Abstract
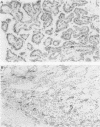
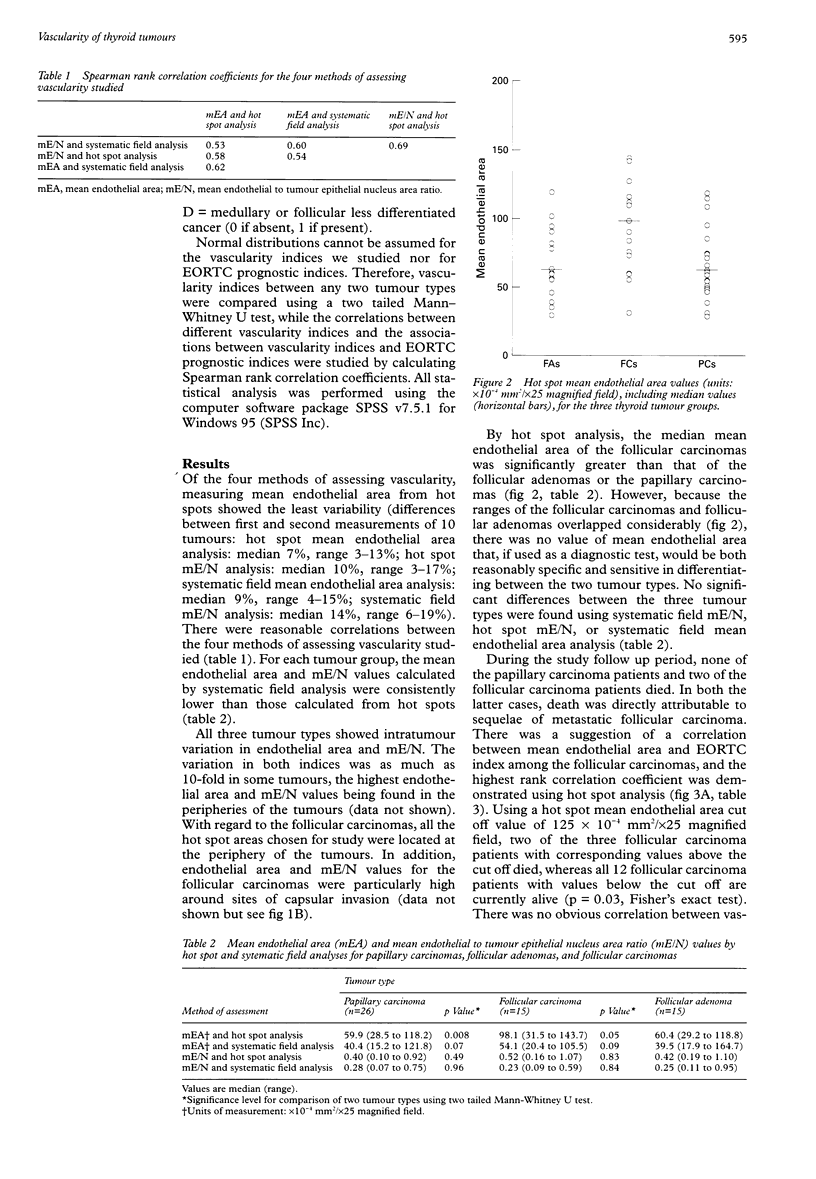
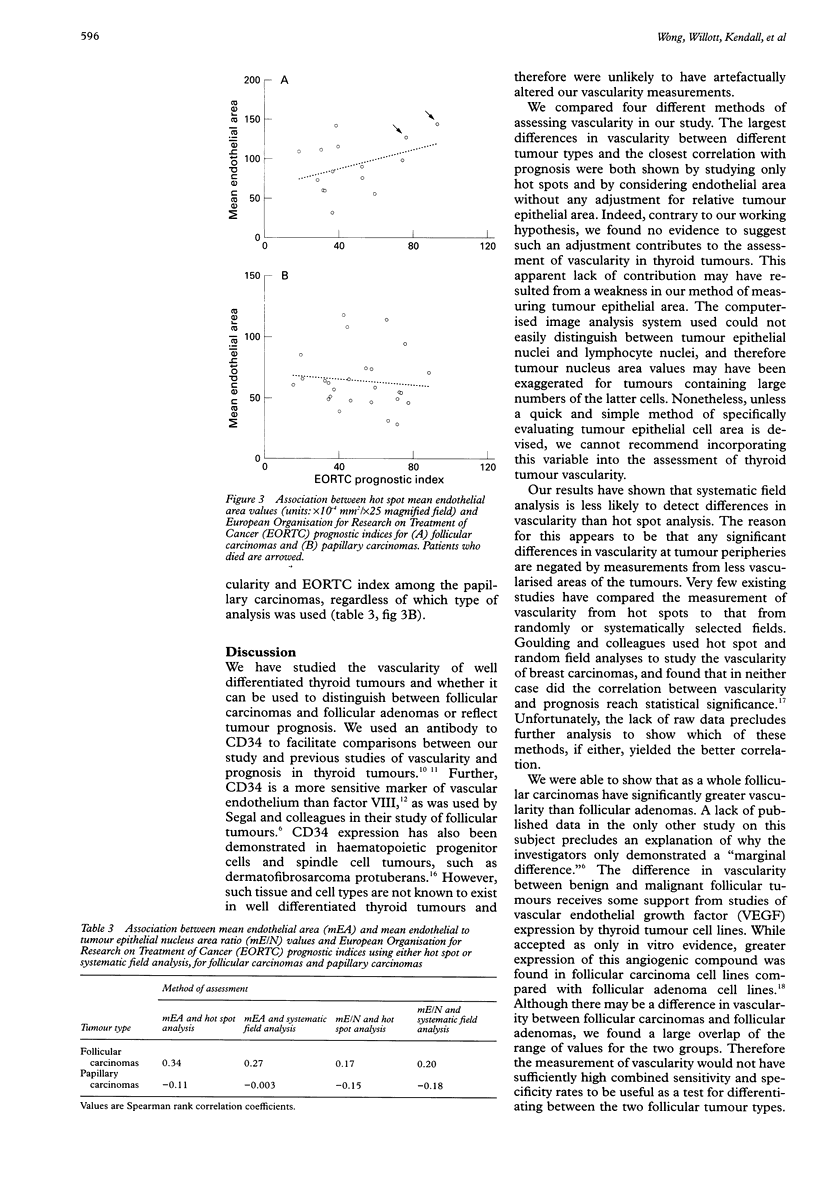
AIMS: To determine whether the measurement of vascularity can be used to differentiate follicular adenomas from follicular carcinomas or to reflect the prognosis of follicular carcinomas and papillary carcinomas of the thyroid gland, and to compare four methods of assessing vascularity. METHODS: Tissue sections from 26 papillary carcinomas, 15 follicular adenomas, and 15 follicular carcinomas were stained with an antibody to CD34. A computerised image analysis system was used to calculate, for each tumour, mean endothelial areas and the mean endothelium to tumour epithelial nucleus area ratio from 10 systematically selected fields across one dimension of the tumour ("systematic field" analysis) or from the three most vascularised fields of the tumour ("hot spot" analysis). A European Organisation for Research on Treatment of Cancer (EORTC) prognostic index was calculated for each papillary carcinoma and follicular carcinoma. RESULTS: Significant differences in vascularity between the three tumour groups could only be shown by comparing mean endothelial area values measured from hot spots. While the hot spot median mean endothelial area of follicular carcinomas was significantly greater than that of follicular adenomas, there was a large overlap between the two groups. For follicular carcinomas, higher hot spot mean endothelial area values were related to worse prognosis as indicated by the EORTC prognostic indices. No association between vascularity and prognosis was found for the papillary carcinomas, regardless of the method of assessing vascularity. CONCLUSIONS: Measuring endothelial area from hot spots using a computerised image analysis system is a sensitive method of assessing the vascularity of thyroid tumours. While vascularity measurement cannot be recommended as a practical tool for differentiating between malignant and benign follicular tumours, the suggestion that vascularity may reflect prognosis for follicular carcinomas deserves further study.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbareschi M., Gasparini G., Morelli L., Forti S., Dalla Palma P. Novel methods for the determination of the angiogenic activity of human tumors. Breast Cancer Res Treat. 1995;36(2):181–192. doi: 10.1007/BF00666039. [DOI] [PubMed] [Google Scholar]
- Byar D. P., Green S. B., Dor P., Williams E. D., Colon J., van Gilse H. A., Mayer M., Sylvester R. J., van Glabbeke M. A prognostic index for thyroid carcinoma. A study of the E.O.R.T.C. Thyroid Cancer Cooperative Group. Eur J Cancer. 1979 Aug;15(8):1033–1041. doi: 10.1016/0014-2964(79)90291-3. [DOI] [PubMed] [Google Scholar]
- Cohen P. R., Rapini R. P., Farhood A. I. Expression of the human hematopoietic progenitor cell antigen CD34 in vascular and spindle cell tumors. J Cutan Pathol. 1993 Feb;20(1):15–20. doi: 10.1111/j.1600-0560.1993.tb01243.x. [DOI] [PubMed] [Google Scholar]
- Fontanini G., Vignati S., Pacini F., Pollina L., Basolo F. Microvessel count: an indicator of poor outcome in medullary thyroid carcinoma but not in other types of thyroid carcinoma. Mod Pathol. 1996 Jun;9(6):636–641. [PubMed] [Google Scholar]
- Fox S. B., Leek R. D., Weekes M. P., Whitehouse R. M., Gatter K. C., Harris A. L. Quantitation and prognostic value of breast cancer angiogenesis: comparison of microvessel density, Chalkley count, and computer image analysis. J Pathol. 1995 Nov;177(3):275–283. doi: 10.1002/path.1711770310. [DOI] [PubMed] [Google Scholar]
- Fox S. B. Tumour angiogenesis and prognosis. Histopathology. 1997 Mar;30(3):294–301. doi: 10.1046/j.1365-2559.1997.d01-606.x. [DOI] [PubMed] [Google Scholar]
- Goldenberg J. D., Portugal L. G., Wenig B. L., Ferrer K., Wu J. C., Sabnani J. Well-differentiated thyroid carcinomas: p53 mutation status and microvessel density. Head Neck. 1998 Mar;20(2):152–158. doi: 10.1002/(sici)1097-0347(199803)20:2<152::aid-hed9>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Goulding H., Abdul Rashid N. F., Robertson J. F., Bell J. A., Elston C. W., Blamey R. W., Ellis I. O. Assessment of angiogenesis in breast carcinoma: an important factor in prognosis? Hum Pathol. 1995 Nov;26(11):1196–1200. doi: 10.1016/0046-8177(95)90193-0. [DOI] [PubMed] [Google Scholar]
- Hruban R. H., Huvos A. G., Traganos F., Reuter V., Lieberman P. H., Melamed M. R. Follicular neoplasms of the thyroid in men older than 50 years of age. A DNA flow cytometric study. Am J Clin Pathol. 1990 Nov;94(5):527–532. doi: 10.1093/ajcp/94.5.527. [DOI] [PubMed] [Google Scholar]
- Jiang W. G., Puntis M. C., Hallett M. B. Molecular and cellular basis of cancer invasion and metastasis: implications for treatment. Br J Surg. 1994 Nov;81(11):1576–1590. doi: 10.1002/bjs.1800811107. [DOI] [PubMed] [Google Scholar]
- Leon S. P., Folkerth R. D., Black P. M. Microvessel density is a prognostic indicator for patients with astroglial brain tumors. Cancer. 1996 Jan 15;77(2):362–372. doi: 10.1002/(SICI)1097-0142(19960115)77:2<362::AID-CNCR20>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Pavlopoulos P. M., Konstantinidou A. E., Agapitos E., Kavantzas N., Nikolopoulou P., Davaris P. A morphometric study of neovascularization in colorectal carcinoma. Cancer. 1998 Nov 15;83(10):2067–2075. [PubMed] [Google Scholar]
- Ramani P., Bradley N. J., Fletcher C. D. QBEND/10, a new monoclonal antibody to endothelium: assessment of its diagnostic utility in paraffin sections. Histopathology. 1990 Sep;17(3):237–242. doi: 10.1111/j.1365-2559.1990.tb00713.x. [DOI] [PubMed] [Google Scholar]
- Tuccari G., Barresi G. Immunohistochemical demonstration of ceruloplasmin in follicular adenomas and thyroid carcinomas. Histopathology. 1987 Jul;11(7):723–731. doi: 10.1111/j.1365-2559.1987.tb02686.x. [DOI] [PubMed] [Google Scholar]
- Vesalainen S., Lipponen P., Talja M., Alhava E., Syrjänen K. Tumor vascularity and basement membrane structure as prognostic factors in T1-2MO prostatic adenocarcinoma. Anticancer Res. 1994 Mar-Apr;14(2B):709–714. [PubMed] [Google Scholar]
- Vierbuchen M., Schröder S., Uhlenbruck G., Ortmann M., Fischer R. CA 50 and CA 19-9 antigen expression in normal, hyperplastic, and neoplastic thyroid tissue. Lab Invest. 1989 May;60(5):726–732. [PubMed] [Google Scholar]
- Viglietto G., Maglione D., Rambaldi M., Cerutti J., Romano A., Trapasso F., Fedele M., Ippolito P., Chiappetta G., Botti G. Upregulation of vascular endothelial growth factor (VEGF) and downregulation of placenta growth factor (PlGF) associated with malignancy in human thyroid tumors and cell lines. Oncogene. 1995 Oct 19;11(8):1569–1579. [PubMed] [Google Scholar]