Abstract
Elevated microglial/macrophage-associated biomarkers in the cerebrospinal fluid of infant victims of abusive head trauma (AHT) suggest that these cells play a role in the pathophysiology of the injury. In a model of AHT in 11-day-old rats, 3 impacts (24 hours apart) resulted in spatial learning and memory deficits and increased brain microglial/macrophage reactivity, traumatic axonal injury, neuronal degeneration, and cortical and white-matter atrophy. The antibiotic minocycline has been effective in decreasing injury-induced microglial/macrophage activation while simultaneously attenuating cellular and functional deficits in models of neonatal hypoxic ischemia, but the potential for this compound to rescue deficits after impact-based trauma to the immature brain remains unexplored. Acute minocycline administration in this model of AHT decreased microglial/macrophage reactivity in the corpus callosum of brain-injured animals at 3 days postinjury, but this effect was lost by 7 days postinjury. Additionally, minocycline treatment had no effect on traumatic axonal injury, neurodegeneration, tissue atrophy, or spatial learning deficits. Interestingly, minocycline-treated animals demonstrated exacerbated injury-induced spatial memory deficits. These results contrast with previous findings in other models of brain injury and suggest that minocycline is ineffective in reducing microglial/macrophage activation and ameliorating injury-induced deficits following repetitive neonatal traumatic brain injury.
Keywords: Abusive head trauma, Cognition, Inflammation, Microglia, Neurodegeneration, Pediatric.
INTRODUCTION
Inflicted injury or abuse is one of the leading causes of traumatic brain injury (TBI) in infants under 1 year of age (1). The annual incidence for abusive head trauma (AHT) in this age group is estimated to be in the range of 20 to 30 cases per every 100,000 patients (2); survivors of early-childhood TBI often develop cognitive and behavioral deficits well into adulthood (3–6). Whereas subdural hemorrhages are particularly indicative of AHT (7–9), subarachnoid hemorrhage, epidural hematomas, edema, and contusions are also prevalent in injured infant brains (10). Importantly, the presence of chronic subdural hematomas, cerebral atrophy, and ex vacuo ventriculomegaly suggests that these children incur multiple TBIs as a result of repeated instances of abuse (10–12).
The initial models of abusive head trauma utilized unrestrained shaking or rapid rotations of the head (13–17). Repetitive shaking in lambs and neonatal rodents resulted in axonal injury, glial reactivity, retinal tearing, and hemorrhage (13, 14, 17). In piglets, 2 rotational injuries exacerbated traumatic axonal injury and resulted in cognitive deficits (15, 16). With the recognition that impact is an important component of AHT (18), impact-based models have been developed. In 11-day-old rat pups, 2 impacts to the intact skull exacerbated traumatic axonal injury and astrogliosis compared to a single impact, whereas 3 impacts accelerated traumatic axonal injury and resulted in ex vacuo ventriculomegaly (19). In juvenile (35-day-old) rats, 2 impacts exacerbated axonal injury, astrocytic reactivity, and cognitive deficits (20). Collectively, these data underscore the importance of the utility of multiple animal models of AHT.
A number of studies have evaluated biomarkers in the cerebrospinal fluid of infants and children subjected to inflicted or accidental TBI (21). Compared to accidental TBI, victims of AHT have increased levels of macrophage/microglial-associated proteins and neurochemicals such as interleukins (IL) 4 and 12 and quinolinic acid (22, 23). Few studies have evaluated microglia/macrophage activation in pediatric TBI models. In a neonatal mouse model of TBI, microglial reactivity was present in the same areas as degenerating neurons (24). In contrast, the microglial/macrophage response has been extensively documented following hypoxic-ischemic brain injury in neonatal rats (25–27); importantly, the microglial/macrophage response in 9-day-old animals was increased compared with animals that were injured on postnatal day 30 (28). The neuroinflammatory cascade and microglial reactivity have, therefore, been identified as targets for therapeutic action in both adult and pediatric TBI (29, 30). During development, microglia play a crucial role in removing apoptotic cells and dysfunctional synapses, so it is not uncommon to observe activated microglia/macrophage profiles in an uninjured neonatal animal (31, 32). Limiting microglial activation in a developing injured brain needs to be evaluated carefully because it may negatively impact the normal developmental functions of microglia.
Minocycline is a broad-spectrum tetracycline antibiotic that is effective in reducing brain damage in multiple models of brain injury across the age spectrum (33, 34). It reduces microglial activation and proliferation, thereby attenuating injury-induced deficits in adult models of TBI (35–37). In rat models of neonatal hypoxic ischemia (HI), minocycline also decreased microglial activation and subsequently rescued injury-induced tissue atrophy, myelination deficits, oligodendrocyte cell death, and locomotor activity deficits (38–40). However, the efficacy of minocycline in neonatal brain injury may be limited. Thus, minocycline administration was not effective in rescuing injury-induced cell death in brains that were more severely injured (39). In a model of focal cerebral ischemia in the neonatal rat brain, minocycline was only able to decrease injury volume 24 hours after the insult but had no effect 7 days after injury, indicating the transient nature of the neuroprotection (41). In a mouse model of neonatal HI, minocycline exacerbated injury-induced tissue damage (42). It must be noted that most of these studies used a single dose; therefore, the limited efficacy may be attributed to an incorrect dose/dosing paradigm.
To date, there have been no studies specifically evaluating the efficacy of minocycline in a model of pediatric TBI. The present study sought to test the hypothesis that minocycline administration following repetitive TBI in the neonate rat will ameliorate post-traumatic cellular pathology and spatial learning deficits by reducing microglial/macrophage reactivity.
MATERIALS AND METHODS
Brain Injuries and Drug Administration
Eleven-day-old male and female Sprague-Dawley rat pups (Charles River, Wilmington, MA) (n = 43) were injured using an electrically-driven impact device (eCCI, Custom Design and Fabrication, Richmond, VA), as previously described (19). Injuries (3 impacts spaced 24 hours apart) were conducted using a metal-tip impactor centered over the exposed midline suture at a depth of 2 mm and a velocity of 5 m/s. As an analgesic, carprofen (0.1 mg/kg, Rimadyl™, Pfizer Animal Health, New York, NY) was administered subcutaneously after each impact. Immediately following the third impact, animals received a 45 mg/kg dose of minocycline hydrochloride (Sigma, St. Louis, MO) (n = 22) or PBS (phosphate-buffered saline), 0.2 ml/kg vehicle (n = 21) via an intraperitoneal injection. Animals subsequently received injections every 12 hours for 3 days for a total of 6 injections (45 mg/kg/injection or 0.2 ml/kg/injection). This dose was based on the efficacy in models of HI and stroke in neonate animals (38–41). The dosing paradigm reflected the short half-life of minocycline in rodents (2–3 hours) (43) and has been successful in reducing brain damage following trauma and/or stroke (34, 37, 44, 45). Sham-injured animals (n = 26) received incisions under anesthesia on all 3 days, followed by subcutaneous injections of Rimadyl. Sham-injured animals also received either vehicle (n = 12) or minocycline (n = 14) injections.
Tissue Collection and Preparation for Histology and Immunohistochemistry
At 3, 7, and 21 days after the third injury, separate groups of brain-injured (3 days: 5 vehicle, 5 minocycline; 7 days: 5 vehicle, 6 minocycline; 21 days: 11 vehicle, 11 minocycline) and sham-injured animals (3 days: 3 vehicle, 4 minocycline; 7 days: 3 vehicle, 4 minocycline; 21 days: 6 vehicle, 6 minocycline) were killed and the brains were processed for histology (19). Twelve sets of coronal sections (45-μm thick, 12–14 sections per set) were collected for each animal. Adjacent sets of sections were mounted on gelatin-coated slides and stained for Fluoro-Jade B (FJB) (46) or Nissl-myelin (2% Cresyl violet and 0.2% Cyanine R). Additional separate sets of sections were evaluated for microglia/macrophages by immunohistochemistry using antibodies to ionized calcium-binding adaptor molecule 1 ([Iba1], Wako, Richmond, VA, 1:20,000) or CD68 (Clone ED1, AbD Serotec, Raleigh, NC, 1:500) and traumatic axonal injury using a polyclonal antibody to the C-terminal end of amyloid precursor protein ([APP], Zymed, San Francisco, CA, 1:2,000). For anti-APP immunohistochemistry, antigen retrieval was executed by incubation with 10 mM sodium citrate (pH 6.5) in a 60ºC water bath for 20 minutes. Primary antibody binding was detected using biotinylated donkey anti-rabbit IgG (Jackson ImmunoResearch, West Grove, PA, 1:1,000 for APP and 1:500 for Iba1) or biotinylated donkey anti-mouse IgG (Jackson ImmunoResearch, 1:500). Antibody binding was visualized using the ABC Elite System with diaminobenzidine (Vector Laboratories, Burlingame, CA).
All quantifications were performed in 3 nonadjacent sections between 2 and 5 mm posterior to bregma. Quantification of pathology in the cortex and corpus callosum included the area between the cingula and extended to approximately 2 mm on either side of the cingula. Analysis in the thalamus was limited to the regions of staining (dorsolateral thalamus and lateral geniculate nucleus). FJB-positive profiles were manually counted in the cortex (18 high-power fields [HPF]) and thalamus (12 HPF) of brain-injured animals and presented as an average number of profiles per HPF. Analysis of APP-labeled sections was conducted using the grid analysis, as previously described (47). Measurements of the areas of the corpus callosum and cortex were taken from Nissl-myelin stained sections, as previously described (48). Because of the density of Iba1 and ED1 labeling, clear cellular bodies could not be distinguished to conduct reliable cell counting. For this reason, we used a thresholding approach from digitized images as described by Donnelly et al (49), and the labeled area was divided by the corresponding total area measured in the Nissl-myelin stained sections. For the thalamus, the labeled area was divided by the total area of the analyzed image. The hippocampus was analyzed between 2 and 5 mm posterior to bregma.
Spatial Learning and Memory Assessment
Spatial learning was assessed on days 7 to 10 after the third injury (n = 12 sham-injured, 11 brain-injured vehicle, 11 brain-injured minocycline), as previously described (46); the intertrial interval was 15 minutes for each rat. On day 11 postinjury, animals were tested for retention of the location of the platform (spatial memory) in 2 probe trials of 60 seconds duration each and the times spent in the zone closest to the former location of the platform and the periphery of the pool was measured. Following the probe trials, animals were subjected to a visible platform trial in which the top inch of the platform was exposed and a flag was adhered to the top of the platform. These data were analyzed in terms of latency to the platform to detect if the animals had any type of visual deficits (which have been observed following focal and diffuse trauma to the neonate animal [50, 51]).
Statistical Analysis
All data are presented as mean ± SD (standard deviation). Vehicle-treated sham-injured animals and minocycline-treated sham-injured animals showed no differences on any analyses and were, therefore, combined into 1 group. All statistics were performed using Statistica 7 (StatSoft, Tulsa, OK). Areas of corpus callosum and cortex and the percent area of Iba1 or ED1 labeling were evaluated using a factorial ANOVA with injury status (sham-injured, brain-injured vehicle-treated, and brain-injured minocycline-treated) and time after injury (3, 7, and 21 days) used for between-subject comparison. Analysis of APP and FJB labeling were compared using independent samples t tests. For analyses of the spatial learning, a repeated measures ANOVA was used to compare the latencies to the platform over 4 learning days between the 3 injury groups. A 1-way ANOVA was used to compare the times spent in the platform and peripheral zones during the probe trials and the latencies in the visible platform trial. Animals that did not find the visible platform were excluded from all statistical analyses of spatial learning, probe, and visible platform data (1/12 sham-injured, 4/11 brain-injured vehicle, 4/11 brain-injured minocycline). When appropriate, post hoc analyses were performed using the Newman-Keuls test and a value of p ≤ 0.05 was considered significant for all analyses.
RESULTS
Minocycline Does Not Affect the Extent of Iba1 Immunoreactivity
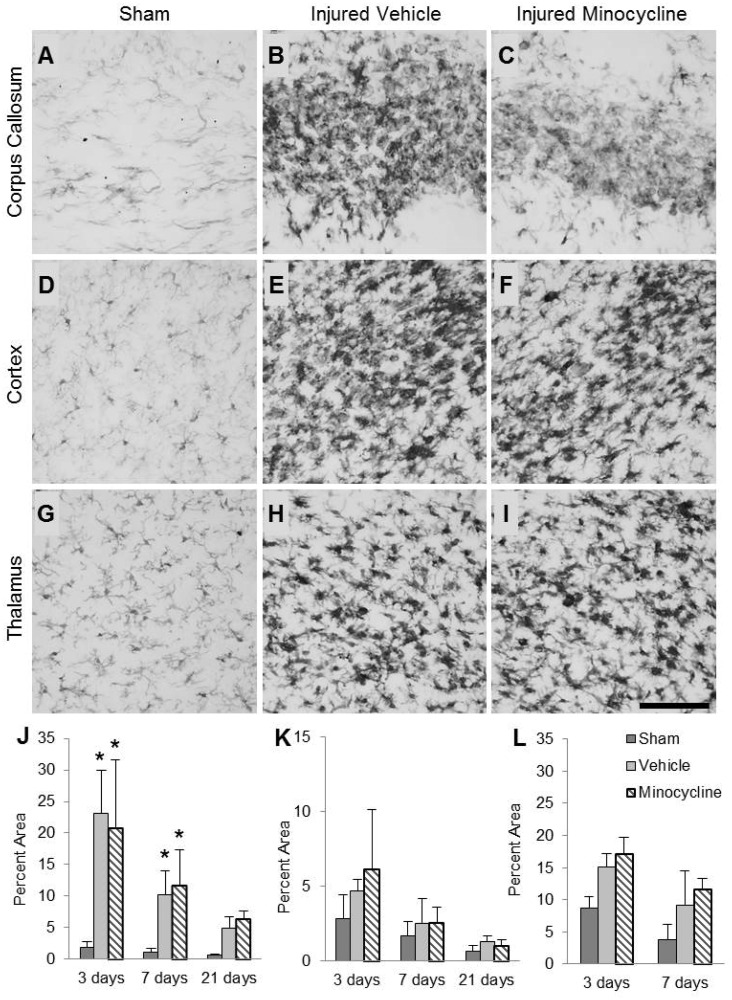
Microglia/macrophages in the corpus callosum of sham-injured animals appeared flat with elongated cell bodies and long processes indicative of a resting phenotype (Fig. 1A). Repetitive TBI resulted in activation of microglia/macrophages, as indicated by an increase in the extent of Iba1 immunoreactivity and an enlargement of the cell bodies (Fig. 1B, C). Increased Iba1 labeling was observed between 0.8 and 6 mm posterior to bregma, extended 2 mm lateral to each cingulum and was present throughout the thickness of the corpus callosum. Quantification of the area of staining revealed an injury effect (F2,42 = 34.66, p < 0.001), a time effect (F2,42 = 24.63, p < 0.001), and an interaction effect between time and injury (F4,42 = 5.36, p < 0.01). Post hoc analysis revealed that brain-injured animals had significantly increased areas of staining compared to the corresponding sham-injured groups at both 3 and 7 days, but not at 21 days (Fig. 1J; p < 0.001). Additionally, brain-injured vehicle-treated animals demonstrated decreased areas of staining at 7 (p < 0.001) and 21 days (p < 0.001) compared to 3 days postinjury, indicating that microglial activation in the white matter decreases over time. There was no effect of minocycline treatment at any time postinjury.
FIGURE 1.
Minocycline does not reduce microglia/macrophage activation induced by repeated brain trauma. (A–I) Representative photomicrographs illustrate ionized calcium-binding adaptor molecule 1 (Iba1)-labeled microglia/macrophages in the corpus callosum (A–C), cortex (D–F), and thalamus (G–I) of sham-injured (A, D, G), brain-injured vehicle-treated (B, E, H), and brain-injured minocycline-treated animals (C, F, I) at 3 days postinjury. Note the increase in Iba1 immunoreactivity and the cell density in the injured brain sections. (J–L) Quantification of the area labeled with Iba1 above threshold in the corpus callosum (J), cortex (K), and thalamus (L) expressed as a percent of the total area of the corresponding region from Nissl-myelin stained sections. *p < 0.05 compared to sham-injured values at the corresponding time point. Scale bar in (I) = 50 μm.
Microglia/macrophages in the cortex of sham-injured animals appeared rounded, but still resembled the resting phenotype with clearly visible processes (Fig. 1D). In brain-injured animals, regardless of treatment, there was a characteristic pattern of dense immunoreactivity (Fig. 1E, F), which extended from the cingulum toward the midline encompassing the agranular retrosplenial cortex, parts of the frontal cortex (rostral), and parts of the medial occipital cortex (caudal); increased immunoreactivity in the cortex was observed between 2 and 6 mm posterior to bregma. Although quantification of the area of labeling revealed an effect of injury (F2,42 = 4.25, p = 0.02), post hoc analysis did not reveal any significant differences between sham- and brain-injured groups (Fig. 1K). However, an overall effect of time postinjury was observed (F2,42 = 21.21, p < 0.001) and the post hoc analysis revealed that the area of labeling was significantly greater at 3 days compared to 7 (p < 0.001) and 21 days postinjury (p < 0.001) and at 7 days compared to 21 days following injury (p < 0.05). Minocycline treatment had no effect on Iba1 labeling in the cortex at any time postinjury.
Similar to the cortex, microglia/macrophages in the thalamus of sham-injured animals predominantly exhibited a resting phenotype (Fig. 1G). Iba1 immunoreactivity was increased in both brain-injured groups compared to the sham-injured group (Fig. 1H, I). Microglial/macrophage reactivity was restricted to lateral aspects including the laterodorsal nucleus (rostral) and parts of the lateral geniculate nucleus (caudal) and was only observed up to 7 days postinjury. Quantification revealed an effect of injury (F2,29 = 29.70, p < 0.001) and time (F1,29 = 35.25, p < 0.001, Fig. 1L). Post hoc analysis of the injury effect revealed that both groups of brain-injured animals showed significantly greater areas of thalamic Iba1 labeling vs sham-injured animals (Fig. 1L; p < 0.001). Minocycline treatment did not affect this injury-induced increase in Iba1 labeling in the thalamus. No increase in Iba1 staining was observed in the hippocampus of brain-injured animals (data not shown).
Treatment With Minocycline Decreases ED1 Labeling in the Corpus Callosum
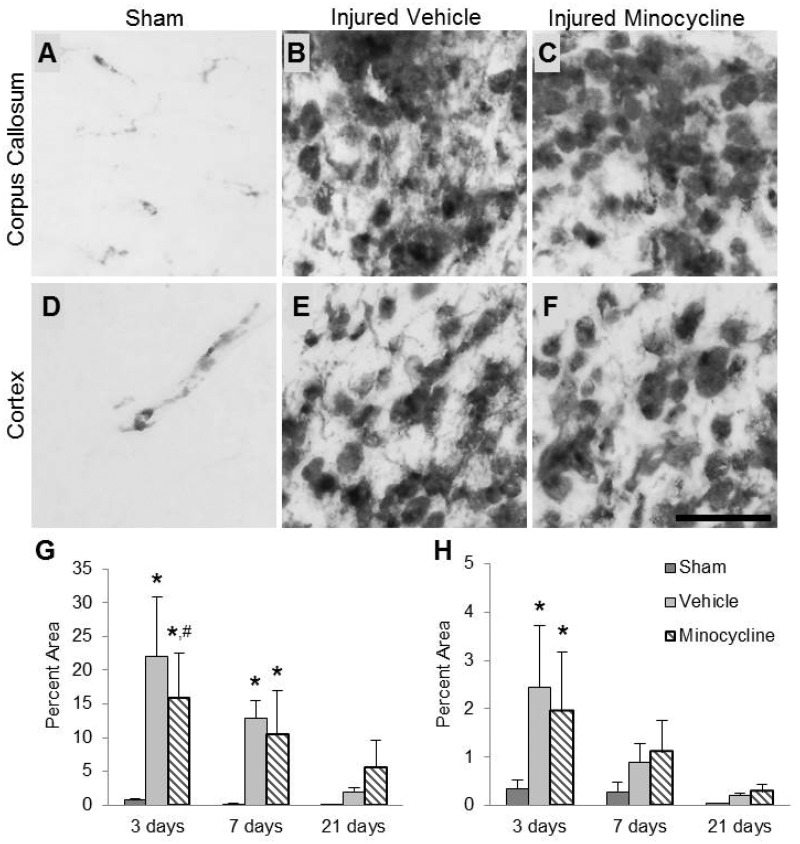
ED1 labeling was minimally observed in the corpus callosum of sham-injured animals (Fig. 2A) but was much more extensive in the brain-injured animals (Fig. 2B, C). ED1-positive cells took on a rounded amoeboid morphology with very few, if any, visible processes. The topography and pattern of ED1 immunoreactivity in this region was similar to the pattern for Iba1 staining in the corpus callosum. Quantification of the area of staining revealed an injury effect (F2,35 = 24.85, p < 0.001), a time effect (F2,35 = 17.73, p < 0.001), and an interaction effect (F4,35 = 4.98, p < 0.01) (Fig. 2G). Post hoc analysis of the injury effect revealed that the ED1 immunoreactivity was significantly increased in both brain-injured groups compared to the sham-injured group (p < 0.001). Post hoc analysis of the time effect revealed that injured animals had significantly larger areas of staining at both 3 (vehicle, p < 0.001; minocycline, p < 0.001) and 7 days (vehicle, p < 0.05; minocycline, p < 0.01) postinjury, but this effect was lost at 21 days. Interestingly, a treatment effect was observed at 3 days postinjury with minocycline-treated brain-injured animals exhibiting a mild decrease in the area of ED1 labeling compared to vehicle-treated brain-injured animals (p = 0.05).
FIGURE 2.
Minocycline reduces ED1-labeled microglia/macrophages in the corpus callosum of brain-injured animals. (A–F) Representative photomicrographs illustrate ED1-labeled microglia/macrophages in the corpus callosum (A–C) and cortex (D–F) of sham-injured (A, D), brain-injured vehicle-treated (B, E), and brain-injured minocycline-treated animals (C, F) at 3 days postinjury. Note the relative paucity of ED1-labeled cells in sham-injured brains and the marked increase in the cell size and intensity of ED1 immunoreactivity in the brain-injured animals. (G, H) Quantification of area labeled with ED1 above threshold (as described in Materials and Methods) in the corpus callosum (G) and cortex (H) expressed as percent of the total area of the corresponding region from Nissl-myelin stained sections. *p < 0.05 compared to the sham-injured values at the corresponding time point; #p = 0.05 compared to the brain-injured vehicle-treated group at the corresponding time point. Scale bar in (F) = 25 μm.
In sham-injured animals, ED1 staining was present in the cortex around what appeared to be small blood vessels (Fig. 2D). In brain-injured animals, ED1-labeled cells were rounded and had very few visible processes (Fig. 2E, F). Quantitative analysis of the area of immunoreactivity revealed an injury effect (F2,35 = 8.57, p < 0.001), a time effect (F2,35 = 16.44, p < 0.001), and an interaction effect between injury and time (F4,35 = 2.76, p < 0.05) (Fig. 2H). Post hoc analysis of the injury effect indicated that brain-injured animals, irrespective of treatment, possessed significantly larger areas of staining compared to sham-injured animals (p < 0.01). Post hoc analysis of the time effect revealed that labeling was significantly decreased at 7 (p < 0.001) and 21 days (p < 0.001) compared to 3 days postinjury, and an additional significant decrease in area stained was observed between 7 and 21 days postinjury (p < 0.05). The post hoc analysis for the interaction effect revealed that both groups of injured animals were different from the corresponding sham-injured group at 3 days postinjury (Fig. 2H; vehicle, p < 0.01; minocycline, p < 0.001), but no such intragroup differences were found at 7 or 21 days postinjury; there was no effect of treatment at any time point. In the thalamus, ED1 labeling was only present in the laterodorsal nucleus 2.0 mm posterior to bregma at 3 days postinjury (data not shown). There was no discernible ED1 labeling in the hippocampus.
Minocycline Does Not Affect Traumatic Axonal Injury
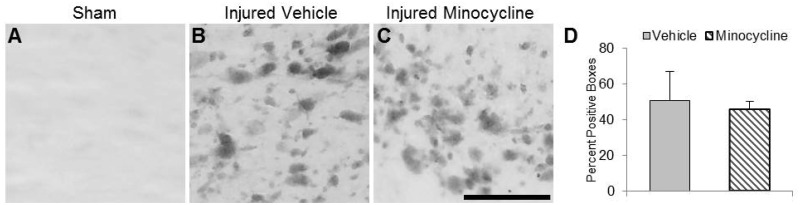
There was no observable APP labeling in sham-injured animals (Fig. 3A). Intra-axonal APP labeling in the white matter of brain-injured animals primarily appeared as terminal bulbs, suggesting that the axons had disconnected and/or retracted (Fig. 3B, C). These APP-positive profiles were present in the cingulum and dorsal portions of the corpus callosum in rostral sections (0.5–2 mm posterior to bregma) and through both dorsal and ventral portions of the fiber bundle more caudally (2–5.5 mm), indicating that APP labeling was increased under the impact site. Axonal APP labeling was present in the corpus callosum at 3 days postinjury and was not visible at 7 days postinjury. Quantitative grid analyses revealed no significant difference in the extent of APP labeling between brain-injured vehicle-treated and minocycline-treated animals (Fig. 3D). No APP-positive profiles were observed in the thalamus at any time postinjury (data not shown).
FIGURE 3.
Minocycline does not reduce the extent of traumatic axonal injury in the corpus callosum of brain-injured animals. (A–C) Representative photomicrographs illustrate intra-axonal accumulation of amyloid precursor protein (APP) in the corpus callosum of brain-injured vehicle-treated (B) and brain-injured minocycline-treated (C) animals at 3 days postinjury; note the absence of immunoreactivity in sham-injured animals (A). (D) Quantification of APP-labeled profiles using the grid analysis method. Scale bar in (C) = 25 μm.
Minocycline Does Not Affect Neurodegeneration
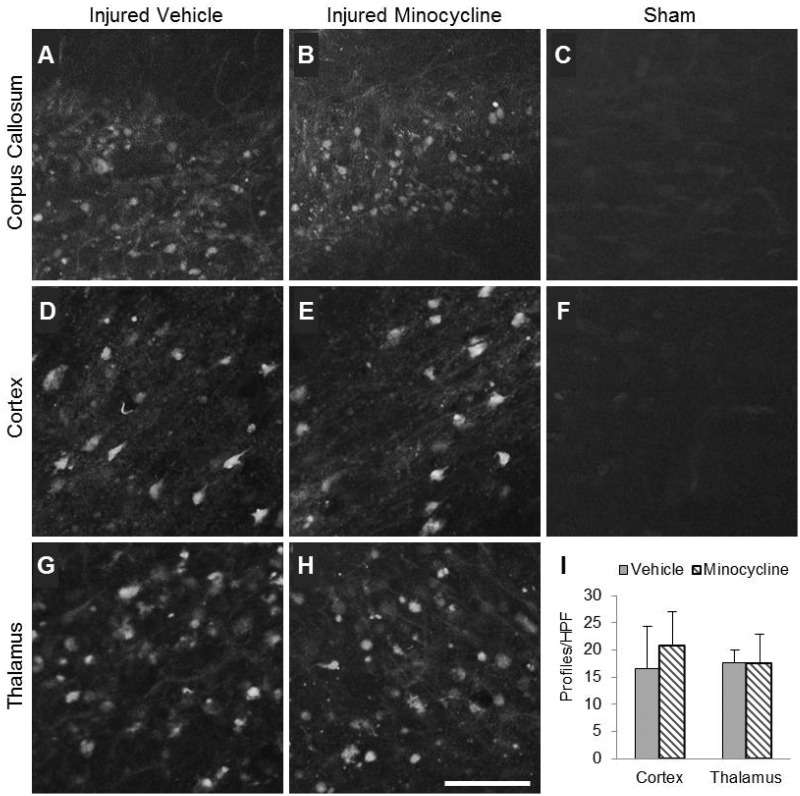
In the corpus callosum, FJB-positive profiles were punctate and diffuse, suggestive of axonal degeneration (Fig. 4A, B). Labeling in the corpus callosum was extensive at 3 days postinjury and much less at the 7 and 21 day time points (not shown). Minocycline appeared to have no effect on the extent of labeling when compared to vehicle-treated injured animals (Fig. 4A, B). FJB-positive profiles in the cortex exhibited neuronal morphology (Fig. 4D, E) and were present in the agranular retrosplenial cortex, the frontal cortex (rostral), and the occipital cortex (caudal). Labeled profiles were visible at 3 days after injury (Fig. 4D, E) but not at 7 or 21 days postinjury (data not shown). However, numbers of FJB-positive profiles in the cortex did not differ between vehicle- and minocycline-treated injured animals (Fig. 4I). FJB labeling in the injured thalamus exhibited a mix of diffuse, punctate labeling (axonal) and cellular labeling (Fig. 4G, H). FJB-positive profiles were present in the laterodorsal thalamus (rostral) and the lateral geniculate nucleus (caudal) up to 21 days. Treatment with minocycline did not affect the number of FJB-positive profiles at 3 days postinjury (Fig. 4I) or at later times (data not shown). Labeled profiles were not present in the hippocampus (data not shown). No FJB-positive profiles were visible in any region of the sham-injured brain (Fig. 4C, F).
FIGURE 4.
Minocycline does not reduce the extent of Fluoro-Jade B (FJB) labeling in brain-injured animals. (A–H) Representative photomicrographs illustrate Fluoro-Jade B labeling in the corpus callosum (A–C), cortex (D–F), and thalamus (G, H) of brain-injured vehicle-treated (A, D, G), brain-injured minocycline-treated (B, E, H), and sham-injured animals (C, F) at 3 days postinjury. Note the absence of FJB reactivity in sham-injured animals (C, F). (I) Quantification of FJB-positive profiles in each high-power field. Scale bar in (H) = 50 μm.
Minocycline Does Not Affect Tissue Loss
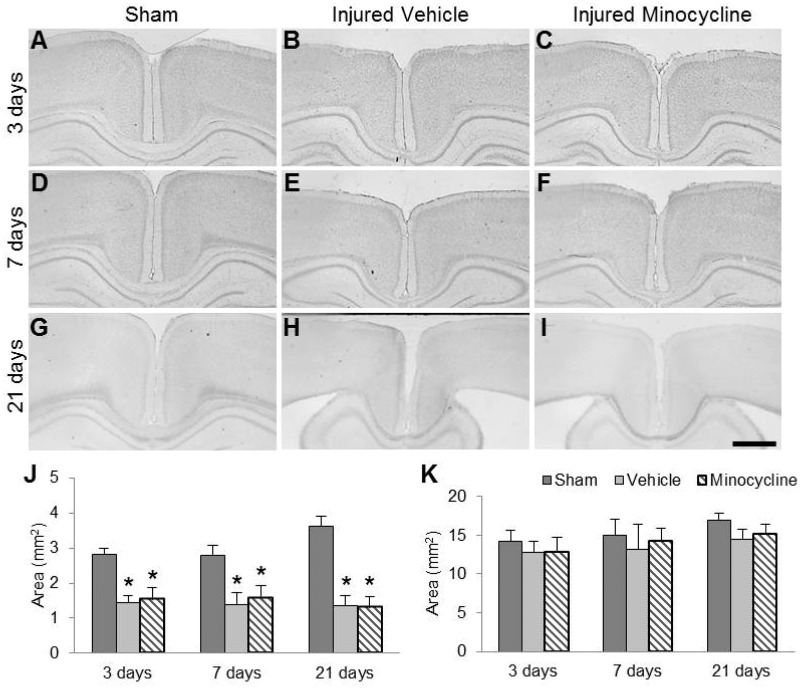
Repeated impacts to the skull of the 11-day-old rat did not result in any overt tears in the white or gray matter, and there was no evidence of lesion or cavitation (Fig. 5). Area measurements of the subcortical white-matter tracts between the 2 cingula revealed an injury effect (F2,47 = 218.04, p < 0.001); post hoc analysis indicated that brain-injured animals had significantly decreased white matter areas compared to sham-injured animals (p < 0.001). There was also an interaction effect between injury and time (F4,47 = 8.88, p < 0.01); post hoc analysis revealed that brain-injured animals within each time point had significantly decreased areas compared to their corresponding sham-injured animals (Fig. 5J; p < 0.001). There was no difference between the vehicle-treated and minocycline-treated brain-injured groups at any time point. Cortical area analysis at 3, 7, and 21 days postinjury revealed a significant effect of injury (F2,47 = 6.20, p < 0.01) and time (F2,47 = 8.22, p < 0.001) (Fig. 5K). Post hoc analysis of the injury effect revealed that brain-injured animals had significantly lower cortical areas vs sham-injured animals (vehicle, p < 0.01; minocycline, p < 0.05), but there was no difference between vehicle- and minocycline-treated brain-injured animals. Analysis of the time effect revealed that cortical area was significantly increased at 21 days compared to 3 (p < 0.01) and 7 (p < 0.05) days, which may be a consequence of developmental age.
FIGURE 5.
Minocycline does not reverse injury-induced decrease in corpus callosum area. (A–I) Representative Nissl-myelin stained sections from sham-injured (A, D, G), brain-injured vehicle-treated (B, E, H), and brain-injured minocycline-treated animals (C, F, I) at 3 days (A–C), 7 days (D–F), and 21 days postinjury (G–I). Note the appearance of enlarged ventricles at 21 days postinjury in brain-injured animals (H, I). Quantification of the area of the corpus callosum (J) and cortex (K). *p < 0.001 compared to sham-injured values at the corresponding time point. Scale bar in (I) = 1000 μm.
Minocycline Exacerbates Spatial Retention Deficits
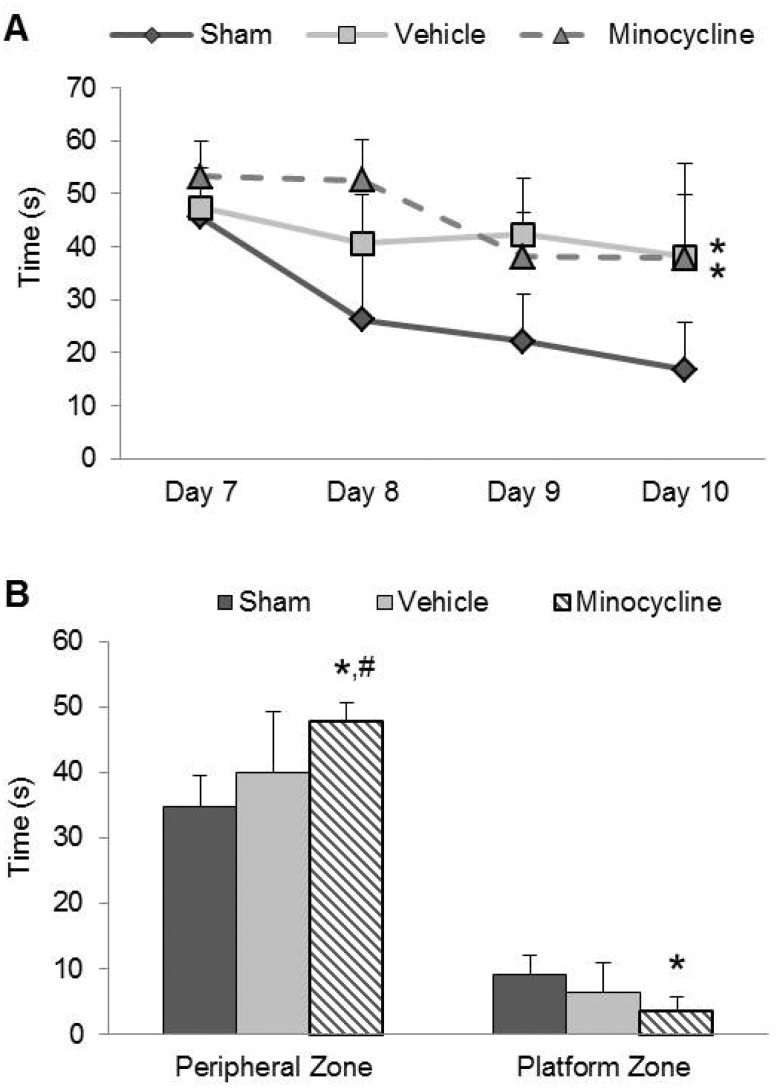
Brain-injured animals had significantly longer latencies to find the hidden platform in the spatial learning task compared to sham-injured animals in the second week following injury (Fig. 6A). Analysis of the latencies revealed an injury effect (F2,66 = 20.13, p < 0.001), a time effect (learning days) (F3,66 = 15.24, p < 0.001), and an interaction effect (F6,66 = 2.86, p < 0.05). Post hoc analysis of the injury effect revealed that both vehicle- and minocycline-treated brain-injured animals displayed increased escape latencies when compared to the latencies of sham-injured animals (p < 0.001). Post hoc analysis of the interaction effect confirmed this injury effect and revealed that the latencies of vehicle-treated brain-injured animals did not differ from the latencies of minocycline-treated brain-injured animals. In the probe trials, brain-injured animals spent significantly more time in the periphery of the maze (F2,22 = 9.83, p < 0.001) and significantly less time in the zone corresponding to the platform location (F2,22 = 6.44, p < 0.01) (Fig. 6B). Post hoc analysis revealed that there was no difference in time spent in either zone between sham-injured animals and vehicle-treated brain-injured animals. In contrast, brain-injured minocycline-treated animals spent significantly more time in the peripheral zone (p < 0.001) and less time in the platform zone (p < 0.01) than the sham-injured animals. Additionally, brain-injured minocycline-treated animals spent more time in the peripheral zone than brain-injured vehicle-treated animals (p < 0.05). During the visible platform trial, an injury effect was observed (F2,22 = 4.01, p < 003), and post hoc analysis indicated that both vehicle-treated brain-injured animals (p = 0.05) and minocycline-treated brain-injured animals (p = 0.06) had difficulty locating the exposed platform compared to the sham-injured animals, suggesting a visual deficit. Despite this deficit, brain-injured animals were able to find the hidden platform (time effect vide supra) and demonstrated searching behavior through the 4 days of learning and during the probe trial.
FIGURE 6.
Minocycline does not attenuate injury-induced spatial learning deficits but exacerbates retention deficits. (A) Latency to the platform in the spatial learning task using the Morris water maze illustrates the deficit in the time taken to reach the hidden platform in brain-injured animals. (B) Times spent in the periphery of the maze and the area surrounding the platform location during the probe trial. *p < 0.01 compared to sham-injured animals; #p < 0.05 compared to brain-injured vehicle-treated animals.
DISCUSSION
The results of the present study demonstrate that minocycline administered to neonatal rats subjected to repetitive brain trauma reduced the extent of ED1-labeled microglia/macrophages in the corpus callosum but had no effect on the extent of traumatic axonal injury or axonal degeneration. This effect was observed immediately after the cessation of minocycline treatment and did not extend to the 7- or 21-day time points. Furthermore, the extent of microglia/macrophage activation in the cortex and thalamus and the associated neurodegeneration were unaffected by minocycline treatment. Whereas injury-induced deficits in spatial learning were not reversed, minocycline treatment exacerbated the spatial retention deficits observed in brain-injured animals. Collectively, these data suggest that minocycline may not be an effective therapeutic intervention for neonatal animals in the acute period following repetitive TBI (Table).
TABLE.
Summary of Results
| Group | Iba1 | ED1 | Traumatic Axonal Injury | FJB | Tissue Loss | Spatial Learning/Memory |
|---|---|---|---|---|---|---|
| Vehicle-treated, brain-injured animals | Increased in corpus callosum, cortex, and thalamus | Increased in corpus callosum and in the cortex | APP(+) accumulation in the corpus callosum at 3 days only | Neuronal staining in the cortex, axonal staining in the corpus callosum, and both neuronal and axonal staining in the thalamus | Decreased area of corpus callosum and cortex | Deficits in acquisition and retention |
| Minocycline-treated, brain-injured animals | No difference from vehicle-treated animals | Decreased in corpus callosum at 3 days only compared with vehicle-treated animals | No difference from vehicle-treated animals | No difference from vehicle-treated animals | No difference from vehicle-treated animals | No difference from vehicle-treated animals in acquisition; exacerbated deficits in retention |
Iba1, ionized calcium-binding adapter molecule 1; ED1, rat homologue of CD68; APP, amyloid precursor protein; FJB, Fluoro-Jade B.
Minocycline has never been used in a rodent model of neonatal TBI, but systemic administration of minocycline has been effective in reducing injury-induced increases in microglial/macrophage activation in models of adult TBI and spinal cord injury (SCI) and in neonatal HI (39, 40, 44, 52–54). Using a combination of immunohistochemistry with antibodies to proteins such as Iba1, F4/80, and CD11b or lectin histochemistry and morphology (resting, intermediately-reactive, and amoeboid), minocycline administration was observed to reduce the number of activated microglia/macrophages following HI in the neonatal rat (39, 40, 52, 55, 56) or closed-head injury in the adult mouse (44, 54); interestingly, minocycline only decreased the number of activated cells and did not affect the number of intermediately reactive cells following TBI (44). Because of the extensive and robust nature of microglial activation observed in the brain-injured animals in the present study, cell counts could not be performed. Instead, an alternate approach based on a threshold of staining intensity was taken that encompasses changes in cell number, morphology (cells becoming larger), and alterations in the expression of the protein/cell-surface marker (49). Whereas this approach has been successful in determining an effect of minocycline on injury-induced microglia/macrophage activation using Iba-1 immunohistochemistry (54), the lack of an overall effect using this approach in the present study may reflect the relative insensitivity of the technique.
Alternatively, the antibody to CD68 (ED1) selectively labels activated (amoeboid) cells by binding to a glycosylated protein that is present in the membranes of phagosomes and lysosomes that are indicative of a phagocytic phenotype (57). Minocycline has been reported to reduce ED1-labeled cells in the cortex and white-matter tracts following HI in the neonatal rat (39, 58). Similarly, activated microglia/macrophages labeled with human alveolar macrophage-56 (HAM-56) were reduced following minocycline treatment following SCI in the adult rat (53). In the present study, the decrease in the area of ED1 immunoreactivity following minocycline treatment was limited to the corpus callosum and did not extend to the cortex. Similar regional differences in acute minocycline treatment after neonatal HI were observed, wherein the numbers of Iba1-labeled activated microglia/macrophages were decreased in the thalamus and the dorsal raphe but not the frontal cortex (59). Although the majority of preclinical studies indicate that acute treatment with minocycline does reduce microglia/macrophage activation, a study did demonstrate that minocycline was ineffective in reducing the number of ED1-labeled activated microglia in the cortex after stroke in the neonate rat (41).
In the current study, minocycline treatment did not affect injury-induced neurodegeneration and/or tissue atrophy in the cortex or thalamus. This might explain the visual deficit (which was not affected by minocycline treatment) observed in the repetitively brain-injured animals in the current study. Indeed, FJB-positive neurons were observed in the lateral geniculate nucleus of the thalamus and the occipital cortex, that is, areas that are directly involved in visual pathways (60). In addition, damage to the optic nerve that has been reported following repetitive TBI in adult mice (61) might also contribute to the visual deficit and warrants further investigation. In contrast, minocycline treatment has been successful in reducing lesion area in the cortex in models of adult TBI, juvenile asphyxia, and neonatal stroke (37, 38, 44, 56, 62). The success of minocycline in reducing cellular degeneration is mixed, however, with a study in a model of adult TBI reporting that the effect of minocycline was transient (44) and another wherein treatment with minocycline after neonatal HI in the mouse resulted in an increase in tissue loss (42). Minocycline has been reported to limit apoptotic cell death in animal models of several neurodegenerative diseases (63, 64), SCI (65), cardiac arrest (56), and neonatal HI (39, 40, 52), in addition to decreasing levels of activated caspase-3 (38). In contrast, minocycline was not effective in reducing the number of apoptotic cells following TBI in the adult mouse (44). In the present study, despite the attenuation of activated microglial/macrophage reactivity in the corpus callosum, minocycline had no effect on axonal injury and degeneration or white-matter tissue loss in the brain-injured animals. By contrast, in neonatal rodent HI brain injury, minocycline attenuated both microglial/macrophage reactivity and the loss of myelin and white-matter volume (39, 40, 52, 58). Following TBI in adult mice, acute administration of minocycline was associated with a reduction in microglial activation, which accompanied an attenuation of tissue loss in the corpus callosum but had no effect on trauma-induced increase in axonal injury (35, 66). Although microglial activation shares a spatiotemporal relationship with axonal injury/degeneration, ultrastructural or functional analyses do not demonstrate a clear cause-and-effect mechanism in adult rodent TBI models (67, 68).
Given the absence of any overt neuroprotective effects, it is not surprising that postinjury minocycline treatment was ineffective in reducing spatial learning and -retention deficits. Brain-injured animals, irrespective of treatment, did not learn the task efficiently and, therefore, had difficulty in the consolidation phase, as reflected in the deficit in the probe trial. In a model of central fluid percussion injury in the adult rat, spatial learning and memory deficits were observed in the absence of overt pathology in the hippocampus (69). These deficits, however, were associated with alterations in long-term potentiation (LTP) in the hippocampus (70). While there was no overt pathology present in the hippocampus, other brain regions that are thought to be involved in spatial learning and memory were affected by the injury. In the present study, brain-injured animals demonstrated neurodegeneration in the retrosplenial cortex and the laterodorsal nucleus of the thalamus; lesions in these areas result in spatial learning deficits in the Morris-water-maze task (71–73).
Moreover, the exacerbation of the retention deficit observed in the minocycline-treated animals suggests that by altering microglial-derived soluble mediators such as IL 1β, 6, and 18 and tumor necrosis factor (TNF) (44, 74–76), minocycline may compound injury-induced deficits in synaptic plasticity. It has been demonstrated that these ILs regulate maintenance of LTP (77, 78), while TNF can upregulate surface expression of AMPA (α-amino-3-hydorxy-5-methyl-4-isozazoleproprionic) receptors and strengthen LTP (79). However, it is unclear if this theory holds true in the injured brain because cytokines levels are significantly increased following injury (80). For example, pathologic increases in IL-1β can inhibit both LTP and the acquisition and retention of a spatial memory task (81, 82). Alternatively, the reduction in the number of phagocytic microglia/macrophages in the corpus callosum may have prevented the removal of cellular debris (eg, myelin fragments or apoptotic oligodendrocytes), thereby preventing white-matter repair (83, 84). In contrast to our observations in neonatal rats, minocycline treatment in adult brain-injured mice has been observed to reduce impairments in the novel object-recognition task (85). Minocycline treatment has also been effective in reducing injury-induced deficits in noncognitive behaviors such as locomotion, hyperactivity, and anxiety-like behavior following either TBI in adult mice or HI in the neonate rat (35, 37, 40).
One potential caveat to the current study is that we only used 1 dose (45 mg/kg/injection) and 1 dosing paradigm (every 12 hours for 3 days). The dose and dosing paradigms were chosen based on their efficacy and use in several models of brain injury in both adult and neonatal animals (34, 37, 44, 56, 86). Future efforts will focus on continuing the dosing to the later time points, which has demonstrated success (52). Nevertheless, the apparent lack of efficacy of minocycline in neonatal brain trauma raises the possibility that this compound may be acting on anti-inflammatory subsets of microglia/macrophages. Very few studies have investigated the effect of minocycline on microglial/macrophage polarization into M1 (pro-inflammatory) and M2 (anti-inflammatory) phenotypes. Although minocycline was successful in decreasing M1 pro-inflammatory markers while leaving M2 markers unaffected in a mouse model of amyotrophic lateral sclerosis (87), it has been reported to increase pro-inflammatory markers in animals that exhibit depressive-like behavior (88). Based on the observation that depletion of microglia at the time of injury exacerbated tissue loss following stroke (89), it is tempting to suggest that pro-inflammatory mediators released in acute post-traumatic period may be necessary for neuroprotection after neonatal TBI. Future efforts will also be directed at determining the appropriate dosing paradigm and the cellular mechanisms, such as cytokine synthesis and release and cellular activity, underlying the efficacy of minocycline as an effective intervention for repeated TBI in immature animals.
REFERENCES
- 1.Bishop NB. Traumatic brain injury: A primer for primary care physicians. Curr Probl Pediatr Adolesc Health Care 2006;36:318–31 [DOI] [PubMed] [Google Scholar]
- 2.Parks SE, Annest JL, Hill HA, et al. Pediatric Abusive Head Trauma: Recommended Definitions for Public Health Surveillance and Research. Atlanta, GA: Centers for Disease Control and Prevention, 2012 [Google Scholar]
- 3.Barlow KM, Thomson E, Johnson D, et al. Late neurologic and cognitive sequelae of inflicted traumatic brain injury in infancy. Pediatrics 2005;116:e174–85 [DOI] [PubMed] [Google Scholar]
- 4.Bonnier C, Nassogne MC, Evrard P. Outcome and prognosis of whiplash shaken infant syndrome; late consequences after a symptom-free interval. Dev Med Child Neurol 1995;37:943–56 [DOI] [PubMed] [Google Scholar]
- 5.Duhaime AC, Christian C, Moss E, et al. Long-term outcome in infants with the shaking-impact syndrome. Pediatr Neurosurg 1996;24:292–8 [DOI] [PubMed] [Google Scholar]
- 6.Ewing-Cobbs L, Prasad MR, Kramer L, et al. Late intellectual and academic outcomes following traumatic brain injury sustained during early childhood. J Neurosurg 2006;105:287–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matschke J, Voss J, Obi N, et al. Nonaccidental head injury is the most common cause of subdural bleeding in infants <1 year of age. Pediatrics 2009;124:1587–94 [DOI] [PubMed] [Google Scholar]
- 8.Myhre MC, Grogaard JB, Dyb GA, et al. Traumatic head injury in infants and toddlers. Acta Paediatr 2007;96:1159–63 [DOI] [PubMed] [Google Scholar]
- 9.Reece RM, Sege R. Childhood head injuries: Accidental or inflicted? Arch Pediatr Adolesc Med 2000;154:11–5 [PubMed] [Google Scholar]
- 10.Dias MS, Backstrom J, Falk M, et al. Serial radiography in the infant shaken impact syndrome. Pediatr Neurosurg 1998;29:77–85 [DOI] [PubMed] [Google Scholar]
- 11.Ewing-Cobbs L, Prasad M, Kramer L, et al. Acute neuroradiologic findings in young children with inflicted or noninflicted traumatic brain injury. Child Nerv Sys 2000;16:25–33; Discussion 34 [DOI] [PubMed] [Google Scholar]
- 12.Jenny C, Hymel KP, Ritzen A, et al. Analysis of missed cases of abusive head trauma. JAMA 1999;281:621–6 [DOI] [PubMed] [Google Scholar]
- 13.Bonnier C, Mesples B, Carpentier S, et al. Delayed white matter injury in a murine model of shaken baby syndrome. Brain Pathol 2002;12:320–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finnie JW, Blumbergs PC, Manavis J, et al. Neuropathological changes in a lamb model of non-accidental head injury (the shaken baby syndrome). J Clin Neurosci 2012;19:1159–64 [DOI] [PubMed] [Google Scholar]
- 15.Friess SH, Ichord RN, Ralston J, et al. Repeated traumatic brain injury affects composite cognitive function in piglets. J Neurotrauma 2009;26:1111–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raghupathi R, Mehr MF, Helfaer MA, et al. Traumatic axonal injury is exacerbated following repetitive closed head injury in the neonatal pig. J Neurotrauma 2004;21:307–16 [DOI] [PubMed] [Google Scholar]
- 17.Smith SL, Andrus PK, Gleason DD, et al. Infant rat model of the shaken baby syndrome: Preliminary characterization and evidence for the role of free radicals in cortical hemorrhaging and progressive neuronal degeneration. J Neurotrauma 1998;15:693–705 [DOI] [PubMed] [Google Scholar]
- 18.Christian CW, Block R; Committee on Child Abuse and Neglect; American Academy of Pediatrics. Abusive head trauma in infants and children. Pediatrics 2009;123:1409–11 [DOI] [PubMed] [Google Scholar]
- 19.Huh JW, Widing AG, Raghupathi R. Basic science; repetitive mild non-contusive brain trauma in immature rats exacerbates traumatic axonal injury and axonal calpain activation: A preliminary report. J Neurotrauma 2007;24:15–27 [DOI] [PubMed] [Google Scholar]
- 20.Prins ML, Hales A, Reger M, et al. Repeat traumatic brain injury in the juvenile rat is associated with increased axonal injury and cognitive impairments. Dev Neurosci 2010;32:510–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papa L, Ramia MM, Kelly JM, et al. Systematic review of clinical research on biomarkers for pediatric traumatic brain injury. J Neurotrauma 2013;30:324–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amick JE, Yandora KA, Bell MJ, et al. The Th1 versus Th2 cytokine profile in cerebrospinal fluid after severe traumatic brain injury in infants and children. Pediatr Crit Care Med 2001;2:260–4 [DOI] [PubMed] [Google Scholar]
- 23.Berger RP, Heyes MP, Wisniewski SR, et al. Assessment of the macrophage marker quinolinic acid in cerebrospinal fluid after pediatric traumatic brain injury: Insight into the timing and severity of injury in child abuse. J Neurotrauma 2004;21:1123–30 [DOI] [PubMed] [Google Scholar]
- 24.Tong W, Igarashi T, Ferriero DM, et al. Traumatic brain injury in the immature mouse brain: Characterization of regional vulnerability. Exp Neurol 2002;176:105–16 [DOI] [PubMed] [Google Scholar]
- 25.Cowell RM, Xu H, Galasso JM, et al. Hypoxic-ischemic injury induces macrophage inflammatory protein-1alpha expression in immature rat brain. Stroke 2002;33:795–801 [DOI] [PubMed] [Google Scholar]
- 26.Ivacko JA, Sun R, Silverstein FS. Hypoxic-ischemic brain injury induces an acute microglial reaction in perinatal rats. Pediatr Res 1996;39:39–47 [DOI] [PubMed] [Google Scholar]
- 27.McRae A, Gilland E, Bona E, et al. Microglia activation after neonatal hypoxic-ischemia. Brain Res Dev Brain Res 1995;84:245–52 [DOI] [PubMed] [Google Scholar]
- 28.Ferrazzano P, Chanana V, Uluc K, et al. Age-dependent microglial activation in immature brains after hypoxia- ischemia. CNS Neurol Disord Drug Targets 2013;12:338–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loane DJ, Byrnes KR. Role of microglia in neurotrauma. Neurotherapeutics 2010;7:366–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Potts MB, Koh SE, Whetstone WD, et al. Traumatic injury to the immature brain: Inflammation, oxidative injury, and iron-mediated damage as potential therapeutic targets. NeuroRx 2006;3:143–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paolicelli RC, Bolasco G, Pagani F, et al. Synaptic pruning by microglia is necessary for normal brain development. Science 2011;333:1456–8 [DOI] [PubMed] [Google Scholar]
- 32.Pont-Lezica L, Bechade C, Belarif-Cantaut Y, et al. Physiological roles of microglia during development. J Neurochem 2011;119:901–8 [DOI] [PubMed] [Google Scholar]
- 33.Elewa HF, Hilali H, Hess DC, et al. Minocycline for short-term neuroprotection. Pharmacotheraphy 2006;26:515–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Plane JM, Shen Y, Pleasure DE, et al. Prospects for minocycline neuroprotection. Arch Neurol 2010;67:1442–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Homsi S, Piaggio T, Croci N, et al. Blockade of acute microglial activation by minocycline promotes neuroprotection and reduces locomotor hyperactivity after closed head injury in mice: A twelve-week follow-up study. J Neurotrauma 2010;27:911–21 [DOI] [PubMed] [Google Scholar]
- 36.Kovesdi E, Kamnaksh A, Wingo D, et al. Acute minocycline treatment mitigates the symptoms of mild blast-induced traumatic brain injury. Front Neurol 2012;3:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanchez Mejia RO, Ona VO, Li M, et al. Minocycline reduces traumatic brain injury-mediated caspase-1 activation, tissue damage, and neurological dysfunction. Neurosurgery 2001;48:1393–9; Discussion 9-401 [DOI] [PubMed] [Google Scholar]
- 38.Arvin KL, Han BH, Du Y, et al. Minocycline markedly protects the neonatal brain against hypoxic-ischemic injury. Ann Neurol 2002;52:54–61 [DOI] [PubMed] [Google Scholar]
- 39.Cai Z, Lin S, Fan LW, et al. Minocycline alleviates hypoxic-ischemic injury to developing oligodendrocytes in the neonatal rat brain. Neuroscience 2006;137:425–35 [DOI] [PubMed] [Google Scholar]
- 40.Fan LW, Lin S, Pang Y, et al. Minocycline attenuates hypoxia-ischemia-induced neurological dysfunction and brain injury in the juvenile rat. Eur J Neurosci 2006;24:341–50 [DOI] [PubMed] [Google Scholar]
- 41.Fox C, Dingman A, Derugin N, et al. Minocycline confers early but transient protection in the immature brain following focal cerebral ischemia-reperfusion. J Cereb Blood Flow Metab 2005;25:1138–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsuji M, Wilson MA, Lange MS, et al. Minocycline worsens hypoxic-ischemic brain injury in a neonatal mouse model. Exp Neurol 2004;189:58–65 [DOI] [PubMed] [Google Scholar]
- 43.Andes D, Craig WA. Animal model pharmacokinetics and pharmacodynamics: A critical review. Int J Antimicrob Agents 2002;19:261–8 [DOI] [PubMed] [Google Scholar]
- 44.Bye N, Habgood MD, Callaway JK, et al. Transient neuroprotection by minocycline following traumatic brain injury is associated with attenuated microglial activation but no changes in cell apoptosis or neutrophil infiltration. Exp Neurol 2007;204:220–33 [DOI] [PubMed] [Google Scholar]
- 45.Xue M, Mikliaeva EI, Casha S, et al. Improving outcomes of neuroprotection by minocycline: Guides from cell culture and intracerebral hemorrhage in mice. Am J Pathol 2010;176:1193–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huh JW, Widing AG, Raghupathi R. Midline brain injury in the immature rat induces sustained cognitive deficits, bihemispheric axonal injury and neurodegeneration. Exp Neurol 2008;213:84–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DiLeonardi AM, Huh JW, Raghupathi R. Impaired axonal transport and neurofilament compaction occur in separate populations of injured axons following diffuse brain injury in the immature rat. Brain Res 2009;1263:174–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Creed JA, DiLeonardi AM, Fox DP, et al. Concussive brain trauma in the mouse results in acute cognitive deficits and sustained impairment of axonal function. J Neurotrauma 2011;28:547–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Donnelly DJ, Gensel JC, Ankeny DP, et al. An efficient and reproducible method for quantifying macrophages in different experimental models of central nervous system pathology. J Neurosci Methods 2009;181:36–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raghupathi R, Huh JW. Diffuse brain injury in the immature rat: Evidence for an age-at-injury effect on cognitive function and histopathologic damage. J Neurotrauma 2007;24:1596–608 [DOI] [PubMed] [Google Scholar]
- 51.Huh JW, Raghupathi R. Chronic cognitive deficits and long-term histopathological alterations following contusive brain injury in the immature rat. J Neurotrauma 2007;24:1460–74 [DOI] [PubMed] [Google Scholar]
- 52.Carty ML, Wixey JA, Colditz PB, et al. Post-insult minocycline treatment attenuates hypoxia-ischemia-induced neuroinflammation and white matter injury in the neonatal rat: A comparison of two different dose regimens. Int J Dev Neurosci 2008;26:477–85 [DOI] [PubMed] [Google Scholar]
- 53.Festoff BW, Ameenuddin S, Arnold PM, et al. Minocycline neuroprotects, reduces microgliosis, and inhibits caspase protease expression early after spinal cord injury. J Neurochem 2006;97:1314–26 [DOI] [PubMed] [Google Scholar]
- 54.Ng SY, Semple BD, Morganti-Kossmann MC, et al. Attenuation of microglial activation with minocycline is not associated with changes in neurogenesis after focal traumatic brain injury in adult mice. J Neurotrauma 2012;29:1410–25 [DOI] [PubMed] [Google Scholar]
- 55.Leonardo CC, Eakin AK, Ajmo JM, et al. Delayed administration of a matrix metalloproteinase inhibitor limits progressive brain injury after hypoxia-ischemia in the neonatal rat. J Neuroinflammation 2008;5:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tang M, Alexander H, Clark RS, et al. Minocycline reduces neuronal death and attenuates microglial response after pediatric asphyxial cardiac arrest. J Cereb Blood Flow Metab 2010;30:119–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Damoiseaux JG, Dopp EA, Calame W, et al. Rat macrophage lysosomal membrane antigen recognized by monoclonal antibody ED1. Immunology 1994;83:140–7 [PMC free article] [PubMed] [Google Scholar]
- 58.Lechpammer M, Manning SM, Samonte F, et al. Minocycline treatment following hypoxic/ischaemic injury attenuates white matter injury in a rodent model of periventricular leucomalacia. Neuropathol App Neurobiol 2008;34:379–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wixey JA, Reinebrant HE, Spencer SJ, et al. Efficacy of post-insult minocycline administration to alter long-term hypoxia-ischemia-induced damage to the serotonergic system in the immature rat brain. Neuroscience 2011;182:184–92 [DOI] [PubMed] [Google Scholar]
- 60.Peters A, Feldman ML. The projection of the lateral geniculate nucleus to area 17 of the rat cerebral cortex. I. General description. J Neurocytol 1976;5:63–84 [DOI] [PubMed] [Google Scholar]
- 61.Tzekov R, Quezada A, Gautier M, et al. Repetitive mild traumatic brain injury causes optic nerve and retinal damage in a mouse model. J Neuropathol Exp Neurol 2014;73:345–61 [DOI] [PubMed] [Google Scholar]
- 62.Homsi S, Federico F, Croci N, et al. Minocycline effects on cerebral edema: Relations with inflammatory and oxidative stress markers following traumatic brain injury in mice. Brain Res 2009;1291:122–32 [DOI] [PubMed] [Google Scholar]
- 63.Wang X, Zhu S, Drozda M, et al. Minocycline inhibits caspase-independent and -dependent mitochondrial cell death pathways in models of Huntington's disease. Proc Natl Acad Sci USA 2003;100:10483–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu S, Stavrovskaya IG, Drozda M, et al. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature 2002;417:74–8 [DOI] [PubMed] [Google Scholar]
- 65.Teng YD, Choi H, Onario RC, et al. Minocycline inhibits contusion-triggered mitochondrial cytochrome c release and mitigates functional deficits after spinal cord injury. Proc Natl Acad Sci USA 2004:101;3071–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Siopi E, Cho AH, Homsi S, et al. Minocycline restores sAPPalpha levels and reduces the late histopathological consequences of traumatic brain injury in mice. J Neurotrauma 2011;28:2135–43 [DOI] [PubMed] [Google Scholar]
- 67.Bennett RE, Brody DL. Acute reduction of microglia does not alter axonal injury in a mouse model of repetitive concussive traumatic brain injury. J Neurotrauma 2014;31:1647–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kelley BJ, Lifshitz J, Povlishock JT. Neuroinflammatory responses after experimental diffuse traumatic brain injury. J Neuropathol Exp Neurol 2007;66:989–1001 [DOI] [PubMed] [Google Scholar]
- 69.Lyeth BG, Jenkins LW, Hamm RJ, et al. Prolonged memory impairment in the absence of hippocampal cell death following traumatic brain injury in the rat. Brain Res 1990;526:249–58 [DOI] [PubMed] [Google Scholar]
- 70.Miyazaki S, Katayama Y, Lyeth BG, et al. Enduring suppression of hippocampal long-term potentiation following traumatic brain injury in rat. Brain Res 1992;585:335–9 [DOI] [PubMed] [Google Scholar]
- 71.Harker KT, Whishaw IQ. Impaired spatial performance in rats with retrosplenial lesions: Importance of the spatial problem and the rat strain in identifying lesion effects in a swimming pool. J Neurosci 2002;22:1155–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van Groen T, Kadish I, Wyss JM. The role of the laterodorsal nucleus of the thalamus in spatial learning and memory in the rat. Behav Brain Res 2002;136:329–37 [DOI] [PubMed] [Google Scholar]
- 73.Vann SD, Wilton K, Muir LA, et al. Testing the importance of the caudal retrosplenial cortex for spatial memory in rats. Behav Brain Res 2003;140:107–18 [DOI] [PubMed] [Google Scholar]
- 74.Henry CJ, Huang Y, Wynne A, et al. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflammation 2008;5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Krady JK, Basu A, Allen CM, et al. Minocycline reduces proinflammatory cytokine expression, microglial activation, and caspase-3 activation in a rodent model of diabetic retinopathy. Diabetes 2005;54:1559–65 [DOI] [PubMed] [Google Scholar]
- 76.Wasserman JK, Schlichter LC. Minocycline protects the blood-brain barrier and reduces edema following intracerebral hemorrhage in the rat. Exp Neurol 2007;207:227–37 [DOI] [PubMed] [Google Scholar]
- 77.del Rey A, Balschun D, Wetzel W, et al. A cytokine network involving brain-borne IL-1beta, IL-1ra, IL-18, IL-6, and TNFalpha operates during long-term potentiation and learning. Brain Behav Immun 2013;33:15–23 [DOI] [PubMed] [Google Scholar]
- 78.McAfoose J, Baune BT. Evidence for a cytokine model of cognitive function. Neurosci Biobehav Rev 2009;33:355–66 [DOI] [PubMed] [Google Scholar]
- 79.Beattie EC, Stellwagen D, Morishita W, et al. Control of synaptic strength by glial TNFalpha. Science 2002;295:2282–5 [DOI] [PubMed] [Google Scholar]
- 80.Ziebell JM, Morganti-Kossmann MC. Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurother 2010;7:22–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ross FM, Allan SM, Rothwell NJ, et al. A dual role for interleukin-1 in LTP in mouse hippocampal slices. J Neuroimmunol 2003;144:61–7 [DOI] [PubMed] [Google Scholar]
- 82.Trofimov AN, Zubareva OE, Simbirtsev AS, et al. The influence of neonatal interleukin-1beta increase on the formation of adult rats' spatial memory. Neurosci Behav Physiol 2014;44:359–64 [PubMed] [Google Scholar]
- 83.Neumann H, Takahashi K. Essential role of the microglial triggering receptor expressed on myeloid cells-2 (TREM2) for central nervous tissue immune homeostasis. J Neuroimmunol 2007;184:92–9 [DOI] [PubMed] [Google Scholar]
- 84.Takahashi K, Rochford CD, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med 2005;201:647–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Siopi E, Llufriu-Daben G, Fanucchi F, et al. Evaluation of late cognitive impairment and anxiety states following traumatic brain injury in mice: The effect of minocycline. Neurosci Lett 2012;511:110–5 [DOI] [PubMed] [Google Scholar]
- 86.Buller KM, Carty ML, Reinebrant HE, et al. Minocycline: A neuroprotective agent for hypoxic-ischemic brain injury in the neonate? J Neurosci Res 2009;87:599–608 [DOI] [PubMed] [Google Scholar]
- 87.Kobayashi K, Imagama S, Ohgomori T, et al. Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis 2013;4:e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Burke NN, Kerr DM, Moriarty O, et al. Minocycline modulates neuropathic pain behaviour and cortical M1-M2 microglial gene expression in a rat model of depression. Brain Behav Immun 2014;42:147–56 [DOI] [PubMed] [Google Scholar]
- 89.Faustino JV, Wang X, Johnson CE, et al. Microglial cells contribute to endogenous brain defenses after acute neonatal focal stroke. J Neurosci 2011;31:12992–3001 [DOI] [PMC free article] [PubMed] [Google Scholar]