Abstract
Transactivating responsive sequence (TAR) DNA-binding protein 43-kDa (TDP-43) pathology has been described in various brain diseases, but the full anatomical distribution and clinical and biological implications of that pathology are incompletely characterized. Here, we describe TDP-43 neuropathology in the basal forebrain, hypothalamus, and adjacent nuclei in 98 individuals (mean age, 86 years; median final mini-mental state examination score, 27). On examination blinded to clinical and pathologic diagnoses, we identified TDP-43 pathology that most frequently involved the ventromedial basal forebrain in 19 individuals (19.4%). As expected, many of these brains had comorbid pathologies including those of Alzheimer disease (AD), Lewy body disease (LBD), and/or hippocampal sclerosis of aging (HS-Aging). The basal forebrain TDP-43 pathology was strongly associated with comorbid HS-Aging (odds ratio = 6.8, p = 0.001), whereas there was no significant association between basal forebrain TDP-43 pathology and either AD or LBD neuropathology. In this sample, there were some cases with apparent preclinical TDP-43 pathology in the basal forebrain that may indicate that this is an early affected area in HS-Aging. We conclude that TDP-43 pathology in the basal forebrain is strongly associated with HS-Aging. These results raise questions about a specific pathogenetic relationship between basal forebrain TDP-43 and non-HS-Aging comorbid diseases (AD and LBD).
Keywords: Aging, Alzheimer disease, Basal forebrain, Frontotemporal lobar degeneration, Hippocampal sclerosis, TDP-43.
INTRODUCTION
Pathologic transactivating responsive sequence (TAR) DNA-binding protein 43-kDa (TDP-43)-immunopositive inclusions were initially recognized in frontotemporal lobar degeneration (FTLD) with ubiquinated inclusions and in amyotrophic lateral sclerosis (ALS) (1, 2); however, subsequent studies of this pathologic biomarker have found evidence of much broader implications. TDP-43 protein is redistributed from the nuclei into cytoplasmic and neuritic components in many disease states. This redistribution is observed in both neurons and glia, often in patterns that correlate with clinical and/or genetic findings (3, 4). The mechanisms by which pathologic TDP-43 induces cellular dysfunction remain under investigation (5).
In addition to FTLD-TDP and ALS, pathologic deposition of TDP-43 has been recognized in hippocampal sclerosis of aging (HS-Aging) (6), Alzheimer disease (AD) (7), Alexander disease (8), corticobasal degeneration (9), and chronic traumatic encephalopathy (10), among other diseases. In aged patients, TDP-43 pathology is associated with cognitive decline (11), particularly in episodic and working memory domains (12). Another study of cognitively normal, aged patients identified TDP-43 deposition in 36.4% of patients, typically in the form of neurites in the mesial temporal lobe, associated with argyrophilic grain disease (AGD) (13). Many prior studies examining TDP-43 proteinopathy in aging-related disorders have focused on structures of the mesial temporal lobe (amygdala, subiculum, hippocampal sector CA1, granule cells of dentate gyrus) or neocortex. Thus, it remains an open question whether TDP-43 proteinopathy is seen in common age-related brain diseases in the basal forebrain, hypothalamus, and adjacent deep nuclei.
There are several reasons to hypothesize that TDP-43 pathology would be present in the basal forebrain in brain diseases of aging. First, neurons of the mesial temporal lobe (which are most commonly affected in the aforementioned TDP-43 proteinopathies) project via several pathways to the basal forebrain/hypothalamus, including via the ventral amygdalofugal pathway and stria terminalis (from basolateral and corticomedial nuclear groups of amygdala, respectively) and fornix (pyramidal cells of hippocampus and subiculum) (14–16). Second, the basal forebrain and amygdala are contiguous with one another across the endorhinal sulcus (entorhinal in some sources) in the region of the olfactory cortex (encompassing anterior perforated substance, olfactory tubercle, and piriform cortex) (17). Dorsomedial temporal lobe and basal forebrain are also contiguous with one another via components of “extended” amygdala within the basal forebrain itself (bed nucleus of the stria terminalis, sublenticular extended amygdala) (18) and laterally at the transition region between cells of the ventral claustrum (claustrum diffusa) and dorsal amygdala (15). Finally, neurons of the basal forebrain (and hypothalamus) are susceptible to accumulation of neurodegenerative disease-associated proteins in AD (19), AGD (20), and Lewy body diseases (LBDs) (21) and have recently been implicated in primary TDP-43 proteinopathies including those on the FTLD-ALS spectrum (22).
The present study was carried out to determine whether the basal forebrain, hypothalamus, and adjacent deep nuclei harbor TDP-43 pathology in aged humans and whether the TDP-43 is associated with particular aging-related disease states (HS-Aging, AD, and LBD). For this study, we considered the basal forebrain to encompass those structures not recognized as being part of another group of nuclei (eg, amygdala, thalamus, basal ganglia, and hypothalamus). This would include structures (further detailed in “Materials and Methods”) adjacent to and ventral to anterior commissure, extending rostrally and ventrally to include the primary olfactory area in the region of the anterior perforated substance (a region sometimes referred to as the olfactory tubercle). We hypothesized that a subset of participants would have TDP-43 pathology in this region for the above-described reasons. Because prior studies have investigated the importance of TDP-43 with comorbid AD neuropathology and profound cognitive impairment (23), we also sought to evaluate the importance of this pathology in a cohort that included both cognitively normal and impaired patients, with only a minority that were severely impaired. Here, we describe the frequency, severity, topography, and potential cognitive sequelae of basal forebrain TDP-43 pathology in 98 individuals for whom detailed clinical and pathologic data are available.
MATERIALS AND METHODS
Identification of Pathologic Materials
These studies were carried out with the approval of the Institutional Review Boards at Houston Methodist Hospital (IRB-2-0114-0013) and the University of Kentucky. Formalin-fixed brain tissue was retrieved from the brain bank at the University of Kentucky Alzheimer’s Disease Center (UK-ADC) Neuropathology Core. Information on this study cohort and autopsy protocols have been published (24, 25). Ninety-eight cases were selected for study based on the gross examination of archived, fixed (“wet”) brain tissue by 2 study authors (MDC and PTN). Working together, these authors identified and submitted representative sections from the formalin-fixed tissues of each of these 98 cases. These included an “anterior” section including elements of basal forebrain (detailed further below) at the level of anterior commissure, with basal ganglia and internal capsule, as well as a “posterior” section at the level of the mammillary bodies, lateral and posterior hypothalamic areas, and anterior thalamus. Sections adjacent to these (ie, immediately rostral, caudal, or between) were taken if the target anterior or posterior sections were not available. One or both sections frequently had elements of claustrum, external and extreme capsules, and insula. Embedding of these sections into 196 blocks (2 per patient) was performed at the UK-ADC.
Histology and Immunohistochemistry
The formalin-fixed paraffin-embedded tissue samples were further processed at Houston Methodist Hospital. Briefly, 5 serial 4- to 5-µm sections were cut for each block, mounted on Plus-coated slides, and dried at 60 °C. Sequential levels for each block were stained with hematoxylin and eosin (H&E) for anatomic localization and by immunohistochemistry for nonphosphorylated TDP-43 antibody (ProteinTech, Rosemont, IL; 10782-2-AP, polyclonal antiserum, 1:200). TDP-43 staining was performed on a BenchMark ULTRA platform (Ventana Medical Systems, Inc., Tucson, AZ) with appropriate controls. The remaining slides were archived for repeat staining if necessary.
Clinical and Neuropathologic Data
The protocols of the UK-ADC for obtaining cognitive and clinical data, as well as pathologic analysis, have previously been described (24, 26, 27). Clinical and whole brain pathologic data were examined in this study only after the blinded pathologic analysis. Clinical variables included patient age, sex, years of education, neurologic diagnosis, presence/absence of cognitive impairment, and mini-mental state examination (MMSE) scores (28). MMSE at the time of enrollment in the UK-ADC protocol and the last available score were available; percent changes from baseline MMSE were also calculated. Clinical Dementia Rating (CDR) data (global CDR, as well as memory, orientation, and judgment domains) were also available (29). Pathologic and genetic variables included brain weight, Braak stage (30), CERAD neuritic plaque score (31), and APOE genotype. All neuropathologies identified on whole brain examination (HS, LBD, AGD, FTLD, vascular brain injury, cerebral amyloid angiopathy, or no significant pathologic alteration) were recorded. HS-Aging was defined pathologically as described elsewhere (32–34). Tissue processing and neuropathologic assessments performed on the UK-ADC cases were as previously described (32). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards and in accordance with National Institutes of Health/Alzheimer’s Association 2012 guidelines (34).
Anatomic Regions of Interest
Twenty-three regions of interest (ROIs) were examined in the basal forebrain, hypothalamus, and adjacent deep nuclear structures. These were identified on both H&E and TDP-43 stains on sequentially cut tissue sections for each tissue block. At the outset of analysis, ROIs that could be defined on H&E examination include the subcallosal gyrus (SCG); lateral septal area (LSA); large neurons of the medial septal nucleus and vertical limb of the nucleus of the diagonal band (NDB); ventral striatum and bed nucleus of the stria terminalis (VS/BNST); dorsal caudate nucleus (CD); globus pallidus and ventral pallidum (GP/VP); olfactory area including piriform cortex in the region of anterior perforated substance (PFM); Islands of Calleja and interface islands comprising clusters of small neurons and/or granule cells (IsC); other neurons of substantia innominata, including nucleus basalis of Meynert and nucleus of the horizontal limb of the diagonal band (SI/NBM); putamen, including fundus of the putamen (PT); claustrum and ventral claustrum (CLST); insula (INS); supraoptic nucleus of the hypothalamus (SON); paraventricular nucleus of the hypothalamus (PVN); accessory/intermediate nuclei of the hypothalamus (ACS/INT); medial hypothalamus, including preoptic area, ventromedial and dorsomedial nuclear groups, and the posterior hypothalamic area (HYP/MED); mammillary bodies (MB); large neurons of the lateral hypothalamic area, tuberomammillary nucleus, and lateral tuberal nucleus (HYP/LAT); floor of the third ventricle, infundibulum, and median eminence (HYP/FLR); medial nuclei of thalamus (THL/MED, including anterior, lateral dorsal, and dorsomedial nuclei); lateral nuclei of the thalamus (THL/LAT, lateral to the internal medullary lamina); and periventricular thalamic neurons (THL/PV). Although it was not a focus of this study, a portion of dorsal amygdala, including the transition area between amygdala and piriform cortex/anterior perforated substance around the endorhinal sulcus (AMY), was present in some cases. These ROIs at the level of an “anterior” section are shown in Figure 1 (top row).
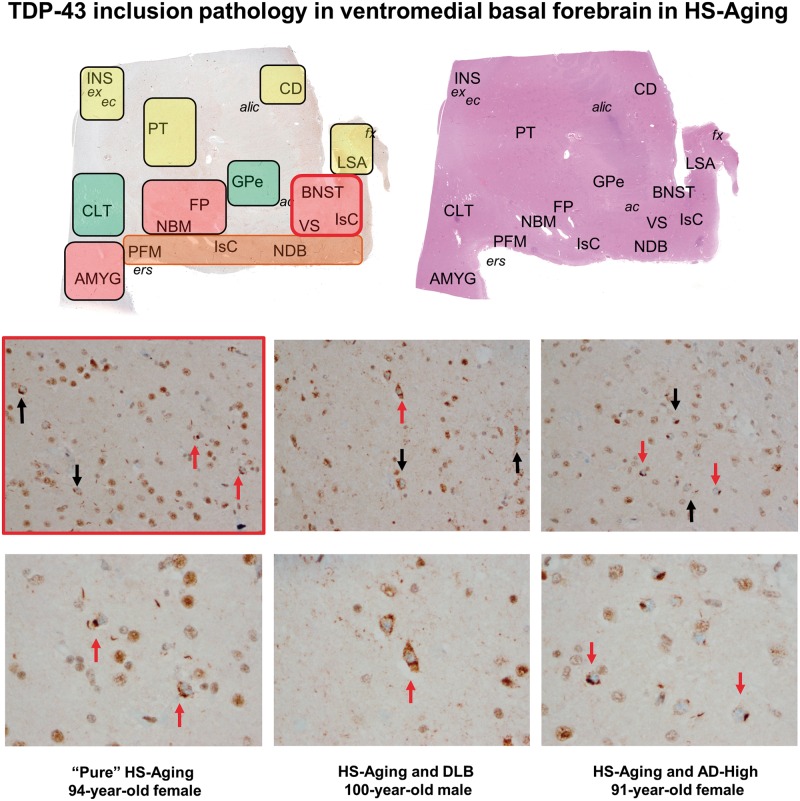
FIGURE 1.
Regions-of-interest (ROI) in the “anterior” sections of this study with the topography of TDP-43 inclusion pathology in the ventromedial basal forebrain. Hematoxylin and eosin (top right) and TDP-43-immunostained (top left) sections of a 65-year-old patient with dementia but without TDP-43 inclusion pathology are used to illustrate ROI across all individuals. Shaded rectangles (left) summarize severity of TDP-43 pathology (red = most severe pathology, orange = moderate, yellow = mild, green = not affected; see also Figure 2 and Figure 2 color bar). The most severe NCI pathology was identified in ventromedial basal forebrain (BNST, VS), fundus of putamen (FP), and amygdala (when present). The middle (200x) and bottom rows (400x) illustrate photomicrographs of 3 different study participants in the region of BNST and VS. Note the striking similarity across all 3 individuals, despite the presence or absence of very different comorbid neuropathologies. Black arrows (middle row) indicate representative NCIs at 200x and red arrows indicate NCIs also shown at 400x (bottom row). ac, anterior commissure; alic, anterior limb of the internal capsule; AMYG, amygdala; BNST, bed nucleus of the stria terminalis (combined with VS in this study, see text); CD, caudate; CLT, ventral portion of the claustrum, near the transition region with dorsal amygdala (AMYG); ec, external capsule; ers, endorhinal sulcus; ex, extreme capsule; FP, fundus of the putamen; fx, fornix; INS, insula; IsC, Islands of Calleja and interface islands comprising small neurons and granule cells; LSA, lateral septal area/nuclei; NBM, nucleus basalis of Meynert, commonly identified adjacent to large-caliber vessels underlying the ventral putamen; NCI, neuronal cytoplasmic inclusion; NDB, nucleus of the diagonal band (of Broca); PFM, piriform, or olfactory, cortex (see text); PT, putamen; SI-NOS, not depicted in the image, but used in this study to indicate neurons of substantia innominata not readily assigned to one of these designated structures; VS, ventral striatum/nucleus accumbens.
Anatomic ROIs Subgrouped Into 5 Regions, Including Basal Forebrain
For data recording, we grouped certain structures within one ROI due to the difficulty on H&E examination alone of distinguishing their boundaries. For example, while BNST is defined as the group of medium-sized neurons dorsal to anterior commissure, medial to LSA, and lateral to the ventricle and LSA (14), in our experience, this region is not always discrete and may be contiguous with VS. For similar reasons, ROIs in the hypothalamus were grouped by location (lateral, medial) unless they could be clearly identified on as neurons of the MB, SON, PVN, or ACS/INT nuclei. Furthermore, not all ROIs were present in all individuals because of limitations of sampling, due in part to the retrospective nature of the study and in part to the inherent anatomic variability across participants within these regions, as described elsewhere (35–37). Therefore, for further analysis, we grouped these into regions of basal forebrain (LSA, VS/BNST, PFM, IsC, NDB, and SI/NBM), basal ganglia (CD, GP/VP, PT, CLST), thalamus (THL/MED, THL/LAT, THL/PV), cortical regions (INS, SCG), and hypothalamus (PVN, SON, MB, ACS/INT, HYP/MED, HYP/LAT, HYP/FLR). At least one ROI component of these regions was present in 100% (basal forebrain), 100% (basal ganglia), 84% (thalamus), 77% (cortical regions), and 66% (hypothalamus) of patient samples.
Assessment of TDP-43 Pathology
Two study authors (MDC and HT) independently reviewed and rated TDP-43 pathology in all 196 TDP-43-immunostained sections (2 per each of 98 participants). The reviewers were blinded to original clinical and pathologic diagnoses made at the UK-ADC. The topography of inclusion pathology was determined using H&E-stained sections from the immediately adjacent level for each block. Inclusions were recorded and classified by morphologic subtype as follows: neuronal cytoplasmic (NCI), neuronal intranuclear (NII), glial cytoplasmic (GCI), and neurites. Re-review of slides was performed to reach consensus as needed. Quantitative measures were performed in each area where TDP-43 pathologies were identified. For quantitation, any cellular TDP-43 inclusions (NCI, GCI) were recorded within 3 consecutive high-power microscopic fields (HPFs) at 400x (0.19625 mm2 per HPF).
Statistical Analyses
Two-sided Mann-Whitney (Wilcoxon rank sum) testing was used to determine whether patients with TDP-43-positive and -negative neuropathology in the ROIs differed with respect to age at death, years of education, MMSE (at enrollment and final MMSE), and in global CDR as well as its memory domain. Similarly, TDP-43-positive and -negative groups were compared with respect to brain weight, Braak stage, and CERAD score. The Fisher exact test was used to determine the strength of association between TDP-43 pathology in the region examined with respect to (a) AD neuropathology, (b) LBD, (c) HS, and (d) normal aging without other significant neuropathology.
Multiple logistic regression analyses were also performed with TDP-43 pathology in the region examined as the dichotomous variable of interest (present/absent) modeled as a combination of predictor variables HS (present/absent), age at death (years), Braak stage (0–VI), and LBD (present/absent). These variables were chosen in the model based on the association with TDP-43 pathology elsewhere in the brain. Statistical analyses were performed using the R software package (38).
RESULTS
Demographic and Clinical Characteristics of the UK-ADC Study Participants
Clinical characteristics are summarized for all 98 individuals in Table 1. Briefly, the study sample comprised 59 women and 39 men with a median age at death of 86 years (range from 48–102 years, first quartile [Q1] of 81, third quartile [Q3] of 91.8). Mean education across all participants was 15.4 years (SD = 2.85). In 66 participants (67.3%), there was a question raised on clinical or neuropsychological testing for cognitive impairment, ranging from possible mild cognitive impairment to dementia. Thirty-two participants (32.6%) were deemed cognitively intact without a question of mild or severe cognitive impairment. The median final MMSE score was 27 across all study participants (Q1 = 24, Q3 = 29), with median values of 24 and 29 in the cognitively impaired and cognitively intact groups, respectively.
TABLE 1.
Clinical and Pathologic Findings in 98 Study Individuals
| Female/male | n = 59/n = 39 |
| Age at death (yrs) (Q1, Q3) | 86 (81, 91.75) |
| Education (yrs) (SD) | 15.4 (2.85) |
| Last MMSE (Q1, Q3) | 27 (24, 29) |
| Brain weight (g) (SD) | 1156.0 (134.89) |
| Median Braak stage (Q1, Q3) | II (I, IV) |
| CERAD score (Q1, Q3) | 1 (0, 2) |
| Cognitively impaired | n = 66 (67.3%) |
| Normal cognition | n = 32 (32.6%) |
CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; MMSE, mini-mental state examination; yrs, years.
Neuropathologic and Genetic Characteristics of the UK-ADC Study Participants
The mean fresh brain weight at the time of autopsy in all study participants was 1,156 g (SD = 134.89). The median Braak score was II (Q1 = I, Q3 = IV), and median CERAD score was 1 (Q1 = 0, Q3 = 2) (Table 1). Nonmutually exclusive pathologic diagnoses in order of descending frequency included AD neuropathology (Braak score ≥ III or CERAD score ≥ 2/moderate) in 51 participants (52.0%), CAA in 40 participants (40.8%), HS in 18 participants (18.4%), LBD/Parkinson's disease in 18 participants (18.4%), vascular brain injury in 18 participants (18.4%), non-AD tauopathy (all with a progressive supranuclear palsy-like distribution) in 4 participants (4.1%), AGD in 4 participants (4.1%), and FTLD (3.1%) (FTLD-TDP in 2 participants and the third with FTLD-tau). No significant pathologic alteration was seen in 26 participants (26.5%). Those participants with no pathologic alteration had a mean age of 83.8 years (SD = 9.2). No study participants had the neuropathology of multiple system atrophy, Pick disease, prion disease, corticobasal degeneration, or CADASIL (cerebral autosomal dominant arteriopathy with leukoencephalopathy).
Apolipoprotein E (APOE) data were also available in all but 3 study participants (n = 95/98). Of those with available data, APOE ϵ3/3 was present in the majority (65.3%), followed by ϵ3/4 (15.8%), ϵ2/3 (12.6%), and ϵ4/4 and ϵ2/4 (both 3.2%). Twenty of 21 participants with a single copy of the ϵ4 allele all demonstrated some level of cognitive impairment (95.2%) with the single exception having an APOE ϵ3/4 genotype (age 68 at the time of death).
TDP-43 Inclusion Pathology in the Basal Forebrain, Hypothalamus, and Deep Nuclei
TDP-43 pathology was present in 19 study participants (19.4%) for the ROIs studied (broadly categorized as basal forebrain, hypothalamus, basal ganglia, thalamus, and cortical regions [insula and SCG]). Within this group of 19 participants, involvement was most frequent in the basal forebrain (17/19 participants, or 89.5%), followed by basal ganglia (11/19 participants, or 57.9%), thalamus (8/17 participants, or 47.1%), hypothalamus (6/14 participants, or 42.9%), and cortical regions (7/18 participants, or 38.9%).
Maximum TDP-43 pathology in 3 fields across all regions varied from 1 to >20 pathologic structures (NCI, GCI, neurites) in 3 HPFs, and the average number of pathologic structures across all ROIs per participant varied from 0.33–14.2. Pathologic findings by ROI, patient, and consensus diagnosis are shown in Figure 2. For these ROIs, across the 19 participants with any TDP-43 pathology, the greatest severity was seen in VS/BNST (average of 9.3 inclusions per 3 HPFs across 19 participants), SCG (8.4); PT, including fundus (5.7); PFM (4.9); INS (4.4); NDB/NBM/SI-NOS (3.8); LSA (3.7); CD (3.2); THL/MED (2.9); IsC (2.8);THL/PV (2.4); and, less commonly, in the HYP/LAT (1.9) and CLST (1.6). Values <1 were seen for THL/LAT, HYP/MED, and GP/VP. No examples of inclusion pathology were seen in the following hypothalamic regions: SON, PVN, ACS/INT, MB, and HYP/FLR.
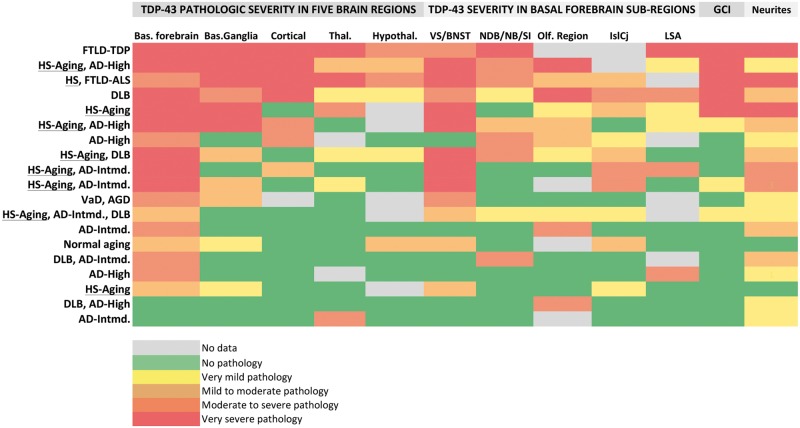
FIGURE 2.
A heatmap of TDP-43 pathologic burden across all regions of interest (columns) by patient and their principal diagnoses (rows). For the 19 participants with TDP-43 pathology in the regions examined in this study (of 98 total participants), the principal and consensus clinical and pathologic diagnoses from the UK-ADC brain bank are provided on the left (row labels). Regions of interest and basal forebrain subregions, as well as glial cytoplasmic inclusion (GCI) and neurite pathology, are represented in columns. Inclusion severity is provided by the color bar at the bottom of the figure. Cases are ranked from top to bottom by overall pathologic severity in the region with the most severe TDP-43 pathology being in the top row (a patient with frontotemporal lobar degeneration [FTLD] with ubiquinated inclusions in the UK-ADC brain bank). HS/HS-Aging cases (n = 9) are underlined. IslCj, Islands of Calleja and interface islands in basal forebrain.
Representative microscopic images of TDP-43 pathology are shown for 3 participants in Fig. 1 (middle and bottom rows). Figure 3 illustrates the pathologic findings in a single patient without a comorbid pathologic diagnosis. TDP-43-positive GCIs were present in 5 of these 19 study participants (including 3 participants with HS or HS-Aging), equivocal in 3 (2 with HS-Aging), and they were not present in the remaining 11 participants. NCI and neurite pathologies were frequently identified together in patients, although TDP-43-positive neurites were more abundant with more severe NCI pathology rather than the opposite (compare Fig. 1 with Fig. 3). Seven participants had a milder, neurite-predominant form of TDP-43 deposition, although this was not seen in association with HS or HS-Aging (Fig. 4). One patient whose pathology is depicted in the sixth row of the heatmap in Fig. 2 had NIIs in a portion of dorsal amygdala included in the section studied. This patient also had NCIs and neurites in affected regions and was equivocal for GCI pathology (Fig. 2). NII pathology was not otherwise identified.
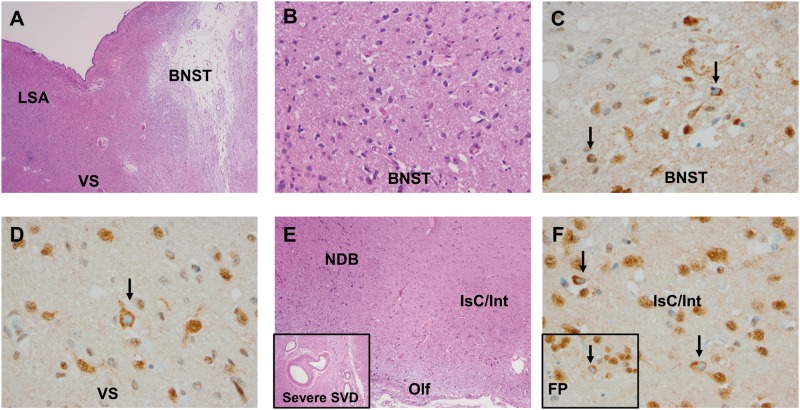
FIGURE 3.
Pathology in the basal forebrain, including vascular brain injury (microinfarct) and severe arteriolosclerosis with concomitant mild TDP-43 neuronal cytoplasmic inclusion (NCI) pathology, in a nondemented 86-year-old female study participant. (A) Hematoxylin and eosin (H&E) section demonstrates a remote microinfarct in the bed nucleus of the stria terminalis (BNST) (20x, 2x objective). (B–D) H&E and TDP-43 immunostain of the BNST (B, at 200x, and C, at 400x, respectively) and adjacent ventral striatum (VS) (D, 400x) in the same patient. Black arrows indicate NCI pathology. (E) Basal forebrain lateral and ventral to that shown in (A) with the pial surface (bottom of image, 40x), anterior perforated substance/olfactory cortex (Olf), large neurons in the nucleus of the diagonal band (NDB), and small groups of neurons termed interface islands (Isc/Int). Inset at the lower left shows severe small vessel disease (SVD) adjacent to the anterior commissure in this patient. (F) TDP-43-positive NCI pathology (arrows) in interface islands, as well as focally in the adjacent fundus of the putamen (inset, lower left) (400x). FP, fundus of the putamen; LSA, lateral septal area/nuclei.
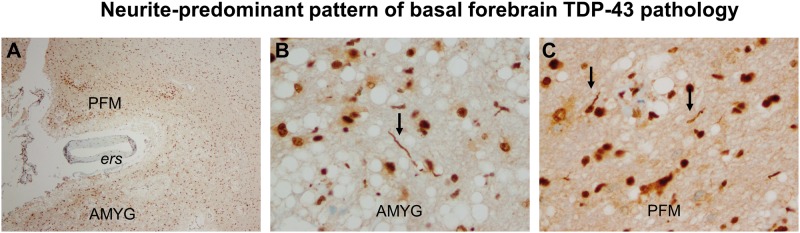
FIGURE 4.
Neurite-predominant TDP-43 pathology in components of the basal forebrain. In addition to the patterns of neuronal cytoplasmic inclusion (NCI) pathology shown in Figures 1 and 3, the brain of this 78-year-old female with intermediate AD pathology shows an additional pattern in 7 of 19 individuals, ie, a neurite-predominant form. (A) The corticomedial region of the amygdala (AMYG) and adjacent piriform cortex (PFM) are shown, separated by the endorhinal sulcus (ers) containing an artery (40x). (B, C) TDP-43-reactive neurites are indicated in panels B (AMYG) and C (PFM) by arrows. Panels B and C are photographed at 400x.
Clinicopathologic Associations of Basal Forebrain TDP-43 Pathology
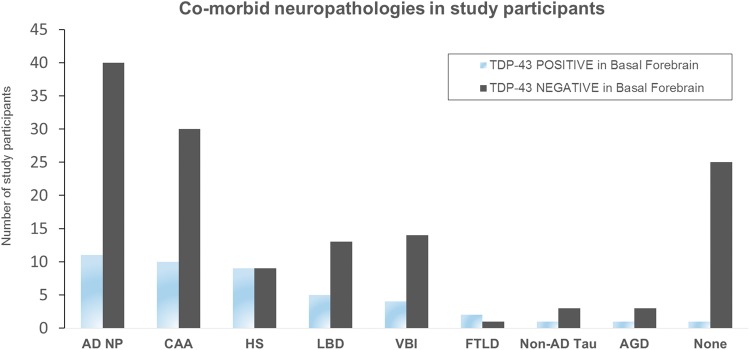
Consensus clinical-pathologic diagnoses for the 19 participants with TDP-43 pathology in any study ROI are shown in Fig. 2, ranked from top to bottom by the severity of TDP-43 inclusion pathology. Eleven patients had intermediate or advanced (high) AD neuropathology, 9 had HS, 5 had LBD spectrum pathology, 2 had FTLD-TDP, and 1 had AGD with vascular dementia. Only one patient of the 19 was cognitively intact without other significant neuropathologic findings (Fig. 3). The numbers of patients with and without TDP-43 pathology in the basal forebrain by comorbid neuropathologies are shown in Fig. 5. Eleven of 51 individuals (21.6%) with AD neuropathology (defined here as Braak stage ≥III or neuritic plaque score of moderate or greater) had TDP-43 pathology in the region investigated versus 78.4% without. Likewise, the percentage of participants with TDP-43 pathology in the basal forebrain by comorbid diagnoses were 66.7% for FTLD (2 of 3), 50.0% for HS (9 of 18), 27.8% for LBD (5 of 18), 25.0% for CAA (10 of 40), 25% of non-AD tauopathies (1 of 4), 25% of AGD (1 of 4), 22.2% for VBI (4 of 18), and 3.8% of normal cases (1 of 26). A very strong and significant association between HS and TDP-43 pathology in basal forebrain and adjacent deep nuclei was identified (p = 0.001, odds ratio = 6.8). In contrast, no significant association with AD neuropathology (odds ratio 1.3, p = 0.62) or LBD-spectrum pathology (odds ratio 1.8, p = 0.33) was identified. A negative association also was seen between TDP-43 pathology in the region and the presence of no pathologic diagnosis (p = 0.02, odds ratio = 0.12). Five of the 9 HS participants with basal forebrain TDP-43 had hippocampal TDP-43 data available in the UK-ADC database, and the remaining 4 cases were stained for TDP-43 in the course of this study. All 9 of these participants had hippocampal TDP-43 pathology.
FIGURE 5.
Comorbid neuropathologies in study participants, with and without TDP-43 pathology in the basal forebrain and adjacent structures. AD NP, individuals with intermediate or greater neurofibrillary pathology (Braak stage >III), and/or CERAD neuritic plaque frequency ≥ moderate. AGD, argyrophilic grain disease; CAA, cerebral amyloid angiopathy; HS, hippocampal sclerosis; FTLD, frontotemporal lobar degeneration; LBD, Lewy body disease; NON-AD TAU, individuals with a progressive supranuclear palsy-like distribution of neurofibrillary tangle pathology; NONE, individuals without significant brain pathology on comprehensive neuropathologic examination; VBI, vascular brain injury.
Study participants with and without TDP-43 pathology in the regions examined significantly differed with respect to last available MMSE score, the memory domain of the CDR, and global CDR, but not in the MMSE at study entry (Table 2). Participants did not differ significantly with respect to age at death, Braak stage, CERAD score, or other group comparisons. Logistic regression analysis showed a significant effect of HS on the presence/absence of TDP-43 pathology in the basal forebrain (regression coefficient = 2.2, p = 0.00052, confidence intervals, 0.99, 3.54), but not a significant effect of age, Braak stage, or LBD.
TABLE 2.
Clinical and Pathologic Features of TDP-43-Positive and TDP-43-Negative Groups
| Feature | TDP-43-Positive | TDP-43-Negative | p Value |
|---|---|---|---|
| Age at death (yrs) (Q1, Q3) | 90.0 (82.5, 92.5) | 85.0 (81.0, 91.0) | 0.37 |
| Education (yrs) (SD) | 15.1 (4.33) | 15.5 (2.4) | 0.69 |
| Last MMSE (Q1, Q3) | 24 (19.8, 28.0) | 28 (24, 29) | 0.02a,b |
| First MMSE (Q1, Q3) | 27.5 (26, 29.8) | 29 (28, 30) | 0.15a |
| CDR, Memory (Q1, Q3) | 1.0 (0.5, 1.0) | 0.5 (0.0, 1.0) | 0.03a,b |
| CDR, Global (Q1, Q3) | 0.5 (0.5, 1.0) | 0.5 (0.0, 1.0) | 0.04a,b |
| Brain weight (g) (SD) | 1112.11 (119.60) | 1166.52 (136.9) | 0.094 |
| Braak stage (Q1, Q3) | 3.0 (1.0, 4.5) | 2.0 (1.0, 3.0) | 0.38 |
| CERAD score (Q1, Q3) | 1.0 (0.0, 2.0) | 1.0 (0.0, 2.0) | 0.5 |
CDR, Clinical Dementia Rating; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; MMSE, mini-mental state examination; yrs, years.
aThe values of FTLD individuals were not included in this analysis as 2 such individuals were in the TDP-43-positive group.
bSignificant differences.
DISCUSSION
To our knowledge, this is the first large study of TDP-43 deposition in the myriad structures comprising the basal forebrain, hypothalamus, and adjacent subcortical nuclei across a variety of neurodegenerative diseases (24). Three major findings emerged from this study. First, HS-Aging is significantly associated with TDP-43 deposition in the basal forebrain. Second, TDP-43 pathology in this brain region is not associated with brain aging and/or normal cognition but is instead strongly associated with cognitive impairment. With regard to diagnostic significance, this means that samples of the brain region studied here at autopsy may be a useful addition to a limited panel of TDP-43 staining. This is because cases positive for pathology are very likely to be associated with both significant neuropathology in other regions and cognitive impairment. Third, and most critically, the pattern of TDP-43 pathology observed is identical in pure HS-Aging participants and HS-Aging participants with intermediate or high AD neuropathology or with DLB. In other words, in older individuals, it appears that TDP-43 pathology is likely to be associated with HS-Aging rather than being a secondary proteinopathy of a different disease process.
HS is characteristically defined as neuronal loss and gliosis in the hippocampus and the subiculum in the absence of correspondingly severe neurofibrillary tangle pathology (32, 34, 39). This alteration is present in up to 20% of patients with dementia (40) and results in an impairment of episodic memory in a disease process that has been referred to as HS-Aging (41). The pathologic bases for the disease are not entirely known, although a significant association with cerebrovascular arteriolosclerosis has recently been shown (42). HS-Aging is also frequently identified in association with TDP-43 pathology in the region of the medial temporal lobe and is strongly associated with small vessel disease (26). Specifically, Amador-Ortiz et al identified hippocampal TDP-43 pathology that did not colocalize with tau-immunoreactive lesions in 70% of patients with HS (6). In a larger study, a higher rate of TDP-43 pathology in HS-Aging (89.9%) was identified versus a frequency of hippocampal TDP-43 of 9.7% in patients without HS-Aging (6, 32, 43).
By expanding the spectrum of TDP-43 pathology in HS-Aging described in these earlier studies, we have identified TDP-43 pathology in 19 participants (median age of 90 years), 9 of whom (47%) had diagnoses in the UK-ADC brain bank of HS or HS-Aging. Extra-hippocampal TDP-43 pathology in HS has been described previously (6), but, to our knowledge, this is the first such description that links HS-Aging with TDP-43 proteinopathy in structures of the basal forebrain. This finding is particularly relevant because HS related to aging is present in anywhere from 5%–30% of persons over the age of 90 years (33). Not surprisingly, our positive cases included 2 participants with “ubiquitinopathies” (FTLD-ALS spectrum, 1 with comorbid HS pathology). The remaining HS-Aging cases consisted instead of 2 patients with pure HS-Aging and 6 with HS-Aging and comorbid pathology (AD-intermediate, AD-high, or LBD). Moreover, all cases with HS, pure HS-Aging or HS-Aging plus comorbid diagnoses (AD, LBD) had TDP-43 positive NCIs and frequently neurites and GCIs, with the most affected region being ventromedial basal forebrain (BNST, VS, IsC). This was in contrast to a sparse, neurite-only pattern with few or, more typically, no NCIs/GCIs, similar to a pattern described in the hippocampus in normal aging (13), and seen in patients with only AD and/or LBD pathologies as well as the one patient with AGD. Aside from the individuals with FTLD-ALS-spectrum disease, it is unlikely the pathology we describe here could be identified as significant neuronal loss and/or gliosis in the basal forebrain on H&E examination without reference to hippocampal sections. However, TDP-43 pathology in HS-Aging is not always associated with neuronal loss and gliosis. This has been demonstrated in individuals with characteristic HS-Aging by H&E stain who have TDP-43 deposition in the contralateral hippocampus in the absence of neuronal loss and gliosis (32).
Basal forebrain TDP-43 pathology was also identified in participants with HS-Aging and AD-intermediate (Braak stages III, IV) or AD-high pathology (Braak stages V, VI) (Fig. 5). Among all cases included in the current study, however, there was not a significant relationship between TDP-43 pathology and either AD or LBD pathologies specifically (unlike HS-Aging). Although 11 of the 19 basal forebrain-positive cases had AD-intermediate pathology or greater, this represented only 21.6% of all participants with AD-intermediate or AD-high pathology. Moreover, as described above, when basal forebrain TDP-43 pathology was present in AD participants with HS-Aging, this was indistinguishable from pure HS-Aging or HS-Aging with LBD. This raises an important question about the relationship(s) between TDP-43 pathology and the combination of amyloid and tau pathology collectively termed AD neuropathologic change. It has been suggested that an important pathologic relationship exists between AD neuropathologic change and TDP-43 proteinopathy, at least in the mesial temporal lobe; these AD plus TDP-43 participants are significantly older at death, demonstrate greater hippocampal atrophy, and are more severely impaired on cognitive testing (44). Basal forebrain and ventral striatum TDP-43 pathology has also been described in approximately 20%–30% of patients with AD neuropathology in a study of patients with advanced AD neuropathology (the vast majority of TDP-43-positive and -negative groups having Braak stages V and VI) (23). The TDP-43-positive cases of that study (in any region), included up to 42% and 43% of patients, respectively, with HS-Aging versus 3%–10% of TDP-43-negative cases. The number of AD patients in that study with TDP-43 pathology in basal forebrain/ventral striatum and comorbid HS-Aging is not provided. Hu et al also found that the amygdala, followed by the amygdala and hippocampus, represented the most frequent sites of TDP-43 NCIs in participants with high-stage AD (Braak V and VI) (45). Our results, in tandem with these earlier findings (older age, association with hippocampal atrophy), suggest the possibility that HS-Aging accounts for a significant component of this AD plus TDP-43 relationship, at least within the region of basal forebrain. Further studies are warranted to clarify this relationship in patients with high AD neuropathology (with and without comorbid HS), the relationship of basal forebrain and hippocampal TDP-43 in these subgroups, and the relationship of these findings to clinical presentation.
Another intriguing possibility is that basal forebrain TDP-43 pathology reflects an as yet undescribed stage in the “sequential, regional” progression of TDP-43 deposition in limbic or limbic plus neocortical brain regions (7, 45). Though much remains to be examined in this brain region, circuits of basal forebrain organization are postulated that involve the hippocampus (eg, hippocampal-septal area-diagonal band) (46). This suggests an alternate possibility that these seemingly disparate brain regions share susceptibility to the deposition of TDP-43 protein in HS-Aging. Relative to this point, structures of the “extended amygdala” (eg, BNST and sublenticular extended amygdala, or SLEA) have been described in the basal forebrain based on morphologic and chemical neuroanatomic analyses (37, 46). The link between HS-Aging and basal forebrain TDP-43 pathology, independent of AD neuropathology, may thus reflect functional and anatomic links between these two regions or shared neuronal susceptibilities based on developmental relationships.
As one may expect, cognitively normal elderly patients typically have brain pathologies that are milder than those seen in patients with dementia (12). Recent studies have established TDP-43 pathology as an additional factor that may lower the overall threshold for cognitive impairment. Arnold et al identified TDP-43 pathology in the mesial temporal lobe in up to 36.4% of cognitively normal elderly patients, most frequently in the form of thick neurites (13). We did identify a neurite-predominant pattern in 7 of 19 participants, but these individuals were not cognitively normal. Longitudinal studies have also demonstrated that deposition of TDP-43 is significantly associated with rapid cognitive decline in aging (12). The finding here that TDP-43 pathology in the basal forebrain and adjacent nuclei is associated with cognitive impairment and is not part of normal cerebral aging is consistent with this observation. We found that individuals with basal forebrain TDP-43 pathology had significantly lower scores on the last MMSE available prior to death (24 vs 28), and significant reductions in the global score on the clinical dementia rating scale and in the memory subcategory. This is in keeping with findings that HS-Aging itself results in an amnestic disorder, resulting in “probable” or “possible” clinical AD diagnoses in 68% and 15% of patients, respectively, although HS-Aging patients typically have intact verbal fluency and better overall cognitive ability (41).
The one patient whom we did identify with successful cerebral aging in the consensus diagnosis of the UK-ADC participant database had features suggestive of possible early cognitive impairment. This 86-year-old woman lacked AD neuropathology and had a reduced MMSE score (final score of 28, entry score of 30) and hippocampal TDP-43 pathology without morphologic HS on routine stains. The pathology in this patient was similar to that seen in individuals with clinically confirmed dementia and pathologic HS-Aging (Fig. 3). This provides additional evidence that the TDP-43 proteinopathy in the basal forebrain is not secondary to AD pathology (7), but may instead represent a separate pathologic process strongly liked to HS-Aging itself, including in an early symptomatic or prodromal form. Confirmation of these findings and their role in early cognitive impairment will require systematic screening in a greater number of early symptomatic and/or asymptomatic individuals. For this purpose, a TDP-43-immunostained section of basal forebrain elements (LSA, BNST, VS, magnocellular neurons, olfactory region, and ventral putamen) at the level of the anterior commissure, anterior limb of the internal capsule, and globus pallidus might be of utility in a limited battery of TDP-43 stains performed in autopsies of aged individuals.
There are limitations of the present study, including the intrinsically retrospective nature of the study design. The study was conducted with archived sections available or with supplemental sections that could be generated from stored tissues. In rare instances, involving older archived cases, one or more anatomic regions were not available in the original material, and no additional tissue was available for sampling. In addition, the study cohort was not a population-based sample but rather representative cases from the UK-ADC Brain Bank. Finally, we did not have the phosphorylated TDP-43 antibody available for use in this study, although this may further increase the sensitivity of similar and follow-up studies in the future.
Despite these limitations, we have identified a pattern of basal forebrain pathology that is common in patients with comorbid HS-Aging. Further, to our knowledge, this is the largest study systematically examining this critical brain region across an entire spectrum of diseases, including controls, AD, and LBD. The fact that TDP-43 evaluation was performed blinded to original brain bank diagnoses is an additional strength of the study. Additional factors to be considered in future studies will be whether extrahippocampal pathology in HS-Aging is uniquely linked to genetic alterations, including polymorphisms in ATP-binding cassette, subfamily C member 9 (ABCC9) (47), TMEM106B, and granulin (GRN), and C9orf72 expansion (48), as well as the role of small vessel disease in this brain region with respect to TDP-43 (42).
In summary, we systematically screened components of the basal forebrain, hypothalamus, and adjacent deep nuclei in 98 individuals of whom approximately one-third were cognitively normal at last examination. A strong association was observed between comorbid HS-Aging and TDP-43 pathology in ventromedial basal forebrain and surrounding structures, frequently with neurites and GCIs. This association was observed independent of other contexts (pure HS-Aging or comorbid with AD or LBD). Among the cohort was a single individual, exhibiting seemingly successful cerebral aging, that had a milder but otherwise identical pattern of pathology, possibly representing an early or prodromal stage of HS-Aging (TDP-43 inclusions were present in hippocampus without H&E findings of HS). These findings expand our understanding of HS-Aging, raising the question as to whether some or most individuals manifest an extensive, septal-basal forebrain-hippocampal form of TDP-43 proteinopathy. Our results also expand our understanding of TDP-43 pathology in association with other comorbid illnesses and call into question whether this is truly a secondary phenomenon in all cases, particularly with AD or LBD, or whether it is an independent process driven largely by the disease currently termed HS-Aging. Extension and confirmation of these findings, and whether the definition of HS-Aging itself needs to be expanded, awaits further studies.
ACKNOWLEDGMENTS
At the University of Kentucky Sanders-Brown Center on Aging, we are grateful for the technical assistance of Ms. Sonya Anderson and Ms Ela Patel. At Houston Methodist Hospital, we are grateful for the technical assistance of Ms Candice Hamilton and Ms Sandra Steptoe.
REFERENCES
- 1.Mackenzie IR, Bigio EH, Ince PG, et al. Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Ann Neurol 2007;61:427–34 [DOI] [PubMed] [Google Scholar]
- 2.Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 2006;314:130–3 [DOI] [PubMed] [Google Scholar]
- 3.Dickson DW, Josephs KA, Amador-Ortiz C. TDP-43 in differential diagnosis of motor neuron disorders. Acta Neuropathol 2007;114:71–9 [DOI] [PubMed] [Google Scholar]
- 4.Mackenzie IR, Neumann M, Baborie A, et al. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol 2011;122:111–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ling JP, Pletnikova O, Troncoso JC, et al. TDP-43 repression of nonconserved cryptic exons is compromised in ALS-FTD. Science 2015;349:650–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amador-Ortiz C, Lin WL, Ahmed Z, et al. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer's disease. Ann Neurol 2007;61:435–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Josephs KA, Murray ME, Whitwell JL, et al. Staging TDP-43 pathology in Alzheimer's disease. Acta Neuropathol 2014;127:441–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker AK, Daniels CM, Goldman JE, et al. Astrocytic TDP-43 pathology in Alexander disease. J Neurosci 2014;34:6448–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kouri N, Oshima K, Takahashi M, et al. Corticobasal degeneration with olivopontocerebellar atrophy and TDP-43 pathology: An unusual clinicopathologic variant of CBD. Acta Neuropathol 2013;125:741–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKee AC, Gavett BE, Stern RA, et al. TDP-43 proteinopathy and motor neuron disease in chronic traumatic encephalopathy. J Neuropathol Exp Neurol 2010;69:918–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson PT, Abner EL, Schmitt FA, et al. Modeling the association between 43 different clinical and pathological variables and the severity of cognitive impairment in a large autopsy cohort of elderly persons. Brain Pathol 2010;20:66–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson RS, Yu L, Trojanowski JQ, et al. TDP-43 pathology, cognitive decline, and dementia in old age. JAMA Neurol 2013;70:1418–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnold SJ, Dugger BN, Beach TG. TDP-43 deposition in prospectively followed, cognitively normal elderly individuals: Correlation with argyrophilic grains but not other concomitant pathologies. Acta Neuropathol 2013;126:51–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carpenter MB. Core Text of Neuroanatomy. 3rd ed Baltimore: Williams & Wilkins; 1985 [Google Scholar]
- 15.Crosby EC, Humphrey T, Lauer EW. Correlative Anatomy of the Nervous System. New York: MacMillan; 1962 [Google Scholar]
- 16.Mai JK, Paxinos G. The Human Nervous System. 3rd ed Amsterdam: Elsevier Academic Press; 2012 [Google Scholar]
- 17.Crosby EC, Humphrey T. Studies of the vertebrate telencephalon. II. The nuclear pattern of the anterior olfactory nucleus, tuberculum olfactorium and the amygdaloid complex in adult man. J Comp Neurol 1941;74:309–52 [Google Scholar]
- 18.Yilmazer-Hanke DM. Amygdala In: Mai JK, Paxinos G, eds. The Human Nervous System. Amsterdam: Elsevier Academic Press; 2012:759–835 [Google Scholar]
- 19.Schultz C, Ghebremedhin E, Braak H, et al. Neurofibrillary pathology in the human paraventricular and supraoptic nuclei. Acta Neuropathol 1997;94:99–102 [DOI] [PubMed] [Google Scholar]
- 20.Braak H, Braak E. Cortical and subcortical argyrophilic grains characterize a disease associated with adult onset dementia. Neuropathol Appl Neurobiol 1989;15:13–26 [DOI] [PubMed] [Google Scholar]
- 21.Braak H, Del Tredici K, Rub U, et al. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 2003;24:197–211 [DOI] [PubMed] [Google Scholar]
- 22.Cykowski MD, Takei H, Schulz PE, et al. TDP-43 pathology in the basal forebrain and hypothalamus of patients with amyotrophic lateral sclerosis. Acta Neuropathol Commun 2014;2:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Josephs KA, Whitwell JL, Weigand SD, et al. TDP-43 is a key player in the clinical features associated with Alzheimer's disease. Acta Neuropathol 2014;127:811–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmitt FA, Nelson PT, Abner E, et al. University of Kentucky Sanders-Brown healthy brain aging volunteers: Donor characteristics, procedures and neuropathology. Curr Alzheimer Res 2012;9:724–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson PT, Jicha GA, Schmitt FA, et al. Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: Neuritic plaques and neurofibrillary tangles “do count” when staging disease severity. J Neuropathol Exp Neurol 2007;66:1136–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ighodaro ET, Jicha GA, Schmitt FA, et al. Hippocampal sclerosis of aging can be segmental: Two cases and review of the literature. J Neuropathol Exp Neurol 2015;74:642–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Markesbery WR, Schmitt FA, Kryscio RJ, et al. Neuropathologic substrate of mild cognitive impairment. Arch Neurol 2006;63:38–46 [DOI] [PubMed] [Google Scholar]
- 28.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psych Res 1975;12:189–98 [DOI] [PubMed] [Google Scholar]
- 29.Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 1993;43:2412–4 [DOI] [PubMed] [Google Scholar]
- 30.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991;82:239–59 [DOI] [PubMed] [Google Scholar]
- 31.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology 1991;41:479–86 [DOI] [PubMed] [Google Scholar]
- 32.Nelson PT, Schmitt FA, Lin YS, et al. Hippocampal sclerosis in advanced age: Clinical and pathological features. Brain 2011;134:1506–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nelson PT, Smith CD, Abner EL, et al. Hippocampal sclerosis of aging, a prevalent and high-morbidity brain disease. Acta Neuropathol 2013;126:161–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: A practical approach. Acta Neuropathol 2012;123:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saper CB. Hypothalamus In: Mai JK, Paxinos G, eds. The Human Nervous System. Amsterdam: Elsevier Academic Press; 2012:548–83 [Google Scholar]
- 36.Braak H, Braak E. The hypothalamus of the human adult: Chiasmatic region. Anat Embryol 1987;175:315–30 [DOI] [PubMed] [Google Scholar]
- 37.Haber SN, Adler A, Bergman H. The basal ganglia In: Mai JK, Paxinos G, eds. The Human Nervous System. Amsterdam: Elsevier Academic Press; 2012:678–738 [Google Scholar]
- 38.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2015 [Google Scholar]
- 39.Dickson D, Weller RO. Neurodegeneration: The Molecular Pathology of Dementia and Movement Disorders. 2nd ed Chichester, West Sussex: Wiley-Blackwell: International Society of Neuropathology; 2011 [Google Scholar]
- 40.Pao WC, Dickson DW, Crook JE, et al. Hippocampal sclerosis in the elderly: Genetic and pathologic findings, some mimicking Alzheimer disease clinically. Alzheimer Dis Assoc Disord 2011;25:364–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brenowitz WD, Monsell SE, Schmitt FA, et al. Hippocampal sclerosis of aging is a ley Alzheimer's disease mimic: Clinical-pathologic correlations and comparisons with both Alzheimer's Disease and non-tauopathic frontotemporal lobar degeneration. J Alzheimer Dis 2014;39:691–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neltner JH, Abner EL, Baker S, et al. Arteriolosclerosis that affects multiple brain regions is linked to hippocampal sclerosis of ageing. Brain 2014;137:255–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zarow C, Sitzer TE, Chui HC. Understanding hippocampal sclerosis in the elderly: Epidemiology, characterization, and diagnostic issues. Curr Neurol Neurosci Rep 2008;8:363–70 [DOI] [PubMed] [Google Scholar]
- 44.Josephs KA, Whitwell JL, Knopman DS, et al. Abnormal TDP-43 immunoreactivity in AD modifies clinicopathologic and radiologic phenotype. Neurology 2008;70:1850–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu WT, Josephs KA, Knopman DS, et al. Temporal lobar predominance of TDP-43 neuronal cytoplasmic inclusions in Alzheimer disease. Acta Neuropathol 2008;116:215–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alheid GF. Extended amygdala and basal forebrain. Ann N Y Acad Sci 2003;985:185–205 [DOI] [PubMed] [Google Scholar]
- 47.Nelson PT, Estus S, Abner EL, et al. ABCC9 gene polymorphism is associated with hippocampal sclerosis of aging pathology. Acta Neuropathol 2014;127:825–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murray ME, Bieniek KF, Banks Greenberg M, et al. Progressive amnestic dementia, hippocampal sclerosis, and mutation in C9ORF72. Acta Neuropathol 2013;126:545–54 [DOI] [PMC free article] [PubMed] [Google Scholar]