Abstract
Azoospermic factor c (AZFc) deletions are the underlying cause in 10% of azoo- or severe oligozoospermia. Through extensive molecular analysis the precise genetic content of the AZFc region and the origin of its deletion have been determined. However, little is known about the effect of AZFc deletions on the functionality of germ cells at various developmental steps. The presence of normal, fertilization-competent sperm in the ejaculate and/or testis of the majority of men with AZFc deletions suggests that the process of differentiation from spermatogonial stem cells (SSCs) to mature spermatozoa can take place in the absence of the AZFc region. To determine the functionality of AZFc-deleted spermatogonia, we compared in vitro propagated spermatogonia from six men with complete AZFc deletions with spermatogonia from three normozoospermic controls. We found that spermatogonia of AZFc-deleted men behave similar to controls during culture. Short-term (18 days) and long-term (48 days) culture of AZFc-deleted spermatogonia showed the same characteristics as non-deleted spermatogonia. This similarity was revealed by the same number of passages, the same germ cell clusters formation and similar level of genes expression of spermatogonial markers including ubiquitin carboxyl-terminal esterase L1 (UCHL1), zinc finger and BTB domain containing 16 (ZBTB16) and glial cell line-derived neurotrophic factor family receptor alpha 1 (GFRA1), as well as germ cell differentiation markers including signal transducer and activator of transcription 3 (STAT3), spermatogenesis and oogenesis specific basic helix-loophelix 2 (SOHLH2), v-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog (KIT) and synaptonemal complex protein 3 (SYCP3). The only exception was melanoma antigen family A4 (MAGEA4) which showed significantly lower expression in AZFc-deleted samples than controls in short-term culture while in long-term culture it was hardly detected in both AZFc-deleted and control spermatogonia. These data suggest that, at least in vitro, spermatogonia of AZFc-deleted men are functionally similar to spermatogonia from non-deleted men. Potentially, this enables treatment of men with AZFc deletions by propagating their SSCs in vitro and autotransplanting these SSCs back to the testes to increase sperm counts and restore fertility.
Keywords: AZFc, spermatogonia, SSC, infertility
Introduction
Azoospermic factor c (AZFc) deletions are a common molecular cause of spermatogenic failure with a frequency of about 10% in men with non-obstructive azoospermia or severe oligozoospermia (Vogt et al., 1996; Kuroda-Kawaguchi et al., 2001). The AZFc region contains five multicopy gene families namely Deleted in azoospermia (DAZ), Chromo domain on Y (CDY1), Basic charge Y-linked, 2 (BPY2), Golgi autoantigen Golgin subfamily A2 like Y (GOLGA2LY) and Chondroitin sulfate proteoglycan 4 like Y (CSPG4LY) (Kuroda-Kawaguchi et al., 2001; Skaletsky et al., 2003). These genes are all expressed exclusively or predominantly in the testis and are therefore thought to play a role in spermatogenesis (Skaletsky et al., 2003). However, the precise function of AZFc genes in humans is unknown. Most of our knowledge on the function of AZFc genes comes from animal studies (Gromoll et al., 1999; Kostova et al., 2002; Vera et al., 2002; Richardson et al., 2009). In Cynomolgus monkey for instance, cyn DAZ/DAZL (the homolog of human DAZ) is expressed in spermatogonia, spermatocytes, round and elongated spermatids and its associated protein is suggested to shuttle RNA between nucleus and cytoplasm (Gromoll et al., 1999; Vera et al., 2002). Cyn CDY1 is only expressed in round and elongated spermatids and it is suggested to be involved in chromatin binding and fatty acid oxidation (Kostova et al., 2002). In more distant species such as drosophila and mouse, genetic knockout studies have demonstrated that homologs of human DAZ are essential for male fertility (Eberhart et al., 1996; Ruggiu et al., 1997; VanGompel and Xu, 2010). BPY2, mostly studied in human (Tse et al., 2003; Wong et al., 2004), is expressed in the nuclei of spermatogonia, spermatocytes and round spermatids which suggests that BPY2 is likely to function throughout male germ cell development (Tse et al., 2003). However, the function of GOLGA2LY and CSPG4LY is still largely unknown.
In humans, AZFc deletions always cause either oligozoospermia or azoospermia (Vogt et al., 1996). In 70% of azoospermic men with AZFc deletions, mature sperm can be found in their testes upon testicular sperm extraction (TESE) (Silber et al., 1998; Oates et al., 2002; Choi et al., 2004; Gambera et al., 2010). Spermatozoa retrieved from men with AZFc deletions are fertilization competent and, when used for ICSI (intracytoplasmic sperm injection), produce viable embryos and pregnancy at similar rates as spermatozoa of men without AZFc deletions (Page et al., 1999; Liu et al., 2013). In several cases AZFc deletions have even been transmitted to offspring via natural conception (Toth et al., 2001; Patsalis et al., 2002; Kuhnert et al., 2004; Minor et al., 2007; Zhu et al., 2010). Histological evaluation of testes in men with AZFc deletion indicates the presence of a heterogeneous phenotype of Sertoli cell only (SCO) tubules, tubules with maturation arrest (MA) and tubules with normal spermatogenesis (Luetjens et al., 2002). All together, these observations indicate that in the majority of men with an AZFc deletion there is continuous spermatogenesis up to spermatozoa albeit to a much lesser extent (30–75%) potentially as a result of a low number of spermatogonia or a lower efficiency of differentiation (Hopps et al., 2003; Ferras et al., 2004; Stouffs et al., 2005; Patrat et al., 2010; Kim et al., 2012; Zhang et al., 2013).
In this study we aimed to determine the functional capacity of spermatogonia from men with AZFc deletions. We compared the behavior of spermatogonia in culture as well as the expression levels of specific spermatogonial and germ cell differentiation markers in cultured spermatogonia of AZFc-deleted men to cultured spermatogonia of men without an AZFc deletion with normal spermatogenesis.
Materials and Methods
Patients
AZFc-deleted patients were selected from our cohort of 2206 consecutively included male partners of subfertile couples, who were referred to the Center for Reproductive Medicine of Academic Medical Center (Amsterdam, the Netherlands) from January 2000 until December 2012. We identified 19 men with a complete AZFc deletion and of six of these men (AMC1295, AMC1426, AMC1835, AMC2200, URO0099 and URO0155) we had enough spare testicular tissue from a TESE procedure to investigate the characteristics of spermatogonia in vitro. As controls we used healthy testicular tissue from three prostate cancer patients who underwent bilateral orchidectomy as part of their treatment (URO0034, URO0059 and URO0077). All these controls had normal spermatogenesis as determined by histology and all were confirmed to have an intact AZFc region. Testis tissue of these control men has also been used in previous studies (Chikhovskaya et al., 2013; Nickkholgh et al., 2014).
Ethical approval
From all men, patients and controls, we obtained informed consent to use their spare tissue for research purposes. According to Dutch law, spare human tissues can be used for research without approval of a medical ethical committee as none of the patients had to undergo any additional intervention to obtain the material for this research.
Histological evaluation
Part of the testis biopsy from all men were fixed in diluted Bouin's solution, processed as paraffin blocks and cut in 5 µm sections. For each patient one slide was stained by conventional hematoxylin and eosin and seminiferous tubules were graded using the Johnsen score (Johnsen, 1970). In this classification system, at least 40 seminiferous tubules are evaluated and individually scored in a range from one to ten. Transversal tubules in this section with complete spermatogenesis with >10 spermatozoa without apoptotic cells are given a score of 10 and with apoptotic cells in the lumen are given a score of 9, SCO is scored as 2 and sclerotic tubules without Sertoli cells are given a score of 1.
Sequence-tagged sites (STS) deletion confirmation
To confirm the presence or absence of AZFc deletions, testicular tissue DNA was extracted using QIAamp DNA Mini Kit (51304, Qiagen, Germany). Multiplex PCR was performed using six STS markers: sY142, sY1191, sY1197, sY1201, sY1206 and sY1291 as previously described (Repping et al., 2003).
Testicular cell isolation and culture
Testicular cells were isolated and cultured as previously described (Sadri-Ardekani et al., 2009). The input of tissue varied depending on the amount of material that was left over from the TESE procedure (range 150–300 µl). Briefly, after two steps enzymatic digestion and overnight plating, floating cells were collected, plated in uncoated plastic dishes and cultured in supplemented stempro-34 (Invitrogen, USA) in 5% CO2 at 37°C. The cells were passaged every 7–10 days. The formation of germ line stem cell clusters (GSCs) in culture was checked. Cultured cells were harvested at 18 days (short-term culture) and 48 days (long-term culture) of culture for magnetic assisted cell sorting (MACS) to enrich for germ cells or SSCs for further expression analyses.
Magnetic assisted cell sorting
Short-term and long-term cultured cells were labeled with biotin conjugated anti-integrin alpha 6 (ITGA6) (313604, Biolegend, USA) to enrich for SSCs (ITGA6+ cells) (Nickkholgh et al., 2014) or APC conjugated anti-HLA I (human leukocyte antigen I, 555555, BD Pharmingen, USA) to enrich for germ cells at all steps of development including spermatogonia and SSCs (HLA− cells) (Hutter and Dohr, 1998). MACS was performed according to the manufacturer's protocol (Miltenyi Biotec, Inc., the Netherlands). Briefly, after trypsinization, cells were washed in minimum essential media (MEM) and incubated for 30 min at 4°C with the primary antibodies for ITGA6 or HLA I and subsequently with microbeads conjugated with anti-biotin (130-090-458, Miltenyi biotech, the Netherlands) or anti-APC (130-090-855, Miltenyi biotech, the Netherlands), respectively, for 15 min at 4°C and sorted using the mini MACS (MS) column separator (130-042-201, Miltenyi biotech, the Netherlands). Cells were snap-frozen in a cell pellet and stored at −80°C for RNA extraction later.
For MACS sorting we used all cultured testicular cells and the average retrieved percentage of HLA− cells was 45.6 ± 4.4% and for ITGA6+ this was 13.2 ± 3.1%.
Quantitative real-time polymerase chain reaction
RNA was extracted from the cell pellet of the sorted human short-term and long-term cultured testicular cells using the RNeasy Mini kit (74104, Qiagen, Germany). Copy DNA (cDNA) was synthesized from an equal amount of RNA of all samples using random primers and M-MLV reverse transcriptase (28025-021, Invitrogen, USA). Although we used various amount of tissue for culture, after culture, sorting and RNA isolation an equal amount of RNA was used as input for the gene expression analyses.
To investigate whether AZFc genes were expressed in spermatogonia in our culture system, expression of BPY2, CDY1, DAZ1, GOLGA2LY and CSPG4LY was checked in MACS sorted ITGA6+ short-term cultured (18 days) and long-term cultured (48 days) testicular cells of control men. PCR products were visualized using gel electrophoresis.
To compare the degree of possible spermatogonial differentiation to more advanced germ cells during culture of testicular cells of AZFc-deleted patients with that of controls, expression levels of spermatogonial markers (ubiquitin carboxyl-terminal esterase L1 (UCHL1), zinc finger and BTB domain containing 16 (ZBTB16), glial cell line-derived neurotrophic factor family receptor alpha 1 (GFRA1) and melanoma antigen family A4 (MAGEA4)) and germ cell differentiation markers signal transducer and activator of transcription 3 (STAT3), spermatogenesis and oogenesis specific basic helix-loop-helix 2 (SOHLH2), v-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog (KIT) and synaptonemal complex protein 3 (SYCP3) were determined using quantitative real-time polymerase chain reaction (qPCR) in cultured testicular cells that were HLA− MACS sorted for germ cells.
All quantitative PCR reactions were probe based using Universal probes (Exicon probes, Roche diagnostic, Germany) and all primer sets were selected from the universal probe library (see Table I) of Roche-applied science (www.roche-applied-science.com) and optimized on RNA from human testis tissue. The specificity of the primers was confirmed by sequencing the obtained PCR product. Real-time PCR was performed in the Lightcycler 480 Real time PCR system (Roche diagnostic, USA) in 384 well plates using the following program: 15 min at 95°C, 50 cycles of 15 s at 95°C, 20 s at 55°C, 20 s at 72°C. The total volume for each PCR reaction including Absolute PCR master mix (AB-1132, Abgene, Germany) was 10 µl. Polymerase (RNA) II polypeptide A (POLR2A) was selected as reference gene (Radonic et al., 2004). All samples were run in triplicate. The mean Ct values for each transcript were normalized to that of the reference gene and comparison between short- and long-term cultured AZFc-deleted and control samples were calculated with the ΔΔCT method. In cases where no gene expression was observed, the Ct value was assumed 50 cycles (the number of total performed cycles).
Table I.
Primer sets and specific universal probe for detecting specific transcripts in quantitative real-time PCR.
| Gene symbol | Transcript ID | Primer set | Probe no. | Product size |
|---|---|---|---|---|
| BPY2 | NM_004678.2 | Fw: cgtgcaggacaggatcatta Rev: tgccctctgtaagcagcac |
#43 | 67 bp |
| CDY1 | NM_004680.2 | Fw: ggccaatgagagagagtgtga Rev:gctttatatcccaacagaggtatttt |
#73 | 96 bp |
| DAZ1 | NM_004081.5 | Fw: atcacgccgaatcctgtaac Rev: tggagatggttgagtttgga |
#84 | 73 bp |
| GOLGA2LY2 | ENST00000398377.3 | Fw: ggccatgcatcatctgcta Rev: cagggccactgctagttctc |
#36 | 64 bp |
| CSPG4LY | NR_001554.2 | Fw: gagaggcagctgagatcagaa Rev: cggctccgagatgatgaa |
#78 | 62 bp |
| POL2A | ENST00000322644 | Fw: ttgtgcaggacacactcaca Rev:caggaggttcatcacttcacc |
#1 | 83 bp |
| PLZF | NM_001018011 | Fw: gcacagttttcgaaggagga Rev: cagaagacggccatgtca |
#80 | 78 bp |
| UCHL1 | NM_004181.4 | Fw: cctgaagacagagcaaaatgc Rev:aaatggaaattcaccttgtcatct |
#27 | 110 bp |
| GFRA1 | NM_005264.4 | Fw: gtcgggcaatacacacctct Rev: gcagccattgattttgtgg |
#11 | 93 bp |
| MAGA4 | NM_001011548 | Fw: cccaggctctataaggagacaag Rev: cagcaggcaagagtgcag |
#61 | 143 bp |
| c-KIT |
NM_000222.2 NM_001093772.1 |
Fw: ctttcctcgcctccaagaat Rev: gtgatccgaccatgagtaagg |
#71 | 76 bp |
| SOHLH2 | NM_017826.2 | Fw: ctgtggagctcctcctgct Rev: cccacagtgacatctccaacta |
#43 | 146 bp |
| STAT3 | NM_213662.1 | Fw: ctgcctagatcggctagaaaac Rev: ccctttgtaggaaactttttgc |
#25 | 112 bp |
| SYCP3 | ENST00000392924.1 | Fw: tttgtttcagcagtgggattt Rev:tcttttgttgctgtcgaaacat |
#73 | 86 bp |
Statistics
One way ANOVA with Tukey–Kramer post hoc test was used to compare the expression of different markers in AZFc-deleted samples with controls after 18 days and 48 days of culture. A P-value <0.05 was considered to be significant.
Results
Confirmation of AZFc deletion in testicular tissue
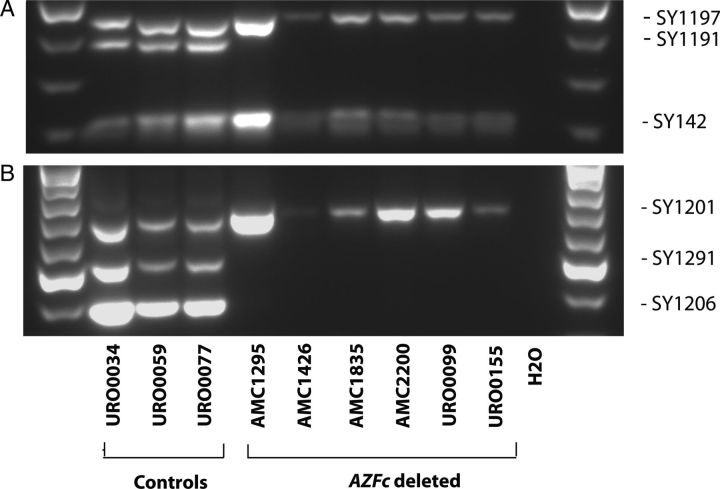
The initial diagnosis of an AZFc deletion was based on the absence of STS markers SY1191, SY1206 and SY1291 and the presence of SY142, SY1197 and SY1201 in blood. In order to rule out germ line mosaicism (i.e. the presence of an AZFc deletion in blood but not in testis) we investigated the presence of the same STS markers in testis derived DNA. We confirmed the presence of the deletion in testicular tissue from all six patients and the absence of the deletion in all three controls (Fig. 1).
Figure 1.
Confirmation of the absence of the complete AZFc region in testis tissues of all AZFc-deleted patients. The complete absence or presence of the AZFc region in testicular genomic DNA of all patients and controls was performed in a multiplex PCR by testing six STS markers. Primer set 1 contains sY142, sY1191 and sY1197. Primer set 2 contains sY1206, sY1291 and sY1201. Absence of sY1191, sY1206 and sY1291 in the presence of sY142, sY1197 and sY1201 (LANES 4–9) confirmed the AZFc deletion in our azoospermic patients. All controls had no deletion (LANES 1–3).
Histological evaluation of testicular tissue
We determined the Johnsen score of 40–70 tubules in testes section of each AZFc-deleted and non-deleted control men. From the six AZFc-deleted patients, one patient showed only SCO tubules in the examined biopsy sections, while all others showed a mixed phenotype of which three showed SCO and maturation arrest and two SCO with maturation arrest and full spermatogenesis (Table II). The percentage of SCO tubules in the scored biopsy varied between 18 and 100% in the evaluated sections (Table II). Upon TESE, sperm was found in two out of six patients (URO0099 with 18% SCO tubules and URO0155 with 56% SCO tubules). In testis sections of controls, we evaluated 58–122 tubules per control. Almost all tubules showed elongated spermatids and hardly any SCO tubules were observed (Table II).
Table II.
Johnsen score and number of germ line stem cell clusters (GSCs) in culture.
|
AZFc-deleted patients | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient code | Johnsen score* |
Counted tubules | Average Johnsen score | SCO tubules (%) | Sperm found in TESE | NO. of GSCs in short-term culture | NO. of GSCs in long-term culture | ||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||||||||
| 1 | AMC1295 | 40 | 40 | 2.0 | 100 | No | 0 | >20 | |||||||||
| 2 | AMC1426 | 8 | 30 | 3 | 5 | 46 | 2.1 | 65 | No | 0 | >20 | ||||||
| 3 | AMC1835 | 21 | 8 | 20 | 2 | 51 | 3.1 | 41 | No | 0 | >200 | ||||||
| 4 | AMC2200 | 68 | 2 | 70 | 2 | 97 | No | 0 | 0 | ||||||||
| 5 | URO0099 | 19 | 11 | 2 | 9 | 6 | 4 | 6 | 2 | 1 | 60 | 3.4 | 18 | Yes | 0 | 0 | |
| 6 | URO0155 | 29 | 7 | 10 | 5 | 1 | 52 | 2.8 | 56 | Yes | 0 | 4 | |||||
| Controls | |||||||||||||||||
| Patient code | Johnsen score* |
Counted tubules | Average Johnsen score | SCO tubules (%) | Sperm found in TESE | NO. of GSCs in short-term culture | NO. of GSCs in long-term culture | ||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||||||||
| 1 | URO0034 | 1 | 1 | 7 | 33 | 53 | 27 | 122 | 8.7 | 0 | NA | 0 | 3 | ||||
| 3 | URO0059 | 2 | 2 | 2 | 20 | 27 | 5 | 58 | 8.4 | 0 | NA | 0 | >100 | ||||
| 2 | URO0077 | 3 | 5 | 4 | 35 | 36 | 15 | 98 | 8.4 | 0 | NA | 0 | >20 | ||||
SCO, Sertoli cell only; TESE, testicular sperm extraction; GSCs, germ line stem cell clusters.
*Johnsen Score per tubular cross section: Score 1: No seminiferous epithelium, Score 2: no germ cells, only presence of Sertoli cells, Score 3: only presence of Spermatogonia, Score 4: no spermatids, 1–10 spermatocytes, Score 5: no spermatids, presence of ≥10 spermatocytes, Score 6: no elongated spermatids, presence of 1–10 round spermatids, Score 7: no elongated spermatids, presence of ≥10 round spermatids, Score 8: presence of 1–10 elongating spermatids, Score 9: ≥10 elongated spermatids, disorganized epithelium with released immature and apoptotic cells in the lumen, Score 10: presence of ≥10 spermatozoa without immature cells in lumen.
Testicular cell culture
To study the behavior of testicular cells of AZFc-deleted patients and controls during short-term culture (18 days) and long-term culture (48 days), the number of germline stem cell-clusters (GSCs) formed in culture was evaluated. During short-term culture, testicular cells of all patients and controls underwent two passages and no cluster formation was observed in any of the cultures. During long-term (48 days) culture, testicular cells of AZFc-deleted patients went through 5–6 passages and GSCs were observed in four out of six patients, while testicular cells of controls went through 6–7 passages and GSCs were present in all of them. Whenever GSC formation was observed, it was first observed at the end of the third week. In two out of three controls and two out of six patients >20 GSCs were formed, while the number of GSCs in the other two cultures (one patient and one control) was <10 (Table II).
Expression of AZFc genes in ITGA6 sorted spermatogonia during culture
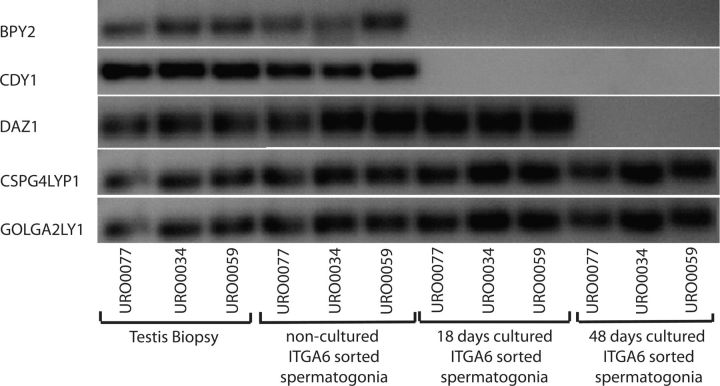
We determined whether AZFc genes are expressed in non-cultured human spermatogonia and found that all five AZFc genes were expressed in non-cultured spermatogonia of all three controls. During culture the expression of GOLGA2LY and CSPG4LY expression was maintained throughout the entire culture period, while the expression of BPY2 and CDY1 was lost already in short-term culture and the expression of DAZ was maintained in short-term culture but lost in long-term culture (Fig. 2).
Figure 2.
Expression of Basic charge Y-linked, 2 (BPY2), Chromo domain on Y (CDY1), Deleted in azoospermia (DAZ), Golgi autoantigen Golgin subfamily A2 like Y (GOLGA2LY) and Chondroitin sulfate proteoglycan 4 like Y (CSPG4LY) in testicular tissue; non-cultured; short-term (18 days) cultured and long-term (48 days) cultured spermatogonia from three men with no deletion of AZFc (URO0077, URO0034 and URO0059). Except for total testicular tissue, MACS sorted ITGA6+ cells were used.
Expression of spermatogonial genes in HLA− germ cells during culture
We next examined the expression of spermatogonial markers in HLA− germ cells during short-term and long-term culture in all patients and controls. We quantified the expression of UCHL1, GFRA1, ZBTB16 (also known as promyelocytic leukemia zinc finger ortholog (PLZF)) and MAGE A4 in HLA− germ cells. During culture similar expression levels of UCHL1, GFRA1 and ZBTB16 were detected in testicular cell cultures of AZFc-deleted and control men (Fig. 3A). The expression level of MAGE A4 was low in controls at short-term culture but AZFc-deleted samples showed a significantly lower expression in comparison to controls. In long-term culture, expression of MAGEA4 in both AZFc-deleted and control samples was hardly detectable (Fig. 3A).
Figure 3.
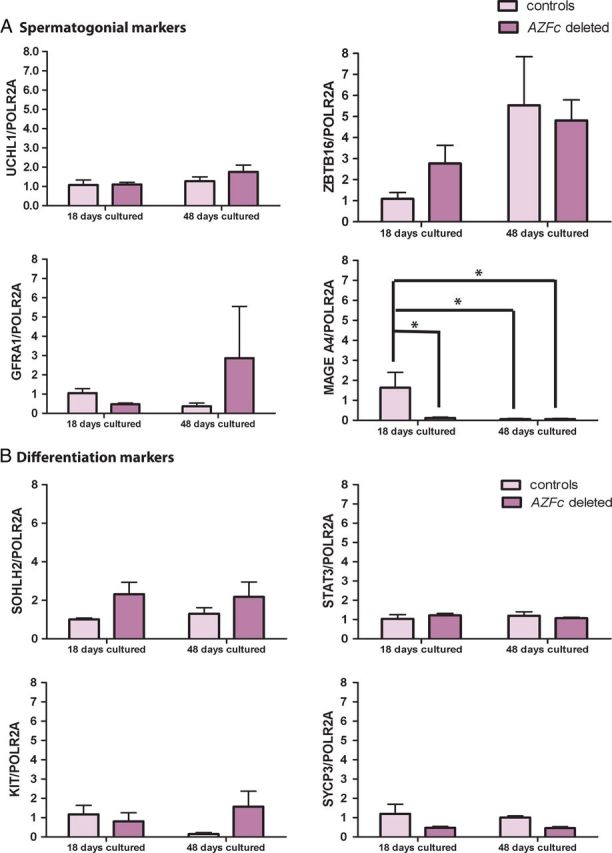
Relative gene expression of germ cell markers in non-deleted (controls) and AZFc-deleted short-term and long-term cultured and MACS sorted HLA− germ cells. (A) Spermatogonial marker expression: ubiquitin carboxyl-terminal esterase L1 (UCHL1), zinc finger and BTB domain containing 16 (ZBTB16), GDNF family receptor alpha 1 (GFRA1) and melanoma antigen family A4 (MAGEA4). (B) Germ cell differentiation marker expression: signal transducer and activator of transcription 3 (STAT3), spermatogenesis and oogenesis specific basic helix-loop-helix 2 (SOHLH2), v-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog (KIT) and synaptonemal complex protein 3 (SYCP3). Polymerase (RNA) II polypeptide A (POLR2A) was used as the reference gene. Data expressed as mean ± SEM compared with the short-term or long-term cultured control cells. Asterisks highlight significant differences with P< 0.05.
Expression of genes associated with spermatogonial differentiation
To investigate whether AZFc deletions enhance or reduce differentiation of spermatogonia, we determined the expression of markers for early and later steps of spermatogonial differentiation in cultured and HLA− sorted germ cells of AZFc-deleted patients and that of non-deleted controls. Spermatogenesis and oogenesis specific basic helix-loop-helix 2 (SOHLH2) and signal transducer and activator of transcription 3 (STAT3), both important markers for early differentiation (Toyoda et al., 2009), were equally expressed in cultured germ cells of AZFc-deleted patients and controls in short- and long-term cultures (Fig. 3B). Although, the mean expression of SOHLH2 in AZFc-deleted germ cells was slightly higher than in controls, the difference was not significant.
v-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog (KIT), another spermatogonial differentiation marker showed relatively low expression and synaptonemal complex protein 3 (SYCP3), known as a late meiotic marker, showed very limited (almost no expression) expression in short-term and long-term culture of germ cells of both AZFc-deleted patients and controls. Statistical analysis showed no significant difference in expression of both markers in cultured germ cells of AZFc-deleted men compared with that of control men and no difference in expression levels between short- and long-term cultured HLA− germ cells (Fig. 3B).
Discussion
In this study, we showed that spermatogonia of azoospermic patients with an AZFc deletion behave similar in culture and are indistinguishable in terms of expression of markers for undifferentiated spermatogonia (ZBTB16, UCHL1, GFRA1) and germ cell differentiation (SOHLH2 and STAT3, KIT and SYCP3) when compared with spermatogonia of men without an AZFc deletion.
Five genes located in the AZFc region are most likely translated into protein, including BPY2 and DAZ that are known to be expressed in human spermatogonia (Menke et al., 1997; Vogt et al., 2008). In the current study, for the first time, we have demonstrated that all AZFc genes are expressed in freshly isolated non-cultured human spermatogonia. During in vitro propagation, expression of two of these genes (BPY2 and CDY1) is lost within 3 weeks and expression of DAZ is lost within 7 weeks of culture. Although we still do not know what the impact of losing the expression of some genes during culture is, at least it is apparent that these genes are not essential in survival of SSCs in vitro. The function of GOLGA2LY and CSPG4LY in human testis is unknown (Navarro-Costa et al., 2010) but the steady-state expression of these genes in spermatogonia during in vitro human SSC culture suggests that they exert their function in these cells. What is true for all AZFc genes based on the results described here is that they are not crucial for proliferation of spermatogonia at least in vitro.
Although some inter-patient variation was observed, the expression of early spermatogonial markers UCHL1 (Luo et al., 2006; Wang et al., 2006), ZBTB16 (Costoya et al., 2004) and GFRA1 (Buageaw et al., 2005; Gassei et al., 2010) was similar in AZFc-deleted as in non-deleted spermatogonia in both early and late culture. This again suggests that the presence of AZFc genes is not critical for survival and propagation of SSCs. Among spermatogonial markers, the expression of MAGEA4 was lost during long-term culture in all samples including non-deleted controls. It is well known that cells change their expression profile in response to an altering microenvironment especially when taken from an in vivo to an in vitro environment (Sandberg and Ernberg, 2005). The expression of MAGEA4 in short-term culture was low in controls and AZFc-deleted samples showed significantly lower expression. The earlier loss of MAGEA4 expression in spermatogonia of AZFc-deleted men is of unknown significance but the loss of MAGEA4 during long-term culture suggests that MAGEA4 is not required for SSC maintenance and proliferation at least in vitro.
In histological evaluation, the overall Johnsen score was 2.6 ± 0.6 for men with AZFc deletions and one out of the six studied patients showed a complete SCO tubular phenotype in the examined biopsy (17%), three out of six showed maturation arrest (50%) and 2 out of 6 (33%) showed normal spermatogenesis (at least in some tubules). In two out of six patients (33%) sperm was successfully retrieved upon TESE. The Johnsen score for men with AZFc deletions was reported in only one other study that evaluated nineteen AZFc-deleted patients. In this study the Johnsen score was 5.1 ± 1.7 which is higher than the score observed in our study possibly reflecting the variable phenotype of men with AZFc deletions (Zhang et al., 2013). Multiple studies have reported sperm retrieval rate and testicular histology in men with AZFc deletions. In these studies the sperm retrieval rate with TESE varied between 30 and 87% and the frequencies for the three histological phenotypes varied between 16 and 58% for complete SCO, 6 and 65% for maturation arrest and 6 and 38% for normal spermatogenesis (Oates et al., 2002; Hopps et al., 2003; Ferras et al., 2004; Stouffs et al., 2005; Patrat et al., 2010; Kim et al., 2012; Zhang et al., 2013). The corresponding rates in our study all fall within these ranges.
It has been suggested that AZFc deletions cause a progressive phenotype in which patients transition gradually from oligozoospermia to azoospermia with increasing frequency of SCO tubules (Simoni et al., 1997). To investigate whether a progressive SCO phenotype is the result of SSCs depletion due to increased SSCs differentiation, we investigated the expression of markers for differentiating germ cells, i.e. SOHLH2 (Hao et al., 2008; Toyoda et al., 2009), STAT3 (Oatley et al., 2010; Kaucher et al., 2012), KIT (Rossi et al., 2008) and the meiosis marker SYCP3 (Aarabi et al., 2006; Shi et al., 2013). The expression of all four markers was very low and similar in spermatogonia from AZFc-deleted and that of control men at all-time points in culture. This indicates that AZFc deletions do not affect the differentiation rate of spermatogonia, at least not in vitro.
Isolated testicular cells were cultured using our previously described testicular cell culture system (Sadri-Ardekani et al., 2009). In this culture system, we take advantage of accompanying somatic cells as feeder cells that provide the suitable microenvironment for propagating spermatogonial cells including SSCs. As the behavior of AZFc-deleted spermatogonia in culture was not different from controls, we might conclude that also the microenvironment provided by (AZFc-deleted) somatic cells in culture is similar in AZFc-deleted men and controls. Thus, somatic cells in the testis appear not to be affected by AZFc deletions in respect to supporting spermatogonial maintenance and proliferation. One patient (AMC 1295) with SCO showed stem cell clusters upon culture. Although all tubules examined with histology demonstrated SCO, it is still very well possible those other parts of the testis of this man still contain spermatogonia and thus, spermatogonia could have been included in the biopsy that was used for testicular cell culture. The opposite is also true in our hands: we have experienced that in some cases with full spermatogenesis, SSCs could be identified in culture by expression markers while no GSCs were formed in culture. This reflects the heterogeneous nature of our testicular cell culture system.
The heterogeneous cell population in our culture system makes it difficult to directly analyze specific cell types. We relied on membrane specific markers to purify spermatogonia or germ cells. Specifically, we used ITGA6 MACS to enrich for cultured human spermatogonia including SSCs (Nickkholgh et al., 2014). Similarly, we used MACS HLA− germ cells (Anderson et al., 1984; Kurpisz et al., 1986; Jassim et al., 1989) to study the potential enhanced spermatogonial differentiation to more advanced germ cells. As equal amounts of RNA for all samples were used for RT–PCR and results were normalized against a reference housekeeping gene, similar gene expression levels most likely reflect similar gene expression per cell in AZFc-deleted samples and in controls.
Since spermatogonia in culture do not appear to be affected by AZFc deletions and AZFc-deleted spermatogonia can progress fully to spermatozoa in vivo, increasing the number of spermatogonia in culture might be a way to directly treat men with AZFc deletions. Such treatment would involve a testicular biopsy, propagation of SSCs in vitro and subsequent transfer of these propagated SSCs to the patients' testis. These transferred SSCs would then home to their niche in the testis, colonize the testis and then initiate self-renewal and differentiation. Eventually this would then revert the azoospermia phenotype to oligozoo- or even normozoospermia. If successful, this treatment would prevent the use of ICSI with its associated risks, costs, burden and possible long-term health effects on offspring (Alukal and Lamb, 2008; Chambers et al., 2009; Okun and Sierra, 2014). Of course, the SSC transplantation technique is not yet clinically applicable (Struijk et al., 2013) but it will likely be in the near future. Besides treating men with AZFc deletions, SSC transplantation could theoretically also be used to treat other cases in which there is a shortage of spermatogonia in vivo as long as the existing spermatogonia are fully functional. The experiments described in this study could provide a roadmap for determining whether such treatment would work in these cases.
In conclusion, the current study demonstrates that AZFc-deleted spermatogonia behave very similar to normal spermatogonia in vitro and show equal levels in expression of spermatogonial and differentiation markers as spermatogonia from non-deleted control men. This indicates that treatment of men with AZFc deletions by propagating their SSCs in vitro and autotransplanting them back to the testis is potentially feasible treatment option to restore their fertility.
Authors' roles
B.N.: study design, obtaining results, data analysis, writing manuscript. C.M.K. and S.K.M.v.D.: obtaining results, critical discussion. A.M.M.v.P.: study design, obtaining results, supervising data analysis, editing manuscript. S.R.: study idea, study design, critical discussion, editing manuscript and corresponding author.
Funding
This study was supported by a VIDI grant to S.R. (ZonMw VIDI-grant 91796362).
Conflict of interest
None declared.
Acknowledgement
We thank Dr Madelon van Wely for statistical advice and Maryam Nazm Bojnordi for technical assistance.
References
- Aarabi M, Modarressi MH, Soltanghoraee H, Behjati R, Amirjannati N, Akhondi MM. Testicular expression of synaptonemal complex protein 3 (SYCP3) messenger ribonucleic acid in 110 patients with nonobstructive azoospermia. Fertil Steril 2006;86:325–331. [DOI] [PubMed] [Google Scholar]
- Alukal JP, Lamb DJ. Intracytoplasmic sperm injection (ICSI)—what are the risks? Urol Clin North Am 2008;35:277–288, ix-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DJ, Narayan P, DeWolf WC. Major histocompatibility antigens are not detectable on post-meiotic human testicular germ cells. J Immunol 1984;133:1962–1965. [PubMed] [Google Scholar]
- Buageaw A, Sukhwani M, Ben-Yehudah A, Ehmcke J, Rawe VY, Pholpramool C, Orwig KE, Schlatt S. GDNF family receptor alpha1 phenotype of spermatogonial stem cells in immature mouse testes. Biol Reprod 2005;73:1011–1016. [DOI] [PubMed] [Google Scholar]
- Chambers GM, Sullivan EA, Ishihara O, Chapman MG, Adamson GD. The economic impact of assisted reproductive technology: a review of selected developed countries. Fertil Steril 2009;91:2281–2294. [DOI] [PubMed] [Google Scholar]
- Chikhovskaya JV, van Daalen SK, Korver CM, Repping S, van Pelt AM. Mesenchymal origin of multipotent human testis-derived stem cells in human testicular cell cultures. Mol Hum Reprod 2013;20:155–167. [DOI] [PubMed] [Google Scholar]
- Choi JM, Chung P, Veeck L, Mielnik A, Palermo GD, Schlegel PN. AZF microdeletions of the Y chromosome and in vitro fertilization outcome. Fertil Steril 2004;81:337–341. [DOI] [PubMed] [Google Scholar]
- Costoya JA, Hobbs RM, Barna M, Cattoretti G, Manova K, Sukhwani M, Orwig KE, Wolgemuth DJ, Pandolfi PP. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat Genet 2004;36:653–659. [DOI] [PubMed] [Google Scholar]
- Eberhart CG, Maines JZ, Wasserman SA. Meiotic cell cycle requirement for a fly homologue of human Deleted in Azoospermia. Nature 1996;381:783–785. [DOI] [PubMed] [Google Scholar]
- Ferras C, Fernandes S, Marques CJ, Carvalho F, Alves C, Silva J, Sousa M, Barros A. AZF and DAZ gene copy-specific deletion analysis in maturation arrest and Sertoli cell-only syndrome. Mol Hum Reprod 2004;10:755–761. [DOI] [PubMed] [Google Scholar]
- Gambera L, Governini L, De Leo V, Luddi A, Morgante G, Tallis V, Piomboni P. Successful multiple pregnancy achieved after transfer of frozen embryos obtained via intracytoplasmic sperm injection with testicular sperm from an AZFc-deleted man. Fertil Steril 2010;94:2330 e2331–2333. [DOI] [PubMed] [Google Scholar]
- Gassei K, Ehmcke J, Dhir R, Schlatt S. Magnetic activated cell sorting allows isolation of spermatogonia from adult primate testes and reveals distinct GFRa1-positive subpopulations in men. J Med Primatol 2010;39:83–91. [DOI] [PubMed] [Google Scholar]
- Gromoll J, Weinbauer GF, Skaletsky H, Schlatt S, Rocchietti-March M, Page DC, Nieschlag E. The Old World monkey DAZ (Deleted in AZoospermia) gene yields insights into the evolution of the DAZ gene cluster on the human Y chromosome. Hum Mol Genet 1999;8:2017–2024. [DOI] [PubMed] [Google Scholar]
- Hao J, Yamamoto M, Richardson TE, Chapman KM, Denard BS, Hammer RE, Zhao GQ, Hamra FK. Sohlh2 knockout mice are male-sterile because of degeneration of differentiating type A spermatogonia. Stem Cells 2008;26:1587–1597. [DOI] [PubMed] [Google Scholar]
- Hopps CV, Mielnik A, Goldstein M, Palermo GD, Rosenwaks Z, Schlegel PN. Detection of sperm in men with Y chromosome microdeletions of the AZFa, AZFb and AZFc regions. Hum Reprod 2003;18:1660–1665. [DOI] [PubMed] [Google Scholar]
- Hutter H, Dohr G. HLA expression on immature and mature human germ cells. J Reprod Immunol 1998;38:101–122. [DOI] [PubMed] [Google Scholar]
- Jassim A, Ollier W, Payne A, Biro A, Oliver RT, Festenstein H. Analysis of HLA antigens on germ cells in human semen. Eur J Immunol 1989;19:1215–1220. [DOI] [PubMed] [Google Scholar]
- Johnsen SG. Testicular biopsy score count—a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones 1970;1:2–25. [DOI] [PubMed] [Google Scholar]
- Kaucher AV, Oatley MJ, Oatley JM. NEUROG3 is a critical downstream effector for STAT3-regulated differentiation of mammalian stem and progenitor spermatogonia. Biol Reprod 2012;86:164, 161–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Choi HW, Park SY, Song IO, Seo JT, Lee HS. Molecular and cytogenetic studies of 101 infertile men with microdeletions of Y chromosome in 1,306 infertile Korean men. J Assist Reprod Genet 2012;29:539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostova E, Rottger S, Schempp W, Gromoll J. Identification and characterization of the cynomolgus monkey chromodomain gene cynCDY, an orthologue of the human CDY gene family. Mol Hum Reprod 2002;8:702–709. [DOI] [PubMed] [Google Scholar]
- Kuhnert B, Gromoll J, Kostova E, Tschanter P, Luetjens CM, Simoni M, Nieschlag E. Case report: natural transmission of an AZFc Y-chromosomal microdeletion from father to his sons. Hum Reprod 2004;19:886–888. [DOI] [PubMed] [Google Scholar]
- Kuroda-Kawaguchi T, Skaletsky H, Brown LG, Minx PJ, Cordum HS, Waterston RH, Wilson RK, Silber S, Oates R, Rozen S, et al. The AZFc region of the Y chromosome features massive palindromes and uniform recurrent deletions in infertile men. Nat Genet 2001;29:279–286. [DOI] [PubMed] [Google Scholar]
- Kurpisz M, Fernandez N, Szymczynski G, Kowalik I. The non-mature sperm cells: evaluation of surface markers and interaction in in vitro cultures. Arch Immunol Ther Exp (Warsz) 1986;34:101–109. [PubMed] [Google Scholar]
- Liu XH, Qiao J, Li R, Yan LY, Chen LX. Y chromosome AZFc microdeletion may not affect the outcomes of ICSI for infertile males with fresh ejaculated sperm. J Assist Reprod Genet 2013;30:813–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luetjens CM, Gromoll J, Engelhardt M, Von Eckardstein S, Bergmann M, Nieschlag E, Simoni M. Manifestation of Y-chromosomal deletions in the human testis: a morphometrical and immunohistochemical evaluation. Hum Reprod 2002;17:2258–2266. [DOI] [PubMed] [Google Scholar]
- Luo J, Megee S, Rathi R, Dobrinski I. Protein gene product 9.5 is a spermatogonia-specific marker in the pig testis: application to enrichment and culture of porcine spermatogonia. Mol Reprod Dev 2006;73:1531–1540. [DOI] [PubMed] [Google Scholar]
- Menke DB, Mutter GL, Page DC. Expression of DAZ, an azoospermia factor candidate, in human spermatogonia. Am J Hum Genet 1997;60:237–241. [PMC free article] [PubMed] [Google Scholar]
- Minor A, Wong EC, Harmer K, Ma S. Molecular and cytogenetic investigation of Y chromosome deletions over three generations facilitated by intracytoplasmic sperm injection. Prenat Diagn 2007;27:743–747. [DOI] [PubMed] [Google Scholar]
- Navarro-Costa P, Goncalves J, Plancha CE. The AZFc region of the Y chromosome: at the crossroads between genetic diversity and male infertility. Hum Reprod Update 2010;16:525–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickkholgh B, Mizrak SC, Korver CM, van Daalen SK, Meissner A, Repping S, van Pelt AM. Enrichment of spermatogonial stem cells from long-term cultured human testicular cells. Fertil Steril 2014;102:1700–1707. [DOI] [PubMed] [Google Scholar]
- Oates RD, Silber S, Brown LG, Page DC. Clinical characterization of 42 oligospermic or azoospermic men with microdeletion of the AZFc region of the Y chromosome, and of 18 children conceived via ICSI. Hum Reprod 2002;17:2813–2824. [DOI] [PubMed] [Google Scholar]
- Oatley JM, Kaucher AV, Avarbock MR, Brinster RL. Regulation of mouse spermatogonial stem cell differentiation by STAT3 signaling. Biol Reprod 2010;83:427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun N, Sierra S. Pregnancy outcomes after assisted human reproduction. J Obstet Gynaecol Can 2014;36:64–83. [DOI] [PubMed] [Google Scholar]
- Page DC, Silber S, Brown LG. Men with infertility caused by AZFc deletion can produce sons by intracytoplasmic sperm injection, but are likely to transmit the deletion and infertility. Hum Reprod 1999;14:1722–1726. [DOI] [PubMed] [Google Scholar]
- Patrat C, Bienvenu T, Janny L, Faure AK, Fauque P, Aknin-Seifer I, Davy C, Thiounn N, Jouannet P, Levy R. Clinical data and parenthood of 63 infertile and Y-microdeleted men. Fertil Steril 2010;93:822–832. [DOI] [PubMed] [Google Scholar]
- Patsalis PC, Sismani C, Quintana-Murci L, Taleb-Bekkouche F, Krausz C, McElreavey K. Effects of transmission of Y chromosome AZFc deletions. Lancet 2002;360:1222–1224. [DOI] [PubMed] [Google Scholar]
- Radonic A, Thulke S, Mackay IM, Landt O, Siegert W, Nitsche A. Guideline to reference gene selection for quantitative real-time PCR. Biochem Biophys Res Commun 2004;313:856–862. [DOI] [PubMed] [Google Scholar]
- Repping S, Skaletsky H, Brown L, van Daalen SK, Korver CM, Pyntikova T, Kuroda-Kawaguchi T, de Vries JW, Oates RD, Silber S, et al. Polymorphism for a 1.6-Mb deletion of the human Y chromosome persists through balance between recurrent mutation and haploid selection. Nat Genet 2003;35:247–251. [DOI] [PubMed] [Google Scholar]
- Richardson TE, Chapman KM, Tenenhaus Dann C, Hammer RE, Hamra FK. Sterile testis complementation with spermatogonial lines restores fertility to DAZL-deficient rats and maximizes donor germline transmission. PLoS One 2009;4:e6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi P, Lolicato F, Grimaldi P, Dolci S, Di Sauro A, Filipponi D, Geremia R. Transcriptome analysis of differentiating spermatogonia stimulated with kit ligand. Gene Expr Patterns 2008;8:58–70. [DOI] [PubMed] [Google Scholar]
- Ruggiu M, Speed R, Taggart M, McKay SJ, Kilanowski F, Saunders P, Dorin J, Cooke HJ. The mouse Dazla gene encodes a cytoplasmic protein essential for gametogenesis. Nature 1997;389:73–77. [DOI] [PubMed] [Google Scholar]
- Sadri-Ardekani H, Mizrak SC, van Daalen SK, Korver CM, Roepers-Gajadien HL, Koruji M, Hovingh S, de Reijke TM, de la Rosette JJ, van der Veen F, et al. Propagation of human spermatogonial stem cells in vitro. JAMA 2009;302:2127–2134. [DOI] [PubMed] [Google Scholar]
- Sandberg R, Ernberg I. The molecular portrait of in vitro growth by meta-analysis of gene-expression profiles. Genome Biol 2005;6:R65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi YQ, Zhuang XJ, Xu B, Hua J, Liao SY, Shi Q, Cooke HJ, Han C. SYCP3-like X-linked 2 is expressed in meiotic germ cells and interacts with synaptonemal complex central element protein 2 and histone acetyltransferase TIP60. Gene 2013;527:352–359. [DOI] [PubMed] [Google Scholar]
- Silber SJ, Alagappan R, Brown LG, Page DC. Y chromosome deletions in azoospermic and severely oligozoospermic men undergoing intracytoplasmic sperm injection after testicular sperm extraction. Hum Reprod 1998;13:3332–3337. [DOI] [PubMed] [Google Scholar]
- Simoni M, Gromoll J, Dworniczak B, Rolf C, Abshagen K, Kamischke A, Carani C, Meschede D, Behre HM, Horst J, et al. Screening for deletions of the Y chromosome involving the DAZ (Deleted in AZoospermia) gene in azoospermia and severe oligozoospermia. Fertil Steril 1997;67:542–547. [DOI] [PubMed] [Google Scholar]
- Skaletsky H, Kuroda-Kawaguchi T, Minx PJ, Cordum HS, Hillier L, Brown LG, Repping S, Pyntikova T, Ali J, Bieri T, et al. The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature 2003;423:825–837. [DOI] [PubMed] [Google Scholar]
- Stouffs K, Lissens W, Tournaye H, Van Steirteghem A, Liebaers I. The choice and outcome of the fertility treatment of 38 couples in whom the male partner has a Yq microdeletion. Hum Reprod 2005;20:1887–1896. [DOI] [PubMed] [Google Scholar]
- Struijk RB, Mulder CL, van der Veen F, van Pelt AM, Repping S. Restoring fertility in sterile childhood cancer survivors by autotransplanting spermatogonial stem cells: are we there yet? Biomed Res Int 2013;2013:903142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth A, Tardy EP, Gombos S, Hajdu K, Batorfi J, Krausz C. AZFc deletion detected in a newborn with prenatally diagnosed Yq deletion. Prenat Diagn 2001;21:253–255. [DOI] [PubMed] [Google Scholar]
- Toyoda S, Miyazaki T, Miyazaki S, Yoshimura T, Yamamoto M, Tashiro F, Yamato E, Miyazaki J. Sohlh2 affects differentiation of KIT positive oocytes and spermatogonia. Dev Biol 2009;325:238–248. [DOI] [PubMed] [Google Scholar]
- Tse JY, Wong EY, Cheung AN, O WS, Tam PC, Yeung WS. Specific expression of VCY2 in human male germ cells and its involvement in the pathogenesis of male infertility. Biol Reprod 2003;69:746–751. [DOI] [PubMed] [Google Scholar]
- VanGompel MJ, Xu EY. A novel requirement in mammalian spermatid differentiation for the DAZ-family protein Boule. Hum Mol Genet 2010;19:2360–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera Y, Dai T, Hikim AP, Lue Y, Salido EC, Swerdloff RS, Yen PH. Deleted in azoospermia associated protein 1 shuttles between nucleus and cytoplasm during normal germ cell maturation. J Androl 2002;23:622–628. [PubMed] [Google Scholar]
- Vogt PH, Edelmann A, Kirsch S, Henegariu O, Hirschmann P, Kiesewetter F, Kohn FM, Schill WB, Farah S, Ramos C, et al. Human Y chromosome azoospermia factors (AZF) mapped to different subregions in Yq11. Hum Mol Genet 1996;5:933–943. [DOI] [PubMed] [Google Scholar]
- Vogt PH, Falcao CL, Hanstein R, Zimmer J. The AZF proteins. Int J Androl 2008;31:383–394. [DOI] [PubMed] [Google Scholar]
- Wang YL, Liu W, Sun YJ, Kwon J, Setsuie R, Osaka H, Noda M, Aoki S, Yoshikawa Y, Wada K. Overexpression of ubiquitin carboxyl-terminal hydrolase L1 arrests spermatogenesis in transgenic mice. Mol Reprod Dev 2006;73:40–49. [DOI] [PubMed] [Google Scholar]
- Wong EY, Tse JY, Yao KM, Lui VC, Tam PC, Yeung WS. Identification and characterization of human VCY2-interacting protein: VCY2IP-1, a microtubule-associated protein-like protein. Biol Reprod 2004;70:775–784. [DOI] [PubMed] [Google Scholar]
- Zhang F, Li L, Wang L, Yang L, Liang Z, Li J, Jin F, Tian Y. Clinical characteristics and treatment of azoospermia and severe oligospermia patients with Y-chromosome microdeletions. Mol Reprod Dev 2013;80:908–915. [DOI] [PubMed] [Google Scholar]
- Zhu XB, Liu YL, Zhang W, Ping P, Cao XR, Liu Y, Huang YR, Li Z. Vertical transmission of the Yq AZFc microdeletion from father to son over two or three generations in infertile Han Chinese families. Asian J Androl 2010;12:240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]