Abstract
T cell-mediated inflammatory myopathies (polymyositis [PM] and inclusion body myositis [IBM]) sometimes arise in conjunction with HIV infection; however, it is not understood whether PM and IBM arising in the context of HIV (HIV-PM and HV-IBM) differ from PM and IBM arising sporadically in HIV-negative individuals (sPM and sIBM). Here, we report the largest series of T cell-mediated inflammatory myopathies from HIV-infected patients (19 biopsies from 15 subjects); 5 cases were pathologically classified as PM (HIV-PM) and 14 as IBM (HIV-IBM). As with sporadic cases, quantitative immunohistochemistry for LC3, p62, and TDP-43 showed significantly greater percentage of stained fibers (% FS) in HIV-IBM compared to HIV-PM samples; however, there was no significant difference in % FS for any of the three markers between HIV-associated and sporadic cases. Despite histologic similarities between HIV-IBM and sIBM but in concordance with prior case reports, patients with HIV-IBM were significantly younger at diagnosis than patients with sIBM; in contrast, the mean age of HIV-PM and sPM patients was not significantly different. In summary, HIV-PM and HIV-IBM are morphologically similar to sPM and sIBM; thus, it remains unclear why patients with HIV-IBM, in contrast to patients with sIBM, sometimes show clinical improvement in response to immunosuppressive therapy.
Keywords: HIV, Inclusion body myositis, LC3, p62, Polymyositis, TDP-43.
INTRODUCTION
Inclusion body myositis (IBM) and polymyositis (PM) are T-cell-mediated inflammatory myopathies that arise sporadically in most cases. Clinically, both diseases are broadly characterized by proximal muscle weakness, although IBM patients often additionally display weakness of distal muscle groups (finger extensors, wrist extensors, and ankle dorsiflexors) and can show prominent quadriceps atrophy that is more pronounced than atrophy in other proximal muscle groups. IBM typically presents in individuals older than 50 years with symptoms unresponsive to immunosuppressive therapy, whereas PM often occurs in younger patients and is almost always highly responsive to steroids. Both IBM and PM are characterized pathologically by the presence of T-cell-predominant inflammatory infiltrates. An important pathologic feature that differentiates IBM from PM is the presence of rimmed vacuoles (basophilic granular material surrounding empty-appearing spaces) and chronic myopathic changes such as endomysial fibrosis and muscle-fiber size variation (1–3). Histologically, PM lacks the rimmed vacuoles and chronic features seen in IBM.
Interestingly, both IBM and PM have been noted to occur in association with HIV infection. In 1996, Dalakas and Cupler reported 3 cases of IBM, 2 of which occurred in association with HIV and 1 in a patient with HTLV-1 (human T-cell lymphotropic virus-1) (4). The authors postulated a possible immune-related etiology and noticed that the 2 HIV-infected patients had an earlier age of onset (37 years old for both cases) and higher elevation of muscle enzymes than typical. Otherwise, these HIV-associated cases of IBM (hereafter designated HIV-IBM) were clinically identical to sporadic IBM (sIBM). Pathologically, the 2 HIV-IBM muscle biopsies appeared identical to sIBM and showed inflammation, rimmed vacuoles, and fiber-size variation, but lacked 15- to 18-nm tubulofilamentous inclusions on ultrastructural analysis (4). (While tubulofilamentous inclusions used to be required for the histopathologic diagnosis of IBM [5, 6], they are now recognized as a specific but insensitive pathologic feature of the disease [7].) Since that time, there have been 6 additional reported cases of HIV-IBM (8–10); the most comprehensive study (4 cases) demonstrated clonal expansion of viral-specific CD8-positive T cells (8).
The occurrence of PM in HIV-infected individuals (designated HIV-PM) has been more commonly reported, including 2 series of 13 to 14 patients each (11, 12). These cases appear to be clinically and pathologically similar to sporadic PM (sPM), although it was noted that a higher proportion of cases in the HIV-PM cohort showed normal or near-normal creatine kinase (CK) levels (13).
The objective of this study was to characterize the histopathology of HIV-related T-cell-mediated inflammatory myopathies, HIV-PM and HIV-IBM, and to determine whether quantitative immunohistochemistry for LC3, p62, and/or TDP-43 could be used as a diagnostic tool to differentiate either HIV-PM from HIV-IBM or to differentiate HIV-IBM or HIV-PM from their sporadic counterparts. We evaluated the largest series of biopsies of T-cell-mediated inflammatory myopathies from HIV-infected patients to date (19 biopsies from 15 patients). The retrospective and tissue-focused nature of this study necessitated a focus on the histologic features, patient demographics, and quantitative immunohistochemistry for autophagic and protein aggregation markers rather than the limited (and variable) clinical information available for these patients. By including the 4 patients with repeat biopsies separated by multiyear intervals, we provide a measure of insight in the possible progression of the disease on the PM-IBM spectrum.
MATERIALS AND METHODS
Ethics Statement
The University of California San Francisco (UCSF) Committee on Human Research (CHR) reviewed and approved the design of this study. Given a minimal potential for harm to study participants and the noninvasive nature of the research, the informed consent requirement was waived by the CHR. This report includes no individually identifiable patient information.
Patients
As with our previous study (14), we performed a computerized search of the UCSF pathology database; the search spanned the interval between 1990 and 2014 and was limited to inflammatory myopathies in patients with confirmed HIV infection who lacked diagnosis of any systemic or autoimmune disease. Candidate cases (for which archival formalin-fixed, paraffin-embedded [FFPE] tissue was available) were reviewed and classified into subgroups by a board-certified neuromuscular pathologist (M.M.), who was aware of the HIV diagnosis but was blinded to all other clinical history and previous diagnoses. Pathologic classification was made after review of all available original light-microscopy slides (hematoxylin and eosin [H&E] stain of the FFPE material and H&E, modified trichrome, ATPase [pH 9.4], reduced nicotinamide adenine dinucleotide-tetrazolium reductase [NADH-TR], succinic dehydrogenase [SDH], and cytochrome oxidase [COX] stains of the frozen material). Typically, major histocompatibility complex 1 (MHC-1) immunoperoxidase stain of the frozen material and CD3, CD20, and CD8 immunoperoxidase stains of either the FFPE or frozen material were also available and, if available, were used for classification based on the diagnostic criteria reported in our earlier study (14) and summarized in Supplementary Data Table 1. Group assignment was based solely on morphologic features; therefore, we did not match participants by age, sex, or other demographic variables.
Immunohistochemical Staining and Quantification
Immunoperoxidase staining for LC3, p62, and TDP-43 was performed on FFPE sections using previously established staining protocols (14), which were shown to yield comparable results on frozen and FFPE muscle tissue (15). In brief, mouse anti-LC3 monoclonal antibody clone 5F10 (Nanotools, Munich, Germany) and guinea pig anti-p62 polyclonal antibody (Progen Bioteknik, Heidelberg, Germany) were used at 1:100 dilutions; rabbit anti-TDP-43 polyclonal antibody (Proteintech, Chicago, IL) was used at a 1:1000 dilution. All staining was performed on an automated stainer following antigen retrieval. Quantification was performed using a bright-field light microscope as described (14), with the investigator blinded to diagnosis/group classification.
Imaging
Images were taken with a DP72 digital camera on a BX41 bright-field light microscope using cellSens Entry 1.6 software (all by Olympus Scientific Solutions, Waltham, MA) and were edited with Adobe Photoshop CS5 Version 12.1.32 (Adobe Systems, San Jose, CA).
Electron Microscopy
For electron microscopy, glutaraldehyde-fixed tissue was obtained for all cases with repeat biopsies except for biopsy 3a, for which glutaraldehyde-fixed tissue was not available. Ultrathin (80 nm) sections of the glutaraldehyde-fixed, Epon-embedded tissue were stained with 2% uranyl acetate. Sections were examined in a Tecnai G212 transmission electron microscope (FEI, Hillsboro, OR) at 80 kV, with images obtained with an Orca HR digital camera (Hamamatsu Photonics, Hamamatsu City, Japan). An average of 21 digital images were obtained and analyzed for each biopsy (range: 12–33 images).
Statistical Methods
Data were analyzed with Prism 5 statistical software (GraphPad Software, San Diego, CA). For comparison of HIV-PM and HIV-IBM cases with their sporadic counterparts, we used data from our previously published sPM and sIBM cohorts (14). All tests were 2-tailed with α = 0.05.
RESULTS
Histology
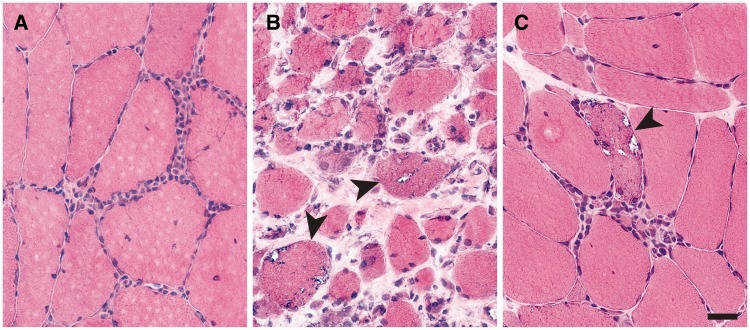
A search of the UCSF pathology database (1990–2014) for cases of T-cell-mediated inflammatory myopathies in patients with a known diagnosis of HIV yielded 19 biopsies from 15 patients (4 patients had repeat biopsies). Due to the variable amount of detail in the available clinical histories, diagnosis/group classification was based solely on morphologic criteria (14). Five biopsies showed classic sPM findings (endomysial lymphocytic infiltrates without rimmed vacuoles or chronic myopathic features, Fig. 1A) and were classified histologically as HIV-PM. Eleven biopsies demonstrated classic sIBM morphology (endomysial inflammation, rimmed vacuoles, endomysial fibrosis, and fiber-size variation, Fig. 1B) and were classified histologically as HIV-IBM. Three biopsies showed a constellation of features not observed in the previously studied sporadic cases; while lacking chronic myopathic features, they showed both well-developed endomysial inflammation and abundant rimmed vacuoles (Fig. 1C). These 3 biopsies were classified histologically as “IBM without chronic features” (Table 1; for classification criteria, see Supplementary Data Table 1) and were included as a subset of the HIV-IBM group for quantitative analyses.
FIGURE 1.
Histology of HIV-associated inflammatory myopathies. (A–C) HIV-polymyositis (HIV-PM) cases showed endomysial lymphocytic infiltrates but no rimmed vacuoles or chronic myopathic features (A, representative image from biopsy #9a). The majority of HIV-inclusion body myositis (HIV-IBM) cases demonstrated classic features of sporadic IBM including rimmed vacuoles, endomysial fibrosis, and significant fiber-size variation (B, representative image from biopsy #12). A minority (3 of 14) of HIV-IBM cases showed pathognomonic rimmed vacuoles but lacked endomysial fibrosis or prominent fiber-size variation (C, representative image from biopsy #2). Arrowheads mark fibers with rimmed vacuoles. Scale bar, 20 µm.
TABLE 1.
Patient Demographic and Clinical Dataa
| Patient ID | Sex | Biopsy ID | Age (y) | Biopsy Site | Creatine Kinase (CK) (U/L) | Available Clinical History | Histologic Classification |
|---|---|---|---|---|---|---|---|
| 1 | M | 1 | 57 | L quadriceps | ∼700 | Proximal weakness for 3 y | IBM |
| 2 | M | 2 | 67 | R biceps | 3360 | Progressive hand and proximal leg weakness | IBM w/o chronic features |
| 3 | M | 3a | 55 | L quadriceps | 494 | Proximal weakness/atrophy; responsive to steroids | IBM |
| 3b | 60 | R quadriceps | 500 | 6 months of worsening weakness (on steroids) | IBM | ||
| 4 | F | 4 | 48 | L biceps | 559 | Diffuse muscle pain, no weakness | IBM w/o chronic features |
| 5 | F | 5 | 44 | L quadriceps | 2488 | Proximal weakness and atrophy for 1 y | PM |
| 6 | M | 6 | 55 | L quadriceps | 1000–1800 | Quadriceps weakness for 1 y | IBM |
| 7 | M | 7 | 48 | R quadriceps | 1322 | Proximal weakness | IBM |
| 8 | F | 8a | 44 | R quadriceps | 1965 | Weakness, quadriceps > proximal arm | PM |
| 8b | 46 | R biceps | >1000 | No note of weakness; increasing CK | PM | ||
| 9 | M | 9a | 49 | R quadriceps | 1000–6000 | No note of weakness; on statin | PM |
| 9b | 56 | L quadriceps | NA | Progressive weakness | IBM | ||
| 10 | M | 10 | 34 | L deltoid | NA | Diffuse weakness | IBM |
| 11 | M | 11 | 44 | L quadriceps | NA | Weakness and wasting for months | IBM |
| 12 | M | 12 | 49 | R biceps | 1000–2000 | 4–5 y of increasing leg weakness | IBM |
| 13 | M | 13 | 46 | L biceps | “increased” | Proximal weakness, losing ability to walk | IBM w/o chronic features |
| 14 | M | 14a | 59 | NA | “increased” | Quadriceps weakness and atrophy | PM |
| 14b | 69 | L biceps | 300–500 | Widespread atrophy and weakness | IBM | ||
| 15 | M | 15 | 44 | R quadriceps | 1000–2900 | Proximal and finger flexor weakness for 2 y | IBM |
IBM, inclusion body myositis; F, female; L, left; M, male; NA, not available; PM, polymyositis; R, right; y, year.
Available demographic and clinical information is provided for each biopsy; for subjects with repeat biopsies, 2 sets of data (a, b) are included.
Study Participant Characteristics
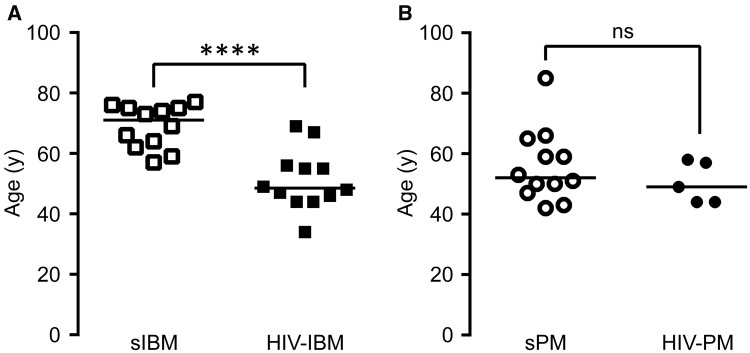
The available demographic and clinical data for all patients are shown in Table 1. Overall, available clinical information was limited. Of the 19 biopsies included in the study, 13 were consult cases for which the clinical information was restricted to the brief summaries in pathology reports. Six biopsies came from patients treated at UCSF prior to the introduction of the comprehensive electronic medical record, resulting in a large variation in the available clinical data. Eighty percent of the patients (12 of 15) were male, and 79% of the biopsies (15 of 19) were from male patients; this percentage is similar to the percentage of males (89%) among all HIV-positive cases in the state of California (1983–present), as reported by the California Department of Public Health (16). Age at diagnosis was significantly lower for patients with HIV-IBM than patients with sIBM (51.2 ± 9.9 vs 68.9 ± 7.1 years; mean ± SD; t test, p < 0.001; Fig. 2A). In contrast, the mean ages of HIV-PM and sPM patients were not significantly different (50.4 ± 3.0 vs 55.8 ± 3.5 years, p = 0.36; Fig. 2B). More than half of patients in the HIV-IBM group (7 of 12) were younger than 50 at first diagnosis; the youngest was 34 years old (patient #10 in Table 1). Plasma CK levels at the time of biopsy were available for 16 biopsies (Table 1). Given the incompleteness of the data and variations in the reporting precision, statistical analysis of this parameter was not possible; however, CK > 1000 U/L was reported in 4 of 5 patients in the HIV-PM group (for the fifth patient, the CK level was described as “increased”) and in half of the patients (4 of 8) in the HIV-IBM group.
FIGURE 2.
Age at diagnosis. (A) Patients were diagnosed with HIV-inclusion body myositis (HIV-IBM) at significantly younger ages than patients with sporadic IBM (sIBM). (B) There was no significant difference in age at diagnosis between patients with HIV-polymyositis (HIV-PM) and sporadic PM (sPM). Each biopsy is represented with a symbol; the lines designate group means. For patients with repeat biopsies, the age at initial diagnosis was used for either condition; thus, patients #9 and #14 were included in both HIV-PM and HIV-IBM groups. The data for sporadic cases were reported previously (14). ****p < 0.0001.
Immunohistochemical Staining
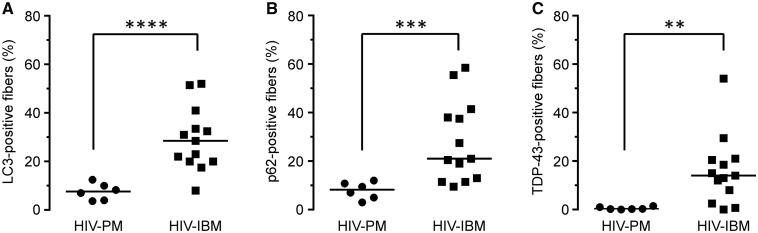
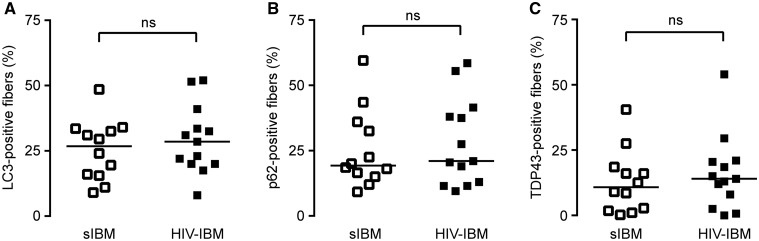
We and others have previously shown that quantitative immunohistochemistry for autophagic and protein aggregation markers LC3, p62, and TDP-43 can be used as a diagnostic aid to distinguish sIBM from sPM cases (14, 17–19). To evaluate whether this was also true for HIV-IBM and HIV-PM cases, we performed the same 3 stains on the current cohort of 19 HIV-PM/IBM muscle biopsies. Qualitatively, the pattern of staining for all 3 markers was indistinguishable between sporadic and HIV-associated cases (not shown). Paralleling our findings for sPM and sIBM, we found that the percentage of fibers staining (% FS), was significantly lower in HIV-PM than HIV-IBM for all 3 markers tested (% FSLC3: 7.6 ± 1.4 vs 29.3 ± 3.6%, p < 0.0001; % FSp62: 7.9 ± 1.4 vs 28.0 ± 4.6%, p < 0.001; % FSTDP-43: 0.5 ± 0.2 vs 16.1 ± 4.0%, p < 0.01; mean ± SEM, t test with Welch’s correction, Fig. 3). In agreement with our qualitative impression, there was no significant difference in % FS for any of the 3 markers between HIV-IBM and sIBM cohorts (Fig. 4) or between HIV-PM and sPM cohorts (Supplementary Data Fig. 1). Interestingly, the 3 cases of HIV-IBM without chronic features showed variable % FS that was at the lower end of the HIV-IBM distribution (% FSLC3: 8%, 17.5%, and 22%; % FSp62: 9.5%, 21%, and 21%; % FSTDP-43: 2.5%, 8%, and 14%).
FIGURE 3.
Quantitative immunohistochemistry for LC3, p62, and TDP-43: HIV-polymyositis (HIV-PM) to HIV-inclusion body myositis (HIV-IBM) comparison. (A–C) The percentage of (A) LC3-, (B) p62-, and (C) TDP-43-positive fibers was significantly higher in the HIV-IBM than the HIV-PM group. Each biopsy is represented with a symbol; the lines designate group means. **p < 0.01; ***p < 0.001; ****p < 0.0001.
FIGURE 4.
Quantitative immunohistochemistry for LC3, p62, and TDP-43: sporadic inclusion body myositis (sIBM) to HIV-inclusion body myositis (HIV-IBM) comparison. (A–C) There was no significant difference in the percentage of (A) LC3-, (B) p62-, and (C) TDP-43-positive fibers between patients with sIBM and HIV-IBM; the sIBM cohort data were reported previously (14). Each biopsy is represented with a symbol; the lines designate group means.
Repeat Biopsies
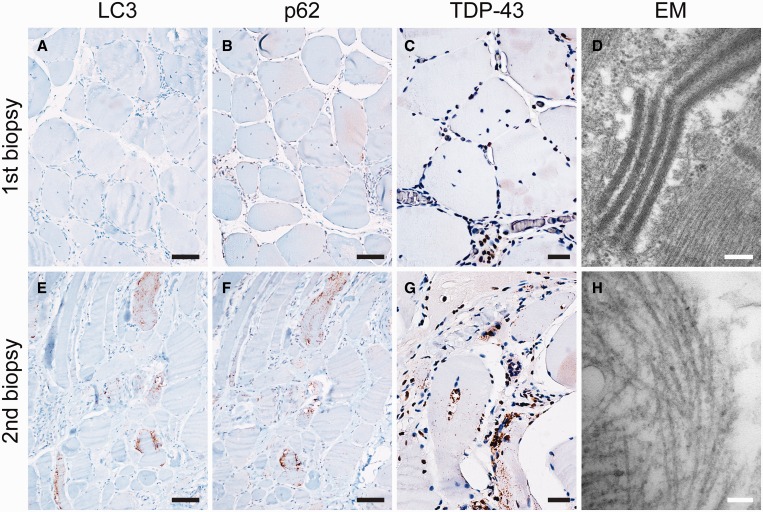
Four patients (#3, #8, #9, and #14 in Table 1) had repeat biopsies, allowing us to examine differences in pathology following a multiyear interval (Table 2). Patients #9 and #14 showed a similar and interesting pattern, with initial biopsy (at ages 49 and 59 years, respectively) diagnostic of HIV-PM and second biopsy (at ages 56 and 69 years, respectively) diagnostic of HIV-IBM. Representative images from patient #14 are shown in Figure 5. Quantitation of fibers positive for LC3, p62, and TDP-43 was in agreement with histologic findings: initial biopsies showed % FSLC3 of 7% and 4%, % FSp62 of 7% and 10%, and % FSTDP-43 of 0% and 1%, while second biopsies showed % FSLC3 of 41% and 52%, % FSp62 of 42% and 59%, and % FSTDP-43 of 30% and 54% (Table 2). A single patient (#3) had both initial biopsy (at age 55) and second biopsy (at age 60) diagnostic of HIV-IBM (Supplementary Data Fig. 2). Quantitation showed initial biopsy % FSLC3 of 31%, % FSp62 of 12%, and % FSTDP-43 of 1%, while the second biopsy showed % FSLC3 of 20%, % FSp62 of 19%, and % FSTDP-43 of 21% (Table 2). Finally, the fourth patient (#8) had repeat biopsies separated by 2 years (at ages 44 and 46 years), both of which showed features of HIV-PM (initial biopsy: % FSLC3 of 10%, % FSp62 of 5%, and % FSTDP-43 of 0.2%; the second biopsy: % FSLC3 of 4%, % FSp62 of 3%, and % FSTDP-43 of 2%).
TABLE 2.
Repeat Biopsies
| Biopsy ID | Morphologic Classification | LC3 (% FS) | p62 (% FS) | TDP-43 (% FS) | Tubular Filaments |
|---|---|---|---|---|---|
| 3a | IBM | 31 | 12 | 1 | NA |
| 3b (5 y later) | IBM | 20 | 19 | 21 | yes |
| 8a | PM | 10 | 5 | 0 | NA |
| 8b (2 y later) | PM | 4 | 3 | 2 | NA |
| 9a | PM | 7 | 7 | 0 | equivocal |
| 9b (7 y later) | IBM | 41 | 42 | 30 | yes |
| 14a | PM | 4 | 10 | 0 | no |
| 14b (10 y later) | IBM | 52 | 59 | 54 | yes |
% FS, percent fibers staining; IBM, inclusion body myositis; NA, not available; PM, polymyositis; y, years.
FIGURE 5.
Immunohistochemical and ultrastructural analysis of repeat biopsies from patient #9. (A–D) The first biopsy at age 49 years demonstrated morphologic features of polymyositis (PM); (E–H) the second biopsy (7 years later) demonstrated morphologic features of inclusion body myositis (IBM). Immunohistochemical staining showed increased staining for LC3 ([E] vs [A]), p62 ([F] vs [B]), and TDP-43 ([G] vs [C]) in the second biopsy; the percentages of fibers positive for each marker are listed in Table 2. On ultrastructural analysis, the first biopsy showed mitochondrial crystalline arrays (D) but no 15- to 18-nm tubulofilamentous inclusions; these inclusions, diagnostic for IBM, were present in the second biopsy (H). Scale bars: A, B, E, F, 50 µm; C, G, 20 µm; D, 200 nm; H, 100 nm.
Thus, 3 of 4 patients with repeat biopsies (#3, #9, and #14) had second biopsies that showed florid IBM with notably increased % FSTDP-43. We were surprised to see that 2 of these patients appeared to progress from HIV-PM to HIV-IBM and, therefore, undertook ultrastructural analysis to confirm HIV-IBM using the specific (although insensitive) IBM finding of the presence of 15- to 18-nm tubulofilamentous inclusions on electron microscopy. Due to cost constraints, we examined only biopsies from these 3 patients; no glutaraldehyde-fixed tissue was available for 1 specimen (patient #3, first biopsy). For all 3 patients, the second biopsies (diagnosed as HIV-IBM) showed clear evidence of diagnostic tubulofilamentous inclusions (Fig. 5H); in contrast, no definitive tubular filaments were present in the 2 initial biopsies evaluated by electron microscopy (Fig. 5D).
DISCUSSION
We report the largest pathologic series of T-cell-mediated inflammatory myopathies in HIV-positive patients, with a total of 19 biopsies from 15 patients. We found that all HIV-PM cases showed histologic features identical to sPM. In contrast, HIV-IBM cases divided into 2 subgroups had 11 of 14 cases showing features identical to sIBM, with 3 of 14 cases showing a novel histologic pattern characterized by the presence of frequent rimmed vacuoles but absence of chronic myopathic features (Fig. 1C). Paralleling the previously published findings for sPM and sIBM (14) and in agreement with these histologic findings, quantitative immunohistochemistry for LC3, p62, and TDP-43 showed significant differences in % FS between HIV-PM and HIV-IBM biopsies (Fig. 3). On the other hand, there was no significant difference in % FS between the HIV-associated cases and their sporadic counterparts (Fig. 4; Supplementary Data Fig. 1). Thus, this work upholds the utility of quantitative immunostaining for LC3, p62, and TDP-43 in distinguishing PM from IBM, regardless of the underlying cause.
In agreement with the impression garnered from initial case reports (4, 8–10), we found that HIV-IBM was diagnosed at a significantly younger age than sIBM (Fig. 2). In contrast, there was no difference in the age of diagnosis between HIV-PM and sPM cases, which is also consistent with the prior literature (11, 12). Why IBM occurs at a younger age in HIV-positive individuals remains an intriguing question for future research. Recent reports demonstrate that CD4-positive regulatory T cells, known to be dysregulated in HIV (20), play a critical role in suppressing muscle inflammation and injury and facilitating muscle repair (21, 22). Thus, dysregulation of regulatory T cells in HIV leading to decreased muscle repair is one possible mechanism by which HIV-IBM might arise in a younger patient population than sIBM and would be a useful direction for follow-up research.
A unique feature of the current series is the inclusion of the 4 patients with repeat biopsies (Table 2). Two of 3 patients initially diagnosed with HIV-PM showed florid IBM in the second biopsy (Fig. 5); the third patient showed features of HIV-PM in both samples, with a 2-year time interval between the 2 biopsies. The fourth patient was diagnosed with HIV-IBM on both biopsies; whereas the 2 samples showed a comparable degree of LC3 and p62-immunostaining, the second biopsy demonstrated a much higher degree of TDP-43 immunopositivity (Supplementary Data Fig. 2; Table 2). Notably, the second biopsies from all 3 patients diagnosed with HIV-IBM showed clear evidence of diagnostic tubulofilamentous inclusions, while both initial biopsies diagnosed as HIV-PM showed no tubular filaments. It is well recognized that muscle pathology can vary from site to site; these repeat biopsies were not taken from the same muscle. Despite this caveat, our findings support the notion that PM over time can progress to IBM, at least in the HIV-positive individuals; limited evidence suggests that this may also be true in some cases of sporadic IBM (23). It has been demonstrated that IBM pathology can occur in conjunction with long-standing inflammatory disorders such as systemic lupus erythematosus and systemic sclerosis; thus, the possibility that IBM pathology can occur following long-standing HIV is worth additional study (24, 25).
It would be particularly interesting to elucidate the relationship between clinical response to treatment and biopsy pathology/immunohistochemistry. Our dataset is limited by variable documentation of clinical features, but we have some information about the patients with repeat biopsies. For the 2 individuals whose first biopsy showed HIV-PM and second biopsy showed HIV-IBM, one (patient #9) reported a continuous decline in function with prominent muscle atrophy at second biopsy. The other (patient #14) showed apparently stable symptoms for many years, with a more rapid decline 6 months prior to his second biopsy. Interestingly, patient #3, initially diagnosed with HIV-IBM, was steroid-responsive after his first biopsy and remained so until approximately 6 months prior to his second biopsy; while both biopsies showed a high degree of LC3 and p62 immunopositivity, there was a marked increase in the degree of TDP-43 immunostaining in the second biopsy. These findings, though correlative, support the previously proposed hypothesis that, in the setting of inflammatory myopathies, TDP-43 is a specific but late marker of IBM (14, 26, 27). Thus, it would be very interesting to determine whether TDP-43 staining can predict steroid responsiveness in a larger cohort of patients with either HIV-related or sporadic inflammatory myopathies.
Supplementary Material
ACKNOWLEDGMENTS
We thank Mr King Chu (UCSF Brain Tumor Research Center Tissue Core) and Mr Scott Allen (UCSF Neuromuscular Pathology Laboratory) for immunohistochemistry and electron microcopy support, respectively. In addition, we are grateful to Ms Christine Lin for help with figure preparation.
REFERENCES
- 1.Dalakas MC. Sporadic inclusion body myositis–diagnosis, pathogenesis and therapeutic strategies. Nat Clin Pract Neurol 2006;2:437–47 [DOI] [PubMed] [Google Scholar]
- 2.Dalakas MC. Review: An update on inflammatory and autoimmune myopathies. Neuropathol Appl Neurobiol 2011;37:226–42 [DOI] [PubMed] [Google Scholar]
- 3.Greenberg SA. Inclusion body myositis. Curr Opin Rheumatol 2011;23:574–8 [DOI] [PubMed] [Google Scholar]
- 4.Dalakas MC, Cupler EJ. Neuropathies in HIV infection. Baillieres Clin Neurol 1996;5:199–218 [PubMed] [Google Scholar]
- 5.Griggs RC, Askanas V, DiMauro S, et al. Inclusion body myositis and myopathies. Ann Neurol 1995;38:705–13 [DOI] [PubMed] [Google Scholar]
- 6.Hilton-Jones D, Miller A, Parton M, et al. Inclusion body myositis: MRC Centre for Neuromuscular Diseases, IBM workshop, London, 13 June 2008. Neuromuscul Disord 2010;20:142–7 [DOI] [PubMed] [Google Scholar]
- 7.Lloyd TE, Mammen AL, Amato AA, et al. Evaluation and construction of diagnostic criteria for inclusion body myositis. Neurology 2014;83:426–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalakas MC, Rakocevic G, Shatunov A, et al. Inclusion body myositis with human immunodeficiency virus infection: Four cases with clonal expansion of viral-specific T cells. Ann Neurol 2007;61:466–75 [DOI] [PubMed] [Google Scholar]
- 9.Lacomis D. Neuromuscular pathology case. J Clin Neuromuscul Dis 2008;10:79–82 [DOI] [PubMed] [Google Scholar]
- 10.Freitas MR, Neves MA, Nascimento OJ, et al. Inclusion body myositis and HIV infection. Arq Neuropsiquiatr 2008;66:428–30 [DOI] [PubMed] [Google Scholar]
- 11.Johnson RW, Williams FM, Kazi S, et al. Human immunodeficiency virus-associated polymyositis: A longitudinal study of outcome. Arthritis Rheum 2003;49:172–8 [DOI] [PubMed] [Google Scholar]
- 12.Heckmann JM, Pillay K, Hearn AP, et al. Polymyositis in African HIV-infected subjects. Neuromuscul Disord 2010;20:735–9 [DOI] [PubMed] [Google Scholar]
- 13.Kenyon C, Pillay K, Heckmann JM. Beware of ‘normal’ creatine kinase levels in HIV-associated polymyositis. S Afr Med J 2010;100:156–7 [DOI] [PubMed] [Google Scholar]
- 14.Hiniker A, Daniels BH, Lee HS, et al. Comparative utility of LC3, p62 and TDP-43 immunohistochemistry in differentiation of inclusion body myositis from polymyositis and related inflammatory myopathies. Acta Neuropathol Commun 2013;1:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee HS, Daniels BH, Salas E, et al. Clinical utility of LC3 and p62 immunohistochemistry in diagnosis of drug-induced autophagic vacuolar myopathies: A case-control study. PLoS One 2012;7:e36221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.California Department of Public Health HASS. HIV/AIDS Surveillance in California. 2014. Last accessed November 17, 2015. http://www.cdph.ca.gov/data/statistics/Documents/HIVSurveillanceReport2013dxBy2014yrenddata.pdf
- 17.Weihl CC, Miller SE, Hanson PI, et al. Transgenic expression of inclusion body myopathy associated mutant p97/VCP causes weakness and ubiquitinated protein inclusions in mice. Hum Mol Genet 2007;16:919–28 [DOI] [PubMed] [Google Scholar]
- 18.Brady S, Squier W, Sewry C, et al. A retrospective cohort study identifying the principal pathological features useful in the diagnosis of inclusion body myositis. BMJ Open 2014;4:e004552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weihl CC, Pestronk A. Sporadic inclusion body myositis: Possible pathogenesis inferred from biomarkers. Curr Opin Neurol 2010;23:482–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chevalier MF, Weiss L. The split personality of regulatory T cells in HIV infection. Blood 2013;121:29–37 [DOI] [PubMed] [Google Scholar]
- 21.Burzyn D, Kuswanto W, Kolodin D, et al. A special population of regulatory T cells potentiates muscle repair. Cell 2013;155:1282–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Villalta SA, Rosenthal W, Martinez L, et al. Regulatory T cells suppress muscle inflammation and injury in muscular dystrophy. Sci Transl Med 2014;6:258ra142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amato AA, Gronseth GS, Jackson CE, et al. Inclusion body myositis: Clinical and pathological boundaries. Ann Neurol 1996;40:581–6 [DOI] [PubMed] [Google Scholar]
- 24.Vemulapalli S, Sharer LR, Hsu VM. Inclusion body myositis in a patient with RNA polymerase III antibody-positive systemic sclerosis. J Rheumatol 2015;42:730–2 [DOI] [PubMed] [Google Scholar]
- 25.Massawi G, Hickling P, Hilton D, et al. Inclusion body myositis evolving in systemic lupus erythrematosus? A case report. Rheumatology 2003;42:1012–4 [DOI] [PubMed] [Google Scholar]
- 26.Girolamo F, Lia A, Amati A, et al. Overexpression of autophagic proteins in the skeletal muscle of sporadic inclusion body myositis. Neuropathol Appl Neurobiol 2013;39:736–49 [DOI] [PubMed] [Google Scholar]
- 27.Temiz P, Weihl CC, Pestronk A. Inflammatory myopathies with mitochondrial pathology and protein aggregates. J Neurol Sci 2009;278:25–9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.