Abstract
MicroRNAs (miRNAs), small non-coding RNAs, can regulate post-transcriptional gene expressions and silence a broad set of target genes. miRNAs, aberrantly expressed in cancer cells, play an important role in modulating gene expressions, thereby regulating downstream signaling pathways and affecting cancer formation and progression. Oncogenes or tumor suppressor genes regulated by miRNAs mediate cell cycle progression, metabolism, cell death, angiogenesis, metastasis and immunosuppression in cancer. Recently, miRNAs have emerged as therapeutic targets or tools and biomarkers for diagnosis and therapy monitoring in cancer. Since miRNAs can regulate multiple cancer-related genes simultaneously, using miRNAs as a therapeutic approach plays an important role in cancer therapy. However, one of the major challenges of miRNA-based cancer therapy is to achieve specific, efficient and safe systemic delivery of therapeutic miRNAs In vivo. This review discusses the key challenges to the development of the carriers for miRNA-based therapy and explores current strategies to systemically deliver miRNAs to cancer without induction of toxicity.
Keywords: miRNA, Gene delivery, In vivo delivery, Cancer therapy, Nanotechnology
1. Introduction
MicroRNAs (miRNAs), distinct from high-molecular-weight microsomal RNA, are small non-coded strands of RNAs discovered in a decade [1]. Many studies aid in the development of miRNA-based therapy for clinical applications. Nowadays, many of the monoclonal antibodies (mAbs) and small molecule inhibitors serve as effective cancer therapeutics in the clinic. However, there are some limitations with regard to the specificity of inhibitors and capability of antibodies to access intracellular targets.
1.1 . Limitations of current cancer therapies
Conventional chemotherapy, which disrupts the functions of cell organelles such as the mitochondria, cytoskeleton, inhibits the key enzyme activity to block DNA replication, mRNA transcription or translation, or directly damages DNA to stop the proliferation of cancer cells and induces toxicity in cancer cells. However, the conventional cancer therapeutic agent does not target the cancer cells specifically. It also displays the toxicity in rapidly dividing normal tissues such as the bone marrow and the gastrointestinal tract, resulting in side effects [2]. Therefore, the targeted therapy was developed to specifically block molecular targets regulating tumor formation and progression.
The targets of small molecule inhibitors are usually overexpressed in the cancer cells and located intracellularly. For example, the tyrosine kinase inhibitor, which targets the growth factor receptors or the downstream effectors recently emerged as the systemic therapy for cancer [2–4]. However, the inhibitors sometimes bind to a broad set of receptors or the downstream mediators, leading to reduced specificity and increased toxicity. Thus, monoclonal antibody-based cancer therapy has been established and becomes one of the most efficient and safe strategies for cancer treatment [5]. For example, therapeutic mAbs targeting the ERBB family including epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF) showed significant therapeutic effect when treating patients with solid tumors [6,7]. Recent evidences showed that EGFR-specific antibodies extended patient survival with colorectal cancer [7,8]. Nevertheless, there are multiple hurdles for efficient antibody-based cancer treatment. For instance, physical properties and pharmacokinetics make it difficult for mAbs to penetrate the tumor tissue efficiently and homogeneously. Immune escape due to ineffective FcγR binding and immunosuppressive microenvironment leads to the reduced therapeutic efficacy [9,10].
Besides, neither inhibitors nor monoclonal antibodies can successfully treat cancer – a heterogenic disease – by suppressing a single target. Heterogeneity exists in expression between individual primary lesions, primary and metastatic lesions, and even tumor lesions before and after treatment. Particularly, it has been known tumors can develop resistant mechanisms in response to the treatment. For example, although the high-level target protein expression is detected before treatment, it may be downregulated during and after treatment as part of the resistance development. Furthermore, some cancer cells will develop the compensation mechanisms by activating other survival signaling pathways to overcome the targeted cancer treatment. For example, it has been reported that B-raf inhibitors such as vemurafenib and dabrafenib develop acquired drug resistance via hyperactivation of the PI3K/Akt pathway, leading to increased expression of adipocyte enhancer-binding protein 1 (AEBP1) and activation of NF-κB in melanoma [11]. To this end, the therapeutic response to the targeted agents including small molecule inhibitors and mAbs is usually partial and only causes a transient delay in tumor growth, after which most tumors continue or even accelerate their progression and metastasis [12].
1.2 . Advantages of miRNA-based cancer therapy
miRNAs, on the other hand, can silence target genes efficiently and regulate a broad set of genes of interest simultaneously, which benefits treatment of cancer as a heterogenic disease. It has been shown that targeting a set of related oncogenic genes or pathways simultaneously triggered synergistic therapeutic effect in cancer. In spite of targeting cancer cells only, miRNAs can also target the tumor-promoting stromal cells such as endothelial cells and tumor-associated fibroblasts to inhibit angiogenesis and tumor fibrosis, which are required during tumor formation, progression and metastasis [13–16]. Moreover, miRNAs, as natural antisense nucleotides, showed reduced immune response and low toxicity when compared to plasmid DNA-based gene therapy and protein-based drug molecules. Thus, miRNAs may play a significant role in cancer therapy. As a novel therapeutic strategy, several miRNA modulators have entered the clinical trials. Locked nucleic acid (LNA)-antimir-122 is the first drug to successfully enter Phase II trials for the treatment of hepatitis C virus (HCV) infection [17]. For cancer diagnosis, miRNA-126 targeting VEGF and EGFL7 showed the prognostic value to provide predictive information in relation to the therapeutic outcome of anti-angiogenic agents in metastatic colorectal cancer [18]. Accordingly, miRNA-based therapeutics may serve as promising anti-cancer drugs for the clinical application in cancer therapy if they can be efficiently and safely delivered to cancer cells.
The charged miRNAs have small size and low molecular weight making them possible to be formulated into an effective delivery system and become attractive options for clinical cancer therapy development. To this end, in order to achieve effective gene silencing in cancer cells, the development of strategies for efficient In vivo delivery and escape from blood clearance, enzyme degradation and intracellular trapping, such as an endosome, is required. This review will focus on current challenges and strategies for delivery of miRNAs In vivo through local and systemic or targeted administration. The strategies employed in In vivo miRNA delivery for cancer therapy are summarized in Table 1.
Table 1.
Summary of studies using miRNA for cancer therapy in vivo.
| Vehicle | Targeted miRNA | Antagonist or mimics |
Model | Effect | Reference |
|---|---|---|---|---|---|
| Local Delivery | |||||
| Cholesterol-conjugated 2′-O- methyl-modified |
miR-375 | Mimics | Hepatoma xenograft | Inhibition of tumor growth | [94] |
| Lentiviral vector | let-7 | Mimics | Non-small-cell lung cancer (NSCLC) | Inhibition of the growth of K-ras-dependent lung tumors | [95] |
| Polyethyleneimine (PEI) | miR-145 miR-33a |
Mimics | Colon carcinoma xenograft | Induction of apoptosis, inhibition of tumor growth and downregulation of the oncogenic kinase Pim-1 |
[96] |
| Systemic delivery | |||||
| Seed-targeting tiny LNAs | miR-21 | Antagonist | Breast cancer | Repression of the miR-21 function in tumor | [106] |
| Cationic liposomes | miR-143 | Mimics | Colorectal carcinoma | Inhibition of tumor growth | [109] |
| Lentiviral vectors | miR-15a/16 | Mimics | Chronic lymphocytic leukemia | Restoration of miR-15a/16 expression and inhibition of tumor cell proliferation |
[110] |
| miR-494 | Antagonist | Breast cancer | Inhibition of tumor growth and metastasis | [111] | |
| Adeno-associated viruses (AAVs) |
miR-26a | Mimics | Hepatocellular carcinoma | Inhibition of tumor cell proliferation and induction of apoptosis | [112] |
| Silica nanoparticles | miR-34a | Mimics | Neuroblastoma | Induction of apoptosis, reduction in vascular density of tumors and inhibition of tumor growth |
[101] |
| PEGylated-PLGA | miR-21 | Antagonists | Breast cancer | Efficient delivery of antagomiR-21 and prolonged release | [125] |
| PLGA-penetratin | miR-155 | Antagonists | Lymphoma | Induction of apoptosis and reduction of tumor growth | [126] |
| PEI | miR-145 miR-33a |
Mimics | Colorectal carcinoma | Reduction of tumor cell proliferation and tumor growth | [130] |
| LNP-DP1 | miR-122 | Mimics | Hepatocellular carcinoma | Inhibition of angiogenesis and tumor growth | [132] |
| Cationic DOTMA lipoplexes | miR-133b | Mimics | NSCLC | Increased expression of miR-133b and downregulation of prosurvival gene MCL-1 |
133] |
| miR-29b | Mimics | NSCLC | Reduced expression of the key target oncogenes and inhibition of tumor growth |
[134] | |
| Neutral lipid | miR-34a | Mimics | NSCLC | Inhibition of tumor growth | [136] |
| miR-34a let-7 |
Mimics | NSCLC | Inhibition of tumor cell proliferation and induction of apoptosis | [137] | |
| LPH-PEG-GC4 | miR-34a | Mimics | Lung cancer | Reduction of tumor growth, induction of apoptosis, inhibition of survivin expression and downregulation of pathway |
[102] |
2. Mechanisms of miRNAs
The regulation of gene expression plays an important role in mediating cellular functions. Small RNA molecules, including short interfering RNAs (siRNAs) and miRNAs are effective modulators of gene expression through translation repression, chromatin remodeling or mRNA degradation [19]. After post-transcriptional modifications, the structures of the endogenous mature miRNAs are similar to the exogenous siRNAs. Both siRNAs and miRNAs are processed by the common enzyme — Dicer and incorporated into an active RNA-induced silencing complex (RISC). Subsequently, miRNAs and siRNAs share the same processing mechanism to achieve gene-silencing effect. However, siRNAs are exogenous double-stranded RNAs that bind to the target mRNA sequences via perfect sequence matching. In contrast, miRNAs are endogenous single-stranded RNAs targeting multiple sequences via imperfect pairing, which leads to simultaneous suppression of multiple target genes. It creates an overwhelming advantage when applied to cancer therapy.
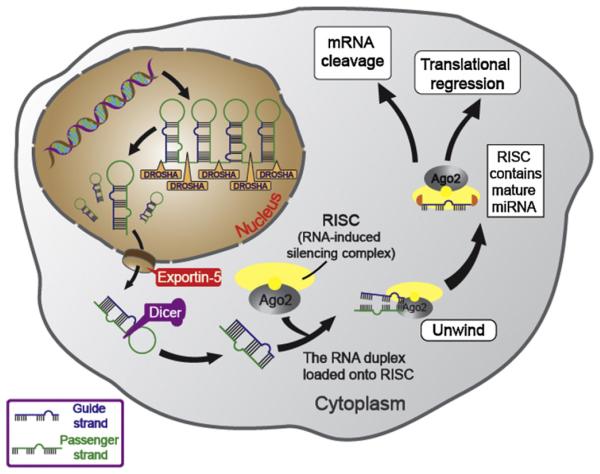
miRNAs, referring to endogenous non-coding RNAs have recently emerged as master regulators of cancer. miRNAs can affect cancer progression through regulating the expression of target genes, which mediate cell cycle progression, metabolism, cell death, angiogenesis, metastasis and immunosuppression in cancer. As illustrated in Fig. 1, miRNAs are derived from 70-nucleotide hairpin-forming miRNA precursors (pre-miRNAs) after long RNA primary transcripts (pri-miRNAs) are cleaved by the ribonuclease III termed DROSHA in the nucleus. Pre-miRNAs are subsequently transported to the cytoplasm by exportin-5 and further converted into double-stranded 18 ~ 25 nt RNAs by Dicer. Mature miRNAs, the short double strand RNAs (dsRNAs), are incorporated into the RISC complex composed of Dicer, many associated proteins, and target mRNAs carrying complementary sequences. One of the strands of mature miRNAs, known as the guide strand, binds to Argonaute (Ago) protein in the RISC complex, while the passenger strand is degraded. By the imperfect base pairing between the guide strands of miRNAs and the 3′ or 5′ untranslated region of the target mRNAs, miRNAs are able to regulate tens to hundreds of mRNAs. Despite only the imperfect base pairing between miRNAs and the 3′ or 5′ untranslated region of the target mRNAs is required for miRNAs to regulate the expressions of target mRNAs, the “seed” region (nucleotides 2 ~ 7 from the 5′ end of the miRNA) of the miRNA still have to be perfectly matched with the complementary mRNA sequence [20]. The miRNA–mRNA interaction suppresses the expression levels of the target genes through mRNA cleavage or translational regression. The gene suppression mechanism depends on the degree of complementarity sites between the miRNAs and mRNAs [21,22]. Extensive base pairing is required for Argonaute2 (Ago2) endonuclease-mediated mRNA cleavage, while moderate base pairing is adequate to achieve translational inhibition [21–23].
Fig. 1.
Schematic representation of the microRNA generation and silencing mechanisms. Hairpin-forming pre-miRNAs are generated by pri-miRNAs, which is cleaved by Drosha. Later, pre-miRNAs are transported into the cytoplasm by exportin-5 and further converted into double-stranded mature miRNAs by Dicer. Mature miRNAs are incorporated into the RISC complex, unwound and annealed to the target mRNAs carrying complementary sequences. miRNAs are able to regulate tens to hundreds of mRNAs via the imperfect base pairing between miRNAs and the 3′ or 5′ untranslated region of the target mRNAs. The miRNA-mRNA interaction silences the target genes through mRNA cleavage or translational inhibition.
Interestingly, some miRNAs generated in the cytoplasm cannot only affect the function of the cell that produces the miRNAs, but also be released into the bloodstream and taken up by other cells to regulate gene expression of the distant target cells. These endogenous miRNAs released into plasma are packaged in microparticles (exosomes, microvesicles, and apoptotic bodies) or bound to RNA-binding proteins such as Ago2 or lipoprotein complexes such as high-density lipoprotein (HDL) to maintain their stabilities [24–26]. It was found that the cell-free miRNAs in plasma could also be detected and expressed in platelets, erythrocytes, and nucleated blood cells [27,28]. For example, of 79 tumor-specific circulating miRNAs, 58% (46 of 79) are highly expressed in one or more blood cell types such as myeloid (e.g., miR-223, miR-197, miR-574-3p, and let-7a), lymphoid blood cells (e.g., miR-150), and red blood cells (e.g., miR-486-5p, miR-451, miR-92a, and miR-16) [29]. Consequently, miRNAs can target various gene expressions, contribute cell-to-cell communication and regulate the key cell signaling pathways to maintain regular functions in normal cells. Thus, abnormal miRNA expressions may participate in cancer formation and progression.
3. miRNAs in cancer
Misexpression or dysfunction of miRNAs is associated with tumor formation and progression via manipulating the oncogenic pathways that influence the processes in tumor progression, such as cell cycle regulation, apoptosis, senescence, metabolism, angiogenesis and metastasis.
3.1. Cell proliferation and cell death
Five groups of miRNAs, including the miR-15a/16 cluster, the miR-17/20 cluster, the miR-221/222 cluster, and the let-7 and miR-34 families, can target cell cycle regulators to control cell cycle checkpoints and progression [30]. Dysfunction of the cell cycle-related miRNAs increases cell proliferation, leading to tumor growth promotion. In contrast, recent study has highlighted miRNAs as anti-apoptotic or pro-apoptotic regulators by targeting various mRNAs associated with key apoptotic pathways in cancer. For instance, miRNA-221/222 cluster targeting p27, PTEN, and PUMA, miRNA-128 targeting BAX and miRNA-17/19 cluster targeting BIM act as anti-apoptotic miRNAs to maintain survival of cancer cells and contribute to drug resistance [31–33]. On the other hand, pro-apoptotic miRNAs 15a/b and 16 targeting BCL2 family serve as anti-cancer guardians [34].
3.2. Metabolism
The miRNA expression profiles associated with metabolism are distinct from normal cells. miRNAs play as key regulators of metabolism in cancer cells to increase nutrient uptake and the accumulation of materials for controlling metabolic flux [35]. For example, miRNAs such as miR-133, miR-138 and miR-150 targeting glucose transporter, miR-33a/b targeting metabolic enzymes to regulate fatty acid metabolism and AMPK pathway and miR-29b targeting amino acid catabolism are involved in modulating cancer cell metabolism and biogenesis to support tumor growth and proliferation [36–39].
3.3. Metastasis and angiogenesis
miRNAs have impact on both intrinsic signaling pathways of cancer cells and interactions between cancer cells and tumor stroma to regulate invasion and metastasis. For instance, miR-200 family and miR-205 are downregulated in various tumors to promote epithelial– mesenchymal transition (EMT) progression and facilitate cancer cell invasion [40]. miR-29b upregulated in breast cancer cells suppresses the expression of MMP2 and MMP9 and consequently triggers extracellular matrix (ECM) remodeling to facilitate cancer cell migration and local invasion [40]. Besides, angiogenesis is required for supporting the formation of both primary and metastatic lesions. miR-424 triggered by hypoxia stabilizes hypoxia-inducible factor-α (HIF-α) and enhances angiogenesis in tumor microenvironment [41]. miR-503 suppresses the expression of angiogenic factors like fibroblast growth factor (FGF) 2 and VEGF-A. miR-503 is downregulated in hepatocellular carcinoma (HCC), resulting in increased angiogenesis and tumorigenesis [42].
3.4. miRNAs in cancer diagnosis and cancer therapy
As we summarized above, the aberrant expressions of miRNAs are associated with tumor formation, progression and metastasis. Moreover, the abnormal expressions of miRNAs are also correlated with the resistant mechanisms to chemotherapy. Thus, miRNAs can serve as both diagnostic and prognostic biomarkers in cancer [43]. For example, miR-155 overexpression and let-7a downregulation are associated with poor disease outcome in lung cancer [44]. For prognostic applications, overexpression of miR-221 and miR-222 are associated with poor therapeutic outcome of anti-estrogenic therapies such as Tamoxifen and Fulvestran, while tumor suppressor miR-205 is responsible for enhanced therapeutic effect of tyrosine kinase inhibitors [45–47]. Many reports have demonstrated the significance of microRNAs as diagnostic and prognostic biomarkers. Besides, miRNAs can also serve as either therapeutic agents or therapeutic targets in cancer. In the following sections, we will further focus on the therapeutic applications of miRNAs.
4. miRNAs: therapeutic agents or therapeutic targets?
According to the functions of miRNAs, cancer types and stages, both antagonists and mimics are developed as miRNA-based therapeutic strategies to achieve tumor regression [48]. miRNA antagonists – single-stranded oligonucleotides with miRNA complementary sequences – are designed to interrupt the miRNA processing as well as RISC assembly, and result in increased expression of the tumor suppressor genes. For example, miR-21, overexpressed in various tumor types, downregulates many tumor suppressor genes regulating cell proliferation, cell death, metastasis and chemoresistance. miR-21 antagonist reverses EMT phenotype and blocks angiogenesis in breast cancer through inactivation of AKT and MAPK pathways [49,50]. The targeted endogenous miRNA can also serve as a noninvasive biomarker for early cancer diagnosis, prediction of response to miRNA antagonist therapy and therapy monitoring.
By contrast with miRNA antagonists, miRNA mimics, known as miRNA replacement therapy, play an opposite role in regulating the expression of target genes. Genomic loss of tumor suppressor miRNAs can be restored by miRNA mimics, which behave like endogenous miRNAs. Moreover, miRNA mimics cannot only provide obvious benefits to those cancer cells with low tumor suppressor miRNA expression levels, but also show therapeutic benefits in cancer with normal miRNA expression levels. For example, the miR-34 family is dysregulated in different cancer types including several epithelial tumors, melanomas, neuroblastomas, leukemias and sarcomas [51]. miR-34 serves as a downstream effector of p53 pathway, which is defective in about half of human cancers and plays an important role in the suppression of tumor development [52]. Therefore, miR-34 is referred as a potential tumor suppressor and a possible therapeutic target. Interestingly, delivery of miR-34 mimics to cancer cells with both reduced and normal expression levels of miR-34 showed growth inhibitory effect. Therefore, miRNA mimics could be a promising treatment for various types and stages of cancer diseases.
During miRNA processing, double-stranded miRNAs are loaded onto the RISC complex and one strand of the miRNAs, the passenger strand, is cleaved by Ago2. Furthermore, the other RNA strand, the guide strand, remains and matches the complementary sequences of the target mRNAs, leading to mRNA cleavage or translational repression. Thus, the information of the miRNA guide strands is more important for designing miRNA antagonists or miRNA mimics.
Both miRNA antagonists and miRNA mimics are low molecular weight oligonucleotides and thus easier to deliver into the target cells compared with large viral vectors or plasmids normally used for gene therapy. However, the lack of correlation between in vitro and In vivo efficacies was observed due to inefficient In vivo delivery of miRNAs. Like other therapeutic oligonucleotides, the delivery of the miRNA antagonists or mimics as cancer therapies encounter several barriers such as poor bioavailability, limited tissue permeability and payload instability.
5. Pharmacokinetics and pharmacodynamics of miRNAs
miRNA antagonists or miRNA mimics, the poly-anionic molecules with low molecular weights, are highly water-soluble and suitable for intravenous and subcutaneous injections. After intravenous administration, the plasma levels of miRNA antagonists or miRNA mimics reduce quickly. They further distribute broadly but later accumulate mostly in the liver and kidney. Tissue concentrations of miRNA antagonists or miRNA mimics in the brain, heart and lung decline rapidly after systemic injection. Nevertheless, tissue concentrations of miRNA-based therapeutics in the liver and kidney remain high and sustained levels up to 24 h after injection [53]. The modified miRNAs show distinct pharmacokinetics from unmodified ones. As unmodified miRNAs, plasma levels of modified miRNA antagonists or miRNA mimics are reduced within hours and accumulated into tissues. However, after entering into cells, the modified miRNAs remain stable that their clearance rate in tissues is reduced, leading to prolonged therapeutic benefit.
The duration of the pharmacological effects (or pharmacodynamics) of miRNA antagonists or miRNA mimics is determined by their retention in the target tissues. On the other hand, the onset of their pharmacological effects is often delayed because of the time delay between internalization of miRNA antagonists/miRNA mimics and regulation of the target proteins. Furthermore, the fact that miRNA-based therapeutics may indirectly mediate the diseased phenotype further delays the pharmacological effect. For example, both miR-122 and mi-208 antagonists have delayed effect on their target cholesterol and β-MHC respectively and cause a postponed change in the disease phenotype [54–56]. To this end, there are still many challenges remaining in developing miRNAs as effective therapeutic agents in cancer.
6. Current challenges in miRNA delivery
Several problems encountered in clinical development of miRNA delivery limit the application of miRNAs as a therapeutic option to treat cancer [57–60] (Fig. 2).
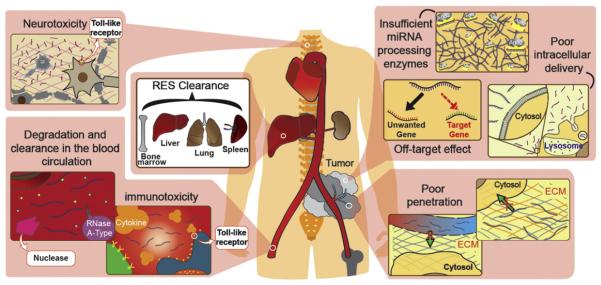
Fig. 2.
Barriers of In vivo miRNA delivery for cancer therapy. The leaky structure and compression of abnormal tumor vessels lead to poor blood perfusion, which reduces the delivery efficacy of naked miRNAs. Extravascular miRNAs encounter the ECM, which blocks the penetration of miRNAs into tumors. Intravascular barriers including enzyme degradation disrupt the unmodified naked miRNAs. Also, miRNAs carried by nanoparticles larger than 100 nm in diameter increase the RES clearance in the liver, spleen, lung and bone marrow, which results in non-specific uptake by innate immune cells such as monocytes and macrophages. Moreover, miRNAs can cause immunotoxicity by triggering secretion of inflammatory cytokines through Toll-like receptors. Neurotoxicity may also be induced via miRNA-bound TLRs. Once miRNAs reach the target tumor cells, the intracellular miRNAs may be trapped in the endosomes and degraded in lysosomes. Off-target effects of miRNA may cause unwanted side effects and insufficient or saturated miRNA processing enzymes may result in deficiency of miRNAs.
6.1. Poor penetration of miRNAs into tumor tissues due to mechanical and biological barriers
The major challenge of miRNA delivery into cancer is to successfully deliver miRNA antagonists or miRNA mimics to the target tumor tissue with efficient penetration of cargos into the tumor. The leaky structure and compression of abnormal tumor vessels lead to poor blood perfusion, which reduces the delivery efficacy of naked miRNAs [61] (Fig. 2). In addition, the slowdown of miRNA diffusion in solid tumors caused by higher interstitial fluid pressure holds the hurdle of miRNA delivery in cancer therapy [62]. The complex extracellular matrix (ECM) also plays an important role to hinder miRNAs from reaching the cancer cells (Fig. 2). For example, the fibrotic microenvironment of pancreatic cancer results in poor diffusion of therapeutic drugs [63, 64]. The nonmalignant cells in the tumor microenvironment also appear to be an important extracellular barrier. For instance, tumor-associated macrophages, neutrophils and monocytes can nonspecifically uptake and trap miRNAs encapsulated in the delivery system [65]. Besides, the blood–brain-barrier (BBB) represents a problem to the miRNA therapy involving cancer in central nervous system. Tight junctions between the brain endothelial cells reduce miRNA diffusion and delivery into brain tumors [66].
6.2. Unmodified miRNA antagonists and miRNA mimics are quickly degraded and cleared in the blood circulation
The other challenge that remains in miRNA delivery is to maintain the stability and integrity of miRNAs in circulation. Naked miRNAs are degraded within seconds by the abundant nucleases such as serum RNase A-type nucleases in the blood (Fig. 2) [67]. In addition, naked miRNAs are cleared rapidly via renal excretion, leading to a short half-life in systemic circulation [68]. miRNAs carried by nanoparticles larger than 100 nm in diameter increase the reticuloendothelial system (RES) clearance in the liver, spleen, lung and bone marrow, which results in non-specific uptake by innate immune cells such as monocytes and macrophages (Fig. 2) [69].
6.3. miRNAs, as other ssRNAs or dsRNAs have the potential to induce immunotoxicity
Systemic miRNA delivery, like other types of nucleic acid, activates innate immune system leading to unexpected toxicities and significant undesirable side effects. Systemic administration of miRNA duplexes can trigger secretion of inflammatory cytokines and type I interferons (IFNs) through Toll-like receptors (TLRs) (Fig. 2). TLRs 3, 7 and 8 are activated by single or double-stranded RNAs (dsRNAs) to drive innate and adaptive immune responses. These TLRs sensing dsRNA molecules in cellular endosomal and lysosomal compartments stimulate the type I interferon (IFN) pathway and trigger cytokine production according to the structure, sequence, and the delivery system [70,71]. IFN induced by the activation of TLRs further upregulates the expressions of TLRs and primes the surrounding immune cells such as monocytes, dendritic cells (DCs), natural killer (NK) cells, and B cells to become more sensitive to RNA stimulation. For example, dsRNAs with uridine- and guanosine-rich sequences up-regulate gene expression of TLRs 3 and 7, and trigger the release of IFN-α, IFN-β, interleukin (IL)-1β, and interleukin (IL)-6 [72]. TLR 8 is activated by dsRNAs containing AU-rich sequences. TLRs are expressed by different types of cells and involved in different immune responses as they bind to the corresponding RNAs. For example, TLR 3 is mainly expressed in mature myeloid DCs. When dsRNAs bind to TLR 3, they activate DCs to secret IL-12, and trigger immune responses more polarized toward CD4+ [73]. TLR 7 is expressed in plasmacytoid DCs and B cells. TLR 8 is expressed in myeloid DCs, monocytes and macrophages. TLR 8 bound to RNAs secrets IL-6 and tumor necrosis factor (TNF).
Although the perspective of immunogenicity of either single-stranded or double-stranded RNAs is well established, the immune response triggered by miRNAs still requires further studies. miR-21 and miR-29a can bind to TLR 7 and TLR 8 as agonists, leading to NF-κB signaling activation and secretion of IL-6 and TNF-α, which locally promote cancer cell growth and metastasis when treating cancer, and may cause systemic immune toxicity [74]. miRNAs may bind to the TLR 7 and TLR 8 via the GU-rich motif (GUUG for miR-21 and GGUU for miR-29a), which is crucial for RNA–TLR recognition [75,76].
However, some miRNAs induced by TLRs, such as miR-146, miR-9, miR-147, miR-21 and miR-155, can block the activation of inflammatory pathways in myeloid cells. For example, miR-146 attenuates inflammation by inhibition of IL-1R-associated kinases 1 and 2, TNFR-associated factor, and the downstream molecules of TLRs. miR-155 suppresses the expression of interleukin-1β and pro-inflammatory protein TAK1-binding protein 2, and decreases the activity of NF-κB transcription factor. Such mechanism leads to an anti-inflammatory effect in human myeloid-derived DCs [77,78]. When the anti-inflammatory miRNAs are delivered as therapeutic agents, they may suppress the systemic immune response instead of causing immune toxicity.
6.4. Neurotoxicity occurs as the result of exposure to miRNAs
When it comes to toxicity induced by miRNA-bound TLRs, neurotoxicity is another cause of concern. Some miRNAs can trigger neurodegeneration and cause neurotoxicity through TLRs (Fig. 2). For example, miRNA let-7b can bind and activate TLR 7 signaling in neurons and induce neurodegeneration [79]. The GU-rich sequence of let-7b displays a binding motif interacting with TLR 7 [80]. Furthermore, it has been shown that the intrathecal injection of let-7b into mice caused significant axonal injury and loss of neurons. Thus, let-7b may cause marked neuron damage and injury. Occurrence of neurotoxicity with exposure to miRNAs might be a problem when using miRNAs for systemic cancer therapy.
6.5. Poor intracellular delivery and aggregation within the endosomes of naked miRNAs result in inefficient gene silencing
Even though some miRNAs can reach the target tumor tissues successfully, there is another challenge remaining unsolved — how to increase the uptake of miRNAs in cancer cells. The endocytosis process also creates a challenge for the intracellular delivery of miRNAs, as most of the miRNAs are trapped in the endosomes and are further transported to late endosomes/lysosomes for degradation (Fig. 2). Strategies to enhance endosomal escape and release therapeutic miRNA cargoes into the cytoplasm to achieve target gene silencing are necessary. Moreover, gene silencing in cancer cells should be sufficiently prolonged to achieve therapeutic benefit.
6.6. Off-target effects of miRNAs
After miRNAs are delivered into the cytoplasm and released from the endosome, one of the biggest issues regarding miRNA therapy is the off-target effect of miRNAs. Since miRNAs are designed to target multiple pathways via imperfect matching with 3′ UTRs, they may cause unwanted gene silencing of the tumor suppressor genes. Such off-target gene silencing may cause potential toxicities and reduced therapeutic effects. The combination strategy can be added into the miRNA therapy to avoid unintended off-target effects [81]. A multifunctional nanoparticle co-delivering miRNA, siRNA and mRNA cocktails to silence several oncogenic pathways and activate the tumor suppressive and off-target pathways simultaneously can minimize unintended side effect and maximize the therapeutic effect.
6.7. Insufficient or saturated miRNA processing enzymes lead to dysfunction of therapeutic miRNAs
One of the important factors to regulate the silencing efficacy of miRNAs is the expression of RISC complex. RISC is a key enzyme that facilitates the mature miRNAs interacting with complementary 3′ UTRs of target mRNAs, enabling miRNAs to regulate the target gene expression [23]. However, in certain cancer cells or under certain tumor microenvironment such as hypoxia, the activity of RISC complex is downregulated [82]. Thus, the tumor suppression miRNAs are dysfunctional. For example, EGFR, a well-characterized oncogene in human cancers, inhibits the function of tumor suppressor miRNAs via phosphorylation and inactivation of argonaute 2 (AGO2) under hypoxic conditions [83]. In addition, generation of mature miRNAs requires Dicer, another key enzyme involved in miRNA processing. In some tumor types such as ovarian cancer, Dicer is downregulated leading to miRNA dysregulation [84]. Moreover, the extrinsic therapeutic miRNA may compete with endogenous miRNA for the processing enzymes, which may lead to decreased expression of mature tumor suppressor miRNA [85,86]. In addition, the saturation of miRNA processing enzymes may also result in the inefficient gene-silencing efficacy of the extrinsic therapeutic miRNA [87]. Thus, the enzyme activity in the target tumors should be carefully evaluated and considered when miRNAs are delivered and introduced as therapeutic agents against cancer. Besides, using mature miRNA or miRNA-mimicking siRNAs as therapeutic agents may minimize the potential disadvantage of miRNA therapy due to over-saturation of the processing enzymes.
Some success has already been achieved in the application of RNA therapeutics including siRNA and miRNA in some topical or localized target tissues (i.e. the eye, the lungs, and the central nervous system) [56,88,89]. However, critical challenges remain before safe and highly efficient miRNA therapeutics can be derived for clinical applications in cancer therapy. Thus, many groups have worked on overcoming those hurdles to achieve safe, specific and efficient miRNA therapy. Several carrier systems and delivery strategies were developed to solve the problems that arise during In vivo delivery of miRNAs for treating cancer. These studies reveal the possibility for regulation of cancer-related gene expression profiles via systemic administration of therapeutic miRNAs or miRNA antagonists. Among various cancer treatments, miRNAs might represent a revolution in cancer therapy.
7. In vivo miRNA delivery strategies for cancer therapy
A lot of work has been done in exploiting and evaluating the features of tumor and tumor microenvironment to improve RNA delivery. There are two classes of strategies for miRNA delivery — local and systemic delivery.
7.1. Local delivery of miRNAs
Effective gene-silencing and anti-tumor effects have been demonstrated via intratumoral injection or local administration of miRNAs with or without carriers. Local delivery of miRNAs can achieve the desired gene silencing due to higher bioavailability. Furthermore, local delivery of miRNAs shows reduced toxicity when compared with systemic delivery. Several local delivery strategies have been developed, ranging from direct intratumoral injection of miRNA vectors to the nanoparticle formulation with surface modification. For example, intracranial delivery of miRNA is developed for treatment of glioblastoma multiforme (GBM) [90]. Topical delivery is another example. For skin disease, topical administration allows the easy accessibility of the target region, a focused delivery and potential for systemic delivery with reduced side effect [91]. Inoue et al. has demonstrated the successful delivery of siRNA via electroporation silenced the target gene expression in the skin [92]. Zheng et al. showed that spherical nucleic acid nanoparticle conjugates (SNA-NCs), gold cores modified by a dense shell of highly oriented, covalently immobilized siRNA, can efficiently penetrate skin and silence the target gene [93]. Topical delivery of 1.5 μM EGFR siRNA incorporated in SNA-NCs to hairless mouse skin almost completely silenced EGFR expression and inhibited downstream MAPK signaling. Although the topical delivery hasn't been applied in miRNA-based therapy yet, we believe it may be a promising delivery system for miRNA-based therapeutic agents in skin cancer.
The modified miRNAs were used for local miRNA delivery. For example, intratumoral administration of cholesterol-conjugated 2′-O-methyl-modified miR-375 mimics could target astrocyte elevated gene-1 (AEG-1) and significantly suppress tumor growth in hepatoma xenograft models [94]. Virus vectors were used to deliver let-7 miRNA into lung cancer. let-7 miRNA family usually serves as tumor suppressor and is downregulated in non-small-cell lung cancer (NSCLC). Trang et al. showed intranasal delivery of lentiviral vector expressing let-7a increased let-7 expression in lung and effectively inhibited the growth of K-ras-dependent lung tumors [95]. They tested the tumor growth inhibition effect in subcutaneously inoculated human H460 lung tumor models — the most aggressively growing NSCLC xenografts. They showed that local delivery of synthetic let-7b triggered a specific inhibitory response and the tumor size could be reduced by 60 to 70% compared with control groups after four continuous treatments at 3-day treatment intervals. Moreover, polymer-based delivery system was applied for local delivery of miRNAs. Loss of miR-145 function in various cancers decreased apoptosis and promoted proliferation. In addition, downregulation of miR-33a in different types of cancers upregulates the oncogenic kinase Pim-1 leading to tumor formation and growth. After local administration of the unmodified miRNAs — miR-145 and miR-33a formulated in low molecular weight polyethyleneimine (PEI)/miRNA complexes, intact miR-145 and miR-33a could be successfully delivered into mouse xenograft colon carcinoma and achieve significant anti-tumor effects [96].
Besides, some siRNAs can act as miRNA antagonists or miRNA inducers delivered intratumorally for cancer therapy. For example, DCAMKL-1 specific siRNA delivered by poly(lactide-co-glycolide)-based nanoparticles (NPs) were injected intratumorally into colorectal cancer xenografts. NP-DCAMK-1 siRNA downregulated proto-oncogene c-Myc and Notch-1 through upregulation of let-7a and miR-144 miRNA expressions in colorectal cancer xenograft model. Intratumoral administration of NP-DCAMKL-1 siRNA into colorectal cancer xenografts led to tumor growth inhibition [97].
Local delivery of miRNAs improved therapeutic effect in cancer therapy, especially in the readily accessible primary tumors such as melanoma, breast cancer or cervical cancer. The advantage of local delivery is more focused delivery of miRNAs into target tumor tissues without non-specific uptake of therapeutic miRNAs by normal healthy organs and undesired toxicity induced by systemic miRNA delivery. However, since local delivery is not an option for late stage metastatic cancer, it has a limited role in miRNA cancer therapy. To this end, it is important to develop systemic delivery system to fill the current need for miRNA cancer therapy.
7.2. Systemic delivery of miRNAs
Significant progress has been made in developing the systemic miRNA delivery strategies to overcome the hurdles of In vivo miRNA delivery and enhance the efficacy of cancer therapy. The first strategy developed for systemic delivery of miRNAs into tumors is to synthesize the chemical modified miRNAs or miRNA antagonists such as anti-miRNA oligonucleotides (AMOs) [98]. Such modifications can prevent miRNA antagonists or miRNA mimics from nuclease degradation in the blood circulation. Moreover, the modified oligonucleotides have higher binding affinity with the target sequence. However, the modified miRNAs require a targeting moiety for intracellular miRNA uptake. In addition, the small modified miRNA molecules may show rapid renal and hepatic clearance resulting in short half-lives and their tumor uptake and biodistribution are still limited.
The second delivery strategy is further established to design the nanoparticle formulation for passive diffusion into tumor tissues based on the enhanced permeability and retention (EPR) effect [99,100]. The leakiness of tumor-associated neovasculature contributes to the EPR effect, by which the nanoparticles with optimal size distribution can accumulate in tumor microenvironment compared with healthy tissues. Although miRNAs formulated in nanoparticles can enhance favorable tissue distribution and tumor localization compared with naked miRNAs, the degree of enhancement is often not sufficient. Thus, the tumor-targeting approach is needed to enhance the miRNA uptake in cancer cells. Third-generation delivery strategy has recently emerged to add surface modifications to the nanoparticles, which allow specific binding to the target cancer cells and facilitate the internalization of the nanoparticles into the cancer cells through receptor-mediated endocytosis [101,102].
As more and more approaches were developed for In vivo delivery of miRNAs, those studies open new opportunities for miRNA-based cancer therapeutics. This review further focuses on current developments in the systemic delivery of miRNAs.
7.2.1. Modified miRNA antagonists or miRNA mimics
Several chemical modifications can enhance the stability of miRNA modulators and improve the systemic delivery efficacy by increasing the resistance to degradation by nucleases in the blood circulation.
7.2.1.1. The 2′ OH group modification
The 2′-OH in the ribose ring is easily attacked by nuclease action. 2′-O-methyl (2′-OMe), 2′-O-methoxyethyl or 2′-fluoro carried by the modified anti-miRNA oligonucleotides show enhanced stability, higher binding affinity with the targeted miRNA and improved gene silencing In vivo compared with unmodified anti-miRNA oligonucleotides [103]. Krützfeldt et al. showed that intravenous administration of antisense 2′-OMe oligoribonucleotides efficiently and specifically silenced the targeted endogenous miRNAs expressed in the liver, lung, kidney, heart, intestine, fat, skin, bone marrow, muscle, ovaries and adrenals in mice. They demonstrated the chemically engineered miRNA antagonist has a potent and long-lasting effect [104].
7.2.1.2. LNA modification
LNA is a conformational RNA analog that specifically interacts with the complementary miRNA with high affinity and “neutralizes” the function of the targeted miRNA (Fig. 3A). LNA oligonucleotides can silence the function of miRNAs In vivo. For example, a LNA-miRNA antagonist was developed to inhibit the function of miR-122 – a miRNA regulating HCV replication – in vitro and In vivo [105]. By systemic administration of the LNA-miRNA antagonist into mice, miR-122 levels were downregulated dose-dependently in the liver and the silencing effect was sustained for several weeks. The study demonstrated the promising potential of the LNA-miRNA antagonist in inhibition of HCV replication and HCC tumor formation.
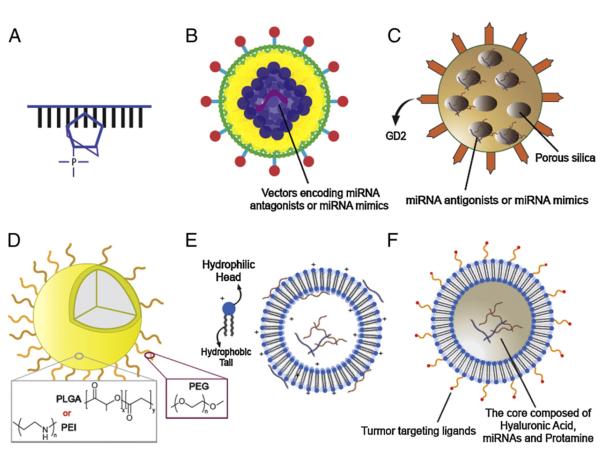
Fig. 3.
Strategies for miRNA delivery In vivo. Many strategies such as modified miRNA antagonists or miRNA mimics, viral vectors, inorganic or organic non-viral delivery systems have been established for delivery of miRNAs for cancer therapy. miRNA antagonists modified with LNAs bind to the targeted miRNAs with high affinity (A). Vectors encoding miRNA antagonists or miRNA mimics can be carried by viral vectors for In vivo delivery (B). Silica nanoparticles modified with GD2 antibody specifically deliver miRNAs into tumors overexpressing GD2 (C). miRNAs can be encapsulated in organic non-viral delivery systems such as PLGA, PEI (D) and liposome based nanoparticle (E) that are modified either with ligands or with antibodies for tumor-targeted delivery (F).
Seed-targeting 8-mer LNA oligonucleotides, termed tiny LNAs are developed as miRNA antagonists to target seed region of miRNAs. Tiny LNAs can simultaneously silence miRNAs within families sharing the same seed region, efficiently eliminate the functions of miRNAs within the families and thus upregulate the target mRNAs. Systemic delivery of anti-miR-21 tiny LNA showed significant repression of the miR-21 luciferase reporter in orthotopic breast tumors [106]. It demonstrated that anti-miR-21 tiny LNA can be successfully delivered to tumor sites and this antagonist molecule can serve as a platform for developing new therapeutic strategies to treat cancer.
7.2.1.3. The passenger strand modification
Modified miRNA mimics can increase the stability and avoid the interferon secretion triggered by TLRs when delivered In vivo [107]. To achieve the maximized protection, the heavier modifications on the passenger strand were designed to protect the duplex miRNA mimics from nuclease degradation and immunotoxicity induction. Such modifications included application of nucleotide analogs, backbone modifications and terminal modifications such as addition of inverted bases and biotin or alkyl groups [48,69]. The advantage of this approach is that the target specificity and silencing activity still remain in the guide strand that is less modified [58,108]. Accordingly, Akao et al. edited the sequences of the passenger strand of the miR-143 duplex and modified the 3′-overhang portion of miR-143, resulting in enhanced efficacy and better stability [109]. Systemic administration of the modified miR-143 showed 15% (low dose) to 50% (high dose) tumor growth inhibition effect on xenografted DLD-1 human colorectal tumor models. Their finding indicated that the tumor suppressor miRNA mimics, whose passenger strands were chemically modified may serve as a potential candidate for cancer treatment.
Although modified miRNA therapeutics have shown enhanced stability In vivo and increased affinity with the target sequence, they fail to show efficient and specific uptake by tumor cells, and elevated accumulation of miRNAs in tumor. Consequently, modified or unmodified miRNA modulators have been engineered by using viral and non-viral carriers. The biocompatible and biodegradable carriers for miRNAs with favorable size and surface modification are designed to improve tumor-specific delivery, achieve immune evasion and reduce toxicity. Both viral and non-viral vectors can serve as carriers for miRNAs.
7.2.2. Viral delivery of miRNAs
Vectors encoding miRNA antagonists or miRNA mimics can be carried by viral vectors (Fig. 3B). Several viruses such as lentiviruses, adenoviruses and adeno-associated viruses (AAVs) can be used to deliver vectors encoding miRNAs into the cell nuclei and efficiently express miRNAs. To aid specific delivery into tumors, the targeting moieties can be added on the viral capsid through genetic manipulation of viral capsid proteins to modulate the affinity between viral vectors and cancer-specific receptors. For example, lentiviral vectors expressing miR-15a/16 were systemic delivered into the de novo New Zealand Black (NZB) mouse model, a naturally occurring age-associated mouse model of chronic lymphocytic leukemia (CLL). Systemic lentiviral delivery of miR-15a/16 restored the expression of the targeted miRNAs and ameliorated disease manifestations of CLL [110]. In addition, miR-494, induced by tumor-derived factors such as TGF-β1, increases tumor accumulation and pro-angiogenesis and metastasis activity of myeloid-derived suppressor cells (MDSCs) in the tumor microenvironment. Intravenous administration of lentivirus containing miR-494 antagonists significantly decreased tumor-infiltrating MDSCs, reduced the activity of MDSCs and inhibited the tumor growth and metastasis in the murine breast cancer model [111]. However, lentiviruses integrate their own reverse transcribed DNAs into the host cellular genome, which may lead to insertional mutagenesis and activation of oncogenic pathways. Thus, non-integrating adenoviruses and AAVs are used as alternative miRNA carriers. Unlike lentiviruses, adenoviruses and AAVs keep their own genomes in episomal form. For example, AAVs were used as tools to perform miRNA replacement therapy for liver cancer and showed promising therapeutic effect. Systemic delivery of miR-26a carried by AAVs showed cell cycle arrest and apoptosis induction in cancer cells and tumor growth inhibition in HCC. Furthermore, systemic administration of AAV-miR-26 displayed undetectable toxicity [112]. It indicated that miRNA replacement therapy using AAVs provides a safe and efficient strategy for cancer therapy.
Recent studies demonstrate exosomes, nano-sized lipid vesicles, produced and released by virus-infected cells can encapsulate and deliver RNA therapeutics into the target cells. Pegtel et al. showed that virus-infected cells packaged virus-encoded RNAs into exosomes and the RNA cargoes were later taken into non-infected target cells [113, 114]. Subsequently, it indicated that exosomes could be exploited for therapeutic miRNA delivery strategies, with increased targeting efficacy [115]. For example, the exosomes derived from viruses specifically targeting liver cells can be engineered to carry therapeutic miRNAs for treating liver cancer [115].
Despite the fact that viral vectors can efficiently deliver miRNA antagonists or miRNA mimics into tumor tissues and regulate target gene expressions, they are more immunogenic and more difficult to scale up manufacturing compared with non-viral delivery systems. Moreover, the possibility of the production of a replication competent virus may cause the potential of pathogenetic disease. For example, some retroviruses develop CNS disease as a consequence of their active replication [116,117]. As discussed above, virus delivery system may generate insertional mutagenesis, leading to upregulation of oncogenes and cancer formation [118]. Thus, although viral vector-mediated miRNA delivery is an attractive option for cancer therapy, the extra care and refinement of this technology are required to minimize the adverse effect. To this end, non-viral carriers such as polymer or lipid-based delivery vehicles are developed as safer and more manufacturable delivery systems for miRNA cancer therapy.
7.2.3. Non-viral delivery of miRNAs
Even though non-viral delivery systems usually show lower transfection efficacy and shorter duration of target gene expression compared with viral vectors, recent studies successfully demonstrated that non-viral carriers with rational design and suitable modifications can also achieve clinically relevant efficiency. The last part of this review is to provide an update and concise perspective on development and applications of non-viral miRNA delivery for cancer therapy.
7.2.3.1. Inorganic nanoparticles-based miRNA delivery
Lately, inorganic materials such as gold, carbon and silica are used to compose a non-viral gene delivery system with controlled size and morphology. The unique physical and chemical properties of inorganic nanoparticles offer potential for development of a biocompatible, non-immunogenic and non-toxic delivery system, which is also easier to scale up manufacturing. Gold nanoparticles functionalized by cysteamine were developed to incorporate therapeutic miRNAs and deliver them into target tumor cells [119]. AuNP-S-polyethylene glycol or AuNP-S-PEG showed high miRNA loading capacity and low toxicity and triggered efficient endosomal release of the cargoes in cancer cells. Furthermore, miRNA containing AuNP-S-PEG showed efficient gene silencing effect and inhibited proliferation of cancer cells.
Another example of inorganic miRNA delivery system is the silica-based vehicle [101]. Silica nanoparticles modified with disialoganglioside GD2 (GD2) antibody specifically deliver miR-34a into neuroblastoma, which overexpresses GD2 (Fig. 3C). Systemic administration of miR-34a containing anti-GD2-silica nanoparticles resulted in increased apoptosis in tumor tissues, a marked reduction in vascular density of tumors and inhibition of tumor growth. However, the inorganic gene delivery system is limited by drawbacks such as lack of cargo protection, low loading efficiency, and inefficient endosomal escape.
7.2.3.2. Polymer-based miRNA delivery
Polymers such as poly(lactic-co-glycolic acid) or PLGA and PEI are commonly used as miRNA carriers for gene therapy in cancer (Fig. 3D). For the purpose of efficient and effective delivery into cancer cells or more specifically, cytosol, many studies have concentrated on designing polymer-based nanoparticles with special modifications. PLGA – which has been studied for many years and well-characterized by its safe, biocompatible, and biodegradable nature – is chosen for making nanoparticles with high production efficiency and stable mechanical property. PLGA-based nanoparticles are capable of sustained release of drugs in cytosol through endo/ lysosomal escape owing to reversal of the surface charge after cellular internalization [120–123]. Furthermore, surface modification of PLGA nanoparticles with PEG greatly increases the circulation time and retention of nanoparticles in tumor sites In vivo [124]. Here are some studies utilizing PLGA-based nanoparticles for delivery of miRNAs as cancer therapy. Antagonists of miR-21 and miR-10b encapsulated in PEGylated-PLGA nanoparticles were systemically injected into mice bearing subcutaneous breast cancer tumor model. It showed that nanoparticles with size about 150 to 200 nm and encapsulation efficiency around 60–70% sustainably released the miRNA antagonists for a long period of time. The fluorescent labeled miR-21 antagonists encaptured in the PLGA nanoparticles still remained in SUM159 triple negative breast cancer cells even 126 h after treatment as detected by flow cytometry. For In vivo experiment, miRNAs delivered by PLGA nanoparticles were accumulated in the tumor tissues 24 h after injection. Thus, the miRNA antagonists containing PEGylated-PLGA nanoparticles may achieve great therapeutic effect In vivo [125]. Babar et al. examined whether the induction or withdrawal of miR-155 expression would cause tumor regression. They showed that PLGA-based nanoparticles, with surface modification of a cell-penetrating peptide, performed a great delivery efficiency and therapeutic effect in a murine lymphoma model via local administration [126]. In the cultured cells, antagonists of miRNAs delivered by the peptide-modified PLGA nanoparticles showed 65% reduction of miR-155 levels, and unmodified nanoparticles yielded 23% reduction. For the therapeutic outcome, treatment of peptide-modified PLGA nanoparticles loaded with scramble control antagonists showed an approximately 10-fold increase in tumor volume compared with starting volume, while treatment of miR-155 antagonists delayed tumor growth to less than an approximately twofold increase compared with starting volume. Furthermore, the nanoparticles were retained in the tumor tissues 2 days after injection. These studies indicate that PLGA-based nanoparticles can be a potential approach for efficient miRNA delivery.
Another widely used material – polyethyleneimine (PEI)– is water-soluble and positively charged. In physiological milieus, the positively charged PEI-based nanoparticles can encapsulate negatively charged DNA, siRNAs or miRNAs by electrostatic interaction. After endocytosis of the nanocomplexes, the strong buffer effect of the complex causes endosome swelling by influx of protons and water through the ‘proton sponge effect’ in endosomes, and subsequently promote endosome destabilization and release of miRNA-encapsulated nanoparticles into cytosol, achieving the gene silencing effect [127–129]. Some studies have provided successful examples of utilizing PEI-based nanoparticles in miRNA delivery. miR145 encapsulated in nanospheres with a short polyurethane and a branched polyethylenimine (PU-PEI) greatly reduced growth of lung adenocarcinoma (LAC) that carries cancer stem cell-like properties. Furthermore, PU-PEI-miR145 in combination with ionizing radiation and cisplatin nearly eradicated metastatic tumor nodules. The study indicated that effective delivery of miR145 can promote inhibition of tumor growth. Furthermore, it shows that PU-PEI nanocarriers have great potential for miRNA-based cancer therapy [130]. Another study also successfully validated efficient systemic delivery of therapeutic miRNAs in cancer via PEI-based formulations. Locally and systemically administrations of miR-145 and miR-33a formulated with low molecular weight PEI showed repression of c-Myc and knockdown of Pim-1 in mouse models of colon carcinoma, respectively [96]. The miR-145 and miR-33a containing PEI delivery system suppressed cancer cell proliferation, increased apoptosis and caused profound reduction of tumor growth.
Synthetic polymers as nanocarriers can be a promising approach in miRNA delivery. Among different polymers, cationic polymers are potent carriers to stabilize and deliver miRNAs due to strong electrostatic interactions with miRNA cargoes and enhanced cellular uptake via negatively charged cell membrane. These novel polymer-based nanoparticles become potential therapeutic agents to treat cancer [131]. However, their systemic toxicity must be carefully evaluated.
7.2.3.3. Lipid-based miRNA delivery
Liposomes, composed of a lipid bilayer and an internal aqueous phase are used to incorporate and deliver cargoes such as chemotherapy drugs and nucleic acids into tumor lesions. Similar to other nucleic acid therapeutics, the lipids such as cationic, anionic, neutral lipids or a mixture thereof can be used to form lipoplexes for miRNA delivery. The lipid carriers can protect miRNAs from degradation and increase the stability of miRNAs in blood circulation. Thus, liposomes have been developed as useful tools to systemically deliver miRNAs. When miRNAs are formulated with cationic lipids, the negatively charged hydrophilic miRNAs bind to the positively charged lipids, which form complexes to enhance the uptake of the incorporated miRNAs through interaction between the positively charged complexes and negatively charged cell membranes (Fig. 3E).
For example, miR-122, which is usually downregulated in HCC, serves as a tumor suppressor miRNA in liver cancer. A cationic lipid nanoparticle - LNP-DP1, composed of 2-dioleyloxy-N,N-dimethyl-3-aminopropane (DOTMA), egg phosphatidylcholine, cholesterol and cholesterol-polyethylene glycol was developed to deliver miR-122 for treatment of HCC. The miR-122 mimic delivered by LNP-DP1 restored the gene downregulation, inhibited angiogenesis and suppressed tumor growth in HCC without induction of systemic toxicity [132]. In addition, miR-133b, which plays an important role in tumor suppression, has been selected as a therapeutic target. Pre-miRNA-133B containing DOTMA lipoplexes led to downregulation of prosurvival gene MCL-1 in target lung cancer cells in vitro [133]. Systemic delivery of pre-miR-133b encapsulated in DOTMA lipoplexes resulted in 30% accumulation and increased mature miR-133b expression in lung. Furthermore, miR-29b was also reported as a tumor suppressor and downregulated in lung cancer tissues [134]. miR-29b delivered systemically via cationic lipoplexes (LPs)-based carriers reduced the expression of the key target oncogenes such as cyclin-dependent protein kinase 6 (CDK6), DNMT3B, and myeloid cell leukemia sequence 1 (MCL1) and blocked tumor growth in NSCLC.
Wu et al. also reported a cationic lipid-based delivery system composed of DOTMA and cholesterol to formulate therapeutic miRNAs for cancer therapy. miR-133b, a tumor suppressor targeting the prosurvival gene MCL-1, regulates cell proliferation and sensitizes the lung cancer cells to chemotherapy. After intravenous administration, lipoplexes tended to accumulate in lung tissue (30%). Furthermore, pre-miR-133b formulated in lipoplexes efficiently increased the expression of mature miR-133b in lung, indicating that cationic lipoplexes may serve as a promising delivery system for miRNA-based therapeutics in lung cancer [133].
Solid lipid nanoparticles (SLNs) were also applied as miRNA carriers for cancer therapy. A SLN system containing cationic lipids was established to deliver miR-34a mimics into cancer stem cells (CSC), which cause tumor growth and drug resistance [135]. Treatment with miR-34a loaded SLNs led to induction of apoptosis in CSCs and increased survival of CSC-bearing mice. These studies showed that cationic lipoplexes could serve as a potential miRNA delivery system for cancer therapy. However, several problems caused by the positively charged lipids such as type I and type II interferon induction and liver toxicity hinder the development and clinical application of cationic lipid-based delivery system [57,58].
To overcome these drawbacks, neutral lipids were used to replace the cationic lipids and serve as the non-toxic carriers for miRNA delivery. Wiggins et al. demonstrated that miR-34a formulated in a neutral lipid-based delivery vehicle achieved significant miRNA accumulation and downregulation of the target genes in the lung tumor tissues in NSCLC xenograft mouse models [136]. Systemic delivery of miR-34a containing vehicles displayed unchanged levels of cytokines and liver enzymes in the blood circulation, indicating the neutral lipid-based delivery system is a safe and non-immunogenic formulation. Furthermore, formulated miR-34a inhibited tumor growth significantly in xenograft mouse models of NSCLC. They further continued the study and expanded the application of the neutral lipid-based delivery system. Trang et al. later demonstrated that systemic delivery of miR-34a or let-7 miRNA mimics in the neutral lipid-based formulation showed a 60% reduction of tumor burden in a K-ras-activated autochthonous mouse model of NSCLC compared with a control miRNA [137]. The study provides a promising delivery platform for the systemic delivery of tumor suppressing miRNAs without inducing toxicity.
Even though neutral lipid-based delivery systems are less toxic than cationic lipid-based formulations, the transfection efficacy of miRNAs incorporated in neutral lipids remains poor. In addition, vehicles composed of neutral lipids have a lower loading efficacy for miRNAs compared with miRNA-cationic liposome complexes. To achieve both – high efficacy and safety – in one formulation, we have developed a nanoparticle formulation – liposome–polycation– hyaluronic acid (LPH) post-inserted DSPE-PEG – to systemically deliver miRNAs into tumor tissues with low toxicity [102]. We modified LPH nanoparticles with GC4 single-chain variable fragment (scFv), a tumor-targeting human monoclonal antibody, to effectively deliver miRNAs to lung metastasis in a syngeneic murine model (Fig. 3F).
In our studies, we found that miR-34a delivered by GC4-targeted nanoparticles significantly silenced the expression of the target protein survivin and inactivated the downstream signaling such as MAPK pathway in the B16F10 lung metastasis. Apoptosis was induced after the treatment with miR-34a containing GC4-targeted nanoparticles in the B16F10 lung metastasis. To increase the anti-tumor effect of the miRNA containing nanoparticles, we further co-delivered miR-34a and multi-targeted siRNA cocktails against c-Myc, MDM2 and VEGF via GC4-targeted nanoparticles. Systemic administration of siRNAs and miR-34a in the GC4-targeted nanoparticles showed a significant inhibition of the metastatic tumor load in the lung. In addition, the pro-inflammatory cytokines and liver enzymes in the blood remained unchanged after treatment with GC4-targeted LPH nanoparticle. Thus, GC4-targeted LPH nanoparticle formulation is a safe and effective vehicle to deliver miRNAs for cancer therapy either at the early stage of primary tumor growth or the late stage of metastasis development.
In conclusion, liposomes were developed as an effective miRNA delivery system with reduced toxicity and side effect. The lipid-based carriers developed to date can facilitate a rapid route for miRNA-based cancer therapy into the clinic.
8. Future perspectives
There has been tremendous improvement in understanding the mechanisms of miRNAs and the development of strategies for miRNA delivery In vivo. However, some problems are still unsolved. One of the main issues is poor cancer tissue permeability. The penetration of miRNA-containing delivery vesicles with or without targeting moieties in the tumor microenvironment still remains inefficient due to heterogeneous tumor perfusion and interstitial fibrosis leading to poor efficacy outcome in the preclinical studies.
The latest generation delivery system is to design a “smart” nanoparticle, whose size and property are changeable due to different microenvironments, conditions or time series. The smart nanoparticles contain multi-component and multi-function carriers leading to controlled release and efficient diffusion of the therapeutic cargoes in tumor tissues. The aim is to change the structures or sizes of smart nanoparticles by using a stimulus property of the tumor microenvironment, such as low pH, low partial oxygen pressure, or high concentrations of proteases. An example is a pH-sensitive nanoparticle formulation. Materials with biodegradable and pH-sensitive properties can be used to formulate a nanoparticle-based miRNA therapy for tumor-specific delivery [138,139]. The structure of pH-sensitive nanoparticles is destabilized and undergoes rapid dissolution when the pH of the surrounding environment is lower than 6.5 and hence facilitated release of the cargoes encapsulated in the nanoparticles within the acidic tumor microenvironment. Another example is an enzyme-sensitive nanoparticle formulation. A linker susceptible to proteases cleavage such as MMP-2 cleavage can be conjugated to the well PEGylated nanoparticles to achieve the efficient tumor penetration of the modified nanoparticles [140]. The size of nanoparticles can be changed from 100 nm to 10 nm after MMP-2 cleavage. There is abundant MMP-2 released at the region where the invasion starts and at the sites of angiogenesis, leading to efficient enzymatic degradation of the modified nanoparticles by MMPs. The size-reduced nanoparticles can more readily penetrate leaky vessels into the dense collagen matrix and release the cargoes such as therapeutic miRNAs into the tumor microenvironment. Thus, miRNAs delivered by the smart nanoparticles may remain sustained gene silencing and promote anti-tumor effects.
In addition to designing tunable nanoparticles, nanoparticles targeting tumor-associated fibroblasts in the tumor stoma provide another therapeutic strategy to modulate tumor microenvironment and inhibit tumor progression. Some miRNAs such as miR-31, 155 and 214 mediating the activation or differentiation of the tumor-associated fibroblasts can serve as a therapeutic target for treating cancer [141–143]. To overcome the stroma-mediated hurdles, miRNAs can be delivered by vesicles modified by the ligands or antibodies directly or indirectly targeting stromal cells via binding to endogenous receptors. One example is to conjugate chemotherapy drugs with albumin-nanoparticles. The albumin-conjugated nanoparticles can bind to secreted protein acidic and rich in cysteine (SPARC) and efficiently deliver the cargoes into both tumor and stromal cells, leading to enhanced tumor accumulation [144–146]. Another example is to develop lipid-based nanoparticles containing either gemcitabine monophosphate or cisplatin targeting tumor-associated fibroblast [147]. The combination of gemcitabine monophosphate nanoparticles and Cisplatin nanoparticles significantly depleted tumor stroma and triggered synergistic anti-tumor effects in a stroma-rich bladder tumor model. The nanoparticle-based combination treatment increased levels of apoptotic cells by approximately 1.3 folds and decreased infiltration of activated tumor-associated fibroblast by more than 87% in the tumor tissue compared with free drugs [147]. Thus, it provides an alternative for cancer treatment without targeting cancer cells directly by developing a stroma-specific delivery system, resulting in the enhanced uptake of those therapeutic cargoes, including miRNAs in the tumor site and significant tumor regression.
Another novel approach is to apply cell-based delivery mechanisms to deliver miRNA mimics and miRNA antagonists for cancer therapies. For example, neural stem cells and mesenchymal stem cells can serve as drug carriers targeting GBM [148]. Mesenchymal stem cells own the advantages such as efficient delivery of the cargos, specific tropism to the target region, ease to amplification and collection and ability to suppress allogeneic responses [149,150]. Thus, mesenchymal stem cells can be used to deliver the therapeutic cargos such as miRNA mimics or miRNA antagonists into the target cancer cells via gap junctional intercellular communication and secreted exosomes [151,152]. Munoz et al. successfully blocked miR-9 expression in GBM by delivery of miR-9 antagonist through MSCs resulting in reversed chemoresistance of GBM cells [153]. It revealed cell-based delivery systems might be the next generation strategies for systemic delivery of miRNA therapeutics to cancer cells.
Moving forward, we believe that miRNA-based therapy will play a key role in cancer therapy in the future. Particularly, personalized cancer medicine can be realized by designing the specific miRNA mimic or antagonist sets for individuals based on individual patient miRNA expression profiles. We expect those new strategies developed can overcome the biological barriers for miRNA delivery and reveal great therapeutic potential of miRNAs in the oncology clinic.
Acknowledgments
This work was supported by NIH grants CA151652, CA151455 and CA149363 to Leaf Huang and by NSC 102-2320-B-007-011-MY2 to Yunching Chen.
Abbreviations
- AAVs
adeno-associated virusesy
- AEBP1
adipocyte enhancer-binding protein 1
- AEG-1
astrocyte elevated gene-1
- Ago
argonaute protein
- Ago2
argonaute2 protein
- AMOs
anti-miRNA oligonucleotides
- BBB
blood–brain-barrier
- CDK6
cyclin-dependent protein kinase 6
- CLL
chronic lymphocytic leukemia
- CSC
cancer stem cells
- DCs
dendritic cells
- dsRNAs
short double strand RNAs
- ECM
extracellular matrix
- EGFR
epidermal growth factor receptor
- EMT
epithelial–mesenchymal transition
- EPR
enhanced permeability and retention
- FGF
fibroblast growth factor
- GBM
glioblastoma multiforme
- HCC
hepatocellular carcinoma
- HCV
hepatitis c virus
- HDL
high-density lipoprotein
- HIF-α
hypoxia-inducible factor-α
- IFN
type I interferon
- IL
interleukin
- LAC
lung adenocarcinoma
- LNA
locked nucleic acid
- LPH
liposome–polycation–hyaluronic acid
- mAbs
monoclonal antibodies
- MCL1
myeloid cell leukemia sequence 1
- MDSCs
myeloid-derived suppressor cells
- miRNA
microRNA
- NPs
nanoparticles
- NSCLC
non-small-cell lung cancer
- PEG
polyethylene glycol
- PEI
polyethyleneimine
- PLGA
poly(lactide-co-glycolide)
- pre-miRNAs
hairpin-forming miRNA precursors
- pri-miRNAs
long RNA primary transcripts
- PU
polyurethane
- RES
reticuloendothelial system
- RISC
RNA-induced silencing complex
- scFv
single-chain variable fragment
- siRNA
short interfering RNA
- SLNs
solid lipid nanoparticles
- SNA-NCs
spherical nucleic acid nanoparticle conjugates
- SPARC
secreted protein acidic and rich in cysteine
- TLRs
Toll-like receptors
- TNF
tumor necrosis factor
- VEGF
vascular endothelial growth factor
Footnotes
This review is part of the Advanced Drug Delivery Reviews theme issue on “miRNAs as targets for cancer treatment: Therapeutics design and delivery”.
References
- [1].Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- [2].Arora A, Scholar EM. Role of tyrosine kinase inhibitors in cancer therapy. J. Pharmacol. Exp. Ther. 2005;315:971–979. doi: 10.1124/jpet.105.084145. [DOI] [PubMed] [Google Scholar]
- [3].Kris MG, Natale RB, Herbst RS, Lynch TJ, Jr., Prager D, Belani CP, Schiller JH, Kelly K, Spiridonidis H, Sandler A, Albain KS, Cella D, Wolf MK, Averbuch SD, Ochs JJ, Kay AC. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA. 2003;290:2149–2158. doi: 10.1001/jama.290.16.2149. [DOI] [PubMed] [Google Scholar]
- [4].Druker BJ, Lydon NB. Lessons learned from the development of an abl tyrosine kinase inhibitor for chronic myelogenous leukemia. J. Clin. Invest. 2000;105:3–7. doi: 10.1172/JCI9083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat. Rev. Cancer. 2012;12:278–287. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- [6].Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R, Patterson SD, Chang DD. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J. Clin. Oncol. 2008;26:1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- [7].Karapetis CS, Khambata-Ford S, Jonker DJ, O'Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S, Price TJ, Shepherd L, Au HJ, Langer C, Moore MJ, Zalcberg JR. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N. Engl. J. Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- [8].Lievre A, Bachet JB, Boige V, Cayre A, Le Corre D, Buc E, Ychou M, Bouche O, Landi B, Louvet C, Andre T, Bibeau F, Diebold MD, Rougier P, Ducreux M, Tomasic G, Emile JF, Penault-Llorca F, Laurent-Puig P. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J. Clin. Oncol. 2008;26:374–379. doi: 10.1200/JCO.2007.12.5906. [DOI] [PubMed] [Google Scholar]
- [9].Pillay V, Gan HK, Scott AM. Antibodies in oncology. N. Biotechnol. 2011;28:518–529. doi: 10.1016/j.nbt.2011.03.021. [DOI] [PubMed] [Google Scholar]
- [10].Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat. Rev. Immunol. 2010;10:317–327. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hu W, Jin L, Jiang CC, Long GV, Scolyer RA, Wu Q, Zhang XD, Mei Y, Wu M. AEBP1 upregulation confers acquired resistance to BRAF (V600E) inhibition in melanoma. Cell Death Dis. 2013;4:e914. doi: 10.1038/cddis.2013.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhu AX, Duda DG, Sahani DV, Jain RK. HCC and angiogenesis: possible targets and future directions. Nat. Rev. Clin. Oncol. 2011;8:292–301. doi: 10.1038/nrclinonc.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Suarez Y, Sessa WC. MicroRNAs as novel regulators of angiogenesis. Circ. Res. 2009;104:442–454. doi: 10.1161/CIRCRESAHA.108.191270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Plummer PN, Freeman R, Taft RJ, Vider J, Sax M, Umer BA, Gao D, Johns C, Mattick JS, Wilton SD, Ferro V, McMillan NA, Swarbrick A, Mittal V, Mellick AS. MicroRNAs regulate tumor angiogenesis modulated by endothelial progenitor cells. Cancer Res. 2013;73:341–352. doi: 10.1158/0008-5472.CAN-12-0271. [DOI] [PubMed] [Google Scholar]
- [15].Enkelmann A, Heinzelmann J, von Eggeling F, Walter M, Berndt A, Wunderlich H, Junker K. Specific protein and miRNA patterns characterise tumour-associated fibroblasts in bladder cancer. J. Cancer Res. Clin. Oncol. 2011;137:751–759. doi: 10.1007/s00432-010-0932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nielsen BS, Jorgensen S, Fog JU, Sokilde R, Christensen IJ, Hansen U, Brunner N, Baker A, Moller S, Nielsen HJ. High levels of microRNA-21 in the stroma of colorectal cancers predict short disease-free survival in stage II colon cancer patients. Clin. Exp. Metastasis. 2011;28:27–38. doi: 10.1007/s10585-010-9355-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Stenvang J, Petri A, Lindow M, Obad S, Kauppinen S. Inhibition of microRNA function by antimiR oligonucleotides. Silence. 2012;3:1. doi: 10.1186/1758-907X-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hansen TF, Christensen R, Andersen RF, Sorensen FB, Johnsson A, Jakobsen A. MicroRNA-126 and epidermal growth factor-like domain 7-an angiogenic couple of importance in metastatic colorectal cancer. Results from the Nordic ACT trial. Br. J. Cancer. 2013;109:1243–1251. doi: 10.1038/bjc.2013.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006;20:515–524. doi: 10.1101/gad.1399806. [DOI] [PubMed] [Google Scholar]
- [20].Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- [21].Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- [22].Yekta S, Shih IH, Bartel DP. MicroRNA-directed cleavage of HOXB8 mRNA. Science. 2004;304:594–596. doi: 10.1126/science.1097434. [DOI] [PubMed] [Google Scholar]
- [23].Macfarlane LA, Murphy PR. MicroRNA: biogenesis, function and role in cancer. Curr. Genomics. 2010;11:537–561. doi: 10.2174/138920210793175895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- [25].Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL, Tait JF, Tewari M. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc. Natl. Acad. Sci. U. S. A. 2011;108:5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat. Cell Biol. 2011;13:423–433. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lawrie CH, Gal S, Dunlop HM, Pushkaran B, Liggins AP, Pulford K, Banham AH, Pezzella F, Boultwood J, Wainscoat JS, Hatton CS, Harris AL. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br. J. Haematol. 2008;141:672–675. doi: 10.1111/j.1365-2141.2008.07077.x. [DOI] [PubMed] [Google Scholar]
- [28].Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X, Li Q, Li X, Wang W, Zhang Y, Wang J, Jiang X, Xiang Y, Xu C, Zheng P, Zhang J, Li R, Zhang H, Shang X, Gong T, Ning G, Wang J, Zen K, Zhang J, Zhang CY. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- [29].Pritchard CC, Kroh E, Wood B, Arroyo JD, Dougherty KJ, Miyaji MM, Tait JF, Tewari M. Blood cell origin of circulating microRNAs: a cautionary note for cancer biomarker studies. Cancer Prev. Res. (Phila.) 2012;5:492–497. doi: 10.1158/1940-6207.CAPR-11-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yu Z, Baserga R, Chen L, Wang C, Lisanti MP, Pestell RG. microRNA, cell cycle, and human breast cancer. Am. J. Pathol. 2010;176:1058–1064. doi: 10.2353/ajpath.2010.090664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Garofalo M, Quintavalle C, Di Leva G, Zanca C, Romano G, Taccioli C, Liu CG, Croce CM, Condorelli G. MicroRNA signatures of TRAIL resistance in human non-small cell lung cancer. Oncogene. 2008;27:3845–3855. doi: 10.1038/onc.2008.6. [DOI] [PubMed] [Google Scholar]
- [32].Xiao C, Srinivasan L, Calado DP, Patterson HC, Zhang B, Wang J, Henderson JM, Kutok JL, Rajewsky K. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat. Immunol. 2008;9:405–414. doi: 10.1038/ni1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lima RT, Busacca S, Almeida GM, Gaudino G, Fennell DA, Vasconcelos MH. MicroRNA regulation of core apoptosis pathways in cancer. Eur. J. Cancer. 2011;47:163–174. doi: 10.1016/j.ejca.2010.11.005. [DOI] [PubMed] [Google Scholar]
- [34].Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, Wojcik SE, Aqeilan RI, Zupo S, Dono M, Rassenti L, Alder H, Volinia S, Liu CG, Kipps TJ, Negrini M, Croce CM. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc. Natl. Acad. Sci. U. S. A. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chen B, Li H, Zeng X, Yang P, Liu X, Zhao X, Liang S. Roles of microRNA on cancer cell metabolism. J. Transl. Med. 2012;10:228. doi: 10.1186/1479-5876-10-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Horie T, Ono K, Nishi H, Iwanaga Y, Nagao K, Kinoshita M, Kuwabara Y, Takanabe R, Hasegawa K, Kita T, Kimura T. MicroRNA-133 regulates the expression of GLUT4 by targeting KLF15 and is involved in metabolic control in cardiac myocytes. Biochem. Biophys. Res. Commun. 2009;389:315–320. doi: 10.1016/j.bbrc.2009.08.136. [DOI] [PubMed] [Google Scholar]
- [37].Koh HJ, Toyoda T, Fujii N, Jung MM, Rathod A, Middelbeek RJ, Lessard SJ, Treebak JT, Tsuchihara K, Esumi H, Richter EA, Wojtaszewski JF, Hirshman MF, Goodyear LJ. Sucrose nonfermenting AMPK-related kinase (SNARK) mediates contraction-stimulated glucose transport in mouse skeletal muscle. Proc. Natl. Acad. Sci. U. S. A. 2010;107:15541–15546. doi: 10.1073/pnas.1008131107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Davalos A, Goedeke L, Smibert P, Ramirez CM, Warrier NP, Andreo U, Cirera-Salinas D, Rayner K, Suresh U, Pastor-Pareja JC, Esplugues E, Fisher EA, Penalva LO, Moore KJ, Suarez Y, Lai EC, Fernandez-Hernando C. miR-33a/b contribute to the regulation of fatty acid metabolism and insulin signaling. Proc. Natl. Acad. Sci. U. S. A. 2011;108:9232–9237. doi: 10.1073/pnas.1102281108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Mersey BD, Jin P, Danner DJ. Human microRNA (miR29b) expression controls the amount of branched chain alpha-ketoacid dehydrogenase complex in a cell. Hum. Mol. Genet. 2005;14:3371–3377. doi: 10.1093/hmg/ddi368. [DOI] [PubMed] [Google Scholar]
- [40].Zhang Y, Yang P, Wang XF. Microenvironmental regulation of cancer metastasis by miRNAs. Trends Cell Biol. 2014;24:153–160. doi: 10.1016/j.tcb.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ghosh G, Subramanian IV, Adhikari N, Zhang X, Joshi HP, Basi D, Chandrashekhar YS, Hall JL, Roy S, Zeng Y, Ramakrishnan S. Hypoxia-induced microRNA-424 expression in human endothelial cells regulates HIF-alpha isoforms and promotes angiogenesis. J. Clin. Invest. 2010;120:4141–4154. doi: 10.1172/JCI42980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhou B, Ma R, Si W, Li S, Xu Y, Tu X, Wang Q. MicroRNA-503 targets FGF2 and VEGFA and inhibits tumor angiogenesis and growth. Cancer Lett. 2013;333:159–169. doi: 10.1016/j.canlet.2013.01.028. [DOI] [PubMed] [Google Scholar]
- [43].Iorio MV, Croce CM. MicroRNA dysregulation in cancer: diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol. Med. 2012;4:143–159. doi: 10.1002/emmm.201100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Yanaihara N, Caplen N, Bowman E, Seike M, Kumamoto K, Yi M, Stephens RM, Okamoto A, Yokota J, Tanaka T, Calin GA, Liu CG, Croce CM, Harris CC. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9:189–198. doi: 10.1016/j.ccr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- [45].Rao X, Di Leva G, Li M, Fang F, Devlin C, Hartman-Frey C, Burow ME, Ivan M, Croce CM, Nephew KP. MicroRNA-221/222 confers breast cancer fulvestrant resistance by regulating multiple signaling pathways. Oncogene. 2011;30:1082–1097. doi: 10.1038/onc.2010.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Miller TE, Ghoshal K, Ramaswamy B, Roy S, Datta J, Shapiro CL, Jacob S, Majumder S. MicroRNA-221/222 confers tamoxifen resistance in breast cancer by targeting p27Kip1. J. Biol. Chem. 2008;283:29897–29903. doi: 10.1074/jbc.M804612200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Iorio MV, Casalini P, Piovan C, Di Leva G, Merlo A, Triulzi T, Menard S, Croce CM, Tagliabue E. microRNA-205 regulates HER3 in human breast cancer. Cancer Res. 2009;69:2195–2200. doi: 10.1158/0008-5472.CAN-08-2920. [DOI] [PubMed] [Google Scholar]
- [48].Bader AG, Brown D, Stoudemire J, Lammers P. Developing therapeutic microRNAs for cancer. Gene Ther. 2011;18:1121–1126. doi: 10.1038/gt.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Han M, Liu M, Wang Y, Chen X, Xu J, Sun Y, Zhao L, Qu H, Fan Y, Wu C. Antagonism of miR-21 reverses epithelial-mesenchymal transition and cancer stem cell phenotype through AKT/ERK1/2 inactivation by targeting PTEN. PLoS One. 2012;7:e39520. doi: 10.1371/journal.pone.0039520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Liu LZ, Li C, Chen Q, Jing Y, Carpenter R, Jiang Y, Kung HF, Lai L, Jiang BH. MiR-21 induced angiogenesis through AKT and ERK activation and HIF-1alpha expression. PLoS One. 2011;6:e19139. doi: 10.1371/journal.pone.0019139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wong MY, Yu Y, Walsh WR, Yang JL. microRNA-34 family and treatment of cancers with mutant or wild-type p53. (Review) Int. J. Oncol. 2011;38:1189–1195. doi: 10.3892/ijo.2011.970. [DOI] [PubMed] [Google Scholar]
- [52].Jansson MD, Lund AH. MicroRNA and cancer. Mol. Oncol. 2012;6:590–610. doi: 10.1016/j.molonc.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wang H, Chiu M, Xie Z, Chiu M, Liu Z, Chen P, Liu S, Byrd JC, Muthusamy N, Garzon R, Croce CM, Marcucci G, Chan KK. Synthetic microRNA cassette dosing: pharmacokinetics, tissue distribution and bioactivity. Mol. Pharm. 2012;9:1638–1644. doi: 10.1021/mp2006483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Weiler J, Hunziker J, Hall J. Anti-miRNA oligonucleotides (AMOs): ammunition to target miRNAs implicated in human disease? Gene Ther. 2006;13:496–502. doi: 10.1038/sj.gt.3302654. [DOI] [PubMed] [Google Scholar]
- [55].Montgomery RL, Hullinger TG, Semus HM, Dickinson BA, Seto AG, Lynch JM, Stack C, Latimer PA, Olson EN, van Rooij E. Therapeutic inhibition of miR-208a improves cardiac function and survival during heart failure. Circulation. 2011;124:1537–1547. doi: 10.1161/CIRCULATIONAHA.111.030932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].van Rooij E, Purcell AL, Levin AA. Developing microRNA therapeutics. Circ. Res. 2012;110:496–507. doi: 10.1161/CIRCRESAHA.111.247916. [DOI] [PubMed] [Google Scholar]
- [57].Pecot CV, Calin GA, Coleman RL, Lopez-Berestein G, Sood AK. RNA interference in the clinic: challenges and future directions. Nat. Rev. Cancer. 2011;11:59–67. doi: 10.1038/nrc2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Pereira DM, Rodrigues PM, Borralho PM, Rodrigues CM. Delivering the promise of miRNA cancer therapeutics. Drug Discov. Today. 2013;18:282–289. doi: 10.1016/j.drudis.2012.10.002. [DOI] [PubMed] [Google Scholar]
- [59].Gondi CS, Rao JS. Concepts in in vivo siRNA delivery for cancer therapy. J. Cell. Physiol. 2009;220:285–291. doi: 10.1002/jcp.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Petrocca F, Lieberman J. Promise and challenge of RNA interference-based therapy for cancer. J. Clin. Oncol. 2011;29:747–754. doi: 10.1200/JCO.2009.27.6287. [DOI] [PubMed] [Google Scholar]
- [61].Stylianopoulos T, Jain RK. Combining two strategies to improve perfusion and drug delivery in solid tumors. Proc. Natl. Acad. Sci. U. S. A. 2013;110:18632–18637. doi: 10.1073/pnas.1318415110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Li C, Li L, Keates AC. Targeting cancer gene therapy with magnetic nanoparticles. Oncotarget. 2012;3:365–370. doi: 10.18632/oncotarget.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Phillips P, Munshi HG. Grippo PJ, editor. Pancreatic stellate cells and fibrosis. Pancreatic Cancer and Tumor Microenvironment, Trivandrum (India) 2012 [Google Scholar]
- [64].Kleger A, Perkhofer L, Seufferlein T. Smarter drugs emerging in pancreatic cancer therapy. Ann. Oncol. 2014 doi: 10.1093/annonc/mdu013. http://dx.doi.org/10.1093/annonc/mdu013. [DOI] [PubMed]
- [65].Castelli DD, Terreno E, Cabella C, Chaabane L, Lanzardo S, Tei L, Visigalli M, Aime S. Evidence for in vivo macrophage mediated tumor uptake of paramagnetic/ fluorescent liposomes. NMR Biomed. 2009;22:1084–1092. doi: 10.1002/nbm.1416. [DOI] [PubMed] [Google Scholar]
- [66].Caffo M, Barresi V, Caruso G, Cutugno M, La Fata G, Venza M, Alafaci C, Tomasello F. Innovative therapeutic strategies in the treatment of brain metastases. Int. J. Mol. Sci. 2013;14:2135–2174. doi: 10.3390/ijms14012135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Raemdonck K, Vandenbroucke RE, Demeester J, Sanders NN, De Smedt SC. Maintaining the silence: reflections on long-term RNAi. Drug Discov. Today. 2008;13:917–931. doi: 10.1016/j.drudis.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Yu B, Zhao X, Lee LJ, Lee RJ. Targeted delivery systems for oligonucleotide therapeutics. AAPS J. 2009;11:195–203. doi: 10.1208/s12248-009-9096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Garzon R, Marcucci G, Croce CM. Targeting microRNAs in cancer: rationale, strategies and challenges. Nat. Rev. Drug Discov. 2010;9:775–789. doi: 10.1038/nrd3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kabilova TO, Meschaninova MI, Venyaminova AG, Nikolin VP, Zenkova MA, Vlassov VV, Chernolovskaya EL. Short double-stranded RNA with immunostimulatory activity: sequence dependence. Nucleic Acid Ther. 2012;22:196–204. doi: 10.1089/nat.2011.0328. [DOI] [PubMed] [Google Scholar]
- [71].Judge AD, Sood V, Shaw JR, Fang D, McClintock K, MacLachlan I. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat. Biotechnol. 2005;23:457–462. doi: 10.1038/nbt1081. [DOI] [PubMed] [Google Scholar]
- [72].Villanueva AI, Kulkarni RR, Sharif S. Synthetic double-stranded RNA oligonucleotides are immunostimulatory for chicken spleen cells. Dev. Comp. Immunol. 2011;35:28–34. doi: 10.1016/j.dci.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Benwell RK, Hruska JE, Fritsche KL, Lee DR. Double stranded RNA-relative to other TLR ligand-activated dendritic cells induce extremely polarized human Th1 responses. Cell. Immunol. 2010;264:119–126. doi: 10.1016/j.cellimm.2010.05.008. [DOI] [PubMed] [Google Scholar]
- [74].Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, Lovat F, Fadda P, Mao C, Nuovo GJ, Zanesi N, Crawford M, Ozer GH, Wernicke D, Alder H, Caligiuri MA, Nana-Sinkam P, Perrotti D, Croce CM. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc. Natl. Acad. Sci. U. S. A. 2012;109:E2110–E2116. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Chen X, Liang H, Zhang J, Zen K, Zhang CY. microRNAs are ligands of Toll-like receptors. RNA. 2013;19:737–739. doi: 10.1261/rna.036319.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Fabbri M, Paone A, Calore F, Galli R, Croce CM. A new role for microRNAs, as ligands of Toll-like receptors. RNA Biol. 2013;10:169–174. doi: 10.4161/rna.23144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Ceppi M, Pereira PM, Dunand-Sauthier I, Barras E, Reith W, Santos MA, Pierre P. MicroRNA-155 modulates the interleukin-1 signaling pathway in activated human monocyte-derived dendritic cells. Proc. Natl. Acad. Sci. U. S. A. 2009;106:2735–2740. doi: 10.1073/pnas.0811073106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Lu F, Weidmer A, Liu CG, Volinia S, Croce CM, Lieberman PM. Epstein–Barr virus-induced miR-155 attenuates NF-kappaB signaling and stabilizes latent virus persistence. J. Virol. 2008;82:10436–10443. doi: 10.1128/JVI.00752-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Lehmann SM, Kruger C, Park B, Derkow K, Rosenberger K, Baumgart J, Trimbuch T, Eom G, Hinz M, Kaul D, Habbel P, Kalin R, Franzoni E, Rybak A, Nguyen D, Veh R, Ninnemann O, Peters O, Nitsch R, Heppner FL, Golenbock D, Schott E, Ploegh HL, Wulczyn FG, Lehnardt S. An unconventional role for miRNA: let-7 activates Toll-like receptor 7 and causes neurodegeneration. Nat. Neurosci. 2012;15:827–835. doi: 10.1038/nn.3113. [DOI] [PubMed] [Google Scholar]
- [80].Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- [81].van Dongen S, Abreu-Goodger C, Enright AJ. Detecting microRNA binding and siRNA off-target effects from expression data. Nat. Methods. 2008;5:1023–1025. doi: 10.1038/nmeth.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Ho JJ, Metcalf JL, Yan MS, Turgeon PJ, Wang JJ, Chalsev M, Petruzziello-Pellegrini TN, Tsui AK, He JZ, Dhamko H, Man HS, Robb GB, Teh BT, Ohh M, Marsden PA. Functional importance of Dicer protein in the adaptive cellular response to hypoxia. J. Biol. Chem. 2012;287:29003–29020. doi: 10.1074/jbc.M112.373365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Sarkar S, Horn G, Moulton K, Oza A, Byler S, Kokolus S, Longacre M. Cancer development, progression, and therapy: an epigenetic overview. Int. J. Mol. Sci. 2013;14:21087–21113. doi: 10.3390/ijms141021087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Di Leva G, Croce CM. The role of microRNAs in the tumorigenesis of ovarian cancer. Front. Oncol. 2013;3:153. doi: 10.3389/fonc.2013.00153. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [85].Rao DD, Vorhies JS, Senzer N, Nemunaitis J. siRNA vs. shRNA: similarities and differences. Adv. Drug Deliv. Rev. 2009;61:746–759. doi: 10.1016/j.addr.2009.04.004. [DOI] [PubMed] [Google Scholar]
- [86].Formstecher E, Reverdy C, Cholay M, Planquette C, Trouplin V, Lehrmann H, Aresta S, Calabrese A, Arar K, Daviet L, Colland F. Combination of active and inactive siRNA targeting the mitotic kinesin Eg5 impairs silencing efficiency in several cancer cell lines. Oligonucleotides. 2006;16:387–394. doi: 10.1089/oli.2006.16.387. [DOI] [PubMed] [Google Scholar]
- [87].Loinger A, Shemla Y, Simon I, Margalit H, Biham O. Competition between small RNAs: a quantitative view. Biophys. J. 2012;102:1712–1721. doi: 10.1016/j.bpj.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Vicentini FT, Borgheti-Cardoso LN, Depieri LV, de Macedo Mano D, Abelha TF, Petrilli R, Bentley MV. Delivery systems and local administration routes for therapeutic siRNA. Pharm. Res. 2013;30:915–931. doi: 10.1007/s11095-013-0971-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Xie J, Xie Q, Zhang H, Ameres SL, Hung JH, Su Q, He R, Mu X, Seher Ahmed S, Park S, Kato H, Li C, Mueller C, Mello CC, Weng Z, Flotte TR, Zamore PD, Gao G. MicroRNA-regulated, systemically delivered rAAV9: a step closer to CNS-restricted transgene expression. Mol. Ther. 2011;19:526–535. doi: 10.1038/mt.2010.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Moller HG, Rasmussen AP, Andersen HH, Johnsen KB, Henriksen M, Duroux M. A systematic review of microRNA in glioblastoma multiforme: micro-modulators in the mesenchymal mode of migration and invasion. Mol. Neurobiol. 2013;47:131–144. doi: 10.1007/s12035-012-8349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Schneider MR. MicroRNAs as novel players in skin development, homeostasis and disease. Br. J. Dermatol. 2012;166:22–28. doi: 10.1111/j.1365-2133.2011.10568.x. [DOI] [PubMed] [Google Scholar]
- [92].Inoue T, Sugimoto M, Sakurai T, Saito R, Futaki N, Hashimoto Y, Honma Y, Arai I, Nakaike S. Modulation of scratching behavior by silencing an endogenous cyclooxygenase-1 gene in the skin through the administration of siRNA. J. Gene Med. 2007;9:994–1001. doi: 10.1002/jgm.1091. [DOI] [PubMed] [Google Scholar]
- [93].Zheng D, Giljohann DA, Chen DL, Massich MD, Wang XQ, Iordanov H, Mirkin CA, Paller AS. Topical delivery of siRNA-based spherical nucleic acid nanoparticle conjugates for gene regulation. Proc. Natl. Acad. Sci. U. S. A. 2012;109:11975–11980. doi: 10.1073/pnas.1118425109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].He XX, Chang Y, Meng FY, Wang MY, Xie QH, Tang F, Li PY, Song YH, Lin JS. MicroRNA-375 targets AEG-1 in hepatocellular carcinoma and suppresses liver cancer cell growth in vitro and in vivo. Oncogene. 2012;31:3357–3369. doi: 10.1038/onc.2011.500. [DOI] [PubMed] [Google Scholar]
- [95].Trang P, Medina PP, Wiggins JF, Ruffino L, Kelnar K, Omotola M, Homer R, Brown D, Bader AG, Weidhaas JB, Slack FJ. Regression of murine lung tumors by the let-7 microRNA. Oncogene. 2010;29:1580–1587. doi: 10.1038/onc.2009.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Ibrahim AF, Weirauch U, Thomas M, Grunweller A, Hartmann RK, Aigner A. MicroRNA replacement therapy for miR-145 and miR-33a is efficacious in a model of colon carcinoma. Cancer Res. 2011;71:5214–5224. doi: 10.1158/0008-5472.CAN-10-4645. [DOI] [PubMed] [Google Scholar]
- [97].Sureban SM, May R, Mondalek FG, Qu D, Ponnurangam S, Pantazis P, Anant S, Ramanujam RP, Houchen CW. Nanoparticle-based delivery of siDCAMKL-1 increases microRNA-144 and inhibits colorectal cancer tumor growth via a Notch-1 dependent mechanism. J. Nanobiotechnol. 2011;9:40. doi: 10.1186/1477-3155-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Lennox KA, Behlke MA. Chemical modification and design of anti-miRNA oligonucleotides. Gene Ther. 2011;18:1111–1120. doi: 10.1038/gt.2011.100. [DOI] [PubMed] [Google Scholar]
- [99].Prabhakar U, Maeda H, Jain RK, Sevick-Muraca EM, Zamboni W, Farokhzad OC, Barry ST, Gabizon A, Grodzinski P, Blakey DC. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013;73:2412–2417. doi: 10.1158/0008-5472.CAN-12-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J. Control. Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- [101].Tivnan A, Orr WS, Gubala V, Nooney R, Williams DE, McDonagh C, Prenter S, Harvey H, Domingo-Fernandez R, Bray IM, Piskareva O, Ng CY, Lode HN, Davidoff AM, Stallings RL. Inhibition of neuroblastoma tumor growth by targeted delivery of microRNA-34a using anti-disialoganglioside GD2 coated nanoparticles. PLoS One. 2012;7:e38129. doi: 10.1371/journal.pone.0038129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Chen Y, Zhu X, Zhang X, Liu B, Huang L. Nanoparticles modified with tumor-targeting scFv deliver siRNA and miRNA for cancer therapy. Mol. Ther. 2010;18:1650–1656. doi: 10.1038/mt.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Davis S, Lollo B, Freier S, Esau C. Improved targeting of miRNA with antisense oligonucleotides. Nucleic Acids Res. 2006;34:2294–2304. doi: 10.1093/nar/gkl183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438:685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- [105].Elmen J, Lindow M, Silahtaroglu A, Bak M, Christensen M, Lind-Thomsen A, Hedtjarn M, Hansen JB, Hansen HF, Straarup EM, McCullagh K, Kearney P, Kauppinen S. Antagonism of microRNA-122 in mice by systemically administered LNA-antimiR leads to up-regulation of a large set of predicted target mRNAs in the liver. Nucleic Acids Res. 2008;36:1153–1162. doi: 10.1093/nar/gkm1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Obad S, dos Santos CO, Petri A, Heidenblad M, Broom O, Ruse C, Fu C, Lindow M, Stenvang J, Straarup EM, Hansen HF, Koch T, Pappin D, Hannon GJ, Kauppinen S. Silencing of microRNA families by seed-targeting tiny LNAs. Nat. Genet. 2011;43:371–378. doi: 10.1038/ng.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Peacock H, Fucini RV, Jayalath P, Ibarra-Soza JM, Haringsma HJ, Flanagan WM, Willingham A, Beal PA. Nucleobase and ribose modifications control immunostimulation by a microRNA-122-mimetic RNA. J. Am. Chem. Soc. 2011;133:9200–9203. doi: 10.1021/ja202492e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Henry JC, Azevedo-Pouly AC, Schmittgen TD. MicroRNA replacement therapy for cancer. Pharm. Res. 2011;28:3030–3042. doi: 10.1007/s11095-011-0548-9. [DOI] [PubMed] [Google Scholar]
- [109].Akao Y, Nakagawa Y, Hirata I, Iio A, Itoh T, Kojima K, Nakashima R, Kitade Y, Naoe T. Role of anti-oncomirs miR-143 and -145 in human colorectal tumors. Cancer Gene Ther. 2010;17:398–408. doi: 10.1038/cgt.2009.88. [DOI] [PubMed] [Google Scholar]
- [110].Kasar S, Salerno E, Yuan Y, Underbayev C, Vollenweider D, Laurindo MF, Fernandes H, Bonci D, Addario A, Mazzella F, Raveche E. Systemic in vivo lentiviral delivery of miR-15a/16 reduces malignancy in the NZB de novo mouse model of chronic lymphocytic leukemia. Genes Immun. 2012;13:109–119. doi: 10.1038/gene.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Liu Y, Lai L, Chen Q, Song Y, Xu S, Ma F, Wang X, Wang J, Yu H, Cao X, Wang Q. MicroRNA-494 is required for the accumulation and functions of tumor-expanded myeloid-derived suppressor cells via targeting of PTEN. J. Immunol. 2012;188:5500–5510. doi: 10.4049/jimmunol.1103505. [DOI] [PubMed] [Google Scholar]
- [112].Kota J, Chivukula RR, O'Donnell KA, Wentzel EA, Montgomery CL, Hwang HW, Chang TC, Vivekanandan P, Torbenson M, Clark KR, Mendell JR, Mendell JT. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137:1005–1017. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL, de Gruijl TD, Wurdinger T, Middeldorp JM. Functional delivery of viral miRNAs via exosomes. Proc. Natl. Acad. Sci. U. S. A. 2010;107:6328–6333. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Zomer A, Vendrig T, Hopmans ES, van Eijndhoven M, Middeldorp JM, Pegtel DM. Exosomes: fit to deliver small RNA. Commun. Integr. Biol. 2010;3:447–450. doi: 10.4161/cib.3.5.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Koppers-Lalic D, Hogenboom MM, Middeldorp JM, Pegtel DM. Virus-modified exosomes for targeted RNA delivery; a new approach in nanomedicine. Adv. Drug Deliv. Rev. 2013;65:348–356. doi: 10.1016/j.addr.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Gardner MB, Henderson BE, Officer JE, Rongey RW, Parker JC, Oliver C, Estes JD, Huebner RJ. A spontaneous lower motor neuron disease apparently caused by indigenous type-C RNA virus in wild mice. J. Natl. Cancer Inst. 1973;51:1243–1254. doi: 10.1093/jnci/51.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Anson DS. The use of retroviral vectors for gene therapy-what are the risks? A review of retroviral pathogenesis and its relevance to retroviral vector-mediated gene delivery. Genet. Vaccines Ther. 2004;2:9. doi: 10.1186/1479-0556-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Selten G, Cuypers HT, Berns A. Proviral activation of the putative oncogene Pim-1 in MuLV induced T-cell lymphomas. EMBO J. 1985;4:1793–1798. doi: 10.1002/j.1460-2075.1985.tb03852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Ghosh R, Singh LC, Shohet JM, Gunaratne PH. A gold nanoparticle platform for the delivery of functional microRNAs into cancer cells. Biomaterials. 2013;34:807–816. doi: 10.1016/j.biomaterials.2012.10.023. [DOI] [PubMed] [Google Scholar]
- [120].Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev. 2007;59(8):718–728. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- [121].Kulkarni R, Moore E, Hegyeli A, Leonard F. Biodegradable poly (lactic acid) polymers. J. Biomed. Mater. Res. 1971;5:169–181. doi: 10.1002/jbm.820050305. [DOI] [PubMed] [Google Scholar]
- [122].Panyam J, Zhou W-Z, Prabha S, Sahoo SK, Labhasetwar V. Rapid endo-lysosomal escape of poly (DL-lactide-co-glycolide) nanoparticles: implications for drug and gene delivery. FASEB J. 2002;16:1217–1226. doi: 10.1096/fj.02-0088com. [DOI] [PubMed] [Google Scholar]
- [123].Vasir JK, Labhasetwar V. Biodegradable nanoparticles for cytosolic delivery of therapeutics. Adv. Drug Deliv. Rev. 2007;59:718–728. doi: 10.1016/j.addr.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].van Vlerken LE, Vyas TK, Amiji MM. Poly (ethylene glycol)-modified nanocarriers for tumor-targeted and intracellular delivery. Pharm. Res. 2007;24:1405–1414. doi: 10.1007/s11095-007-9284-6. [DOI] [PubMed] [Google Scholar]
- [125].Paulmurugan R, Sekar NM, Sekar TV. Biodegradable polymer nanocarriers for therapeutic antisense microRNA delivery in living animals. SPIE BiOS, International Society for Optics and Photonics. 2012:823208–823213. [Google Scholar]
- [126].Babar IA, Cheng CJ, Booth CJ, Liang X, Weidhaas JB, Saltzman WM, Slack FJ. Nanoparticle-based therapy in an in vivo microRNA-155 (miR-155)-dependent mouse model of lymphoma. Proc. Natl. Acad. Sci. 2012;109:E1695–E1704. doi: 10.1073/pnas.1201516109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Boussif O, Lezoualc'h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr J-P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl. Acad. Sci. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Park TG, Jeong JH, Kim SW. Current status of polymeric gene delivery systems. Adv. Drug Deliv. Rev. 2006;58:467–486. doi: 10.1016/j.addr.2006.03.007. [DOI] [PubMed] [Google Scholar]
- [129].Akinc A, Thomas M, Klibanov AM, Langer R. Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. J. Gene Med. 2005;7:657–663. doi: 10.1002/jgm.696. [DOI] [PubMed] [Google Scholar]
- [130].Chiou G-Y, Cherng J-Y, Hsu H-S, Wang M-L, Tsai C-M, Lu K-H, Chien Y, Hung S-C, Chen Y-W, Wong C-I. Cationic polyurethanes-short branch PEI-mediated delivery of Mir145 inhibited epithelial-mesenchymal transdifferentiation and cancer stem-like properties and in lung adenocarcinoma. J. Control. Release. 2012;159:240–250. doi: 10.1016/j.jconrel.2012.01.014. [DOI] [PubMed] [Google Scholar]
- [131].Jones CH, Chen C-K, Ravikrishnan A, Rane S, Pfeifer BA. Overcoming nonviral gene delivery barriers: perspective and future. Mol. Pharm. 2013;10(11):4082–4098. doi: 10.1021/mp400467x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Hsu SH, Yu B, Wang X, Lu Y, Schmidt CR, Lee RJ, Lee LJ, Jacob ST, Ghoshal K. Cationic lipid nanoparticles for therapeutic delivery of siRNA and miRNA to murine liver tumor. Nanomedicine. 2013;9:1169–1180. doi: 10.1016/j.nano.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Wu Y, Crawford M, Yu B, Mao Y, Nana-Sinkam SP, Lee LJ. MicroRNA delivery by cationic lipoplexes for lung cancer therapy. Mol. Pharm. 2011;8:1381–1389. doi: 10.1021/mp2002076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Wu Y, Crawford M, Mao Y, Lee RJ, Davis IC, Elton TS, Lee LJ, Nana-Sinkam SP. Therapeutic delivery of MicroRNA-29b by cationic lipoplexes for lung cancer. Mol. Ther. Nucleic Acids. 2013;2:e84. doi: 10.1038/mtna.2013.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Shi S, Han L, Gong T, Zhang Z, Sun X. Systemic delivery of microRNA-34a for cancer stem cell therapy. Angew. Chem. Int. Ed. Engl. 2013;52:3901–3905. doi: 10.1002/anie.201208077. [DOI] [PubMed] [Google Scholar]
- [136].Wiggins JF, Ruffino L, Kelnar K, Omotola M, Patrawala L, Brown D, Bader AG. Development of a lung cancer therapeutic based on the tumor suppressor microRNA-34. Cancer Res. 2010;70:5923–5930. doi: 10.1158/0008-5472.CAN-10-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Trang P, Wiggins JF, Daige CL, Cho C, Omotola M, Brown D, Weidhaas JB, Bader AG, Slack FJ. Systemic delivery of tumor suppressor microRNA mimics using a neutral lipid emulsion inhibits lung tumors in mice. Mol. Ther. 2011;19:1116–1122. doi: 10.1038/mt.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Liu X, Chen Y, Li H, Huang N, Jin Q, Ren K, Ji J. Enhanced retention and cellular uptake of nanoparticles in tumors by controlling their aggregation behavior. ACS Nano. 2013;7:6244–6257. doi: 10.1021/nn402201w. [DOI] [PubMed] [Google Scholar]
- [139].Shenoy D, Little S, Langer R, Amiji M. Poly(ethylene oxide)-modified poly(beta-amino ester) nanoparticles as a pH-sensitive system for tumor-targeted delivery of hydrophobic drugs. 1. In vitro evaluations. Mol. Pharm. 2005;2:357–366. doi: 10.1021/mp0500420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Wong C, Stylianopoulos T, Cui J, Martin J, Chauhan VP, Jiang W, Popovic Z, Jain RK, Bawendi MG, Fukumura D. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc. Natl. Acad. Sci. U. S. A. 2011;108:2426–2431. doi: 10.1073/pnas.1018382108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Mitra AK, Zillhardt M, Hua Y, Tiwari P, Murmann AE, Peter ME, Lengyel E. MicroRNAs reprogram normal fibroblasts into cancer-associated fibroblasts in ovarian cancer. Cancer Discov. 2012;2:1100–1108. doi: 10.1158/2159-8290.CD-12-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Aprelikova O, Green JE. MicroRNA regulation in cancer-associated fibroblasts. Cancer Immunol. Immunother. 2012;61:231–237. doi: 10.1007/s00262-011-1139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Chou J, Werb Z. MicroRNAs play a big role in regulating ovarian cancer-associated fibroblasts and the tumor microenvironment. Cancer Discov. 2012;2:1078–1080. doi: 10.1158/2159-8290.CD-12-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Neesse A, Frese KK, Chan DS, Bapiro TE, Howat WJ, Richards FM, Ellenrieder V, Jodrell DI, Tuveson DA. SPARC independent drug delivery and antitumour effects of nab-paclitaxel in genetically engineered mice. Gut. 2014;63(6):974–983. doi: 10.1136/gutjnl-2013-305559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Kotowski A, Ma WW. Emerging therapies in pancreas cancer. J. Gastrointest. Oncol. 2011;2:93–103. doi: 10.3978/j.issn.2078-6891.2011.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Neuzillet C, Tijeras-Raballand A, Cros J, Faivre S, Hammel P, Raymond E. Stromal expression of SPARC in pancreatic adenocarcinoma. Cancer Metastasis Rev. 2013;32:585–602. doi: 10.1007/s10555-013-9439-3. [DOI] [PubMed] [Google Scholar]
- [147].Zhang J, Miao L, Guo S, Zhang Y, Zhang L, Satterlee A, Kim WY, Huang L. Synergistic anti-tumor effects of combined gemcitabine and cisplatin nanoparticles in a stroma-rich bladder carcinoma model. J. Control. Release. 2014;182:90–96. doi: 10.1016/j.jconrel.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Auffinger B, Thaci B, Nigam P, Rincon E, Cheng Y, Lesniak MS. New therapeutic approaches for malignant glioma: in search of the Rosetta stone. F1000 Med. Rep. 2012;4:18. doi: 10.3410/M4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Forbes GM, Sturm MJ, Leong RW, Sparrow MP, Segarajasingam D, Cummins AG, Phillips M, Herrmann RP. A phase 2 study of allogeneic mesenchymal stromal cells for luminal Crohn's disease refractory to biologic therapy. Clin. Gastroenterol. Hepatol. 2014;12:64–71. doi: 10.1016/j.cgh.2013.06.021. [DOI] [PubMed] [Google Scholar]
- [150].Rameshwar P. Casting doubt on the safety of “off-the-shelf” mesenchymal stem cells for cell therapy. Mol. Ther. 2009;17:216–218. doi: 10.1038/mt.2008.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Greco SJ, Rameshwar P. Mesenchymal stem cells in drug/gene delivery: implications for cell therapy. Ther. Deliv. 2012;3:997–1004. doi: 10.4155/tde.12.69. [DOI] [PubMed] [Google Scholar]
- [152].Matuskova M, Hlubinova K, Pastorakova A, Hunakova L, Altanerova V, Altaner C, Kucerova L. HSV-tk expressing mesenchymal stem cells exert bystander effect on human glioblastoma cells. Cancer Lett. 2010;290:58–67. doi: 10.1016/j.canlet.2009.08.028. [DOI] [PubMed] [Google Scholar]
- [153].Munoz JL, Bliss SA, Greco SJ, Ramkissoon SH, Ligon KL, Rameshwar P. Delivery of functional anti-miR-9 by mesenchymal stem cell-derived exosomes to glioblastoma multiforme cells conferred chemosensitivity. Mol. Ther. Nucleic Acids. 2013;2:e126. doi: 10.1038/mtna.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]