Abstract
Background:
Increased level of serum macrophage inhibitory cytokine-1 (MIC-1), a member of transforming growth factor-β superfamily, was found in patients with epithelial tumors. This study aimed to evaluate whether serum level of MIC-1 can be a candidate diagnostic and prognostic indicator for early-stage nonsmall cell lung cancer (NSCLC).
Methods:
A prospective study enrolled 152 patients with Stage I–II NSCLC, who were followed up after surgical resection. Forty-eight patients with benign pulmonary disease (BPD) and 105 healthy controls were also included in the study. Serum MIC-1 levels were measured using an enzyme-linked immunosorbent assay, and the association with clinical and prognostic features was analyzed.
Results:
In patients with NSCLC, serum protein levels of MIC-1 were significantly increased compared with healthy controls and BPD patients (all P < 0.001). A threshold of 1000 pg/ml of MIC-1 was found in patients with early-stage (Stage I and II) NSCLC, with sensitivity and specificity of 70.4% and 99.0%, respectively. The serum levels of MIC-1 were associated with age (P = 0.001), gender (P = 0.030), and T stage (P = 0.022). Serum MIC-1 threshold of 1465 pg/ml was found in patients with poor early outcome, with sensitivity and specificity of 72.2% and 66.1%, respectively. The overall 3-year survival rate of NSCLC patients with high serum levels of MIC-1 (≥1465 pg/ml) was lower than that of NSCLC patients with low serum MIC-1 levels (77.6% vs. 94.8%). Multivariate Cox regression survival analysis showed that a high serum level of MIC-1 was an independent risk factor for reduced overall survival (hazard ratio = 3.37, 95% confidential interval: 1.09–10.42, P = 0.035).
Conclusion:
The present study suggested that serum MIC-1 may be a potential diagnostic and prognostic biomarker for patients with early-stage NSCLC.
Keywords: Macrophage Inhibitory Cytokine-1, Nonsmall Cell Lung Cancer, Sensitivity, Specificity
INTRODUCTION
Nonsmall cell lung cancer (NSCLC) is one of the most common cancers worldwide and has a poor prognosis.[1] Surgery remains the most important treatment method for patients with stage I–IIIa NSCLC. Conventional platinum-based adjuvant chemotherapy after surgery for patients with Stage II and III NSCLC can improve the prognosis.[2,3,4,5] However, the current use of postoperative adjuvant therapy for Stage Ia and Ib NSCLC is still controversial.[6] About 40–50% of early-stage (Stage I and II) NSCLC patients experience local recurrence and/or metastasis following surgical treatment, with a 5-year survival rate of about 60–90%.[7,8,9,10,11] The international tumor-node-metastasis (TNM) staging system is an important method to evaluate the extent of lesions of NSCLC; however, differences in prognosis may still exist in patients with similar tumor stage.[12,13] Therefore, if NSCLC patients at high risk of tumor recurrence or metastasis were screened out in the early-stage, more aggressive, systemic, and local treatment could be implemented to prolong patient survival.
Macrophage inhibitory cytokine-1 (MIC-1), a member of transforming growth factor-β superfamily, is involved in the inflammatory response and tissue repair following acute injury.[14] The expression levels of serum MIC-1 have been reported to be increased in patients with different types of cancer, and some authors have suggested that serum MIC-1 may relate to tumor pathogenesis. The expression levels of MIC-1 have been shown to be increased in brain tumors, melanoma, lung cancer, gastrointestinal and pancreatic cancer, colorectal cancer, prostate cancer, and breast cancer.[15,16,17] MIC-1 may have multiple roles in the development of tumors and may be involved in the proliferation, migration, metastasis, and drug resistance.[18] In addition, clinical data have shown that overexpression of MIC-1 protein in tumor cells was associated with a poor prognosis.[19,20] These previous studies indicated that MIC-1 may have potential as a novel biomarker for diagnosis and prognosis as well as a novel therapeutic target for malignant tumors.
In this preliminary prospective cohort study in our center, we chose to use an MIC-1 serum test kit that we had previously developed and validated, to study patients with early-stage NSCLC, who received standard treatment in Cancer Hospital of Chinese Academy of Medical Sciences from 2011 to 2013. This study aimed to evaluate the diagnostic and prognostic values of serum MIC-1 measurement in the early-stage (Stage I and II) NSCLC.
METHODS
Clinical data
This study was approved by the Ethics Committee of Cancer Institute and Hospital, Chinese Academy of Medical Sciences. Written informed consent from all patients was obtained. Adult patients (18 years and older) with early-stage NSCLC were enrolled in this study, who underwent radical resection of primary lung cancer with systematic lymph node dissection from September 2011 to May 2013 in the Department of Thoracic Surgery, Cancer Institute and Hospital, Chinese Academy of Medical Sciences.
The diagnosis of NSCLC in these patients was confirmed postoperatively by cytology and histopathology at the Department of Pathology, Cancer Institute and Hospital, Chinese Academy of Medical Sciences. Stage I and II tumors were defined according to the Union for International Cancer Control TNM staging criteria for lung cancer. Postoperative patient treatment was performed according to the guidelines of the National Comprehensive Cancer Network.
The exclusion criteria included: (1) patients with incomplete image data and undefined clinical stage; (2) suspected or diagnosed metastatic carcinoma, combined with other malignant tumors by abdominal computed tomography (CT)/ultrasound, brain magnetic resonance imaging (MRI)/CT, or whole-body bone scanning; (3) previous history of cancer or preoperative radiotherapy and chemotherapy and other adjuvant therapies; or (4) poor compliance.
The study included a total of 152 qualified patients with Stage I–II NSCLC, 48 patients with benign pulmonary disease (BPD), and 105 healthy controls. BPD patients were diagnosed by cytology and/or histopathology at the Department of Pathology, Cancer Institute and Hospital, Chinese Academy of Medical Sciences. Healthy controls were selected from attendees including our hospital staff and population receiving cancer screening tests at our center, and all physical examinations, serological examination of liver and renal functions, and imaging results were negative.
A volume of 2 ml whole blood sample was collected from each enrolled NSCLC patient before treatment and after admission to the hospital. The blood serum was isolated and frozen at –80°C. Clinical information and results of serum samples were collected and input into the structured database by the physicians who were not directly involved in patient diagnosis, treatment, or sample examination. The patient diagnostic and pathological data were blinded during serum sample collection and biomarker detection.
Enzyme-linked immunosorbent assay for serum macrophage inhibitory cytokine-1
An enzyme-linked immunosorbent assay (ELISA) kit, previously developed by the Biological Testing Center, Cancer Institute and Hospital, Chinese Academy of Medical Sciences and manufactured by Beijing Jin Zijing Biological Technology Co., Ltd., was used. The absorbance value is positively correlated with the MIC-1 level in serum and standard samples, and the MIC-1 concentration in the samples was obtained through the use of standard dose-response curve. A critical reference value for serum levels of MIC-1 was set at 1000 pg/ml.
Postoperative follow-up
Patients were followed up every 6 months following surgery. Routine enhanced chest CT was performed. Abdominal CT/ultrasound, brain MRI/CT, and whole-body bone scanning were conducted according to the clinical situation. The site and time of recurrence or metastasis of tumor and the time and cause of death were recorded. Follow-up data were obtained at outpatient visits and through telephone interviews. The primary outcome was tumor death, and the secondary outcome was tumor recurrence or metastasis. Survival rate was calculated from the day of surgery to the end date of the primary outcome event or the last follow-up (November 2015).
Statistical analysis
Statistical analysis was performed using SPSS version 22.0 (SPSS Inc., Chicago, IL, USA). The data for serum MIC-1 levels and age were shown as mean ± standard deviation (SD). Data from two groups of independent samples were analyzed for homogeneity of variance and compared using independent samples t-test. Multiple independent samples were compared using one-way analysis of variance, counting data were compared with Chi-square test, and categorical data were compared using nonparametric test. The role of serum MIC-1 levels in the diagnosis and prognosis of early-stage NSCLC was evaluated by receiver operating characteristics (ROCs) curves. Survival curves were estimated using the Kaplan-Meier method, and differences between them were evaluated by the log-rank test. Cox proportional hazards model regression test was used to estimate univariate and multivariate hazard ratios (HRs) for prognosis. A P < 0.05 was considered statistically significant.
RESULTS
Baseline demographic data
A total of 152 early-stage NSCLC patients were enrolled in this study, including 89 females (58.5%) and 63 males (41.5%). The mean age of these patients was 58.8 ± 8.2 years (range: 27–79 years). There were 98 patients (64.5%) with Stage I NSCLC and 54 patients (35.5%) with Stage II NSCLC; 116 patients (76.3%) with adenocarcinoma and 36 patients (23.7%) with squamous cell carcinoma; and 38 patients (25.0%) with high- to moderate-grade NSCLC and 114 patients (75.0%) with low-grade NSCLC. There were 105 patients (69.1%) with tumors <3 cm in diameter and 47 patients (30.9%) with tumors ≥3 cm in diameter.
Among 48 BPD patients, 20 (41.7%) were males and 28 (58.3%) were females; the mean age was 53.2 ± 10.1 years (range: 23–75 years). In these BPD patients, histological diagnoses showed that 18 patients (37.5%) had pulmonary inflammation, 13 (27.1%) had tuberculosis, 8 (16.7%) had pulmonary hamartoma, 6 (12.5%) had a benign sclerosing hemangioma, 2 (4.2%) had benign adenomatoid hyperplasia, and 1 patient (2.1%) had a benign lung cyst.
Healthy controls consisted of 105 cases from Cancer Institute and Hospital, Chinese Academy of Medical Sciences, of which 59 (56.2%) were males and 46 (43.8%) were females, with mean age of 55.4 ± 9.3 years (range: 20–79 years). No digestive disease was found, and liver and renal functions were normal.
There was no significant difference among the NSCLC patients, BPD patients, and healthy controls after the comparison of baseline demographic data (P > 0.05).
Diagnostic values of serum macrophage inhibitory cytokine-1 protein level in the early-stage nonsmall cell lung cancer
This study showed that the overall mean serum MIC-1 level of patients with early-stage NSCLC was 1325 ± 848 pg/ml, which was significantly higher than those of BPD patients (848 ± 183 pg/ml; t = 7.164, P < 0.001) and healthy controls (367 ± 207 pg/ml; t = 10.481, P < 0.001).
The studies involved 152 early-stage NSCLC patients and 105 healthy controls to evaluate the diagnostic accuracy of MIC-1. The ROC analyses yielded an area under curve (AUC) value of 0.90 (95% confidence interval [CI]= 0.80–0.94) [Figure 1]. When using 1000 pg/ml as the cutoff value for MIC-1, the optimal sensitivity and specificity were 70.4% and 99.0%, respectively. Multivariate logistic regression analyses on variables including age, gender, and serum MIC-1 showed that MIC-1 was a potential diagnostic biomarker for NSCLC (P < 0.001).
Figure 1.
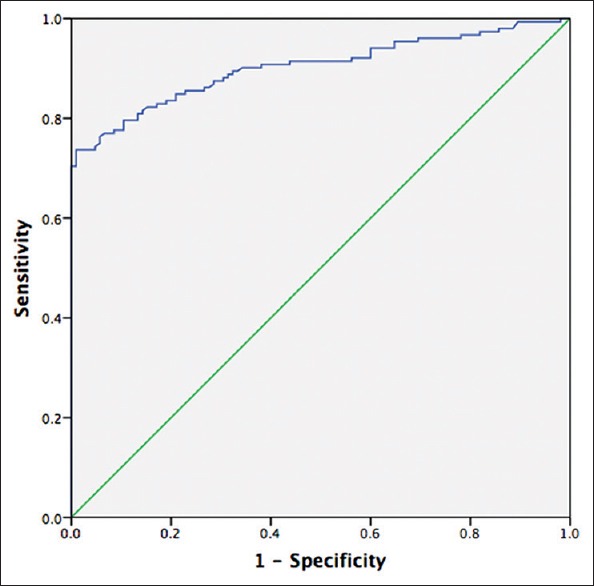
The ROC curves of serum MIC-1 levels for the diagnosis of NSCLC (AUC: 0.90, 95% CI: 0.80–0.94, P < 0.001). ROC: Receiver operating characteristic; MIC-1: Macrophage inhibitory cytokine-1; NSCLC: Nonsmall cell lung cancer; AUC: Area under ROC curve; CI: Confidence interval.
Relationship between serum macrophage inhibitory cytokine-1 level and clinicopathological characteristics in patients with early-stage nonsmall cell lung cancer
In early-stage NSCLC patients, serum levels of MIC-1 were correlated with age (P = 0.001), gender (P = 0.030), and early postoperative death (within 3 years) (P = 0.042) [Table 1].
Table 1.
Comparison of the serum MIC-1 levels before treatment among early-stage NSCLC patients with different clinical and pathological characteristics
| Characteristics | n | Serum levels of MIC-1 (pg/ml), mean ± SD | t | P |
|---|---|---|---|---|
| Age | 1.052 | 0.001 | ||
| <60 years | 64 | 1221 ± 838 | ||
| ≥60 years | 88 | 1666 ± 810 | ||
| Gender | 0.303 | 0.030 | ||
| Female | 89 | 1604 ± 708 | ||
| Male | 63 | 1302 ± 725 | ||
| Tumor size | 0.352 | 0.200 | ||
| <3 cm | 105 | 1427 ± 887 | ||
| ≥3 cm | 47 | 1595 ± 749 | ||
| Smoking history | 0.384 | 0.060 | ||
| <20 years | 83 | 1361 ± 926 | ||
| ≥20 years | 69 | 1620 ± 726 | ||
| Histological type | 1.332 | 0.458 | ||
| Squamous cell | 36 | 1579 ± 730 | ||
| Adenocarcinoma | 116 | 1447 ± 910 | ||
| Stage | 2.420 | 0.141 | ||
| I | 98 | 1035 ± 814 | ||
| II | 54 | 1615 ± 898 | ||
| Differentiation | 0.352 | 0.184 | ||
| Moderate-well | 38 | 1320 ± 1057 | ||
| Poor | 114 | 1532 ± 764 | ||
| T stage | 4.008 | 0.066 | ||
| T1 | 42 | 1224 ± 648 | ||
| T2 | 101 | 1586 ± 924 | ||
| T3 | 9 | 1466 ± 512 | ||
| N stage | 0.252 | 0.193 | ||
| N0 | 124 | 1430 ± 798 | ||
| N1 | 24 | 1675 ± 1461 | ||
| N2 | 4 | 1881 ± 1600 | ||
| Recurrence/metastasis | 0.003 | 0.270 | ||
| No | 118 | 1438 ± 866 | ||
| Yes | 34 | 1618 ± 779 | ||
| Survival | 0.008 | 0.042 | ||
| Yes | 134 | 1428 ± 852 | ||
| No | 18 | 1859 ± 737 |
MIC-1: Macrophage inhibitory cytokine-1; NSCLC: Nonsmall cell lung cancer; SD: Standard deviation.
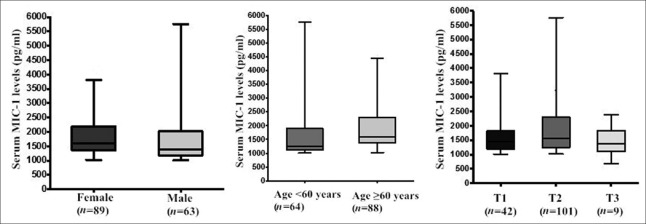
Serum levels of MIC-1 were significantly increased in women (P = 0.001) or elderly patients aged 60 years and older (P < 0.001). Although there was no significant difference in serum MIC-1 levels among stages of NSCLC, further intergroup comparisons showed that MIC-1 level of T2 patients was significantly greater than that of T1 patients (P = 0.022) [Figure 2]. However, MIC-1 levels were not significantly associated with tumor size, histological type, degree of tumor differentiation, stage, lymph node status, and recurrence/metastasis of early-stage NSCLC within 3 years following surgery (P > 0.05).
Figure 2.
Serum levels of MIC-1 in early-stage NSCLC patients with different clinical and pathological features. The serum levels of MIC-1 were associated with older age (P = 0.001), female (P = 0.030), and tumor T2 (P = 0.022, vs. T1 patients). MIC-1: Macrophage inhibitory cytokine-1; NSCLC: Nonsmall cell lung cancer.
Relationship between serum macrophage inhibitory cytokine-1 level and prognosis of early-stage nonsmall cell lung cancer
A total of 152 patients were enrolled in the study with a median follow-up time of 37.6 months. No patients were lost during the follow-up period. Primary outcome (death caused by tumor) occurred in 18 patients (11.8%), and the overall 3-year survival rate was 88.2%; secondary outcome (recurrence or metastasis) occurred in 34 patients (22.4%), of which 7 cases (4.6%) had local recurrence and 27 (17.8%) had distant metastases.
Of those patients who died during the follow-up period, serum MIC-1 levels before treatment (1859 ± 737 pg/ml) were considerably greater than those of the survived patients (1428 ± 852 pg/ml; P = 0.042) [Table 1]. The ROC curve was plotted from the serum MIC-1 level of early-stage NSCLC patients with postoperative outcomes within 3 years, with an AUC of 0.70 (95% CI: 0.59–0.81, P < 0.001) [Figure 3]. When the cutoff value of serum MIC-1 was set at 1465 pg/ml based on the ROC curve, the sensitivity and specificity for prediction of poor patient prognosis during the postoperative follow-up period were 72.2% and 66.1%, respectively.
Figure 3.
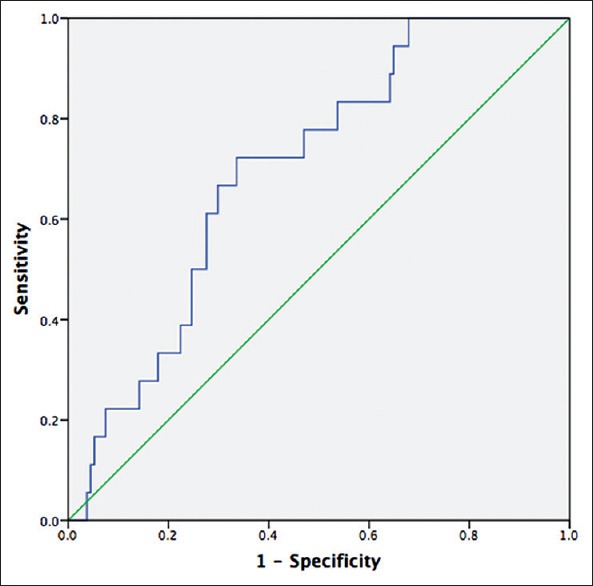
The ROC curves of serum MIC-1 levels for the prediction of postoperative outcomes within 3 years (AUC: 0.70, 95%, CI: 0.59–0.81, P < 0.001). ROC: Receiver operating characteristic; MIC-1: Macrophage inhibitory cytokine-1; AUC: Area under ROC curve; CI: Confidence interval.
Patients were grouped using a serum MIC-1 level of 1465 pg/ml as a criterion [Table 2]. Compared with the low serum MIC-1 group (MIC-1 <1465 pg/ml), the early-stage NSCLC patients in the high serum MIC-1 group (MIC-1 ≥1465 pg/ml) were predominantly female (75.9%) and older age (81.0%) (P < 0.01). There were relatively more long-term smokers in the high serum MIC-1 group, but no statistical difference was found. More patients with tumor diameter ≥3 cm (P = 0.011) and T2 stage (P = 0.034) and stage II (P = 0.026) were found in the high serum MIC-1 group, compared with the low serum MIC-1 group. In the high serum MIC-1 group, more patients with NSCLC died during follow-up, compared with the low serum MIC-1 group (22.4% vs. 5.3%, P = 0.002).
Table 2.
Clinicopathological characteristics of early-stage NSCLC patients with different MIC-1 levels, n (%)
| Factors | Total Patients (n = 152) | Patients with MIC-1 <1465 pg/ml (n = 94) | Patients with MIC-1 ≥1465 pg/ml (n = 58) | Patients with MIC-1 <1465 pg/ml vs. patients with MIC-1 ≥1465 pg/ml | |
|---|---|---|---|---|---|
| χ2 | P | ||||
| Gender | 15.357 | 0.001 | |||
| Male | 63 (41.5) | 49 (52.1) | 14 (24.1) | ||
| Female | 89 (58.5) | 45 (47.9) | 44 (75.9) | ||
| Age | 11.863 | <0.001 | |||
| <60 years | 64 (42.1) | 53 (56.4) | 11 (19.0) | ||
| ≥60 years | 88 (57.9) | 41 (43.6) | 47 (81.0) | ||
| Smoking history | 7.812 | 0.057 | |||
| <20 years | 83 (54.6) | 57 (60.6) | 26 (44.8) | ||
| ≥20 years | 69 (45.4) | 37 (39.4) | 32 (55.2) | ||
| Tumor size | 1.517 | 0.011 | |||
| <3 cm | 105 (69.1) | 72 (76.6) | 33 (56.9) | ||
| ≥3 cm | 47 (30.9) | 22 (23.4) | 25 (43.1) | ||
| Histological type | 4.835 | 0.210 | |||
| Squamous cell | 36 (23.7) | 19 (20.2) | 17 (29.3) | ||
| Adenocarcinoma | 116 (76.3) | 75 (79.8) | 41 (70.7) | ||
| Differentiation | 3.367 | 0.102 | |||
| Moderate-well | 38 (25.0) | 28 (29.8) | 10 (17.2) | ||
| Poor | 114 (75.0) | 66 (70.2) | 48 (82.8) | ||
| T stage | 5.317 | 0.034 | |||
| T1 | 42 (27.6) | 32 (34.0) | 10 (17.2) | ||
| T2 | 101 (66.5) | 57 (60.6) | 44 (75.9) | ||
| T3 | 9 (5.9) | 5 (5.3) | 4 (6.9) | ||
| N stage | 0.763 | 0.156 | |||
| N0 | 124 (81.6) | 80 (85.1) | 44 (75.9) | ||
| N1 | 24 (15.8) | 12 (12.8) | 12 (20.7) | ||
| N2 | 4 (2.6) | 2 (2.1) | 2 (3.4) | ||
| Stage | 0.245 | 0.026 | |||
| I | 98 (64.5) | 67 (71.3) | 31 (53.4) | ||
| II | 54 (35.5) | 27 (28.7) | 27 (46.6) | ||
| Survival | 5.906 | 0.002 | |||
| Yes | 134 (88.2) | 89 (94.7) | 45 (77.6) | ||
| No | 18 (11.8) | 5 (5.3) | 13 (22.4) | ||
| Recurrence/metastasis | 1.942 | 0.107 | |||
| No | 118 (77.6) | 77 (81.9) | 41 (70.7) | ||
| Yes | 34 (22.4) | 17 (18.1) | 17 (29.3) | ||
MIC-1: Macrophage inhibitory cytokine-1; NSCLC: Nonsmall cell lung cancer; *Mann-Whitney U test.
Kaplan-Meier survival curve showed that the 3-year survival rate of patients with serum MIC-1 ≥1465 pg/ml was 77.6%, which was significantly lower than that of patients with serum MIC-1 <1465 pg/ml (94.8%) [Figure 4]. The occurrence of the primary outcome event of death following surgery in the high serum MIC-1 group was earlier (log-rank, P = 0.002); 80% of the primary outcome events occurred 12–18 months following surgery in the low serum MIC-1 group. Death occurred 5–25 months postoperatively in the high serum MIC-1 group, indicating that different follow-up strategies may be needed for the two groups.
Figure 4.
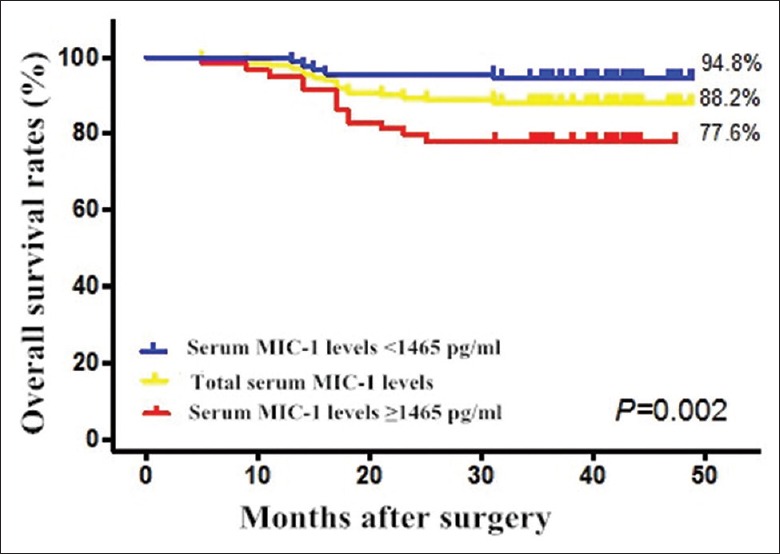
Overall survival rates of patients with low (<1465 pg/ml) and high serum levels of MIC-1 (≥1465 pg/ml) (P = 0.002). MIC-1: Macrophage inhibitory cytokine-1.
Univariate Cox survival analysis indicated that the high mortality risk was significantly associated with high serum levels of MIC-1 (HR: 4.56, 95% CI: 1.62–12.79, P = 0.004). After further elimination of the influencing factors that were potentially related to raised serum MIC-1 levels, such as gender, age, long-term smoking history, tumor size, and tumor stage, the multivariate Cox proportional hazards regression models indicated that high serum MIC-1 level was an independent risk factor for postoperative death (HR = 3.37, 95% CI: 1.09–10.42, P = 0.035) [Table 3]. These results indicated that high serum MIC-1 levels was an independent prognostic marker for predicting poorer overall survival in NSCLC patients.
Table 3.
Association between overall survival after surgery and the predictive factors in patients with early-stage NSCLC
| Items | B | SE | Wald | P | HR | 95% CI |
|---|---|---|---|---|---|---|
| MIC-1 | 1.211 | 0.576 | 4.429 | 0.035 | 3.370 | 1.09–10.42 |
| T stage | 6.675 | 0.036 | ||||
| T1 | 0.723 | 1.107 | 0.427 | 0.514 | 2.060 | 0.24–18.04 |
| T2 | 2.512 | 1.251 | 4.034 | 0.045 | 12.332 | 1.06–143.12 |
| Gender | −0.280 | 0.697 | 0.161 | 0.688 | 0.756 | 0.19–2.96 |
| Age | 1.036 | 0.722 | 2.062 | 0.151 | 2.819 | 0.69–11.60 |
| Smoking | −0.269 | 0.606 | 0.197 | 0.657 | 0.764 | 0.23–2.50 |
| Tumor size | 1.122 | 0.587 | 3.657 | 0.056 | 3.070 | 0.97–9.69 |
NSCLC: Nonsmall cell lung cancer; MIC-1: Macrophage inhibitory cytokine-1; SE: Standard error; HR: Hazard ratio; CI: Confidence interval.
DISCUSSION
In this study, a double antibody sandwich serum ELISA serum MIC-1 test kit was used to conduct a serologic study on early-stage NSCLC patients, BPD patients, and healthy controls. This study found that serum MIC-1 levels in patients with early-stage NSCLC were significantly greater than those of healthy controls (P < 0.001) and BPD patients (P < 0.001). These findings suggested that the serum MIC-1 protein may be involved in occurrence and development of NSCLC and may serve as a tumor biomarker in patients with NSCLC. In addition, when a serum MIC-1 level of 1000 pg/ml was set as a critical value, the sensitivity and specificity for the diagnosis of NSCLC were 70.4% and 99.0%, respectively, indicating that MIC-1 has practical value in the screening and early diagnosis of NSCLC.
Although low-dose computed tomography (LDCT) screening is used to screen out patients with early-stage cancer and to reduce the mortality of lung cancer, false-positive diagnoses still exist.[21,22] Therefore, it is possible that a specific serum marker, such as MIC-1, may assist LDCT in improving early detection rates of NSCLC.
Studies on the serum levels of MIC-1 in patients with early-stage NSCLC showed that the serum MIC-1 levels of females and elderly patients aged 60 years or older were higher than those of males, and young or middle-aged patients, suggesting that MIC-1 levels may be related to age and gender. Serum MIC-1 level in patients with T2 stage was greater than that of T1 stage NSCLC patients, indicating that the serum MIC-1 level could be associated with local invasion of early-stage NSCLC.
Further studies on the prognosis of patients indicated that serum MIC-1 level can be used as a biomarker to determine the prognosis of patients with early-stage NSCLC. The overall 3-year survival rate of patients with serum MIC-1 levels ≥1465 pg/ml was 77.6%, which was lower than that of patients with serum MIC-1 levels <1465 pg/ml (94.8%). After further elimination of the potential influences of factors, such as age, gender, long-term smoking history, tumor size, and degree of local tumor invasion (stage), it was found that high preoperative serum MIC-1 level was an independent risk factor for postoperative death of Stage I–II NSCLC patients. Therefore, preoperative serum MIC-1 level may be of statistically significance for the early screening of high-risk patients with poor prognosis.
When compared with other invasive tests and expensive molecular detection methods, serologic methods are simple, quick, and repeatable. They can also be helpful in preoperative risk stratification of patients, to guide the treatment decisions, and to improve the auxiliary treatments for NSCLC. The time of death following surgery in groups with different serum MIC-1 levels indicated that a postoperative follow-up within 18 months should be done in patients with low serum MIC-1 levels while continuous regular follow-ups are needed for patients with high serum MIC-1 levels.
There are some limitations in this study. First, it is unclear whether high-risk patients with NSCLC will benefit from serum MIC-1 level test, and any treatment guided by the serum MIC-1 levels need be validated by further study. Second, as serum levels of MIC-1 has been described in other benign diseases including chronic obstructive pulmonary disease and acute myocardial infarction, it may be difficult to differentiate whether serum MIC-1 level is specifically related to NSCLC. A large sample size is required to eliminate sampling error because our clinical materials were solely collected from one center. The results of this study should be interpreted with caution. The adjuvant therapy in this early-stage NSCLC population was variable while the relationship between postoperative adjuvant therapy and prognosis was not analyzed. We recommended that further studies be conducted on the application of the results to other patient populations.
In conclusion, the present study suggested that serum MIC-1 level may be a potential diagnostic and prognostic biomarker for patients with early-stage NSCLC.
Financial support and sponsorship
This work was supported by grants from the National Natural Science Foundation of China (No.81502023 and No.81441080), National High-tech R&D Program (863 Program) (No.2008AA02Z415), and Capital Characteristic clinic projects(No.Z12110700102066).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Xin Chen
REFERENCES
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:134. doi: 10.3322/caac.20107. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J International Adjuvant Lung Cancer Trial Collaborative Group. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350:351–60. doi: 10.1056/NEJMoa031644. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 3.Douillard JY, Rosell R, De Lena M, Carpagnano F, Ramlau R, Gonzáles-Larriba JL, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): A randomised controlled trial. Lancet Oncol. 2006;7:719–27. doi: 10.1016/S1470-2045(06)70804-X. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 4.Pisters KM, Evans WK, Azzoli CG, Kris MG, Smith CA, Desch CE, et al. Cancer Care Ontario and American Society of Clinical Oncology adjuvant chemotherapy and adjuvant radiation therapy for stages I-IIIA resectable non small-cell lung cancer guideline. J Clin Oncol. 2007;25:5506–18. doi: 10.1200/JCO.2007.14.1226. doi: 10.1200/JCO.2007.14.1226. [DOI] [PubMed] [Google Scholar]
- 5.Mu JW, Gao SG, Xue Q, Zhao J, Li N, Yang K, et al. A Matched Comparison Study of Uniportal Versus Triportal Thoracoscopic Lobectomy and Sublobectomy for Early-stage Nonsmall Cell Lung Cancer. Chin Med J. 2015;128:2731–5. doi: 10.4103/0366-6999.167298. doi: 10.4103/0366-6999.167298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strauss GM, Herndon JE, 2nd, Maddaus MA, Johnstone DW, Johnson EA, Harpole DH, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol. 2008;26:5043–51. doi: 10.1200/JCO.2008.16.4855. doi: 10.1200/JCO.2008.16.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams DE, Pairolero PC, Davis CS, Bernatz PE, Payne WS, Taylor WF, et al. Survival of patients surgically treated for stage I lung cancer. J Thorac Cardiovasc Surg. 1981;82:70–6. [PubMed] [Google Scholar]
- 8.Nesbitt JC, Putnam JB, Jr, Walsh GL, Roth JA, Mountain CF. Survival in early-stage non-small cell lung cancer. Ann Thorac Surg. 1995;60:466–72. doi: 10.1016/0003-4975(95)00169-l. doi: 10.1016/0003-4975(95)00169-L. [DOI] [PubMed] [Google Scholar]
- 9.Spiro SG, Porter JC. Lung cancer – Where are we today? Current advances in staging and nonsurgical treatment. Am J Respir Crit Care Med. 2002;166:1166–96. doi: 10.1164/rccm.200202-070SO. doi: 10.1164/rccm.200202-070SO. [DOI] [PubMed] [Google Scholar]
- 10.Martini N, Bains MS, Burt ME, Zakowski MF, McCormack P, Rusch VW, et al. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg. 1995;109:120–9. doi: 10.1016/S0022-5223(95)70427-2. doi: 10.1016/S0022-5223(95)70427-2. [DOI] [PubMed] [Google Scholar]
- 11.Kelsey CR, Marks LB, Hollis D, Hubbs JL, Ready NE, D’ Amico TA, et al. Local recurrence after surgery for early stage lung cancer: An 11-year experience with 975 patients. Cancer. 2009;115:5218–27. doi: 10.1002/cncr.24625. doi: 10.1002/cncr.24625. [DOI] [PubMed] [Google Scholar]
- 12.Goldstraw P, Ball D, Jett JR, Le Chevalier T, Lim E, Nicholson AG, et al. Non-small-cell lung cancer. Lancet. 2011;378:1727–40. doi: 10.1016/S0140-6736(10)62101-0. doi: 10.1016/S0140-6736(10)62101-0. [DOI] [PubMed] [Google Scholar]
- 13.Rami-Porta R, Goldstraw P. Strength and weakness of the new TNM classification for lung cancer. Eur Respir J. 2010;36:237–9. doi: 10.1183/09031936.00016210. doi: 10.1183/09031936.00016210. [DOI] [PubMed] [Google Scholar]
- 14.Trombly DJ, Woodruff TK, Mayo KE. Roles for transforming growth factor beta superfamily proteins in early folliculogenesis. Semin Reprod Med. 2009;27:14–23. doi: 10.1055/s-0028-1108006. doi: 10.1055/s-0028-1108006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown DA, Lindmark F, Stattin P, Bälter K, Adami HO, Zheng SL, et al. Macrophage inhibitory cytokine 1: A new prognostic marker in prostate cancer. Clin Cancer Res. 2009;15:6658–64. doi: 10.1158/1078-0432.CCR-08-3126. doi: 10.1158/1078-0432.CCR-08-3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shnaper S, Desbaillets I, Brown DA, Murat A, Migliavacca E, Schluep M, et al. Elevated levels of MIC-1/GDF15 in the cerebrospinal fluid of patients are associated with glioblastoma and worse outcome. Int J Cancer. 2009;125:2624–30. doi: 10.1002/ijc.24639. doi: 10.1002/ijc.24639. [DOI] [PubMed] [Google Scholar]
- 17.Xue H, Lü B, Zhang J, Wu M, Huang Q, Wu Q, et al. Identification of serum biomarkers for colorectal cancer metastasis using a differential secretome approach. J Proteome Res. 2010;9:545–55. doi: 10.1021/pr9008817. doi: 10.1021/pr9008817. [DOI] [PubMed] [Google Scholar]
- 18.Mimeault M, Batra SK. Divergent molecular mechanisms underlying the pleiotropic functions of macrophage inhibitory cytokine-1 in cancer. J Cell Physiol. 2010;224:626–35. doi: 10.1002/jcp.22196. doi: 10.1002/jcp.22196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown DA, Ward RL, Buckhaults P, Liu T, Romans KE, Hawkins NJ, et al. MIC-1 serum level and genotype: Associations with progress and prognosis of colorectal carcinoma. Clin Cancer Res. 2003;9:2642–50. [PubMed] [Google Scholar]
- 20.Zhao L, Lee BY, Brown DA, Molloy MP, Marx GM, Pavlakis N, et al. Identification of candidate biomarkers of therapeutic response to docetaxel by proteomic profiling. Cancer Res. 2009;69:7696–703. doi: 10.1158/0008-5472.CAN-08-4901. doi: 10.1158/0008-5472.CAN-08-4901. [DOI] [PubMed] [Google Scholar]
- 21.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, et al. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Infante M, Cavuto S, Lutman FR, Brambilla G, Chiesa G, Ceresoli G, et al. Arandomized study of lung cancer screening with spiral computed tomography: Three-year results from the DANTE trial. Am J Respir Crit Care Med. 2009;180:445–53. doi: 10.1164/rccm.200901-0076OC. doi: 10.1164/rccm.200901-0076OC. [DOI] [PubMed] [Google Scholar]