National study of cause-specific mortality in rheumatoid arthritis, juvenile chronic arthritis, and other rheumatic conditions: a 20 year followup study. J Rheumatol 2003;30:958–65. [PubMed] [Google Scholar]
- 8.Alamanos Y, Tsifetaki N, Voulgari PV, et al. Epidemiology of primary Sjogren’s syndrome in north-west Greece, 1982–2003. Rheumatology 2006;45:187–91. [DOI] [PubMed] [Google Scholar]
- 9.Horvath IF, Szanto A, Papp G, Zeher M. Clinical course, prognosis, and cause of death in primary Sjogren’s syndrome. J Immunol Res 2014;2014:647507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Routsias JG, Goules JD, Charalampakis G, et al. Malignant lymphoma in primary Sjogren’s syndrome: an update on the pathogenesis and treatment. Semin Arthritis Rheum 2013;43:178–86. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9, W64. [DOI] [PubMed] [Google Scholar]
- 12.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa, ON: Ottawa Hospital Research Institute; 2011. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 13.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brito-Zeron P, Ramos-Casals M, Bove A, Sentis J, Font J. Predicting adverse outcomes in primary Sjogren’s syndrome: identification of prognostic factors. Rheumatology 2007;46:1359–62. [DOI] [PubMed] [Google Scholar]
- 17.Ioannidis JPA, Vassiliou VA, Moutsopoulos HM. Long-term risk of mortality and lymphoproliferative disease and predictive classification of primary Sjogren’s syndrome. Arthritis Rheum 2002;46:741–7. [DOI] [PubMed] [Google Scholar]
- 18.Pertovaara M, Pukkala E, Laippala P, Miettinen A, Pasternack A. A longitudinal cohort study of Finnish patients with primary Sjogren’s syndrome: clinical, immunological, and epidemiological aspects. Ann Rheum Dis 2001;60:467–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Theander E, Manthorpe R, Jacobsson LTH. Mortality and causes of death in primary Sjogren’s syndrome: a prospective cohort study. Arthritis Rheum 2004;50:1262–9. [DOI] [PubMed] [Google Scholar]
- 20.Weng MY, Huang YT, Liu MF, Lu TH. Incidence and mortality of treated primary Sjogren’s syndrome in Taiwan: a population-based study. J Rheumatol 2011;38:706–8. [DOI] [PubMed] [Google Scholar]
- 21.Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjogren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis 2002;61:554–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishishinya MB, Pereda CA, Munoz-Fernandez S, et al. Identification of lymphoma predictors in patients with primary Sjogren’s syndrome: a systematic literature review and meta-analysis. Rheumatol Int 2015;35:17–26. [DOI] [PubMed] [Google Scholar]
- 23.Ramos-Casals M, Brito-Zeron P, Yague J, et al. Hypocomplementaemia as an immunological marker of morbidity and mortality in patients with primary Sjogren’s syndrome. Rheumatology 2005;44:89–94. [DOI] [PubMed] [Google Scholar]
- 24.Voulgarelis M, Ziakas PD, Papageorgiou A, et al. Prognosis and outcome of non-Hodgkin lymphoma in primary Sjogren syndrome. Medicine 2012;91:1–9. [DOI] [PubMed] [Google Scholar]
- 25.Skopouli FN, Dafni U, Ioannidis JP, Moutsopoulos HM.Clinical evolution, and morbidity and mortality of primary Sjögren's syndrome. Semin Arthritis Rheum 2000;29:296–304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.