Abstract
Introduction
Hypogonadism is a growing concern in an aging male population. Historically treated using exogenous testosterone, concerns about possible adverse effects of testosterone have led physicians to seek alternative treatment approaches.
Areas Covered
Enclomiphene citrate is the trans isomer of clomiphene citrate, a non-steroidal estrogen receptor antagonist that is FDA-approved for the treatment of ovarian dysfunction in women. Clomiphene citrate has also been used off-label for many years to treat secondary male hypogonadism, particularly in the setting of male infertility. Here we review the literature examining the efficacy and safety of enclomiphene citrate in the setting of androgen deficiency.
Expert Opinion
Initial results support the conclusion that enclomiphene citrate increases serum testosterone levels by raising luteinizing hormone (LH) and follicle stimulating hormone (FSH) levels, without negatively impacting semen parameters. The ability to treat testosterone deficiency in men while maintaining fertility supports a role for enclomiphene citrate in the treatment of men in whom testosterone therapy is not a suitable option.
Keywords: hypogonadism, testosterone, enclomiphene citrate, selective estrogen receptor modulator
1. Introduction
1.1 Epidemiology of Hypogonadism
Hypogonadism is defined as low serum testosterone levels associated with symptoms including decreased libido, erectile dysfunction, loss of lean muscle mass, loss of vitality, and depression. The most sensitive symptoms supporting a diagnosis of hypogonadism include erectile dysfunction and decreased libido.[1–3] While accurately estimating the prevalence of hypogonadism is challenging, particularly given its varied definitions, in 2006 Mulligan et al. observed that 40% of men over the age of 45 evidenced low serum testosterone levels.[4, 5] Incorporating both serum testosterone levels as well as three symptoms of hypogonadism, the European Male Aging Study (EMAS) reported a prevalence of hypogonadism of only 2.1% in men 40–79 years old, and the Boston Area Community Health Study (BACHS) estimated the prevalence of hypogonadism to be 5.6% among men 30–79 years old when considering both serum testosterone levels and symptoms.[6, 7]
1.2 Pathogenesis of Secondary Hypogonadism
Hypogonadism can be further characterized as primary or secondary due to its etiology. Primary hypogonadism, which results from testicular dysfunction, can be genetic or associated with testicular injury or other insult. Secondary hypogonadism, in contrast, stems from a disruption of the hypothalamic-pituitary-gonadal (HPG) axis.[8] Important causes of secondary hypogonadism include Kallman’s syndrome, obesity, metabolic syndrome and type 2 diabetes. A 2010 follow-up to the EMAS study examined the classification of various types of hypogonadism and whether they could be consistently assigned. The authors distinguished between eugonadal (normal testosterone and LH levels), primarily hypogonadal (2% of study participants with low testosterone and high LH), secondarily hypogonadal (11.8% of participants with low testosterone and normal or low FH) or compensatorily hypogonadal (9.5% of participants with normal testosterone and high LH) men. The study also observed that a body mass index (BMI) of 30 kg/m2 or higher was associated with secondary hypogonadism (P < 0.001).[7] The presence of low serum testosterone levels also increases adiposity, compounding this problem.[9] Testosterone is critical for normal development and function of the male reproductive tract, and plays a role in sexual development, reproductive health and sexual function in the adult male. In 2016, the International Consultation on Sexual Medicine found that hypogonadism can lead to decreased sexual desire in men, which can be ameliorated with treatment, and also reported that estrogens play a minor role in regulating male sexual desire.[10] Testosterone is also important for maintenance of lean muscle mass, bone density and fat distribution in men.[11] A 2016 meta-analysis of studies examining testosterone therapy (TTh) and body composition concluded that exogenous testosterone improves body composition and glycometabolic profiles.[12] Endogenous testosterone is produced primarily in testicular Leydig cells in men, although the adrenal glands also produce small amounts.[13] An estimated 80% of circulating estradiol in men is the product of aromatized testosterone.[14] In a 2013 study, Finkelstein et al. found that when aromatization of testosterone to estradiol was blocked, the observed changes in fat deposition in men could be attributed specifically to decreased estradiol levels, and not the lack of serum testosterone, suggesting an important role for estradiol in homeostasis.[15] Beyond fat composition, estrogens also play a role in bone turnover.[16, 17] Both estrogen and testosterone play a role in sexual function and libido in the male.[15]
1.3 Classical Clinical Management of Hypogonadism
Both primary and secondary hypogonadism are often managed with exogenous testosterone administration. Testosterone is currently the only option for men with primary hypogonadism as well as for men with Kallman’s syndrome. While secondary hypogonadism has historically been managed with TTh, other treatment options are available for appropriately selected patients. The use of TTh in men with both primary and secondary hypogonadism has significantly increased during the past decade, with a large increase in testosterone prescriptions and direct-to-consumer marketing.[18, 19] While testosterone declines in an age-related manner, consistent with an increasing incidence of hypogonadism as a function of age, the Food and Drug Administration (FDA) recently removed idiopathic hypogonadism, which includes age-related hypogonadism, from the list of indications for TTh, recommending that only men with an identifiable cause of hypogonadism (excluding aging) should be prescribed testosterone.[20] Conversely, in 2014, the European Medicines Agency (EMA) concluded that no adverse cardiovascular risk was present in men on TTh and recommended that testosterone should be continued in hypogonadal men with no specific guidelines.[21] Treatment with exogenous testosterone is associated with several adverse sequelae, the most common of which are erythrocytosis, elevated serum estrogen levels, alterations in serum lipids, and infertility.[2] An increased cardiovascular risk in men using exogenous testosterone has also been proposed, although high quality evidence examining this relationship remains lacking.
1.4 Off-Label Strategies for Clinical Management of Hypogonadism
In addition to TTh, off-label pharmacologic strategies to stimulate endogenous testosterone production are available. These include selective estrogen receptor modulators (SERMs), selective androgen receptor modulators (SARMs) and aromatase inhibitors (AIs).[22, 23] AIs can be steroidal or non-steroidal and have progressed through three generations of drugs. In clinical practice, third-generation AIs such as letrozole and anastrozole are used due to their greater specificity for aromatase when compared with first generation drugs, translating to fewer side effects.[24] Suppression of estradiol production increases circulating LH, FSH, and testosterone levels.[25] Clomiphene citrate, the most common SERM used to treat secondary hypogonadism, blocks estrogen binding to receptors in the hypothalamus and increases gonadotropin release, resulting in increased testicular stimulation and testosterone production.[26] SERMs and aromatase inhibitors may be especially useful in treating obesity-related hypogonadism because of the high levels of aromatase in adipose tissue and the influence these drugs have on both the HPG axis and conversion of testosterone to estrogen.[27] Clomiphene may be a useful adjunct to weight loss in obese men, given the 2013 finding that long term weight loss is correlated with an increase in serum testosterone levels.[28] Tamoxifen, a partial estrogen agonist, has also been used to increase gonadotropin levels.[29] While there are numerous studies to support the use of SERMs in the adjunct management of hypogonadism, these are primarily small, retrospective, and uncontrolled. In 2008, the “Recommendations on investigation, treatment and monitoring of late-onset hypogonadism in males” published jointly by several professional societies including European Association of Urology (EAU), concluded that although aromatase inhibitors and SERMs raise testosterone levels, insufficient evidence supporting their use in the setting of hypogonadism exists. [30]
2. Overview of Clomiphene and Enclomiphene
Clomiphene citrate (commercially available as Clomid®) is a SERM that has been used since the 1960s to facilitate ovulation induction. Clomiphene has also been used off-label to raise LH, FSH and testosterone levels in men with secondary or idiopathic hypogonadism, and to raise sperm counts in men with a history of infertility or steroid use.[31] Clomiphene citrate is a mix of two stereoisomers, (cis) zuclomiphene citrate and (trans) enclomiphene citrate.[32] Studies comparing enclomiphene, zuclomiphene and clomiphene found clinically important pharmacologic differences between the three. Zuclomiphene, an estrogen receptor agonist, caused estrogenic side effects often reported with clomiphene use.[33] Additionally, zuclomiphene was found to have a longer half-life (30 days) when compared to clomiphene citrate (10 hours), resulting in persistent side effects lasting beyond the therapeutic effects of the drug.[31, 34] In contrast, enclomiphene was identified as the isomer that raises LH and FSH levels, with a shorter half-life. This led to the hypothesis that treatment with enclomiphene should have more favorable outcomes in treating androgen deficient men with the goal of maintaining fertility, potentially without the side effects associated with clomiphene citrate use and with a more favorable impact on hypogonadal symptoms. While this hypothesis has yet to be proven in hypogonadal men, Gupta et al. found that enclomiphene resulted in more mature ovarian follicles than clomiphene in infertile women.[35]
3. Introduction of the Compound
Enclomiphine citrate (commercial name Androxal®), (E)-2-(p-(2-Chloro-1,2-Diphenylvinyl)phenoxy)triethylamine is an oral, non-steroidal estrogen receptor antagonist that promotes testicular testosterone production by occupying estrogen receptors in the hypothalamus, thereby preventing negative feedback.[31, 36] Enclomiphene citrate is similar to FDA-approved clomiphene citrate, but differs in the absence of the cis isomer, zuclomiphene, found in clomiphene citrate.[37] Enclomiphene is thought to compete with estrogen for estrogen receptor binding sites, limiting suppression of gonadotropin release. In women, this leads to ovulation, and in men it raises testosterone levels by increasing LH and FSH levels. Presently, there are other SERMs on the market including tamoxifen, which is indicated for the treatment breast cancer; raloxifene, which is used to prevent osteoporosis, and clomiphene citrate.[38]
4. Chemistry
Enclomiphene can be synthesized as a single isomer using several approaches. The Horner-Wadsworth-Emmons reaction uses the phosphonates 4-hydroxybenzophenone reacted with N-(2-chloroethyl)-diethylamine to make a phenyl ether which is then refluxed with dimethyl chloro(phenyl methylphosphonate) in tetrahydrofuran to yield enclomiphene with adequate yield and stereo-selectivity. Alternate reactions have also been successfully attempted.[39]
5. Pharmacodynamics, Pharmacokinetics and Metabolism
Enclomiphene is rapidly absorbed and has a half-life of approximately 10 hours. A non-dose-dependent steady-state level was maximized at the 25 mg/day dose.[40] In 2013 Wiehle et al. published a randomized, single-blinded, phase II study enrolling 48 men with testosterone levels <350 ng/dL and low-to-normal LH levels (<12 IU/L) with the goal of developing pharmacodynamic and pharmacokinetic profiles for enclomiphene.[31] In pharmacokinetic studies, it was found that maximum serum drug concentration after a single dose of enclomiphene was achieved 2–3 hours after drug ingestion, followed by first order elimination. There was also evidence of drug accumulation in the tissues, as serum levels did not return to baseline within 24 hours of dosing. This was attributed to either an anti-hormone or undetermined effect on the HPG axis. Additionally, spikes in serum enclomiphene levels did not temporally match LH secretion, which was uniformly elevated, but fluctuated. Overall, the study found that enclomiphene increased testosterone levels while raising FSH and LH levels. These increases persisted for at least 7 days after discontinuing the drug, indicating a longer duration of efficacy than previously anticipated given the drug’s pharmacokinetic profile.[31, 34]
6. Clinical Efficacy
6.1 Phase I and Initial Studies
Initial animal testing of enclomiphene was conducted by Repros Theraputics on baboons. The animals were administered 1.5 mg/kg/day of zuclomiphene, enclomiphene or clomiphene for 12 days with serum hormone measurements at 0, 12 and 19 days.[32, 41, 42]. The study found that zuclomiphene did not significantly raise serum testosterone, but that both enclomiphene and clomiphene did. Surprisingly, baboons treated with zuclomiphene also experienced 22% increases in serum cholesterol levels. While clomiphene increased testosterone levels from a baseline of 170 ng/dl to 559 ng/dl (p=0.03), enclomiphene-treated baboons had an even greater increase in testosterone (170 ng/dl to 1,144 ng/dl, p=0.03) with an 8% reduction in serum cholesterol levels. While the hypothesis that both enclomiphene and clomiphene would raise serum testosterone was supported, no statistically significant increases in LH or FSH levels were observed.
6.2 Phase II Studies
In 2013 Kaminetsky et al. studied enclomiphene in comparison with testosterone in the treatment of hypogonadism in a randomized, open-label, phase IIb study enrolling 12 hypogonadal men. A significant and sustained increase in testosterone levels among the men in both groups was observed at three months of treatment. Among the men receiving testosterone, none had sperm concentrations above 12 million/mL after three months of treatment, in contrast with men receiving enclomiphene citrate, where no participant had a sperm count below 75 million/mL. The mean sperm count among men receiving enclomiphene citrate was 176 million/mL (p=0.004). Additionally, only men on enclomiphene experienced an increase in LH and FSH levels. This study suggested that enclomiphene may represent a better treatment option in hypogonadal men wishing to preserve fertility, but was limited by a small sample size.[43]
A randomized, phase IIb study, double blind for oral dosage, and placebo-controlled by Wiehle et al. in 2014 found similar results. Researchers enrolled 124 male subjects, with 73 completing the study. Inclusion criteria included men diagnosed with secondary hypogonadism using two morning serum testosterone measurements of <250 ng/dL. Men saw significant increases in serum testosterone levels when on testosterone (increase from baseline of 210 ng/dL to 462.6 ng/dL after 3 months, p<0.05), 12.5 mg of enclomiphene citrate (increase from baseline of 217.2 ng/dL to 471.9 ng/dL after 3 months, p<0.05) and 25 mg of enclomiphene citrate (increase from baseline of 209.8 ng/dL to 405.8 ng/dL after 3 months, p<0.05). Men on testosterone were found to have decreases in both LH (3.9 mIU/mL to 1.4 mIU/mL, p<0.05) and FSH (6.0 mIU/mL to 2.4 mIU/mL, p<0.05) over three months of treatment. In contrast, LH and FSH levels increased in men on enclomiphene, with men on 25 mg of enclomiphene seeing the greatest rise in LH (5.3 to 11.9 mIU/mL p<0.05) and FSH (9.4 to 14.9 mIU/mL p<0.05). Similar to the Kaminetsky study, this study also showed preservation of sperm density in men on enclomiphene.[34]
6.3 Phase III Studies
In 2015 Kim et al. examined the effects of enclomiphene on serum hormone levels and sperm counts in two randomized, double-blind, placebo-controlled phase III studies enrolling 265 overweight men 18–60 years old with secondary hypogonadism, comparing men treated with testosterone gel, enclomiphene citrate or placebo. Men in the testosterone gel and enclomiphene citrate groups achieved testosterone levels in the normal range during therapy. In this pooled study, enclomiphene treatment was again found to maintain sperm counts, with an 11.7% change in sperm density in one study group treated with enclomiphene citrate (n=41) and a 15.2% change in the second study group also receiving enclomiphene citrate. In contrast, men in the testosterone groups of both studies showed a marked decrease in sperm counts (−56.6% in one testosterone treated group (n=43) and −32.8% in the other (n=42)). [44]
6.4 Clinical Efficacy
Treatment of hypogonadism is often directed at raising both serum testosterone levels as well as ameliorating hypogonadal symptoms. The efficacy of testosterone in this respect is well established. With regards to impact on the HPG axis, the effects of clomiphene and enclomiphene are well established. In contrast, a paucity of data examining the symptomatic benefits of SERMs in hypogonadal men exists. A 2013 study by Lim et al. followed 5 men with chronic renal failure and androgen insufficiency treated with 100 mg of clomiphene citrate for 5–12 months. All 5 study participants experienced increased libido, sexual potency, and a general sense of well-being. [45] A 2014 study by Ramasamy et al. found no difference in patient satisfaction rates between men on testosterone injections, gels, those on clomiphene citrate, and those receiving no treatment, even though patients on clomiphene and testosterone gels saw a smaller increase in serum testosterone levels compared to those on injectable testosterone.[46] Unfortunately, none of the studies examining the effects of enclomiphene evaluated symptomatic improvement or quality of life using validated metrics. However, it is reasonable to assume that men on enclomiphene would stand to benefit comparably to those taking clomiphene citrate.
7. Safety and Tolerability
While adverse effects of enclomiphene have not been directly studied, enclomiphene appears to be well tolerated with few adverse effects. Of the phase II/III trials of enclomiphene citrate, the reported side effects have included elevated estradiol levels, headache and abdominal discomfort.[31, 32, 37, 43, 44, 47] Clomiphene citrate, more completely studied and FDA-approved, is well tolerated in women. Common side effects include vasomotor flushing, pelvic distension and bloating, nausea and vomiting, breast tenderness, visual disturbances and headaches. Ovarian enlargement and uterine bleeding have also been reported. Anecdotal evidence suggests that long term use of clomiphene in women correlates with increased serum cholesterol, though a direct correlation cannot be made at with current evidence. Less than 1% of women experience acute abdominal pain, increased appetite, constipation, dermatitis, diarrhea, depression, fatigue, hair loss, urinary frequency, insomnia and weight fluctuation when using clomiphene.[38, 47, 48] However, the long-term effects of enclomiphene remain unknown.
8. Regulatory Affairs
Clomiphene was approved by the FDA in 1976 to treat female infertility and is used off-label to treat men with hypogonadism and/or infertility. Clomiphene has not been FDA approved for use in men, and is therefore used off label to treat hypogonadism. Enclomiphene citrate has been under review by the FDA since 2007, when it was not approved for the treatment of secondary hypogonadism because the FDA did not consider increases in testosterone levels alone to be sufficient evidence for a non-testosterone therapy for hypogonadism. The FDA also rejected normalization of LH and quality of life improvements as indications for enclomiphene use. The FDA was willing to reconsider the drug on the basis of fertility (as measured by sperm counts) and for the treatment of metabolic syndrome and obesity-associated hypogonadism, given the positive impact of testosterone therapy on metabolic parameters and visceral obesity. More recently, in November of 2015, a meeting scheduled to review the new drug application (NDA) was canceled because of concerns over the bioanalytical method of validation that arose during the late phases of NDA review.[49] At this point, the future of enclomiphene is uncertain.
9. Conclusion
Enclomiphene citrate is effective in increasing serum testosterone levels in hypogonadal men, as well as maintaining sperm counts.[34, 43] While multiple studies have shown enclomiphene to be superior to testosterone in preservation of semen parameters, to date enclomiphene has not been compared directly with clomiphene, which remains a less expensive generic option. Furthermore, the impact of enclomiphene on hypogonadal symptoms remains unknown, limiting the ability to recommend the drug as therapy for hypogonadism. However, if the effects of enclomiphene on hypogonadal symptoms are comparable to those of clomiphene, then enclomiphene may not have the truly unique impact on hypogonadism that would make it superior.
10. Expert Opinion
Secondary hypogonadism is underdiagnosed, even though treatment has many proven benefits.[30] Hypogonadism is classically managed using exogenous testosterone, although with the recent concerns from the FDA regarding the use of TTh and its putative cardiovascular risk, other options for the treatment of hypogonadism must be explored.[50] SERMs and AIs are effective in raising testosterone levels and maintaining fertility, and may alleviate hypogonadal symptoms as well, although additional studies on symptomatic efficacy are needed given the absence of high-level evidence across primarily retrospective studies. We see the field moving towards alternative treatment strategies for hypogonadism, especially in younger men wishing to maintain fertility and older men who are poor candidates for TTh as a result of other risk factors. Additional studies on the long term safety of testosterone will further determine whether SERMs and AIs are more broadly accepted as treatments for hypogonadism, particularly given that the oral routes of administration of SERMs and AIs are preferable to most other formulations.
Enclomiphene citrate, while incompletely studied, is effective in ameliorating testosterone deficiency and maintaining semen quality, with few apparent adverse effects. The most significant weaknesses among current studies include sample size and a lack of head-to-head comparisons with other SERMs or AIs. While additional work examining the impact of enclomiphene on hypogonadal symptoms is needed, enclomiphene may represent a viable treatment option for hypogonadal young men who desire fertility preservation. In older men, enclomiphene could be used as monotherapy, particularly if the risks of TTh are deemed too high for specific patients.
Future work will need to more definitively determine the symptomatic benefits of enclomiphene. Of particular interest would be randomized control trials comparing the use of enclomiphene, clomiphene and testosterone. A significant barrier to such a study could be the known efficacy of clomiphene and whether the potential reductions in side effects from the single isomer formulation of enclomiphene would justify a more expensive treatment. The FDA’s concerns about enclomiphene could also limit its use, though the limited literature suggests that enclomiphene could be as effective as clomiphene in the treatment of hypogonadism.[20] At this time, clomiphene represents a less expensive, yet effective, alternative in off-label management of hypogonadism in men, whereas the future of enclomiphene in the treatment of male hypogonadism remains unclear.
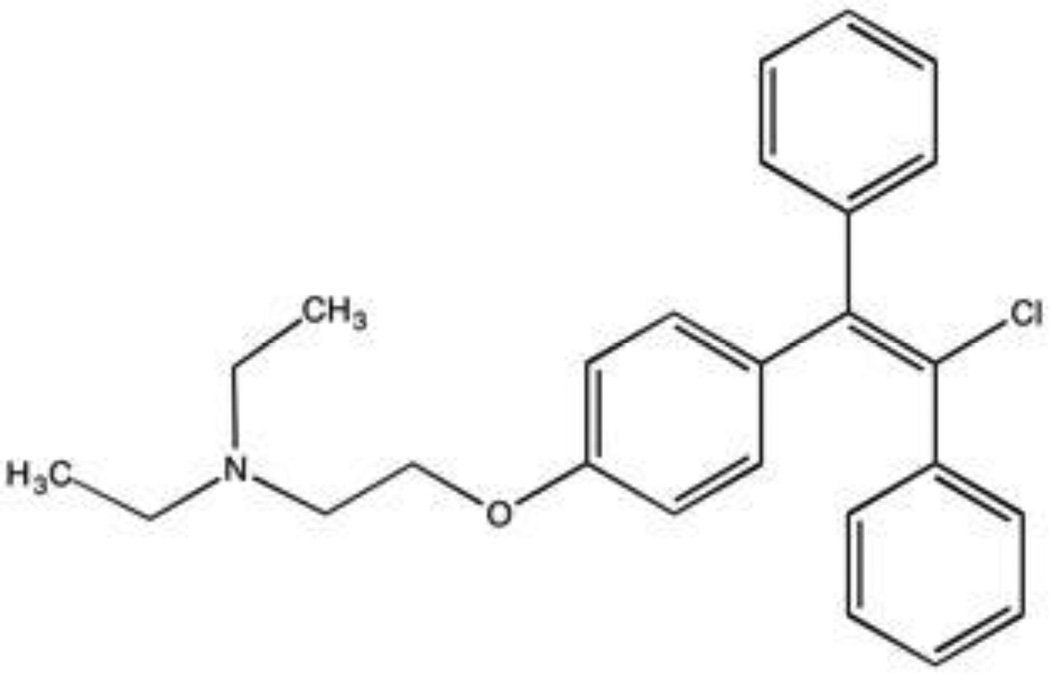
Figure 1.
Chemical structure of Enclomiphene Citrate
Table 1.
Pivotal Enclomiphene Trials
| Trial | Study design | Dosage (mg) |
Sample size |
Duration (months) |
Key Findings |
|---|---|---|---|---|---|
| Wiehle 2013 [31] |
Phase II randomized, single-blind |
6.25 12.5 25 |
44 | 1.5 |
|
| Wiehle 2014 [34] |
Phase IIb randomized, double blind for oral dosage, placebo-controlled |
12.5 25 |
73 | 3 |
|
| Kim 2016 [44] |
Phase III, randomized, double blind |
12.5 25 |
265 | 4 |
|
Box 1. Drug Summary.
| Drug name | Enclomiphene Citrate |
| Phase | I, II and III |
| Indication (specific to discussion) | Secondary male hypogonadism |
| Pharmacology | Selective estrogen receptor modulator |
| Route of administration | Oral |
| Chemical structure | (E)-2-(p-(2-Chloro-1,2-Diphenylvinyl) phenoxy) triethylamine |
| Pivotal trials(s) | Secondary male hypogonadism [31, 34, 40–43] |
Acknowledgments
AW Pastuszak is a National Institutes of Health (NIH) K12 Scholar supported by a Male Reproductive Health Research Career (MHRH) Development Physician-Scientist Award (Grant Number: HD073917-01) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Program. LI Lipshultz is a speaker for American Medical Systems and Repros Therapeutics, he is a clinical investigator for Endo Pharmaceuticals and Repros Therapeutics and he is a consultant for American Medical Systems, Endo Pharmaceuticals, Repros Therapeutics, AbbVie and Lipocine.
Footnotes
Declaration of interest:
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
REFERENCES
- 1.Bhasin S, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536–2559. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 2.Pastuszak AW, et al. Comparison of the Effects of Testosterone Gels, Injections, and Pellets on Serum Hormones, Erythrocytosis, Lipids, and Prostate-Specific Antigen. Sex Med. 2015;3(3):165–173. doi: 10.1002/sm2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rastrelli G, et al. How to define hypogonadism? Results from a population of men consulting for sexual dysfunction. J Endocrinol Invest. 2016;39(4):473–484. doi: 10.1007/s40618-015-0425-1. [DOI] [PubMed] [Google Scholar]
- 4.Mulligan T, et al. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract. 2006;60(7):762–769. doi: 10.1111/j.1742-1241.2006.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corona G, et al. How to recognize late-onset hypogonadism in men with sexual dysfunction. Asian J Androl. 2012;14(2):251–259. doi: 10.1038/aja.2011.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Araujo AB, et al. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab. 2007;92(11):4241–4247. doi: 10.1210/jc.2007-1245. [DOI] [PubMed] [Google Scholar]
- 7.Wu FC, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363(2):123–135. doi: 10.1056/NEJMoa0911101. [DOI] [PubMed] [Google Scholar]
- 8.Surampudi PN, Wang C, Swerdloff R. Hypogonadism in the aging male diagnosis, potential benefits, and risks of testosterone replacement therapy. Int J Endocrinol. 2012;2012:625434. doi: 10.1155/2012/625434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang C, et al. Low testosterone associated with obesity and the metabolic syndrome contributes to sexual dysfunction and cardiovascular disease risk in men with type 2 diabetes. Diabetes Care. 2011;34(7):1669–1675. doi: 10.2337/dc10-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corona G, et al. Endocrinologic Control of Men's Sexual Desire and Arousal/Erection. J Sex Med. 2016;13(3):317–337. doi: 10.1016/j.jsxm.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 11.McBride JA, Carson CC, Coward RM. Diagnosis and management of testosterone deficiency. Asian J Androl. 2015;17(2):177–186. doi: 10.4103/1008-682X.143317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corona G, et al. THERAPY OF ENDOCRINE DISEASE: Testosterone supplementation and body composition: results from a meta-analysis study. Eur J Endocrinol. 2016;174(3):R99–R116. doi: 10.1530/EJE-15-0262. [DOI] [PubMed] [Google Scholar]
- 13.Sanford EJ, et al. The effects of castration on adrenal testosterone secretion in men with prostatic carcinoma. J Urol. 1977;118(6):1019–1021. doi: 10.1016/s0022-5347(17)58283-x. [DOI] [PubMed] [Google Scholar]
- 14.Longcope C, Kato T, Horton R. Conversion of blood androgens to estrogens in normal adult men and women. J Clin Invest. 1969;48(12):2191–2201. doi: 10.1172/JCI106185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finkelstein JS, et al. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. 2013;369(11):1011–1022. doi: 10.1056/NEJMoa1206168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falahati-Nini A, et al. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest. 2000;106(12):1553–1560. doi: 10.1172/JCI10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leder BZ, et al. Differential effects of androgens and estrogens on bone turnover in normal men. J Clin Endocrinol Metab. 2003;88(1):204–210. doi: 10.1210/jc.2002-021036. [DOI] [PubMed] [Google Scholar]
- 18.Handelsman DJ. Global trends in testosterone prescribing, 2000–2011: expanding the spectrum of prescription drug misuse. Med J Aust. 2013;199(8):548–551. doi: 10.5694/mja13.10111. [DOI] [PubMed] [Google Scholar]
- 19.Spitzer M, et al. Risks and benefits of testosterone therapy in older men. Nat Rev Endocrinol. 2013;9(7):414–424. doi: 10.1038/nrendo.2013.73. [DOI] [PubMed] [Google Scholar]
- 20. Food, U. and D. Administration. FDA Drug Safety Communication: FDA cautions about using testosterone products for low testosterone due to aging; requires labeling change to inform of possible increased risk of heart attack and stroke with use. [Accessed June, 2015]; Drugs/DrugSafety/ucm436259.htm. 4 doi: 10.1016/j.juro.2015.06.058. *This change in stance increases the need for SERMs as discussed above.
- 21.Agency EM. PRAC review does not confirm increase in heart problems with testosterone medicines, in Committee recommends medicines can continue to be given for their authorised uses. London: European Medicines Agency; 2014. pp. 1–2. [Google Scholar]
- 22.Coss CC, et al. Selective androgen receptor modulators for the treatment of late onset male hypogonadism. Asian J Androl. 2014;16(2):256–261. doi: 10.4103/1008-682X.122339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crosnoe-Shipley LE, et al. Treatment of hypogonadotropic male hypogonadism: Case-based scenarios. World J Nephrol. 2015;4(2):245–253. doi: 10.5527/wjn.v4.i2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Ronde W, de Jong FH. Aromatase inhibitors in men: effects and therapeutic options. Reprod Biol Endocrinol. 2011;9:93. doi: 10.1186/1477-7827-9-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.T'Sjoen GG, et al. Comparative assessment in young and elderly men of the gonadotropin response to aromatase inhibition. J Clin Endocrinol Metab. 2005;90(10):5717–5722. doi: 10.1210/jc.2005-0982. [DOI] [PubMed] [Google Scholar]
- 26.Surampudi P, Swerdloff RS, Wang C. An update on male hypogonadism therapy. Expert opinion on pharmacotherapy. 2014;15(9):1247–1264. doi: 10.1517/14656566.2014.913022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fejes I, et al. Effect of body weight on testosterone/estradiol ratio in oligozoospermic patients. Arch Androl. 2006;52(2):97–102. doi: 10.1080/01485010500315479. [DOI] [PubMed] [Google Scholar]
- 28.Camacho EM, et al. Age-associated changes in hypothalamic-pituitary-testicular function in middle-aged and older men are modified by weight change and lifestyle factors: longitudinal results from the European Male Ageing Study. Eur J Endocrinol. 2013;168(3):445–455. doi: 10.1530/EJE-12-0890. [DOI] [PubMed] [Google Scholar]
- 29.Tsourdi E, et al. The effect of selective estrogen receptor modulator administration on the hypothalamic-pituitary-testicular axis in men with idiopathic oligozoospermia. Fertil Steril. 2009;91(4 Suppl):1427–1430. doi: 10.1016/j.fertnstert.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Wang C, et al. Investigation, treatment and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA and ASA recommendations. Eur J Endocrinol. 2008;159(5):507–514. doi: 10.1530/EJE-08-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wiehle R, et al. Testosterone Restoration by Enclomiphene Citrate in Men with Secondary Hypogonadism: Pharmacodynamics and Pharmacokinetics. BJU Int. 2013 doi: 10.1111/bju.12363. **Important early study first established pharmodynamic and pharamokinetic paraneters of enclomiphene citrate. Also established ability to increase serum testosterone.
- 32. Hill S, Arutchelvam V, Quinton R. Enclomiphene, an estrogen receptor antagonist for the treatment of testosterone deficiency in men. IDrugs. 2009;12(2):109–119. *Thorough similar review article.
- 33. Huang ES, Miller WL. Estrogenic and antiestrogenic effects of enclomiphene and zuclomiphene on gonadotropin secretion by ovine pituitary cells in culture. Endocrinology. 1983;112(2):442–448. doi: 10.1210/endo-112-2-442. *Early in vitro work pivotal in the development of enclomiphene.
- 34. Wiehle RD, et al. Enclomiphene citrate stimulates testosterone production while preventing oligospermia: a randomized phase II clinical trial comparing topical testosterone. Fertil Steril. 2014;102(3):720–727. doi: 10.1016/j.fertnstert.2014.06.004. **Phase II clinical trial comparing enclomiphene head to head with testosterone.
- 35. Gupta P, Kriplani A. Enclomiphene citrate veresus clomiphene citrate for ovulation induction in women with unexplained infertility. Fertility and Sterility. 2013;3(100):S143. *Study comparing enclomiphene to clomiphene for on label use in women.
- 36.Enclomiphene - MeSH - NCBI. 2014 [Google Scholar]
- 37.CLOMID® (clomiphene citrate tablets USP) Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/016131s026lbl.pdf.
- 38.Dutertre M, Smith CL. Molecular mechanisms of selective estrogen receptor modulator (SERM) action. J Pharmacol Exp Ther. 2000;295(2):431–437. [PubMed] [Google Scholar]
- 39.Cummins CH. Stereospecific Syntheses of Clomiphene and Tamoxifen via Stannylcupration of Diphenylacetylene. Synthetic communications. 1995;25(24):4071–4079. [Google Scholar]
- 40. Wiehle RD, et al. Enclomiphene citrate stimulates serum testosterone in men with low testosterone within 14 days. Journal of Men's Health. 2014;11(4):196–205. **Phase II study showing the impact of a 14 day enclomiphene regiment.
- 41.Podolski J, Wiehle R. Trans-clomiphene for the treatment of benign prostate hypertrophy, prostate cancer, hypgonadism, elevated triglycerides and high cholesterol. 2006 Google Patents. [Google Scholar]
- 42. Wiehle R, Rice K, Garcia W, Willett M, et al. Oral enclomiphene raises total serum testosterone in baboons and hypogonadal men [abstract no. P1212] Intl Society of Endocrinology. 2004;369 **First animal and human trials using enclomiphene to treat male hypogonadism.
- 43. Kaminetsky J, et al. Oral enclomiphene citrate stimulates the endogenous production of testosterone and sperm counts in men with low testosterone: comparison with testosterone gel. J Sex Med. 2013;10(6):1628–1635. doi: 10.1111/jsm.12116. *Phase IIb study enrolling 14 men investigating enclomiphene as an alternative to testosterone.
- 44. Kim ED, McCullough A, Kaminetsky J. Oral enclomiphene citrate raises testosterone and preserves sperm counts in obese hypogonadal men, unlike topical testosterone: restoration instead of replacement. BJU Int. 2016;117(4):677–685. doi: 10.1111/bju.13337. **Phase III trial investigating different dosages of enclomiphene over 16 weeks.
- 45.LIM VS, Fang VS. Restoration of Plasma Testosterone Levels in Uremic Men With Clomiphene Citrate. The Journal of Clinical Endocrinology & Metabolism. 1976;43(6):1370–1377. doi: 10.1210/jcem-43-6-1370. [DOI] [PubMed] [Google Scholar]
- 46.Ramasamy R, et al. Testosterone supplementation versus clomiphene citrate for hypogonadism: an age matched comparison of satisfaction and efficacy. J Urol. 2014;192(3):875–879. doi: 10.1016/j.juro.2014.03.089. [DOI] [PubMed] [Google Scholar]
- 47.Kaminetsky J, Hemani ML. Clomiphene citrate and enclomiphene for the treatment of hypogonadal androgen deficiency. Expert Opin Investig Drugs. 2009;18(12):1947–1955. doi: 10.1517/13543780903405608. [DOI] [PubMed] [Google Scholar]
- 48.Shabsigh A, et al. Clomiphene citrate effects on testosterone/estrogen ratio in male hypogonadism. J Sex Med. 2005;2(5):716–721. doi: 10.1111/j.1743-6109.2005.00075.x. [DOI] [PubMed] [Google Scholar]
- 49.Repros Therapeutics Announces Cancellation of FDA Advisory Committee Meeting to Review Enclomiphene for the Treatment of Secondary Hypogonadism. Available from: http://www.drugs.com/nda/androxal_151029.html. [Google Scholar]
- 50.Aversa A, et al. Effects of testosterone undecanoate on cardiovascular risk factors and atherosclerosis in middle - aged men with late - onset hypogonadism and metabolic syndrome: Results from a 24 - month, randomized, double - blind, placebo - controlled study. The journal of sexual medicine. 2010;7(10):3495–3503. doi: 10.1111/j.1743-6109.2010.01931.x. [DOI] [PubMed] [Google Scholar]