Abstract
Objectives. Neutrophil elastase (NE), a granule-associated enzyme, participates in connective tissue breakdown and promotes cytokine release and specific receptor activation during various inflammatory diseases like RA. NE is increased in the SF and cartilage of RA patients and represents a target for the development of new therapeutic possibilities. The present research aimed to evaluate the preclinical pharmacological profile of the N-benzoylpyrazole derivative EL-17, a potent and selective NE inhibitor, in a rat model of RA.
Methods. Complete Freund’s Adjuvant (CFA) was injected in the tibiotarsal joint and the effect of acute or repeated treatments with EL-17 (1–30 mg/kg by mouth) were evaluated.
Results. On day 14 after CFA injection, a single administration of EL-17 significantly reduced CFA-dependent hypersensitivity to mechanical noxious stimuli and the postural unbalance related to spontaneous pain. To evaluate the preventive efficacy, EL-17 was administered daily starting from the day of CFA treatment. Behavioural measurements performed on days 7 and 14 showed a progressive efficacy of EL-17 against hypersensitivity to mechanical noxious and non-noxious stimuli, as well as a decrease of hind limb weight-bearing alterations. Histological evaluation of the tibiotarsal joint (day 14) demonstrated significant prevention of articular derangement after EL-17 (30 mg/kg) treatment. The protective effects of EL-17 directly correlated with a complete reversion of the plasma NE activity increase induced by CFA.
Conclusions. The NE inhibitor EL-17 relieved articular pain after acute administration. Furthermore, repeated treatment reduced the development of hypersensitivity and protected joint tissue, revealing a disease-modifying profile.
Keywords: polymorphonuclear neutrophil, neutrophil serine proteases, CFA, mechanical and thermal hypersensitivity, elastase activity, joint derangement, TGF-β
Rheumatology key messages
EL-17 decreases neutrophil elastase activity in a rat RA model.
EL-17 reduces adjuvant-induced articular pain in rats.
EL-17 prevents pain progression and joint derangement related to adjuvant-induced arthritis in rats.
Introduction
PMNs play a critical role in host defence against microbial pathogens and secrete a number of proteases involved in the immune response. Nonetheless, prolonged neutrophil accumulation can damage host tissue and has been linked to chronic inflammation, which in turn is thought to contribute to the development of autoimmunity [1, 2]. Thus PMNs are a prominent feature of joint inflammation and cartilage alteration in RA, a chronic systemic inflammatory disease characterized by destructive synovitis, resulting in significant pain, deformity and disability [3].
The major components of PMN azurophilic granules are neutrophil serine proteases (NSPs), including neutrophil elastase (NE), CTG and PR3, which participate in the non-oxidative pathway of intracellular and extracellular pathogen destruction [4]. Neutrophil exposure to inflammatory stimuli evokes the release of these positively charged enzymes [5], which are fully active in a neutral environment [6]. NSPs can proteolytically modify chemokine and cytokine activity, interact with specific cell-surface receptors and potentially participate in neutrophil migration by cleaving adhesion molecules [6]. In particular, NE is able to hydrolyse important connective tissue components, such as elastin, collagens, proteoglycan, fibronectin, laminin and other extracellular matrix proteins components [7]. In the SF and cartilage of patients with RA, PMN elastase activity and expression have been shown to be increased [8, 9].
The physiological activity of NE is regulated by endogenous inhibitors, mainly members of the serine proteinase inhibitor family (serpins) [10], such as α1-protease inhibitor (α1-PI) [11] and α2-macroglobulin [12]. Moreover, endogenous low-molecular weight PIs such as the secretory leucocyte PI elafin and its biologically active precursor trappin-2 have a pivotal role in the control of NSP enzymatic activity [13]. In recent years, continued efforts to identify and optimize novel inhibitors has led to compounds characterized by promising profiles [14] for the treatment of inflammatory diseases such as RA [7, 15]. Sivelestat (ONO-5046), a competitive and selective inhibitor of NE, is currently available for the clinical treatment of acute lung injury associated with systemic inflammatory response syndrome [16]. The efficacy of sivelestat against neuropathic pain was recently described [17], highlighting the benefit of leucocyte elastase inhibitors in persistent pain [18].
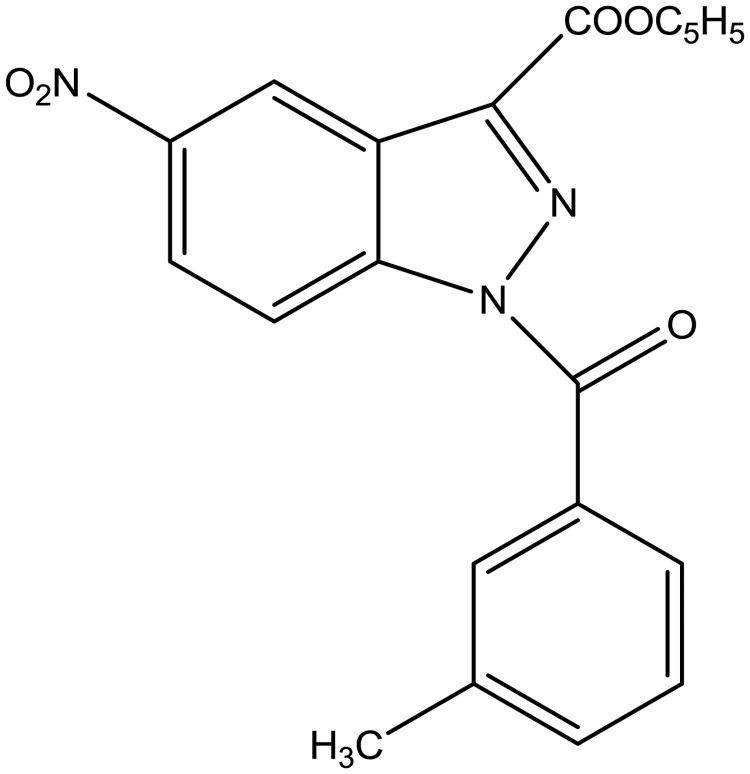
Our interest in this field led us to develop new classes of NE inhibitors with indazole and cinnoline scaffolds [19–21]. The most interesting series, featuring N-benzoylindazoles derivatives, was extensively investigated using structure–activity relationship analysis and biological profiling. Structure–activity relationship analysis clearly demonstrated that the benzoyl substituent at N-1 is essential for activity, while analysis of specificity showed that the new compounds are relatively selective for human neutrophil elastase vs other evaluated proteases. Kinetic experiments indicated that the N-benzoylindazoles act as competitive and pseudo-irreversible inhibitors, and studies of molecular modelling elucidated the effective fit of active compounds into the NE catalytic site. Of the compounds characterized, compound EL-17 (14f of Crocetti et al. [20]), which showed a good balance of potency (IC50 = 20 nM), selectivity and spontaneous hydrolysis, was selected for the present study and is represented in Fig. 1.
Fig. 1.
1-(3-methylbenzoyl)-5-nitro-1H-indazole-3-carboxylic acid ethyl ester
The purpose of the present study was to evaluate the EL-17 profile in a rat model of RA, including measurement of its efficacy against pain and cartilage tissue derangement.
Materials and methods
Animals
Sprague Dawley rats (Harlan, Varese, Italy) weighing ∼200–250 g at the beginning of the experimental procedure were used. Animals were housed in the Centro Stabulazione Animali da Laboratorio (University of Florence) and used at least 1 week after their arrival. Four rats were housed per cage (size 26 cm × 41 cm); animals were fed a standard laboratory diet and tap water ad libitum and kept at 23 ± 1 °C with a 12 h light/dark cycle (light at 7 a.m.). All animal manipulations were carried out according to the European Community guidelines for animal care [DL 116/92, application of the European Communities Council Directive of 24 November 1986 (86/609/EEC)]. The ethical policy of the University of Florence complies with the Guide for the Care and Use of Laboratory Animals of the US National Institutes of Health (NIH Publication No. 85-23, revised 1996; University of Florence assurance number A5278-01). Formal approval to conduct the experiments described was obtained from the Animal Subjects Review Board of the University of Florence. Experiments involving animals have been reported according to Animal Research: Reporting of In Vivo Experiments guidelines [22]. All efforts were made to minimize animal suffering and to reduce the number of animals used.
Complete Freund’s adjuvant-induced arthritis
Articular damage was induced by injection of complete Freund’s adjuvant (CFA; Sigma-Aldrich St Louis, MO, USA), containing 1 mg/ml of heat-killed and dried Mycobacterium tuberculosis in paraffin oil and mannide monooleate, into the tibiotarsal joint [23, 24]. Briefly, the rats were lightly anesthetized by 2% isoflurane, the left leg skin was sterilized with 75% ethyl alcohol and the lateral malleolus located by palpation. A 28-gauge needle was then inserted vertically to penetrate the skin and turned distally for insertion into the articular cavity at the gap between the tibiofibular and tarsal bone until a distinct loss of resistance was felt. A volume of 50 μl of CFA was then injected (day 0). Control rats received 50 μl of saline solution (day 0) in the tibiotarsal joint.
Administration of EL-17
EL-17 was synthesized following the procedure reported [20] and suspended in a 1% solution of carboxymethylcellulose. In the acute protocol, EL-17 (1, 10, 30 and 100 mg/kg) was administered orally once on day 14 after CFA intra-articular (i.a.) injection and the effects were evaluated over time. A second experimental protocol to evaluate the preventive efficacy of EL-17 was performed by administering the molecule orally (10 and 30 mg/kg) daily starting from the day of CFA i.a. injection. Behavioural measurements were conducted on days 7 and 14, 24 h after the last treatment with EL-17. Moreover, on day 14 a new administration of EL-17 was performed in the repeatedly treated animals to measure an eventual additive effect.
Paw-pressure test
The nociceptive threshold in the rat was determined with an analgesimeter (Ugo Basile, Varese, Italy) according to the method described by Leighton et al. [25]. Briefly, constantly increasing pressure was applied to a small area of the dorsal surface of the hind paw using a blunt conical mechanical probe. Mechanical pressure was increased until vocalization or a withdrawal reflex occurred while rats were lightly restrained. Vocalization or withdrawal reflex thresholds were expressed in grams. Rats scoring <40 g or >75 g during the test before drug administration were rejected (25%). For analgesia measures, mechanical pressure application was stopped at 120 g.
Incapacitance test
Weight-bearing changes were measured using an incapacitance apparatus (Linton Instrumentation, Norfolk, UK) to detect changes in postural equilibrium after a hind limb injury [26]. Rats were trained to stand on their hind paws in a box with an inclined plane (65° from horizontal). This box was placed above the incapacitance apparatus. This allowed us to independently measure the weight that the animal applied on each hind limb. The value reported for each animal is the mean of five consecutive measurements. In the absence of hind limb injury, rats applied an equal weight on both hind limbs, indicating postural equilibrium, whereas an unequal distribution of weight on the hind limbs indicated a monolateral decreased pain threshold. Data are expressed as the difference between the weight applied to the limb contralateral to the injury and the weight applied to the ipsilateral limb (Δweight).
Von Frey test
The animals were placed in 20 cm × 20 cm Plexiglas boxes equipped with a metallic mesh floor, 20 cm above the bench. Animals were allowed to habituate themselves to their environment for 15 min before the test. An electronic Von Frey hair unit (Ugo Basile, Varese, Italy) was used: the withdrawal threshold was evaluated by applying forces ranging from 0 to 50 g with a 0.2 g accuracy. A punctate stimulus was delivered to the mid-plantar area of each anterior paw from below the mesh floor through a plastic tip, and the withdrawal threshold was automatically displayed on the screen. The paw sensitivity threshold was defined as the minimum force required to elicit a robust and immediate withdrawal reflex of the paw. Voluntary movements associated with locomotion were not considered as a withdrawal response. Stimuli were applied to each anterior paw at 5 s intervals. Measurements were repeated five times and the final value was obtained by averaging the five measurements [27, 28].
Plantar test
The Hargreaves radiant heat method was carried out as reported by Tao et al. [29]. The rats were placed individually in clear plastic chambers of the Ugo Basile plantar test apparatus for 20 min prior to the experiment for the purpose of adaptation. Heat stimulation was applied at infrared intensity 60 (IR 60) on the plantar surface of the paw with a 30 s cut-off time. The paw withdrawal latency time was measured. Measurements were repeated up to three times and the final value was obtained by averaging the results.
Evaluation of TGF-β concentration
On day 14, venous blood was collected using heparinized syringes (Westmed, Tucson, AZ, USA). The TGF-β concentration (BioLegend, San Diego, CA, USA) was measured by ELISA using a specific anti-rat polyclonal antibody.
Dosage of NE activity
On day 14, venous blood was collected using heparinized syringes (Westmed). The elastase activity was evaluated as described by Yoshimura et al. [30] and Fujimura et al. [31]. Briefly, 180 μl of 0.1 mM elastase substrate (N-methoxysuccinyl-Ala-Ala-Pro-Valp-nitronaliline; Sigma Aldrich, Milan, Italy) solubilized in 0.1 M tris(hydroxymethyl)aminomethane l HCl buffer (pH 8.0) containing 0.5 M NaCl was incubated with 20 μl of plasma at 37 °C for 24 h. The amount of p-nitroanilide produced was measured at 405 nm and considered as the NE activity level.
Histological evaluation
Animals were sacrificed by cervical dislocation. Legs were cut under the knee, flayed and fixed in 4% formaldehyde in PBS for 48 h at room temperature. Subsequently, samples were decalcified by treatment with 0.76 M sodium formate, 1.6 M formic acid solution in H2O for 4 weeks with a change of solution every 7 days. At the end of the decalcification, these samples were dehydrated in alcohol and embedded in paraffin. Sections (6 μm thick) of the tibiotarsal joint and the phlogistic fibrous pannus located around the joint were haematoxylin and eosin stained and analysed qualitatively by two independent observers in a blind fashion.
Statistical analysis
Behavioural measurements were performed on eight rats for each treatment carried out in two different experimental sets. For elastase activity measurements, experiments were performed in triplicate on samples for rats. Results were expressed as mean (s.e.m.) with one-way analysis of variance. A Bonferroni’s significant difference procedure was used as a post hoc comparison. P-values <0.05 or <0.01 were considered significant. Data were analysed using the Origin 9 software (OriginLab, Northampton, MA, USA). For histological qualitative evaluations, two sections for each animal were analysed.
Results
Acute effect of EL-17 on CFA-induced articular pain
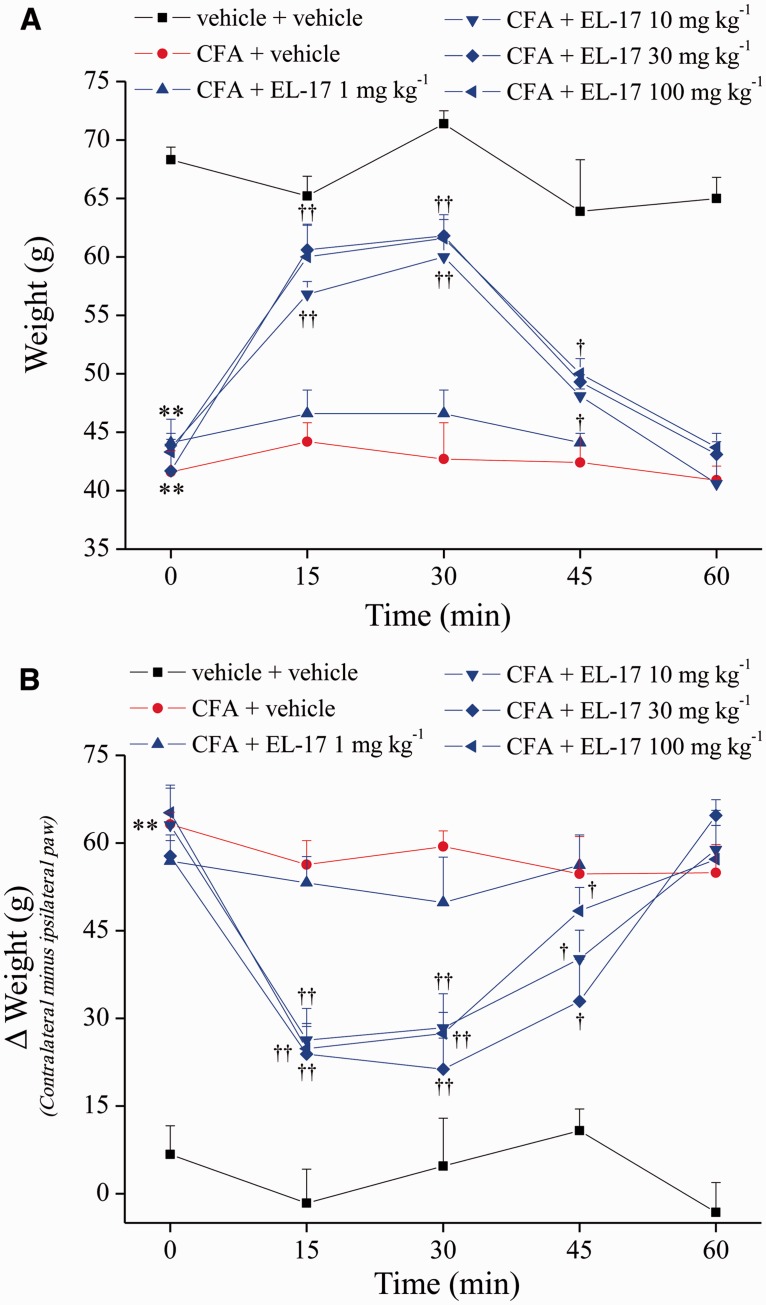
The acute effect of EL-17 was evaluated 14 days after CFA intra-articular treatment. In Fig. 2, hypersensitivity to a mechanical noxious stimulus (paw pressure test) showed that the weight tolerated on the ipsilateral paw in the CFA + vehicle group decreased to 41.6 g (s.e.m. 1.8) with respect to the control (vehicle + vehicle) value of 68.3 g (s.e.m. 1.1). EL-17 administered orally induced a significant effect starting from 10 mg/kg: the pain threshold of the ipsilateral paw increased by 60% 15 min after treatment and by 75% after 30 min. The effect disappeared at 60 min. Thirty and 100 mg/kg EL-17 were also active, showing slightly increased efficacy (Fig. 2A).
Fig. 2.
Acute effect of EL-17
(A) A paw pressure test was performed to evaluate the hypersensitivity to noxious mechanical stimuli. (B) Incapacitance test. The hind limb weight-bearing alterations were measured as postural imbalance related to pain. Data are expressed as the difference between the weight applied on the limb contralateral to the injury and the weight applied on the ipsilateral limb (Δweight). Measurements were performed on day 14 after CFA i.a. administration. EL-17 was suspended in 1% CMC and orally acutely administered and the pain threshold was evaluated over time. Control animals were treated with vehicle. The values represent the mean of eight rats performed in two different experimental sets. **P < 0.01 vs vehicle + vehicle–treated animals; †P < 0.05 and ††P < 0.01 vs the pre-test (time 0 min) of the same group. CFA: Complete Freund’s Adjuvant; CMC: carboxymethylcellulose.
The postural unbalance related to spontaneous pain was evaluated measuring hind limb weight-bearing alterations (incapacitance test). The difference between the weight loaded on the contralateral and the ipsilateral paw was significantly increased in the CFA + vehicle group [63.2 g (s.e.m. 2.1)] compared with the control group [6.7 g (s.e.m. 4.9)] (Fig. 2B). EL-17 10, 30 and 100 mg/kg similarly reduced the Δweight value over time after a single administration, peaking between 15 and 30 min after treatment (unbalance decreased by ∼60%) (Fig. 2B).
Effect of repeated treatment with EL-17 on CFA-induced articular pain
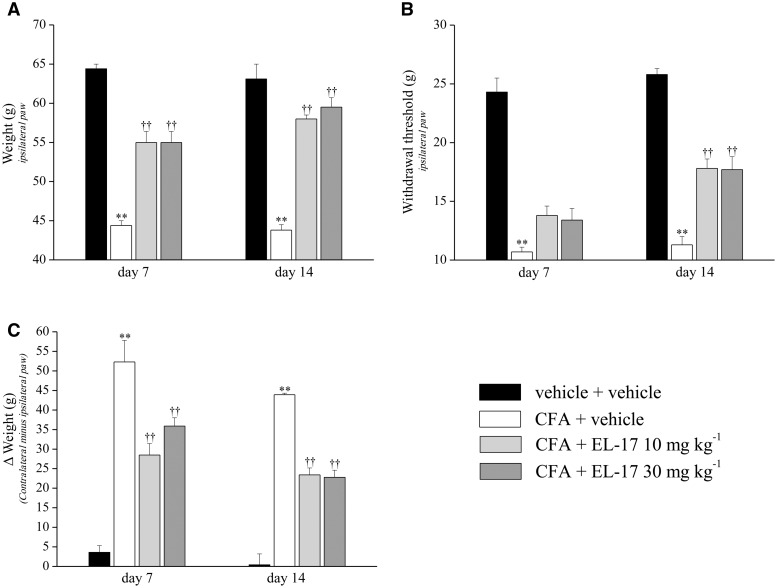
To evaluate the preventive effects of EL-17, a second treatment protocol was applied. The compound was orally administered daily (10 and 30 mg/kg) starting from the same day of CFA i.a. injection and behavioural measurements were performed on days 7 and 14. The CFA + vehicle–treated rats tolerated a paw pressure on the ipsilateral paw of 44.4 g (s.e.m. 0.6) and 43.8 g (s.e.m. 0.7) on days 7 and 14, respectively, compared with control animals [64.4 g (s.e.m. 0.6)] (Fig. 3A). The group treated repeatedly with 10 mg/kg EL-17 showed a pain threshold increase of 53 and 73% on days 7 and 14, respectively, which is consistent with a progressive disease-modifying effect (Fig. 3A). The highest dose (30 mg/kg) showed comparable preventive effects. Values measured on the contralateral paw did not show EL-17 activity on the normal pain threshold (data not illustrated). In addition, the measurements were repeated after a new injection on day 14 (supplementary Table S1, available at Rheumatology Online). Over 60 min, both dosages of EL-17 maintained similar efficacy without additive effects (supplementary Table 1, available at Rheumatology Online).
Fig. 3.
Preventive effect of EL-17
(A) Paw pressure test: response to noxious mechanical stimuli. (B) Von Frey test: withdrawal latency to a non-noxious mechanical stimulus. (C) Incapacitance test: hind limb weight-bearing alterations evaluated as postural imbalance related to pain (Δweight, expressed as the difference between the weight applied on the limb contralateral to the injury and the weight applied on the ipsilateral limb). EL-17 (10 and 30 mg/kg) was suspended in 1% CMC and orally daily administered starting on the day of CFA i.a. injection. Measurements were performed on days 7 and 14. Control animals were treated with vehicle. The values represent the mean of eight rats performed in two different experimental sets. **P < 0.01 vs vehicle + vehicle–treated animals; ††P < 0.01 vs CFA + vehicle–treated animals. CFA: Complete Freund’s Adjuvant; CMC: carboxymethylcellulose.
The pain threshold was also evaluated by the Von Frey test, which employs a mechanical stimulus that does not normally provoke pain (Fig. 3B). The ipsilateral paw withdrawal threshold of the CFA + vehicle group decreased to 13.8 g (s.e.m. 0.8) on day 7 and 13.4 g (s.e.m. 1.0) on day 14, compared with the vehicle + vehicle group at 24.3 g (s.e.m. 1.2). EL-17 treatments significantly prevented CFA-induced hypersensitivity, with progressively increasing efficacy during the treatment. Both dosages increased the paw withdrawal threshold by ∼23% on day 7 and ∼45% on day 14 (Fig. 3B). The effects of repeated treatments with EL-17 were evaluated on CFA-dependent hind limb weight-bearing alterations by the incapacitance test (Fig. 3C). The increased unbalance [50.3 g (s.e.m. 3.5) on day 7 and 57.9 g (s.e.m. 1.9) on day 14, compared with the control value 3.6 g (s.e.m. 1.7)], was reduced on day 7 with 10 mg/kg EL-17 (48%) and 30 mg/kg EL-17 (32%). On day 14, both dosages were able to decrease unbalance by ∼44% (Fig. 3C).
Finally, the pain threshold to thermal noxious stimuli was evaluated by the plantar test, applying a heat stimulation (IR 60) to the injected paw with a 30 s cut-off time. CFA treatment induced a slight alteration on day 7 [6.5 s (s.e.m. 0.5) vs 9.0 (s.e.m. 0.4) for control animals] that vanished on day 14. EL-17 treatment did not appear to alter this response (supplementary Table S2, available at Rheumatology Online).
TGF-β plasmatic levels
CFA i.a. injection was able to induce a plasmatic increase of TGF-β (day 14; Table 1). The repeated treatment with EL-17 did not significantly prevent this systemic alteration (Table 1).
Table 1.
Plasmatic level of TGF-β
| Treatment | TGF-β plasmatic concentration, ng/ml, mean (s.e.m.) |
|---|---|
| Vehicle + vehicle | 30.5 (3.4) |
| CFA + vehicle | 49.5 (4.0)* |
| CFA + EL-17 10 mg/kg | 45.1 (4.7) |
| CFA + EL-17 30 mg/kg | 44.4 (3.9) |
EL-17 (10 and 30 mg/kg) was suspended in 1% CMC and orally daily administered starting on the day of CFA i.a. injection. On day 14, plasma was collected. TGF-β was analysed by ELISA. Each value represents the mean (s.e.m.) of eight rats performed in two different experimental sets.
*P < 0.05 vs vehicle + vehicle–treated animals. CFA: Complete Freund’s Adjuvant.
NE activity measurement
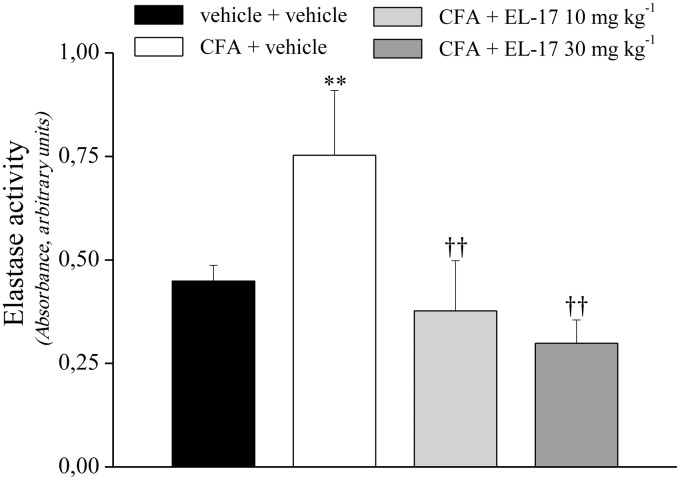
On day 14 after repeated treatments with EL-17, plasma samples were collected from all groups and plasma NE activity was evaluated. The NE enzymatic activity was about doubled in the CFA + vehicle animals compared with the controls (Fig. 4). The plasma NE activity of EL-17-treated rats (both dosages) was comparable to or less than the control rats (Fig. 4), indicating EL-17 effectively inhibited NE in vivo during these treatments.
Fig. 4.
Effects of EL-17 on neutrophil elastase activity in the plasma of CFA-treated rats
Plasma samples were collected on day 14 after repeated treatment with EL-17 (10 and 30 mg/kg orally, daily starting from the day of CFA i.a. injection). The values represent the mean of eight rats performed in two different experimental sets. Measurement for each rat was performed in triplicate. **P < 0.01 vs vehicle + vehicle–treated animals; ††P < 0.01 vs CFA + vehicle–treated animals. CFA: Complete Freund’s Adjuvant.
Morphological analysis of tibiotarsal joint
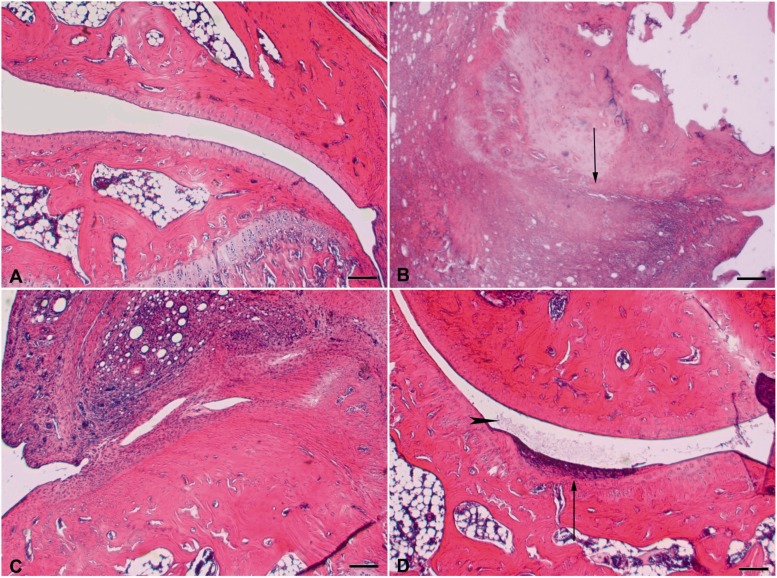
The effect of EL-17 on morphological derangement of the tibiotarsal joint was evaluated in repeatedly treated animals on day 14. As shown in Fig. 5, CFA injection induced a total ablation of the joint space, which appears to be replaced by fibrous tissue. Articular cartilage completely disappeared (CFA + vehicle vs vehicle + vehicle). Animals treated with 10 mg/kg EL-17 showed an ablation of the joint space, which is occupied by fibrous tissue; however, this tissue seems to be slightly more rarefied than observed in animals treated with CFA. Furthermore, we observed cartilage tissue, especially on the tibial side, even though this tissue appears degenerated and damaged. Treatment with the high dose (30 mg/kg) better preserved the joint space, showing similar features to the controls. Nevertheless, tissue residues within the joint space were observable and interpretable as fibrin or actual fibrous tissue. In some cases this fibrous tissue adhered directly to the articular head, producing cartilage loss. Furthermore, articular cartilage still showed some signs of degeneration and seemed to be thinned at some points on the articular surfaces of both tibial and tarsal locations (Fig. 5).
Fig. 5.
Effects of EL-17 on morphological derangement of the tibiotarsal joint
Joints were collected on day 14 after repeated treatment with EL-17 (10 and 30 mg/kg orally, daily starting from the day of CFA i.a. injection). Sections (6 μm) of paraffin-embedded joints were analysed after haematoxylin and eosin staining. Comparative images of the tibiotarsal joint of (A) control animal (vehicle + vehicle); (B) CFA + vehicle (the black arrow indicates a marked joint degeneration with the disappearance of joint space); (C) effect of 10 mg/kg and (D) effect of 30 mg/kg EL-17; small areas of cartilage degeneration are indicated by the black arrow, and the presence of fibrin in the joint space is indicated by the black arrowhead. Bar: 100 μm. Histological evaluations were performed on eight rats for each group (in two different experimental sets) and two sections for each animal were analysed. CFA: Complete Freund’s Adjuvant.
Discussion
This report demonstrates the efficacy of the NE inhibitor EL-17 in relieving articular pain and tissue damage induced by CFA. EL-17 reduces hypersensitivity after a single treatment, and when repeatedly administered, inhibits pain progression during a persistent noxious condition, preventing articular damage.
CFA is able to induce articular damage, which is characterized by diffuse lesions that are similar to an immune reaction towards antigens of joint or connective tissue [32]. The immune response is primarily mediated by T cells, which are responsible for the destruction of collagen. Pathogenesis is characterized by an increase in inflammatory cytokines [33] and neutrophil infiltrate (evaluated as MPO-positive cells [34, 35]), followed by hyperplasia of synoviocytes and formation of the pannus [33]. The inflammatory infiltrate that develops at the level of the damaged articulation contains T lymphocytes activated by specific antigens that inhibit the synthesis of cartilage proteoglycans [36]. The result is a gradual degradation of collagen, matrix and bone. CFA-induced joint damage has similarities with the clinical features of RA, where inflammation of the synovial membrane is followed by damage to the cartilage and bone. In patients, these functional changes appear to contribute to hyperalgesia and spontaneous pain associated with tissue lesions [37]. In our experiments, rats showed progressive development of hypersensitivity to mechanical noxious (hyperalgesia-related measure) and non-noxious (allodynia-related measure) stimuli, as well postural imbalance related to spontaneous pain [38]. Characteristically, the response to thermal (hot) noxious stimuli was reduced, showing a slight decrease of the pain threshold in the plantar test on day 7 that completely disappeared on day 14, indicating low involvement of the thermal nociceptive pathway. CFA evoked a plasmatic increase of the anti-inflammatory cytokine TGF-β that did not achieve protective effects (according to Wu et al. [39]). Moreover, to the best of our knowledge, the present data are the first evidence of a plasma increase of NE activity in the CFA arthritis model. Fourteen days after CFA i.a. injection, NE activity was doubled compared with control animals.
In rats treated with CFA, a single administration of EL-17 reduced pain evoked by noxious stimuli, as well as postural imbalance, suggesting a symptomatic pain relief profile. On the other hand, in the same model of persistent inflammatory articular pain, repeated treatment with EL-17 significantly prevented the development of spontaneous pain and hypersensitivity induced by mechanical noxious and non-noxious stimuli and exhibited increased efficacy over time. The preventive efficacy seems to be related to a disease-modifying effect, since the articular damage clearly present in the histological examination of the tibiotarsal joint was greatly reduced, at least by the higher dose of EL-17. EL-17 did not modify the TGF-β concentration in plasma.
The protective effects of EL-17 were concomitant with a complete reversion of the CFA-dependent NE activity increase measured in the plasma of rats repeatedly treated with both dosages of EL-17. The involvement of NE inhibition in the pharmacodynamics of EL-17 is suggested and further supported by the important role of NSPs the regulation of inflammatory processes [40, 41]. Bank and Ansorge [42] showed that the regulatory functions of these enzymes in local inflammatory processes, as well as their primary catalytic functions and the capacity to attack plasma proteins like immunoglobulins, clotting factors and complement components, are properties that may further contribute to the harmful effects of NSPs in rheumatic diseases. After neutrophil activation at inflammatory sites, NSPs are secreted from granules into the extracellular environment, while a fraction of proteases remained bound in an active form on the external surface of the plasma membrane [43, 44]. Both soluble and membrane-bound NSPs are able to proteolytically regulate the activities of a variety of chemokines and cytokines. In particular, NE participates in the activation of TNF-α, IL-2, IL-8 and epidermal growth factor receptor. NE can also modulate the function of other inflammatory cells, for example, lymphocyte activation, platelet aggregation and PMN influx into the site of inflammation, as well as activate specific cell surface receptors [45, 46]. For example, NE induced acute inflammation and pain in the knee joints of mice by a proteinase-activated receptor 2–dependent mechanism involving activation of a p44/42 MAPK pathway [47]. NE may activate fibroblasts through a proteinase-activated receptor 2 pathway that involves phospholipase C and the nuclear factor κB–releasing chemokines, such as C-X-C motif ligand 8 (CXCL8) and chemokine (C-C motif) ligand 2 (CCL2) [48]. In a subcutaneous air pouch model of inflammation, mice deficient in NE presented decreased neutrophil recruitment and decreased levels of CXCL1 and 2, soluble TNF-α and IL-1β [49]. NE has been shown to induce the expression of cathepsin B and MMP2 [50] by a Toll-like receptor 4 (TLR4)–dependent mechanism. Furthermore, it can directly upregulate the expression of CXCL8 mRNA through a myeloid differentiation primary-response gene 88 (MyD88)/IL-1 receptor–associated kinases/TNF receptor–associated factor 6-dependent pathway that also implicates TLR4 [51, 52].
The multifunctional role of NE suggests a composite activity of NE inhibitors. Thus, although the impairment of NE by EL-17 prevents the hydrolysing activity towards connective tissue components, the indirect modulation of inflammation-related mediators (like proteinase-activated receptors, TLR4 or cytokines) by the NE inhibition also cannot be excluded in the symptomatic and disease-modifying activities of EL-17. Further work with EL-17 is clearly warranted to develop a possible novel opportunity to treat or prevent the progression of RA.
Funding: This research was funded by the Italian Ministry of Instruction, University and Research, by the University of Florence and by a National Institutes of Health IDeA Program grant (GM110732, to M.T.Q.).
Disclosure statement: The authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
- 1.Weiss SJ. Tissue destruction by neutrophils. N Engl J Med 1989;320:365–76. [DOI] [PubMed] [Google Scholar]
- 2.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol 2006;6:173–82. [DOI] [PubMed] [Google Scholar]
- 3.Wright HL, Moots RJ, Edwards SW. The multifactorial role of neutrophils in rheumatoid arthritis. Nat Rev Rheumatol 2014;10:593–601. [DOI] [PubMed] [Google Scholar]
- 4.Korkmaz B, Jenne DE, Gauthier F. Relevance of the mouse model as a therapeutic approach for neutrophil proteinase 3-associated human diseases. Int Immunopharmacol 2013;17:1198–205. [DOI] [PubMed] [Google Scholar]
- 5.Reeves EP, Lu H, Jacobs HL. et al. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature 2002;416:291–7. [DOI] [PubMed] [Google Scholar]
- 6.Pham CTN. Neutrophil serine proteases: specific regulators of inflammation. Nat Rev Immunol 2006;6:541–50. [DOI] [PubMed] [Google Scholar]
- 7.Kakimoto K, Matsukawa A, Yoshinaga M, Nakamura H. Suppressive effect of a neutrophil elastase inhibitor on the development of collagen-induced arthritis. Cell Immunol 1995;165:26–32. [DOI] [PubMed] [Google Scholar]
- 8.Momohara S, Kashiwazaki S, Inoue K, Saito S, Nakagawa T. Elastase from polymorphonuclear leukocyte in articular cartilage and synovial fluids of patients with rheumatoid arthritis. Clin Rheumatol 1997;16:133–40. [DOI] [PubMed] [Google Scholar]
- 9.Ishiguro N, Ito T, Oguchi T. et al. Relationships of matrix metalloproteinases and their inhibitors to cartilage proteoglycan and collagen turnover and inflammation as revealed by analyses of synovial fluids from patients with rheumatoid arthritis. Arthritis Rheum 2001;44:2503–11. [DOI] [PubMed] [Google Scholar]
- 10.Potempa J, Korzus E, Travis J. The serpin superfamily of proteinase inhibitors: structure, function, and regulation. J Biol Chem 1994;269:15957–60. [PubMed] [Google Scholar]
- 11.Loebermann H, Tokuoka R, Deisenhofer J, Huber R. Human α1-proteinase inhibitor: crystal structure analysis of two crystal modifications, molecular model and preliminary analysis of the implications for function. J Mol Biol 1984;177:531–57. [PubMed] [Google Scholar]
- 12.Heutinck KM, ten Berge IJ, Hack CE, Hamann J, Rowshani AT. Serine proteases of the human immune system in health and disease. Mol Immunol 2010;47:1943–55. [DOI] [PubMed] [Google Scholar]
- 13.Zani ML, Nobar SM, Lacour SA. et al. Kinetics of the inhibition of neutrophil proteinases by recombinant elafin and pre-elafin (trappin-2) expressed in Pichia pastoris. Eur J Biochem 2004;271:2370–8. [DOI] [PubMed] [Google Scholar]
- 14.Sjö P. Neutrophil elastase inhibitors: recent advances in the development of mechanism-based and nonelectrophilic inhibitors. Future Med Chem 2012;4:651–60. [DOI] [PubMed] [Google Scholar]
- 15.Herbert JM, Frehel D, Rosso MP. et al. Biochemical and pharmacological activities of SR 26831, a potent and selective elastase inhibitor. J Pharmacol Exp Ther 1992;260:809–16. [PubMed] [Google Scholar]
- 16.Iwata K, Doi A, Ohji G. et al. Effect of neutrophil elastase inhibitor (sivelestat sodium) in the treatment of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS): a systematic review and meta-analysis. Intern Med 2010;49:2423–32. [DOI] [PubMed] [Google Scholar]
- 17.Vicuña L, Strochlic DE, Latremoliere A. et al. The serine protease inhibitor SerpinA3N attenuates neuropathic pain by inhibiting T cell-derived leukocyte elastase. Nat Med 2015;21:518–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weyer AD, Stucky CL. Repurposing a leukocyte elastase inhibitor for neuropathic pain. Nat Med 2015;21:429–30. [DOI] [PubMed] [Google Scholar]
- 19.Crocetti L, Giovannoni MP, Schepetkin IA. et al. Design, synthesis and evaluation of N-benzoylindazole derivatives and analogues as inhibitors of human neutrophil elastase. Bioorg Med Chem 2011;19:4460–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crocetti L, Schepetkin IA, Cilibrizzi A. et al. Optimization of N-benzoylindazole derivatives as inhibitors of human neutrophil elastase. J Med Chem 2013;56:6259–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giovannoni MP, Schepetkin IA, Crocetti L. et al. Cinnoline derivatives as human neutrophil elastase inhibitors. J Enzyme Inhib Med Chem 2015;21:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. J Pharmacol Pharmacother 2010;1:94–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butler SH, Godefroy F, Besson JM, Weil-Fugazza J. A limited arthritic model for chronic pain studies in the rat. Pain 1992;48:73–81. [DOI] [PubMed] [Google Scholar]
- 24.Di Cesare Mannelli L, Bani D, Bencini A. et al. Therapeutic effects of the superoxide dismutase mimetic compound MnIIMe2DO2A on experimental articular pain in rats. Mediators Inflamm 2013;2013:905360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leighton GE, Rodriguez RE, Hill RG, Hughes J. κ-Opioid agonist produce antinociception after i.v. and i.c.v. but not intrathecal administration in the rat. Br J Pharmacol 1988;93:553–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bove SE, Calcaterra SL, Brooker RM, Huber CM, Guzman RE. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthr Cartil 2003;11:821–30. [DOI] [PubMed] [Google Scholar]
- 27.Di Cesare Mannelli L, Bonaccini L, Mello T. et al. Morphologic features and glial activation in rat oxaliplatin-dependent neuropathic pain. J Pain 2013;14:1585–600. [DOI] [PubMed] [Google Scholar]
- 28.Sakurai M, Egashira N, Kawashiri T. et al. Oxaliplatin-induced neuropathy in the rat: involvement of oxalate in cold hyperalgesia but not mechanical allodynia. Pain 2009;147:165–74. [DOI] [PubMed] [Google Scholar]
- 29.Tao F, Tao YX, Zhao C. et al. Differential roles of neuronal and endothelial nitric oxide synthases during carrageenan-induced inflammatory hyperalgesia. Neuroscience 2004;128:421–30. [DOI] [PubMed] [Google Scholar]
- 30.Yoshimura K, Nakagawa S, Koyama S, Kobayashi T, Homma T. Roles of neutrophil elastase and superoxide anion in leukotriene B4-induced lung injury in rabbit. J Appl Physiol 1985 1994;76:91–6. [DOI] [PubMed] [Google Scholar]
- 31.Fujimura N, Obara H, Suda K. et al. Neutrophil elastase inhibitor improves survival rate after ischemia reperfusion injury caused by supravisceral aortic clamping in rats. J Surg Res 2013;180:e31–6. [DOI] [PubMed] [Google Scholar]
- 32.Bendele A. Animal models of rheumatoid arthritis. J Musculoskelet Neuronal Interact 2001;1:377–85. [PubMed] [Google Scholar]
- 33.Inglis JJ, Nissim A, Lees DM. et al. The differential contribution of tumour necrosis factor to thermal and mechanical hyperalgesia during chronic inflammation. Arthritis Res Ther 2005;7:R807–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung JI, Barua S, Choi BH. et al. Anti-inflammatory effect of low intensity ultrasound (LIUS) on complete Freund’s adjuvant-induced arthritis synovium. Osteoarthritis Cartilage 2012;20:314–22. [DOI] [PubMed] [Google Scholar]
- 35.do Nascimento GC, Leite-Panissi CR. Time-dependent analysis of nociception and anxiety-like behavior in rats submitted to persistent inflammation of the temporomandibular joint. Physiol Behav 2014;125:1–7. [DOI] [PubMed] [Google Scholar]
- 36.Waksman BH. Immune regulation in adjuvant disease and other arthritis models: relevance to pathogenesis of chronic arthritis. Scand J Immunol 2002;56:12–34. [DOI] [PubMed] [Google Scholar]
- 37.Dubner R, Ruda MA. Activity-dependent neuronal plasticity following tissue injury and inflammation. Trends Neurosci 1992;15:96–103. [DOI] [PubMed] [Google Scholar]
- 38.Di Cesare Mannelli L, Cinci L, Micheli L. et al. α-Conotoxin RgIA protects against the development of nerve injury-induced chronic pain and prevents both neuronal and glial derangement. Pain 2014;155:1986–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu Z, Ma HM, Kukita T, Nakanishi Y, Nakanishi H. Phosphatidylserine-containing liposomes inhibit the differentiation of osteoclasts and trabecular bone loss. J Immunol 2010;184:3191–201. [DOI] [PubMed] [Google Scholar]
- 40.Pham CT. Neutrophil serine proteases fine-tune the inflammatory response. Int J Biochem Cell Biol 2008;40:1317–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bakele M, Joos M, Burdi S. et al. Localization and functionality of the inflammasome in neutrophils. J Biol Chem 2014;289:5320–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bank U, Ansorge S. More than destructive: neutrophil-derived serine proteases in cytokine bioactivity control. J Leukoc Biol 2001;69:197–206. [PubMed] [Google Scholar]
- 43.Stockley RA. Neutrophils and protease/antiprotease imbalance. Am J Respir Crit Care Med 1999;160(5 Pt 2):S49–52. [DOI] [PubMed] [Google Scholar]
- 44.Owen CA. Leukocyte cell surface proteinases: regulation of expression, functions, and mechanisms of surface localization. Int J Biochem Cell Biol 2008;40:1246–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wiedow O, Meyer-Hoffert U. Neutrophil serine proteases: potential key regulators of cell signalling during inflammation. J Intern Med 2005;257:319–28. [DOI] [PubMed] [Google Scholar]
- 46.Gu Y, Lee HM, Simon SR, Golub LM. Chemically modified tetracycline-3 (CMT-3): a novel inhibitor of the serine proteinase, elastase. Pharmacol Res 2011;64:595–601. [DOI] [PubMed] [Google Scholar]
- 47.Muley MM, Reid AR, Botz B. et al. Neutrophil elastase induces inflammation and pain in mouse knee joints via activation of proteinase-activated receptor-2. Br J Pharmacol 2016;173:766–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uehara A, Muramoto K, Takada H, Sugawara S. Neutrophil serine proteinases activate human nonepithelial cells to produce inflammatory cytokines through protease-activated receptor 2. J Immunol 2003;170:5690–6. [DOI] [PubMed] [Google Scholar]
- 49.Adkison AM, Raptis SZ, Kelley DG, Pham CT. Dipeptidyl peptidase I activates neutrophil-derived serine proteases and regulates the development of acute experimental arthritis. J Clin Invest 2002;109:363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Geraghty P, Rogan MP, Greene CM. et al. Neutrophil elastase up-regulates cathepsin B and matrix metalloprotease-2 expression. J Immunol 2007;178:5871–8. [DOI] [PubMed] [Google Scholar]
- 51.Walsh DE, Greene CM, Carroll TP. et al. Interleukin-8 up-regulation by neutrophil elastase is mediated by MyD88/IRAK/TRAF-6 in human bronchial epithelium. J Biol Chem 2001;276:35494–9. [DOI] [PubMed] [Google Scholar]
- 52.Devaney JM, Greene CM, Taggart CC. et al. Neutrophil elastase up-regulates interleukin-8 via toll-like receptor 4. FEBS Lett 2003;544:129–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.