Abstract
Objectives Describe daily sleep patterns, sleep quality, and sleep hygiene in 2–5-year-old children newly diagnosed with juvenile idiopathic arthritis (JIA) and their parents in comparison with typically developing (TD) children and parents. Methods Participants (13 JIA, 16 TD parent–child dyads) wore actigraphs for 10 days. Parents completed sleep diaries and sleep hygiene survey. Results Children with JIA had significantly less total sleep time, lower sleep efficiency (SE), and longer naps than TD children. Parents of children with JIA had significantly earlier bedtimes, more wake after sleep onset (WASO) and lower SE than TD parents. Parent–child SE and WASO were interrelated in JIA dyads. Sleep hygiene practices were inconsistent in both groups of children. Conclusions Inadequate amounts of sleep and poor sleep quality were common in parent–child dyads. Early interventions to improve sleep duration and promote sleep hygiene practices may alleviate future sleep problems and improve parent and child well-being.
Keywords: actigraphy, juvenile idiopathic arthritis, parent and child sleep, sleep hygiene, sleep quality
Introduction
Juvenile idiopathic arthritis (JIA) is a chronic inflammatory disease with an unknown etiology. It is one of the common pediatric rheumatologic diseases, characterized with unpredictable episodes of active and inactive disease that impact children’s growth and development (Nordal et al., 2011). An estimated 20% of children with JIA experience lifelong pain and significant disability (Gowdie & Tse, 2012). Children experience joint swelling, stiffness, tenderness and pain, limited range of motion, poor sleep quality (SQ) (e.g., frequent night awakenings, inadequate amount of sleep), obstructive sleep apnea, and daytime sleepiness (Bromberg, Connelly, Anthony, Gil, & Schanberg, 2014; Butbul Aviel et al., 2011; Shyen et al., 2014; Ward et al., 2010). While poor sleep hygiene practices including inconsistent prebedtime activities (e.g., screen time, roughhousing) and lack of a regular bedtime routine and a regular bedtime contribute to inadequate amounts of sleep and impair SQ in typically developing (TD) children (Mindell, Li, Sadeh, Kwon, & Goh, 2015; Sadeh, Mindell, Luedtke, & Wiegand, 2009; Staples, Bates, & Petersen, 2015; Thompson & Christakis, 2005), less is known about young children with JIA. In TD children, poor SQ and inconsistent sleep hygiene practices not only predispose children to later development of behavioral and emotional impairments, but also have a negative impact on physical health and family stress (El-Sheikh, Kelly, Bagley, & Wetter, 2012; Gregory & Sadeh, 2012; Meltzer & Mindell, 2007; Sivertsen et al., 2015; Touchette et al., 2007). These effects may be even more pronounced in children with an underlying chronic condition like JIA, particularly in those with early childhood diagnoses when sleep habits are established.
Although the majority of sleep research in JIA examined school-age children and adolescents, early childhood may be a more critical period in JIA because lifelong sleep habits are forming and rapid growth and development are occurring. Early childhood may provide a more opportune time for early identification and intervention to optimize health outcomes in young children with JIA. Irregular bedtime routines and bedtimes, disruptive nighttime awakenings, and inadequate amounts of sleep in JIA may play a major role in behavioral and emotional development and manifestations of disease-related symptoms. Given the importance of adequate sleep and healthy sleep hygiene, and the paucity of sleep research in young children, this study focused on 2–5-year-olds newly diagnosed with JIA and a comparison group of TD children.
Poor SQ and inconsistent sleep hygiene not only affect young children, but also their parents (Gallagher, Phillips, & Carroll, 2010; Matthews, Neu, Cook, & King, 2014; Meltzer, Sanchez-Ortuno, Edinger, & Avis, 2015). Joint inflammation and pain can interfere with SQ and result in frequent nighttime awakenings for both the child and parent. Young children seek parents for comfort during the night, and irregular bedtime routines and bedtimes and repetitive night awakenings can result in parental fatigue, daytime dysfunction (e.g., daytime sleepiness, anxiety), and work absenteeism (Matthews et al., 2014; Mawani et al., 2013). Several studies report poor SQ in children with a chronic condition (Lewandowski, Ward, & Palermo, 2011; Valrie, Bromberg, Palermo, & Schanberg, 2013), but less is known about the impact of a new chronic diagnosis on parent’s sleep.
The purpose of this study was to describe daily sleep patterns, SQ, and sleep hygiene in 2–5-year-old children newly diagnosed with JIA and their parents in comparison with TD children and parents. Sleep patterns were operationalized as actigraphy measured sleep onset and offset times, total sleep time (TST), and sleep diary-reported bedtimes, wake times, and sleep duration. SQ was operationalized as actigraphy measured wake after sleep onset (WASO) and sleep efficiency (SE), as well as parent report of child’s SQ and parent self-report diary SQ. The aims of the study were to (1) describe parent-report sleep hygiene practices (regular bedtime routine and bedtime) in children with JIA and TD children; (2) describe daily sleep patterns (bedtime, wake time, TST) and SQ (WASO, SE, sleep diary SQ) in young children newly diagnosed with JIA in comparison with TD children; (3) describe daily sleep patterns (bedtime, wake time, TST) and SQ (WASO, SE, sleep diary SQ) in parents of young children newly diagnosed with JIA in comparison with parents of TD children; and (4) examine the relationships between parent and child sleep patterns and SQ and determine whether these relationships differ by group (JIA vs. TD). We hypothesized that (1) in comparison with TD children, parent-reported bedtime routines and bedtimes would be inconsistent in children with JIA; (2) in comparison with TD children, children with JIA would have decreased TST, increased WASO, decreased SE, increased daytime naps, and decreased sleep diary SQ; (3) in comparison with parents of TD children, parents of children with JIA would have decreased TST, increased WASO, decreased SE, and decreased sleep diary SQ; and (4) parent and child TST, WASO, SE, and sleep diary SQ would be positively associated in both groups, with the magnitude of this association being greater in the JIA dyads.
Methods
Participants
From January 2013 through September 2014, 30 children (14 newly diagnosed with JIA and 16 TD children), and 30 respective parents enrolled in the study. Eligibility for children included 2–5 years, and a JIA diagnosis within the past 10 months. Children in both groups were excluded if they had a developmental delay, a comorbid illness (diabetes, asthma, cancer), a diagnosed sleep disorder reported by a parent and medical record (obstructive sleep apnea), or a family history of narcolepsy in a first-degree relative. Parent eligibility criteria included at least 18 years of age and able to speak and read English.
Of the 24 families of children with JIA approached, 14 were enrolled and completed the study protocol (58% response rate). One JIA parent–child dyad data was not included in the analysis owing to loss of actigraphy data (i.e., swimming). Of the 29 families of TD children approached, 16 were enrolled and completed the study protocol (55% response rate). The final sample included 29 parent–child dyads (13 JIA dyads, 16 TD dyads).
Procedures
Approval for this study was obtained from the institutional review board at a large urban children’s hospital in the Pacific Northwest. Children newly diagnosed with JIA were recruited from a pediatric rheumatology clinic. During a clinic visit, children with JIA and their parents were informed about the study; if they expressed interest, the research coordinator explained the purpose of the study and scheduled a home visit.
TD children were recruited through flyers and media advertisements in the communities. Interested parents contacted a member of the team who informed parents about the study, and if interested, a home visit was scheduled. The home visit was scheduled for all families to explain the study protocol and the instructions to complete sleep diaries and questionnaires, and to obtain parent consent and child assent. Actigraphy watches were placed on the child’s ankle and the parent’s nondominant wrist. Parents and children wore the watch and completed sleep diaries for 10 consecutive days. Families were contacted each week to answer questions.
Measures
Sleep Hygiene
Two items from the General Sleep Information survey were used to assess parent reports of sleep hygiene during the previous 1 month. Parent reported “yes” or “no” to the questions “does your child have a regular bedtime routine?” and “does your child have a regular bedtime?”
Actigraphy
Actigraphy is a nonintrusive watch-like device to measure sleep–wake patterns in the natural home environment. Each child and parent wore an actigraph watch for 10 consecutive days (Actiwatch 64TM, Mini-Mitter Philips Respironics, Bend, OR). Actigraphy has an accelerometer that senses the occurrence and degree of motion in all directions. The motion is converted into an electric signal and digitally integrated to derive an activity count. Activity counts were recorded and stored in the actigraph at 1-min intervals. Parents were instructed to press the event marker for their child and themselves to indicate the time they attempted to fall asleep, the time they woke up in the morning, the beginning and end of naps, and when the watch was removed (swimming, bathing). Sleep onset and offset were scored based on the sleep diary and event marker data. After study completion, actigraphy data were downloaded and stored in computer files using the Actiware software for scoring (Mini-Mitter Philips Respironics, Inc.). Activity counts were scored using a weighting algorithm with medium threshold, defined as 40 activity counts per epoch, for both parents and children. This algorithm has high sensitivity to detect sleep and low specificity to detect wake when compared with polysomnography or videosomnography in children with and without chronic conditions (Belanger, Simard, Bernier, & Carrier, 2014; Meltzer, Walsh, Traylor, & Westin, 2012; Sitnick, Goodlin-Jones, & Anders, 2008; Ward, Lentz, Kieckhefer, & Landis, 2011) and in adults (Morgenthaler et al., 2007). Interrater reliability was assessed with two members who individually scored actigraphy data, and the intraclass correlation coefficients were 0.97 for sleep onset and 0.95 for sleep offset.
Actigraphy-derived sleep variables include: (a) sleep onset, defined as the time of the initial three or more consecutive minutes of sleep; (b) sleep offset, defined as the time of the last five or more consecutive minutes of sleep; (c) TST, defined as the total amount of time in minutes during the sleep period scored as sleep; (d) WASO, defined as the number of minutes spent awake after sleep onset occurred; (e) SE, defined as the ratio of the amount of time scored as sleep to total sleep period time, which yields the percentage of sleep within the sleep period; (f) daytime nap, defined as the total amount of time in minutes during the nap period scored as sleep per day; and (g) 24-hr sleep, defined as the total amount of nocturnal TST and daytime naps in minutes.
Sleep Diary
Daily sleep diaries were used in conjunction with actigraphy. Parents completed the sleep diaries for themselves and their child every morning and at bedtime. Parents recorded the time they and their child went to bed, how long it took to fall asleep, number and duration of night awakenings, the time they woke up, SQ, duration of naps, and time and duration of actigraph removal. In addition, evening activities, medications, illnesses, and any unusual events that may have affected child or parental sleep (such as parent out of town) were recorded. Variables derived from the child and parent sleep diary include: (a) bedtime, defined as the time parent recorded in the sleep diary as “falling asleep”; (b) wake time, defined as the time parent recorded in the sleep diary as “waking up”; (c) sleep duration, defined as the total amount of time spent asleep between bedtime and wake time; and (d) SQ, defined as 1 = “terrible” to 9 = “great” (higher numbers indicate better SQ).
Demographic Characteristics
A demographic questionnaire was used to obtain information regarding parent demographics (parent’s age, sex, employment, ethnicity, education, and marital status), child demographics (age, sex), and childcare.
JIA disease duration was measured from the date the child was first diagnosed, and obtained from chart review. Each child was examined by a pediatric rheumatologist at a routine clinic appointment, and criteria for disease activity (active vs. inactive) was assessed. Inactive disease was defined by a physical global assessment (PGA) of disease activity (0 to 10) numerical rating scale; no joints with active arthritis; no active uveitis; no fever, rash, serositis, splenomegaly, or lymphadenopathy attributable to JIA; normal erythrocyte sedimentation rate; and morning stiffness ≤15 min (Wallace, Giannini, Huang, Itert, & Ruperto, 2011).
Statistical Analysis
All analyses were conducted in the statistical software R (R Development Core Team, 2013). Descriptive statistics and graphs were computed for the demographic, actigraphy, and sleep diary data. Actigraphy sleep data with matching diary data were included in the analyses (n = 272 number of days). Two-group independent t-test showed group differences in children’s age (p < .01; see Table I ); therefore, children’s age was included as a covariate in the subsequent analyses. To address Aim 1—describe parent-report sleep hygiene practices (regular bedtime routine and regular bedtime)—descriptive statistics were used to describe the two questions between the two groups.
Table I.
Demographic Characteristics of the Sample
| Variables | Child characteristics |
Variables | Parent characteristics |
|||
|---|---|---|---|---|---|---|
| TD (n = 16) | JIA (n = 13) | TD (n = 16) | JIA (n = 13) | |||
| Age, yearsa | 4.0 (1.14) | 2.73 (0.73) | Age, years | 34.87 (4.19) | 32.75 (4.43) | |
| Sex, n (%) | Sex, n (%) | |||||
| Male | 4 (25%) | 3 (23%) | Male | 2 (13%) | 0 | |
| Female | 12 (75%) | 10 (77%) | Female | 14 (88%) | 13 (100%) | |
| JIA category, n (%) | Education, n (%) | |||||
| Oligoarticular | N/A | 6 (46%) | Some college | 2 (13%) | 6 (46%) | |
| Extended oligoarticular | 1 (8%) | College degree | 10 (63%) | 5 (39%) | ||
| Polyarticular | 6 (46%) | Master’s degree | 4 (25%) | 2 (15%) | ||
| Disease condition, n (%) | Marital status, n (%) | |||||
| Active JIA | N/A | 9 (69%) | Married | 16 (100%) | 11 (85%) | |
| Inactive JIA | 4 (31%) | Divorced | 0 | 2 (15%) | ||
| JIA duration, months | N/A | 6.92 (2.22) | Ethnicity, n (%) | |||
| JIA active Joint (numbers) | N/A | 2.31 (2.14) | Caucasian | 13 (81%) | 10 (77%) | |
| PGA disease activity | N/A | 1.68 (1.87) | Hispanic | 0 | 1 (8%) | |
| Attend childcare, n (%) | 8 (50%) | 6 (46%) | Asian | 3 (19%) | 1 (8%) | |
| Attend kindergarten | 3 (19%) | 0 | Other | 0 | 1 (8%) | |
Note. Data are mean ± SD or n (%). JIA = juvenile idiopathic arthritis; TD = typically developing; PGA = physician global assessment.
aDenote significant group differences.
Multilevel modeling (also known as mixed-effects modeling or hierarchical linear modeling) with restricted maximum likelihood estimation was used to analyze the data to account for the nesting effect of daily actigraphy and sleep diary assessed variables within each participant. The multilevel modeling approach allows for different numbers of observations (days) from each participant, providing a better mechanism to handle missing values compared with deleting all data from participants with missing data (Snijders & Bosker, 2012). Each participant’s sleep data from a particular day were treated as a single data point. Packet “lme4” within R was used to conduct multilevel analyses (Bates, Maechler, Bolker, & Walker, 2015). There were two levels of analysis in the data: “Level 1” referred to the days across the study period within each subject, while “Level 2” was the subject. The subject effect was entered into the models as a random effect. To address Aim 2—describe daily sleep patterns (bedtime, wake time, TST) and SQ (WASO, SE, sleep diary SQ) in children with JIA in comparison with TD children—and Aim 3—describe daily sleep patterns and SQ in parents of children with JIA in comparison with parents of TD children—the group effect (TD coded as 0, JIA coded as 1) and child age were entered as fixed effects, and the outcome variables were the sleep variables of the children and parents, respectively.
In multilevel modeling, it is important to separate the within-subject effect from the across-subject effect. Ignoring this distinction may lead to difficulty in interpreting the results and incorrect conclusions (Mancl, Leroux, & DeRouen, 2000). For Aim 4—examine the relationships between parent and child sleep patterns and SQ in the two groups and whether these relationships differ by group (TD vs. JIA)—the across-subject term was calculated by averaging each child’s sleep variables across the study period (e.g., TSTmean). The within-subject term was calculated as the deviation of the daily value of the child’s sleep variables from the within-subject mean (e.g., TSTdiff = TSTday − TSTmean). First, child sleep variables (within- and across-subject effects), age, and group (TD coded as 0, JIA coded as 1) were entered into the model as fixed effects, and subject as a random effect, to examine the relationships between parent and child sleep variables. For example, a significant child TSTmean predictor of parent TST would indicate that when the child has an increased sleep duration on average, the parent would have an increased sleep duration on average. A significant child TSTdiff predictor of parent TST would indicate that within a dyad, when the child has an increased sleep duration in one night, the parent would have an increased sleep duration that night. Interaction terms with the interaction of within-subject term × group and across-subject term × group were then added to the model. The coefficient of the interaction term would be interpreted as whether there was a group difference in parent–child relationships in the sleep variable examined. For example, a significant group × TSTdiff effect would indicate group differences in the relationships between parent–child TST. If there was a significant interaction, further analysis was performed to calculate the coefficients of the relationships for each of the two groups (TD and JIA).
Results
Sample Characteristics
Table I shows the demographic and clinical characteristics of the participants. The average age for children was 3.4 years. In children newly diagnosed with JIA, the average time between diagnosis and study was 6.9 months. The majority of children with JIA were girls with active disease.
Sleep Hygiene
Of the 16 parents of TD children, 100% (n = 16) reported that their child had a regular bedtime routine, and 88% (n = 14) reported that their child had a regular bedtime. Of the 13 parents of children with JIA, 85% (n = 11) reported that their child had a regular bedtime routine, and 92% (n = 12) reported that their child had a regular bedtime.
Sleep Patterns and SQ
The average number of days of sleep examined with actigraphy was 9.4. Table II shows actigraphy and sleep diary data for parent–child dyads. In children, across the 10-day period, on average, young children with JIA had an estimated 31 min less TST (p < .03), 3% lower SE (p < .03), and 24 min more of daytime naps (p < .01), as measured by actigraphy, than TD children, controlling for child age. The reports from the sleep diary did not show any difference in bedtime, wake time, sleep duration, and SQ between the two groups of children.
Table II.
Actigraphy Sleep and Sleep Diary in Parent–Child Dyads
| Variables | Mean (SD) |
Group fixed effects |
|||||
|---|---|---|---|---|---|---|---|
| TD | JIA | b | SE | t | p | Cohen’s d | |
| Actigraphy child sleep | |||||||
| Sleep onset | 21:17 (0:50) | 21:14 (1:08) | −0.02 | 0.26 | −0.07 | .94 | 0.02 |
| Sleep offset | 7:24 (0:46) | 7:06 (1:03) | −0.34 | 0.24 | −1.41 | .16 | 0.54+ |
| TST (min) | 509 (42) | 483 (72) | −30.65 | 13.89 | −2.21 | .03* | 0.86++ |
| WASO (min) | 82 (24) | 93 (36) | 10.97 | 5.91 | 1.86 | .06 | 0.69+ |
| SE (%) | 84.84 (3.75) | 82.53 (6.05) | −2.51 | 1.14 | −2.2 | .03* | 0.84++ |
| Daytime nap (min) | 19 (37) | 63 (49) | 23.94 | 9.51 | 2.52 | .01** | 1.72++ |
| 24-hr sleep (min) | 528 (52) | 546 (76) | −2.04 | 19.85 | −0.1 | .92 | 0.35 |
| Diary child sleep | |||||||
| Bedtime | 21:01 (0:47) | 21:01 (1:04) | 0.09 | 0.25 | 0.34 | .74 | 0.22 |
| Wake time | 7:24 (0:36) | 7:11 (0:59) | −0.24 | 0.21 | −1.15 | .25 | 0.36 |
| Sleep duration (min) | 623 (47) | 610 (62) | −20.66 | 15.75 | −1.31 | .19 | 0.62+ |
| SQ | 7.80 (1.54) | 6.98 (2.23) | −0.63 | 0.61 | −1.04 | .3 | 0.46 |
| Actigraphy parent sleep | |||||||
| Sleep onset | 23:43 (1:06) | 22:56 (1:34) | −0.86 | 0.37 | −2.35 | .02* | 0.89++ |
| Sleep offset | 7:27 (1:05) | 7:01 (1:10) | −0.33 | 0.3 | −1.13 | .26 | 0.44 |
| TST (min) | 397 (65) | 404 (77) | 4 | 18.04 | 0.22 | .82 | 0.16 |
| WASO (min) | 48 (19) | 67 (41) | 20.02 | 8.33 | 2.4 | .02* | 0.92++ |
| SE (%) | 87.81 (4.47) | 84.28 (6.93) | −3.76 | 1.63 | −2.3 | .02* | 0.88++ |
| Diary parent sleep | |||||||
| Bedtime | 23:30 (1:05) | 22:52 (1:20) | −0.67 | 0.29 | −2.31 | .02* | 0.88++ |
| Wake time | 6:55 (0:57) | 6:46 (1:01) | −0.21 | 0.25 | −0.84 | .40 | 0.31 |
| Sleep duration (min) | 444 (76) | 475 (86) | 31.89 | 18.11 | 1.76 | .08 | 0.72+ |
| SQ | 6.54 (1.47) | 5.74 (2.08) | −0.29 | 0.53 | −0.54 | .59 | 0.56+ |
Note. TD = typically developing; JIA = juvenile idiopathic arthritis; TST = total sleep time; WASO = wake after sleep onset; SE = sleep efficiency; min = minutes; SQ = sleep quality reported in sleep diary; bedtimes and wake times are presented as 24-hr clock.
Group fixed effect was entered as TD-0, JIA-1; child age was entered as a fixed effect.
Cohen’s d was calculated based on the individual sleep data averaged across the study period; +medium effect size, ++large effect size.
*p < .05, **p < .01.
In parents, across the 10-day period, on average, the parents of children with JIA went to sleep 52 min earlier in the evening (p < .02), had 20 min more WASO (p < .02), and 4% lower SE (p < .02), as measured by actigraphy, than the parents of TD children. Consistent with actigraphy, the sleep diary reports also showed that the parents of children with JIA went to sleep 40 min earlier in the evening (p < .02) than the parents of TD children. The reports from the sleep diary did not show any difference in wake time, sleep duration, and SQ between the two groups of parents.
Relationships Between Parent and Child Sleep
To determine whether there was a relationship between parent and child actigraphy and sleep diary variables, regardless of group (TD vs. JIA), child sleep variables (actigraphy measured TST, WASO, SE, and child sleep diary SQ), both within- and across-subject effects, were entered into the model as fixed effects, and parent sleep variables (actigraphy measured TST, WASO, SE, and sleep diary SQ) as outcomes, respectively. Table III shows the relationships between parent and child sleep: for the across-subject effects, parent and child TST and SQ were positively related, suggesting that when a child slept 1 hr more on average, the parent was estimated to sleep 34 min more on average (p < .002). When parents rated their child’s SQ 1 unit higher on average, the parents rated their own SQ 0.71 unit higher on average (p < .001). For the within-subject effects, parent and child TST, WASO, SE, and SQ were positively related (p’s < .01). Thus within a dyad, when a child slept 1 hr more during a night, the parent was estimated to sleep 19 min more that night. When a child had 1 hr more WASO, the parent was estimated to have 10 more min WASO that night. When a child had a 10% increase in SE during a night, the parent was estimated to have a 2% increase in SE that night. When parents rated their child’s sleep 1 unit higher in one night, the parents rated their own sleep 0.52 unit higher that night (See Table III).
Table III.
Fixed Effects of Child Sleep as Predictors of Parent Sleep
| Outcome | Parent TST |
|||
|---|---|---|---|---|
| Fixed effect | b | SE | t | p |
| Child TST mean (across) | 0.56 | 0.18 | 3.08 | .002** |
| Child TST diff (within) | 0.31 | 0.08 | 3.81 | <.001*** |
| Group (JIA) | 0.30 | 0.27 | 1.11 | .27 |
| Age | −0.06 | 0.12 | −0.56 | .58 |
| Outcome |
Parent WASO (min) |
|||
| Fixed effect | b | SE | t | p |
| Child WASO mean (across) | 0.09 | 0.28 | 0.33 | .74 |
| Child WASO diff (within) | 0.17 | 0.05 | 3.32 | .001** |
| Group (JIA) | 16.62 | 10.63 | 1.57 | .12 |
| Age | −1.88 | 4.57 | −0.41 | .68 |
| Outcome |
Parent SE (%) |
|||
| Fixed effect | b | SE | t | p |
| Child SE mean (across) | 0.19 | 0.29 | 0.67 | .51 |
| Child SE diff (within) | 0.17 | 0.06 | 3.03 | .002** |
| Group (JIA) | −3.15 | 2.01 | −1.51 | .13 |
| Age | 0.10 | 0.91 | 0.11 | .91 |
| Outcome |
Parent SQ (%) |
|||
| Fixed effect | b | SE | t | p |
| Child SQ mean (across) | 0.71 | 0.10 | 6.87 | <.001*** |
| Child SQ diff (within) | 0.52 | 0.05 | 9.83 | <.001*** |
| Group (JIA) | 0.16 | 0.33 | 0.49 | .63 |
| Age | 0.31 | 0.14 | 2.18 | .03* |
Note. TST = total sleep time; SE = sleep efficiency; WASO = wake after sleep onset; SQ = sleep quality reported in sleep diary; group fixed effect was entered as TD-0, JIA-1.
*p < .05; **p < .01; ***p < .001.
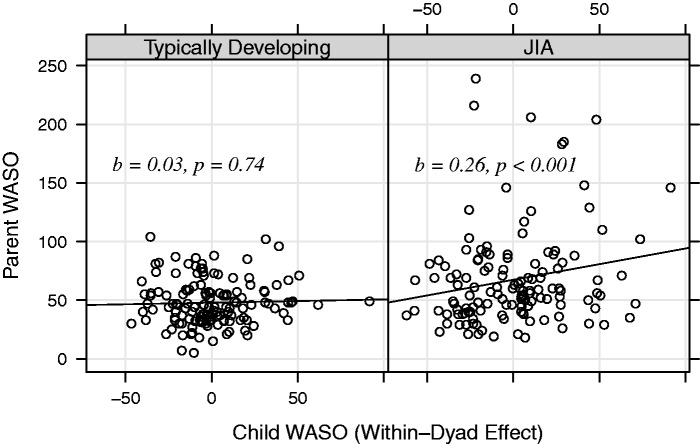
To examine whether the above relationships differed by group (TD vs. JIA dyad), interaction terms (child within-subject term × group and child across-subject term × group) were added to the model. No significant child sleep × group interaction was found for TST (p = .35), but significant within-subject child sleep × group interaction was found for actigraphy-measured WASO (p < .03), SE (p < .003), and sleep diary-measured SQ (p < .02) (Table IV ). To understand the relationships among parent–child WASO, SE, SQ, and group (TD vs. JIA dyad), coefficients of the relationships in the two groups were calculated separately. In JIA dyads, for every 1-hr increase in child WASO, there was an estimated 12-min increase in parent WASO (p < .001) (Figure 1 ). For every 10% decrease in child SE, there was an estimated 3% decrease in parent SE (p < .001). For every 1-unit increase in parental report of child SQ, there was 0.51-unit increase in parent SQ. In TD dyads, for every 1-unit increase in parental report of child SQ, there was 0.51-unit increase in parent self-report SQ; the coefficients for the relationships between parent–child WASO and SE were close to zero and not significant.
Table IV.
Fixed Effects and Interaction Terms (Child Sleep × Group) as Predictors of Parent Sleep
| Outcome |
Parent WASO (min) |
|||
|---|---|---|---|---|
| Fixed effect | b | SE | t | p |
| Child WASO mean (across) | 0.21 | 0.68 | 0.30 | .76 |
| Child WASO diff (within) | 0.03 | 0.08 | 0.33 | .74 |
| Group (JIA) | 30.64 | 64.70 | 0.47 | .64 |
| Child WASO mean × group | −0.16 | 0.75 | −0.22 | .83 |
| Child WASO diff × group | 0.24 | 0.11 | 2.24 | .03* |
| Age | −1.78 | 4.70 | −0.38 | .70 |
| Outcome |
Parent SE (%) |
|||
| Fixed effect | b | SE | t | p |
| Child SE mean (across) | 0.74 | 0.76 | 0.98 | .33 |
| Child SE diff (within) | −0.05 | 0.09 | −0.50 | .62 |
| Group (JIA) | 52.51 | 68.30 | 0.77 | .44 |
| Child SE mean × group | −0.66 | 0.81 | −0.82 | .41 |
| Child SE diff × group | 0.35 | 0.12 | 2.94 | .003** |
| Age | −0.17 | 0.97 | −0.18 | .86 |
| Outcome |
Parent SQ (%) |
|||
| Fixed effect | b | SE | t | p |
| Child SQ mean (across) | 0.40 | 0.16 | 2.55 | .01* |
| Child SQ diff (within) | 0.33 | 0.10 | 3.45 | .001** |
| Group (JIA) | −3.36 | 1.48 | −2.28 | .02* |
| Child SQ mean × group | 0.49 | 0.20 | 2.44 | .02* |
| Child SQ diff × group | 0.28 | 0.11 | 2.43 | .02* |
| Age | 0.37 | 0.13 | 2.78 | .006** |
Note. SE = sleep efficiency; WASO = wake after sleep onset; SQ = sleep quality reported in sleep diary; group fixed effect was entered as TD-0, JIA-1.
*p < .05; **p < .01.
Figure 1.
Interaction between parent–child WASO by group. Note. WASO = wake after sleep onset (minutes); Child WASO for each night was centered at child mean WASO across the 10 days; each circle represents a night, the corresponding x-value is the centered child WASO that night, and the corresponding y-value is the parent WASO that night; Slopes and p-values were calculated using multilevel modeling and controlled for child age.
Discussion
This is one of the first studies that assessed daily sleep patterns and SQ with both actigraphy and diary using multilevel modeling in 2–5-year-old children newly diagnosed with JIA and their parents in comparison with TD children and their parents. Regardless of JIA, our sample of young children obtained inadequate amounts of sleep, poor SQ, and had poor sleep hygiene. Although, parents of children with JIA had earlier bedtimes, their SQ was worse than the parents of TD children. Actigraphy-measured WASO and SE were interrelated in JIA parent–child dyads, but not in TD parent–child dyads. SQ as measured by sleep diaries was interrelated in TD and JIA dyads.
Sleep Patterns and SQ
Average 24-hr sleep was approximately 9 hr in our sample of young children, which is below the recommended 10–13 hr by the National Sleep Foundation (2014) and considerably less than prior reports in young children using actigraphy (Acebo et al., 2005; Staples et al., 2015; Ward, Gay, Anders, Alkon, & Lee, 2008). Previous actigraphy-based studies show that longer daytime naps were associated with shorter nighttime sleep, earlier wake times (Acebo et al., 2005; Jones & Ball, 2014; Ward, Gay, Alkon, Anders, & Lee, 2008), and later sleep onset (Tikotzky & Sadeh, 2001). Contrary to prior research, our study did not support this. Rather our findings were consistent with El-Sheikh, Arsiwalla, Staton, Dyer, & Vaughn (2013) and Lam, Mahone, Mason, & Scharf (2011), who also found inadequate amounts of 24-hr sleep (average 9 hr and 9.4 hr, respectively) as measured by actigraphy in young children (El-Sheikh et al., 2013; Lam et al., 2011). We anticipated that nighttime sleep would be less in JIA due to increased night awakenings, and that daytime naps would compensate for the shorter nighttime sleep durations, but this was not the case. JIA children had longer daytime naps, but the daytime naps did not make up for the inadequate amounts of nighttime sleep. This finding was not explained by JIA disease activity, as sleep patterns and SQ did not differ by active versus inactive disease or active joint count (data not shown). The inadequate amounts of sleep in our sample may be related to poor sleep hygiene practices (discussed below) and/or child temperament (e.g., soothability), family and socio-cultural factors (family stress, socioeconomic status) previously reported in other studies report (El-Sheikh et al., 2013; Jones & Ball, 2014; Molfese et al., 2015; Ward, Gay, Alkon, et al., 2008), but were not included in this pilot study.
Sleep Hygiene
Sleep hygiene practices were inconsistent in both TD and JIA children that may explain the inadequate amounts of sleep observed in our sample. Interestingly, the majority of parents reported that their child had a regular bedtime and a regular bedtime routine, yet there was considerable variability in the actigraphy sleep onset times between days (50–68 min as shown in Table II). This finding may be attributed to the differences in the duration of bedtime routines across the families that can contribute to inadequate amounts of sleep in children and/or parent’s interpretation of “regular” bedtime routine and bedtime. Caring for young children is exhausting and parents may not realize the amount of sleep young children need, the variability in bedtime routines, bedtimes, or the amount of sleep their child obtained. For example, on study completion, we had the opportunity to discuss the sleep findings with parents and review the sleep data from the actigram. Many parents were unaware of the insufficient amounts of sleep their child received and the irregularity in bedtimes. Attending to young children while managing work and family life is demanding for parents, making it plausible that parents may lose sight of the importance of a consistent bedtime routine and sleep needs for their child. Additionally, some parents may need advice on how to implement sleep hygiene practices and manage behaviors associated with sleep (e.g., bedtime resistance) versus the underlying chronic condition (e.g., night awakenings). Although our sample was small, >70% of the parents had a college degree, and our finding highlights the need to routinely ask parents about their child’s sleep habits, including prebedtime activities, consistency in implementing regular bedtime routines and bedtimes, sleep consumption (e.g., nighttime and daytime sleep duration), and location of sleep (home vs. childcare). We did not obtain information on parental sleep hygiene practices, which can contribute to poor sleep hygiene in children. Future studies should obtain information on sleep hygiene practices in both the child and parent, which would provide new knowledge in the development of interventions to promote healthy sleep hygiene practices.
Relationships Between Parent and Child Sleep
Our findings provide preliminary evidence for the interrelations between parent and child sleep in JIA dyads but not in TD dyads, which supports previous studies in parent–child dyads with autism and cancer (Goldman, Wang, & Fawkes, 2014; Matthews et al., 2014). We attribute this finding to parents of children with JIA, as they may be more sensitive to their child’s nighttime awakenings owing to stress, worry, and/or more attentive to their child’s nighttime needs, thus wake up more often than TD parents. Goldman and colleagues (2014) examined actigraphy-based sleep patterns and SQ and parent report of sleep (e.g., “good vs. poor sleeper”) in mother–child dyads with and without autism. SE, sleep fragmentation, and WASO were positively correlated in mother–child dyads with autism who were “poor sleepers,” and TST was positively correlated in TD dyads and mother–child dyads with autism who were “good sleepers.” Another study by Matthews and colleagues also examined sleep in mother–child dyads with and without acute lymphoblastic leukemia (ALL), and found that TST was positively correlated in the mother–child dyads with ALL, but not in control dyads. Although we have few studies for comparison, findings from the above studies suggest that parents caring for children recently diagnosed with a chronic condition are vulnerable to inadequate amounts of sleep and poor SQ. Early interventions tailored to meet the specific sleep health needs of the child and parent caring for a child with a chronic condition are needed to promote parent and child well-being. Interventions to improve sleep early in a child’s disease course may alter later sleep-related comorbidities.
Limitations
Results from this study should be considered in light of its limitations. First, this was a small pilot study, and our findings need replication with a larger sample of parent–child dyads. Multiple comparisons were not adjusted for, with significant p-values set at <.05. Additional studies are needed to provide new knowledge to develop interventions to improve sleep quantity and/or sleep hygiene in parent–child dyads. Second, there may be selection bias in our sample because families in this study may have been concerned about their sleep and/or their child’s sleep before being approached, and thus more likely to participate in the study. Third, this study had a fairly homogeneous sample from two-parent, middle-class families, limiting the generalizability of our findings. Fourth, age was included as a covariate in the analyses; however, the group difference in age may contribute to the difference in children’s sleep (e.g., older children less likely to nap). Lastly, we did not measure light in the bedroom, which may have influenced study findings.
Conclusions
Routinely asking parents about their child’s sleep needs, prebedtime activities, and consistency and duration of bedtime routines during well-child visits is needed to increase parental awareness about the importance of sleep. This information would provide clinicians with new knowledge about how to intervene and work with parents caring for a child with a chronic illness during early versus later development. Parents caring for children with a chronic condition may also need practical advice about how to implement consistent sleep hygiene practices and manage behaviors associated with sleep versus the chronic illness. Disrupted sleep and poor sleep hygiene in parents and children are treatable, and early interventions to improve sleep hygiene practices and promote healthy sleep may alleviate future sleep problems and improve parent and child well-being and daytime function.
Acknowledgments
The authors would like to thank all the families who participated in the study. We thank Ching Hung and Lucas Reichley in the rheumatology clinic for recruiting the participants. We thank Ernie Tolentino, laboratory manager, Jim Rothermel, and sleep laboratory staff, for recording, processing, and scoring of the sleep data. Willada Loch, Casey Guilland, and Megha Santhosh for helping with data collection and processing.
Funding
This work was supported by the National Institute of Health/National Institute of Nursing Research, Center for Research on the Management of Sleep Disturbances (P30 NR011400 to T.M.W.); National Institute of Nursing Research (RO1 NR 012734 to T.M.W.); University of Washington Warren G. Magnuson Scholarship (to W.Y.); University of Washington Hester McLaws Nursing Scholarship (to W.Y.); and Sigma Theta Tau International Psi Chapter-at-Large Small Grant (to W.Y.).
Conflict of interest: None declared.
References
- Acebo C., Sadeh A., Seifer R., Tzischinsky O., Hafer A., Carskadon M. A. (2005). Sleep/wake patterns derived from activity monitoring and maternal report for healthy 1- to 5-year-old children. Sleep , 28, 1568–1577. [DOI] [PubMed] [Google Scholar]
- Bates D., Maechler M., Bolker B., Walker S. (2015). lme4: Linear mixed-effects models using Eigen and S4. (Version R package version 1.1‐9). Retrieved from https://CRAN.R-project.org/package=lme4
- Belanger M. E., Simard V., Bernier A., Carrier J. (2014). Investigating the convergence between actigraphy, maternal sleep diaries, and the child behavior checklist as measures of sleep in toddlers. Front Psychiatry , 5, 158 doi:10.3389/fpsyt.2014.00158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg M. H., Connelly M., Anthony K. K., Gil K. M., Schanberg L. E. (2014). Self-reported pain and disease symptoms persist in juvenile idiopathic arthritis despite treatment advances: An electronic diary study. Arthritis and Rheumatololgy , 66, 462–469. doi:10.1002/art.38223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butbul Aviel Y., Stremler R., Benseler S. M., Cameron B., Laxer R. M., Ota S., Schneider R., Spiegel L., Stinson J. N., Tse S. M., Feldman B. M. (2011). Sleep and fatigue and the relationship to pain, disease activity and quality of life in juvenile idiopathic arthritis and juvenile dermatomyositis. Rheumatology , 50, 2051–2060. doi:10.1093/rheumatology/ker256 [DOI] [PubMed] [Google Scholar]
- El-Sheikh M., Arsiwalla D. D., Staton L., Dyer W. J., Vaughn B. E. (2013). Associations between preschoolers’ daytime and nighttime sleep parameters. Behavioral Sleep Medicine , 11, 91–104. doi:10.1080/15402002.2011.625460 [DOI] [PubMed] [Google Scholar]
- El-Sheikh M., Kelly R. J., Bagley E. J., Wetter E. K. (2012). Parental depressive symptoms and children’s sleep: The role of family conflict. Journal of Child Psychology and Psychiatry , 53, 806–814. doi:10.1111/j.1469‐7610.2012.02530.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher S., Phillips A. C., Carroll D. (2010). Parental stress is associated with poor sleep quality in parents caring for children with developmental disabilities. Journal of Pediatric Psychology , 35, 728–737. doi:10.1093/jpepsy/jsp093 [DOI] [PubMed] [Google Scholar]
- Goldman S. E., Wang L., Fawkes D. B. (2014). Concordance of mother/child sleep patterns using actigraphy: Preliminary findings. Journal of Sleep Disorders - Treatment and Care , 3, 1–11. doi:10.4172/2325‐9639.1000133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowdie P. J., Tse S. M. (2012). Juvenile idiopathic arthritis. Pediatric Clinics of North America , 59, 301–327. doi:10.1016/j.pcl.2012.03.014 [DOI] [PubMed] [Google Scholar]
- Gregory A. M., Sadeh A. (2012). Sleep, emotional and behavioral difficulties in children and adolescents. Sleep Medicine Reviews , 16, 129–136. doi:10.1016/j.smrv.2011.03.007 [DOI] [PubMed] [Google Scholar]
- Jones C. H., Ball H. (2014). Exploring socioeconomic differences in bedtime behaviours and sleep duration in english preschool children. Infant and Child Development , 23, 518–531. doi:10.1002/icd.1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J. C., Mahone E. M., Mason T. B., Scharf S. M. (2011). Defining the roles of actigraphy and parent logs for assessing sleep variables in preschool children. Behavioral Sleep Medicine , 9, 184–193. doi:10.1080/15402002.2011.583906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski A. S., Ward T. M., Palermo T. M. (2011). Sleep problems in children and adolescents with common medical conditions. Pediatric Clinics of North America , 58, 699–713. doi:10.1016/j.pcl.2011.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancl L. A., Leroux B. G., DeRouen T. A. (2000). Between-subject and within-subject statistical information in dental research. Journal of Dental Research , 79, 1778–1781. [DOI] [PubMed] [Google Scholar]
- Matthews E. E., Neu M., Cook P. F., King N. (2014). Sleep in mother and child dyads during treatment for pediatric acute lymphoblastic leukemia. Oncology Nursing Forum , 41, 599–610. doi:10.1188/14.onf.41‐06p [DOI] [PubMed] [Google Scholar]
- Mawani N., Amine B., Rostom S., El Badri D., Ezzahri M., Moussa F., Shyen S., Gueddari S., Wabi M., Shkirat B., Hassouni N. H. (2013). Moroccan parents caring for children with juvenile idiopathic arthritis: Positive and negative aspects of their experiences. Pediatric Rheumatology , 11, 39 doi:10.1186/1546‐0096‐11‐39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer L. J., Mindell J. A. (2007). Relationship between child sleep disturbances and maternal sleep, mood, and parenting stress: A pilot study. Journal of Family Psychology , 21, 67–73. doi:10.1037/0893‐3200.21.1.67 [DOI] [PubMed] [Google Scholar]
- Meltzer L. J., Sanchez-Ortuno M. J., Edinger J. D., Avis K. T. (2015). Sleep patterns, sleep instability, and health related quality of life in parents of ventilator-assisted children. Journal of Clinical Sleep Medicine , 11, 251–258. doi:10.5664/jcsm.4538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer L. J., Walsh C. M., Traylor J., Westin A. M. (2012). Direct comparison of two new actigraphs and polysomnography in children and adolescents. Sleep , 35, 159–166. doi:10.5665/sleep.1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindell J. A., Li A. M., Sadeh A., Kwon R., Goh D. Y. (2015). Bedtime routines for young children: A dose-dependent association with sleep outcomes. Sleep , 38, 717–722. doi:10.5665/sleep.4662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molfese V. J., Rudasill K. M., Prokasky A., Champagne C., Holmes M., Molfese D. L., Bates J. E. (2015). Relations between toddler sleep characteristics, sleep problems, and temperament. Developmental Neuropsychology , 40, 138–154. doi:10.1080/87565641.2015.1028627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenthaler T., Alessi C., Friedman L., Owens J., Kapur V., Boehlecke B., Brown T., Chesson A., Jr, Coleman J., Lee-Chiong T., Pancer J., Swick T. J. (2007). Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: An update for 2007. Sleep , 30, 519–529. [DOI] [PubMed] [Google Scholar]
- National Sleep Foundation. (2014). Children and sleep. Retrieved from https://sleepfoundation.org/sleep-topics/children-and-sleep
- Nordal E., Zak M., Aalto K., Berntson L., Fasth A., Herlin T., Lahdenne P., Nielsen S., Straume B., Rygg M. (2011). Ongoing disease activity and changing categories in a long-term nordic cohort study of juvenile idiopathic arthritis. Arthritis and Rheumatism , 63, 2809–2818. doi:10.1002/art.30426 [DOI] [PubMed] [Google Scholar]
- R Development Core Team. (2013). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; Retrieved from http://www.R-project.org/ [Google Scholar]
- Sadeh A., Mindell J. A., Luedtke K., Wiegand B. (2009). Sleep and sleep ecology in the first 3 years: A web-based study. Journal of Sleep Research , 18, 60–73. doi:10.1111/j.1365‐2869.2008.00699.x [DOI] [PubMed] [Google Scholar]
- Shyen S., Amine B., Rostom S., Badri E. L., Ezzahri D., Mawani M., Moussa N., Gueddari F., Wabi S., Abouqal M. R., Chkirate B., Hajjaj-Hassouni N. (2014). Sleep and its relationship to pain, dysfunction, and disease activity in juvenile idiopathic arthritis. Clinical Rheumatology , 33, 1425–1431. doi:10.1007/s10067‐013‐2409-x [DOI] [PubMed] [Google Scholar]
- Sitnick S. L., Goodlin-Jones B. L., Anders T. F. (2008). The use of actigraphy to study sleep disorders in preschoolers: Some concerns about detection of nighttime awakenings. Sleep , 31, 395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivertsen B., Harvey A. G., Reichborn-Kjennerud T., Torgersen L., Ystrom E., Hysing M. (2015). Later emotional and behavioral problems associated with sleep problems in toddlers: A longitudinal study. JAMA Pediatrics , 169, 575–582. doi:10.1001/jamapediatrics.2015.0187 [DOI] [PubMed] [Google Scholar]
- Snijders T. A. B., Bosker R. J. (2012). Multilevel analysis: An introduction to basic and advanced multilevel modeling (2nd ed.). Los Angeles, CA: SAGE Publications. [Google Scholar]
- Staples A. D., Bates J. E., Petersen I. T. (2015). Ix. Bedtime routines in early childhood: Prevalence, consistency, and associations with nighttime sleep. Monographs of the Society for Research in Child Development , 80, 141–159. doi:10.1111/mono.12149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D. A., Christakis D. A. (2005). The association between television viewing and irregular sleep schedules among children less than 3 years of age. Pediatrics , 116, 851–856. doi:10.1542/peds.2004‐2788 [DOI] [PubMed] [Google Scholar]
- Tikotzky L., Sadeh A. (2001). Sleep patterns and sleep disruptions in kindergarten children. Journal of Clinical Child Psychology , 30, 581–591. doi:10.1207/s15374424jccp3004_13 [DOI] [PubMed] [Google Scholar]
- Touchette E., Petit D., Seguin J. R., Boivin M., Tremblay R. E., Montplaisir J. Y. (2007). Associations between sleep duration patterns and behavioral/cognitive functioning at school entry. Sleep , 30, 1213–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valrie C. R., Bromberg M. H., Palermo T., Schanberg L. E. (2013). A systematic review of sleep in pediatric pain populations. Journal of Developmental and Behavioral Pediatrics , 34, 120–128. doi:10.1097/DBP.0b013e31827d5848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace C. A., Giannini E. H., Huang B., Itert L., Ruperto N. (2011). American College of Rheumatology provisional criteria for defining clinical inactive disease in select categories of juvenile idiopathic arthritis. Arthritis Care and Research , 63, 929–936. doi:10.1002/acr.20497 [DOI] [PubMed] [Google Scholar]
- Ward T. M., Archbold K., Lentz M., Ringold S., Wallace C. A., Landis C. A. (2010). Sleep disturbance, daytime sleepiness, and neurocognitive performance in children with juvenile idiopathic arthritis. Sleep , 33, 252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward T. M., Gay C., Alkon A., Anders T. F., Lee K. A. (2008). Nocturnal sleep and daytime nap behaviors in relation to salivary cortisol levels and temperament in preschool-age children attending child care. Biological Research for Nursing , 9, 244–253. doi:10.1177/1099800407310158 [DOI] [PubMed] [Google Scholar]
- Ward T. M., Gay C., Anders T. F., Alkon A., Lee K. A. (2008). Sleep and napping patterns in 3-to-5-year old children attending full-day childcare centers. Journal of Pediatric Psychology , 33, 666–672. doi:10.1093/jpepsy/jsm102 [DOI] [PubMed] [Google Scholar]
- Ward T. M., Lentz M., Kieckhefer G. M., Landis C. A. (2011). Polysomnography and actigraphy concordance in juvenile idiopathic arthritis, asthma and healthy children. Journal of Sleep Research , 21, 113–121. doi:10.1111/j.1365‐2869.2011.00923.x [DOI] [PMC free article] [PubMed] [Google Scholar]