Abstract
Background:
Neuromyelitis optica spectrum disorder (NMOSD) was long believed to be an aggressive form of multiple sclerosis (MS). This study aimed to describe the clinical features of patients with MS and NMOSD to assist in differential diagnoses in clinical practice.
Methods:
Data including the patients’ serum and cerebrospinal fluid (CSF) tests, image findings, and clinical information from 175 patients with MS or NMOSD at Xuanwu Hospital, Capital Medical University from November 2012 to May 2014 were collected and analyzed retrospectively. An enzyme-linked immunosorbent assay was performed to detect the myelin oligodendrocyte glycoprotein (MOG) autoantibodies in CSF and serum. Cell-based assays were used to detect aquaporin-4-antibody (AQP4-Ab). The Chi-square test was used to compare the categorical variables. Wilcoxon rank sum test was performed to analyze the continuous variables.
Results:
Totally 85 MS patients (49%) and 90 NMOSD patients (51%) were enrolled, including 124 (71%) women and 51 (29%) men. Fewer MS patients (6%) had autoimmune diseases compared to NMOSD (19%) (χ2 = 6.9, P < 0.01). Patients with NMOSD had higher Expanded Disability Status Scale scores (3.5 [3]) than MS group (2 [2]) (Z = −3.69, P < 0.01). The CSF levels of white cell count and protein in both two groups were slightly elevated than the normal range, without significant difference between each other. Positivity of serum AQP4-Ab in NMOSD patients was higher than that in MS patients (MS: 0, NMOSD: 67%; χ2 = 63.9, P < 0.01). Oligoclonal bands in CSF among NMOSD patients were remarkably lower than that among MS (MS: 59%, NMOSD: 20%; χ2 = 25.7, P < 0.01). No significant difference of MOG autoantibodies was found between the two groups.
Conclusion:
The different CSF features combined with clinical, magnetic resonance imaging, and serum characteristics between Chinese patients with MS and NMOSD could assist in the differential diagnosis.
Keywords: Aquaporin-4, Cerebrospinal Fluid, Demyelinating Disease, Multiple Sclerosis, Myelin Oligodendrocyte Glycoprotein, Neuromyelitis Optica Spectrum Disorder, Oligoclonal Bands
INTRODUCTION
Idiopathic inflammatory demyelinating diseases (IIDDs) constitute a group of disorders characterized by inflammatory lesions, those are associated with loss of myelin and eventually axonal damage.[1] Multiple sclerosis (MS) and neuromyelitis optica spectrum disorder (NMOSD) are the most studied subgroups of IIDDs. In patients misdiagnosed with MS, the alternative true diagnosis is often NMOSD, due to the similarities and overlaps among those diseases. Along with serum studies, cerebrospinal fluid (CSF) evaluation is essential in the initial diagnosis.
MS is a primary central nervous demyelinating disease, clinically characterized by relapses, mediated by acute inflammatory lesions in the white matter, followed by a progressive phase, and mediated by axonal and neuronal loss.[1] NMOSD, an inflammatory disease of the central nervous system (CNS), is characterized by severe attacks of myelitis and optic neuritis.[1,2]
Current clinical studies and our clinical practice experience indicate that NMOSD is a more serious clinical condition than MS.[3] Since MS is a completely different pathophysiological process from NMOSD, the treatment strategies differ meaningfully. Some MS immunotherapies appear to aggravate NMOSD conditions; therefore, correct initial diagnosis is crucial for diseases management. Because the initial diagnoses in clinical practice are difficult due to the similarities in patients’ presentation with the two disease groups, we summarized the CSF features combined with clinical, magnetic resonance imaging (MRI), and serum characteristics in Chinese patients with MS and NMOSD to provide clues for differential diagnosis.
METHODS
Patients and diagnostic criteria
Totally 175 consecutive patients with CNS IIDDs hospitalized in Xuanwu Hospital, Capital Medical University (China) from November 2012 to May 2014 were enrolled in this study. MS was diagnosed based on the latest McDonald criteria.[4] The 2015 Updated International Consensus on Diagnostic Criteria for NMOSDs is used for diagnosing NMOSD.[5] The patients’ serum and CSF tests, image findings, and clinical information were retrospectively collected. The study was approved by the Ethnical Committee of Xuanwu Hospital, Capital Medical University.
Magnetic resonance imaging scans
All the brain and spinal MRI were performed on 3.0-T scanners, including axial/sagittal T2-weighted images and T1-weighted images, with or without gadolinium enhancement. The brain scans included axial fluid-attenuated inversion recovery as well.
Cerebrospinal fluid and serum tests
The CSF and serum tests in each group included aquaporin-4-antibodies (AQP4-Ab), myelin oligodendrocyte glycoprotein antibodies (MOG-Ab), myelin basic protein (MBP), specific oligoclonal bands (SOBs), total white blood cell count in CSF, and CSF biochemistry examination.
An enzyme-linked immunosorbent assay (ELISA) was performed to detect CSF and serum antibodies against MOG, with 0.56 and 0.64 as the cutoff values of positivity for CSF and serum, respectively. Cell-based assays (CBAs) were used to detect AQP4-Ab. The immunoenzymatic technique was performed to measure MBP.
Statistical analysis
Categorical variables were expressed as count (percentage) and analyzed by the Chi-square test. Continuous variables were expressed as median (interquartile range [IQR]) due to nonnormal distribution and analyzed by Wilcoxon rank sum test. All statistical analyses were performed using the SAS 9.4 (SAS Institute, Cary, NC, USA). All reported P values are two-sided. P < 0.05 was considered to be statistically significant.
RESULTS
Clinical characteristics
The 175 patients with CNS IIDDs included 85 MS patients (49%) and 90 NMOSD patients (51%) [Table 1]. They were 124 (71%) women and 51 (29%) men. The overall demographic characteristics of the two groups were similar, with no significant differences in gender or age observed [Table 1].
Table 1.
Basic characteristics, clinical manifestation, and auxiliary examination results of patients with MS or NMOSD in this study
| Variables | MS (n = 85) | NMOSD (n = 90) | Statistics | P |
|---|---|---|---|---|
| Gender, male/female | 30/55 | 21/69 | 3.00* | 0.08 |
| Age (years), median (IQR) | 34 (21) | 41 (25) | −1.17† | 0.24 |
| Age at onset (years), median (IQR) | 32 (20) | 36 (24) | −1.29† | 0.20 |
| EDSS at last visit, median (IQR) | 2 (2) | 3.5 (3) | −3.69† | <0.01 |
| Autoimmune disease frequency, n (%) | 5 (6) | 17 (19) | 6.90* | <0.01 |
| MRI findings, n (%) | ||||
| Midbrain lesions frequency | 16 (20) | 6 (7) | 6.90* | <0.01 |
| Pontine lesions frequency | 39 (49) | 22 (25) | 11.00* | <0.01 |
| Medulla lesions frequency | 18 (23) | 24 (27) | 0.39* | 0.53 |
| Periventricular lesions frequency | 52 (66) | 20 (22) | 32.10* | <0.01 |
| Juxtacortical frequency | 29 (37) | 8 (9) | 18.70* | <0.01 |
| Infratentorial lesions frequency | 14 (18) | 3 (3) | 9.50* | <0.01 |
| Long-cord lesions (>3 VS) frequency | 19 (21) | 14 (61) | 14.70* | <0.01 |
| CSF analysis | ||||
| CSF-WBC count (×106/L), median (IQR) | 5 (8) | 5 (8) | 0.46† | 0.64 |
| CSF protein (mg/L), median (IQR) | 320 (290) | 360 (310) | 1.67† | 0.10 |
| AQP4-Ab CSF positive frequency, n (%) | 0 | 30 (46) | 36.40* | <0.01 |
| CSF-SOB frequency, n (%) | 46 (59) | 16 (20) | 25.70* | <0.01 |
| CSF-MBP frequency, n (%) | 9 (19) | 16 (42) | 5.90* | 0.02 |
| CSF-MOG-Ab (IQR) | 0.45 (0.34) | 0.33 (0.51) | 0.37† | 0.72 |
| Serum analysis | ||||
| AQP4-Ab seropositive frequency, n (%) | 0 | 47 (67) | 63.90* | <0.01 |
| Serum MOG-Ab (IQR) | 0.72 (0.81) | 0.47 (0.47) | 2.04† | 0.04 |
*χ2 values; †Z values. MS: Multiple sclerosis; NMOSD: Neuromyelitis optica spectrum disease; IQR: Interquartile range; EDSS: Expanded Disability Status Scale; VS: Vertebral segments; CSF: Cerebrospinal fluid; WBC: White blood cell; AQP4-Ab: Aquaporin-4-antibody; SOB: Specific oligoclonal bands; MBP: Myelin basic protein; MOG-Ab: Myelin oligodendrocyte glycoprotein antibody.
Twenty-two patients (13%) had autoimmune diseases, including Behcet's disease, Hashimoto's thyroiditis, allergic asthma, Sjogren syndrome, Crohn's disease, idiopathic thrombocytopenic purpura, and connective tissue disease (CTD). Fewer MS patients (6%) had autoimmune diseases compared to NMOSD (19%) (χ2 = 6.9, P < 0.01). Paralysis, anesthesia, and decreased eyesight were the most common symptoms among the 175 patients. Expanded Disability Status Scale (EDSS) scores (range in 0.0–10.0, with 0.0 indicates normal neurological exams and 10.0 indicates death) show significant difference among groups: patients with NMOSD have higher EDSS scores (3.5 [3]) than MS group (2 [2]) (Z = −3.69, P < 0.01).
Results of cerebrospinal fluid and serum tests
Routine test and biochemical analysis of cerebrospinal fluid
The CSF results for a routine test and biochemical analysis (including white cell count, CSF protein, glucose, and chloride levels) were available for all MS patients and 82 (91%) patients with NMOSD. CSF white cell count was 5 (8) × 106/L for each group (Z = 0.46, P = 0.64), respectively. CSF protein was 330 (310) mg/L for all samples, 320 (290) mg/L with MS, and 360 (310) mg/L with NMOSD, respectively (Z = 1.67, P = 0.10). CSF glucose was 550 (100) mg/L for all samples, 544 (82) mg/L with MS, and 560 (104) mg/L with NMOSD, respectively (Z = 0.97, P = 0.33). CSF chloride was 116 (5) mmol/L for all samples, 116 (5) mmol/L with MS, and 115 (5) mmol/L with NMOSD, respectively (Z = − 0.15, P = 0.88).
Aquaporin-4-antibody detection in cerebrospinal fluid and serum
AQP4-Ab in CSF and serum was detected from 131 (75%) patients. Totally 47 patients were positive for serum AQP4-Ab by immunohistochemistry: none with MS and 47 with NMOSD (χ2 = 63.9, P < 0.01); while thirty patients were positive for CSF AQP4-Ab: none with MS, thirty patients with NMOSD (χ2 = 36.4, P < 0.01).
Specific oligoclonal bands in cerebrospinal fluid
CSF-SOB frequency in patients with MS was 59%, while it was only 20% with NMOSD (χ2 = 25.7, P < 0.01).
Myelin basic protein in cerebrospinal fluid
CSF samples were available for MBP test in 49 MS and 38 NMOSD patients. No significant difference was found in the CSF MBP positivity between groups: 19% in MS group and 42% in NMOSD group (χ2 = 5.9, P = 0.02).
Myelin oligodendrocyte glycoprotein antibodies in cerebrospinal fluid and serum
MOG-Ab in CSF and serum was detected from 31 patients: 12 had MS and 19 had NMOSD. The median age at symptoms onset of serum MOG-Ab positive patients (serum MOG-Ab level was higher than 0.64) was only 26.5 years (IQR was 17 years). Median serum MOG-Ab level was 0.57, with 0.56 for IQR in the whole sample. Serum MOG-Ab was 0.72 (0.81) in MS group and 0.47 (0.47) in NMOSD group, respectively (Z = 2.04, P = 0.04). CSF MOG-Ab level was 0.45 (0.46) in the whole sample. CSF MOG-Ab was 0.45 (0.34) and 0.33 (0.51) for the MS and NMOSD, respectively (Z = 0.37, P = 0.72). The MS patients who were positive for both CSF-SOB and serum AQP4-Ab were all serum MOG-Ab positive, while the NMOSD patients who were positive for both CSF-SOB and serum AQP4-Ab were serum MOG-Ab negative.
Magnetic resonance imaging findings
In the MS group, 79 patients had available brain MRI results [Figure 1a and 1b]. Sixteen (20%) patients in the MS group had midbrain lesions, 39 (49%) had pontine lesions, 18 (23%) had medullary lesions, 52 (66%) had periventricular lesions, 29 (37%) had juxtacortical lesions, and 14 (18%) had cerebellar lesions. Cervical spinal cord MRI results [available in 83 patients, Figure 2a and 2b] revealed T2 lesions in 47 (of whom 24 patients had spinal lesions that exceeded three vertebral segments) [Table 1]. Thoracic spinal cord MRI results [available in 81 patients, Figure 2a and 2b] revealed T2 lesions in 37 patients (of whom 13 patients had spinal lesions that exceeded three vertebral segments) [Table 1].
Figure 1.
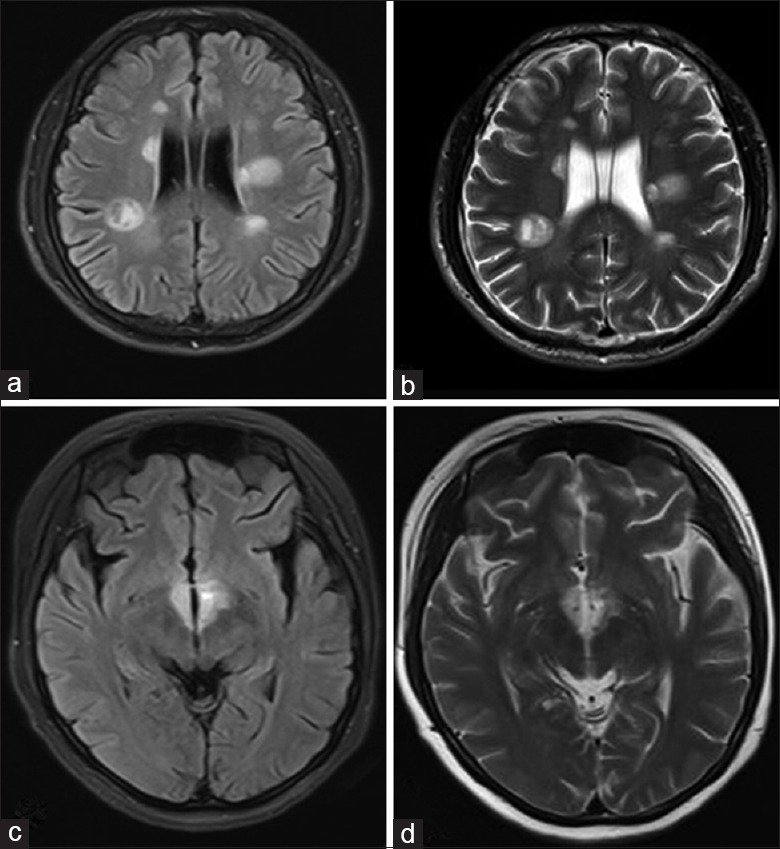
Hallmark brain lesions for multiple sclerosis (a and b) and neuromyelitis optica spectrum disorder (c and d). (a) Axial fluid-attenuated inversion recovery sequence and (b) T2-weighted magnetic resonance imaging showed tumefactive multiple sclerosis lesions, presenting as a “fried egg” in periventricular areas. The yolk is the plaque itself and the white is the surrounding vasogenic edema. Typical neuromyelitis optica spectrum disorder lesions: (c) Fluid-attenuated inversion recovery sequence magnetic resonance imaging with (d) T2-weighted hyperintensities evident in periependymal areas.
Figure 2.
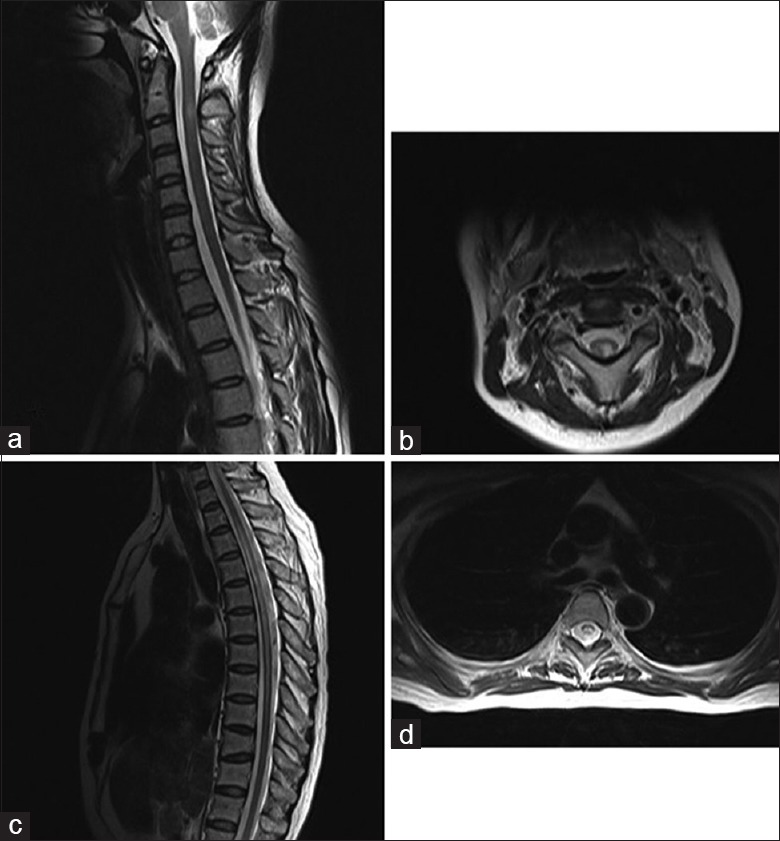
Typical spinal cord lesions for multiple sclerosis (a and b) and neuromyelitis optica spectrum disorder (c and d). (a) The sagittal T2-weighted images showed hyperintensity within the cord with slight swelling at the upper cervical level. (b) T2-weighted axial images of the upper cervical region showed dorsolateral hyperintensity, which is typical in multiple sclerosis. Characteristic lesions for neuromyelitis optica spectrum disorder: (c) Spinal cord T2-weighted magnetic resonance imaging hyperintensities within a longitudinally extensive lesion. (d) Spinal cord magnetic resonance imaging axial T2-weighted image showed the gray matter-dominated involvement of “H”-shaped lesions.
In the NMOSD group, 89 patients had available brain MRI results. Midbrain lesions were found in six (7%) patients and pontine lesions in 22 (25%) cases. Medulla lesions, periventricular lesions, juxtacortical lesions, and cerebellar lesions were found in 24 (27%), 20 (22%), 8 (9%), and 3 (3%) patients, respectively. Only one patient had aqueductal lesions [Figure 1c and 1d]. Cervical spinal cord MRI results [available in 84 patients, Figure 2c and 2d] revealed T2 lesions in 51 (32 patients had spinal lesions that exceeded three vertebral segments) [Table 1]. Thoracic spinal cord MRI results [available in 81 patients, Figure 2c and 2d] revealed T2 lesions in 46 patients (of whom 28 patients had spinal lesions that exceeded three vertebral segments) [Table 1].
DISCUSSION
In this single-center study, we analyzed the clinical, radiological, serum, and CSF findings of patients with CNS demyelinating disease; those were diagnosed as MS and NMOSD at Xuanwu Hospital, Capital Medical University, China.
Females are more prominent in the two kinds of diseases, and this character is more remarkable in NMOSD (female to male ratio was 1.9–7.3:1.0) compared to MS (female to male ratio was 2.57–2.62:1.0).[6,7] Our results in gender ratio failed to reach statistical difference between groups, possibly due to the limited power or potential selection bias. Although both diseases have a female predominance, in most western studies, AQP4-positive NMOSD patients have a female to male ratio between 8 and 9 to 1.[8,9,10] Typically, a distinguishing feature of NMOSD is the later age of symptom onset; patients with NMOSD are on average 10 years older than patients with MS at disease presentation (mean onset at around four decades for NMOSD and three decades for MS).[11] However, our study did not show a significant difference of age at disease onset in the MS and NMOSD group. The age difference was 4 years; median age of symptoms onset in MS group was 32 years old, while in NMOSD group, it was 36 years old [Table 1]. The possible reason could be that this study included only adult patients, while other studies included the whole population.
There were 38 patients with coexisting autoimmune diseases, including Behcet's disease, Hashimoto's thyroiditis, allergic asthma, Sjogren syndrome, Crohn's disease, idiopathic thrombocytopenic purpura, and CTD. The NMOSD suffered more often from autoimmune diseases than the MS patients with a significant difference. These results are also similar with the previous reports: 20–30% of NMOSD patients have been reported to present accompanying autoimmune disorders, and 40–50% of them have coexisting autoantibodies (organ and not organ specific).[11] In particular, AchR-Abs (antibodies against acetylcholine receptor) might serve as a red flag in the differential diagnosis of NMOSD and MS.[12] Although that NMOSD tends to be associated with autoimmune diseases is not fully understood, multiple evidence of the humoral mechanisms in NMOSD lesions were reported.[13]
The primary purpose of the study was to describe the CSF findings in Chinese NMOSD and MS patients at a single site. Slight elevation of CSF protein levels (450–700 mg/L) has been reported in MS patients, while CSF protein level in NMOSD was elevated up to 2900–6400 mg/L during relapses and stayed between 330 and 630 mg/L at the remission phase.[1] CSF protein level was slightly elevated than normal, with average 400 mg/L in MS group and average 483 mg/L in NMOSD group in our findings. CSF pleocytosis is present in around 50% of NMOSD samples.[1] NMO CSF white cell is usually ≥50 × 106/L, while in MS it is <50 × 106/L.[3,14] However, in our study, mean CSF white cell was 10.6 × 106/L in NMOSD group and 7.8 × 106/L in MS group. No significant difference was found between groups regarding CSF white cell count, CSF protein, CSF glucose, or CSF chloride levels. These results may be distinct characteristics of the Chinese population, but this hypothesis still needs to be confirmed by multiple sites studies with larger sample sizes. Total CSF white cell counts and CSF protein levels were generally consistent with what reported previously,[1] except for the CSF protein level and white cell count in NMOSD during relapses which were lower.[1] The levels of CSF white cell counts and CSF protein were slightly elevated than the normal range, indicating inflammatory activity during relapses.
Serum AQP4-Ab positivity was significantly higher in NMOSD (67%) than MS patients (0) (P < 0.001), which is consistent with previous studies.[11] Immunopathological data support the AQP4-Ab association with NMOSD,[11] which is necessary for diagnosing NMOSD.[5] Seventeen patients with NMOSD had AQP4-Ab positive in serum, but negative in CSF. In other words, AQP4-Ab was detectable in 64% CSF samples from AQP4-Ab seropositive patients with NMOSD and in none of the CSF samples from seronegative patients. Therefore, testing for CSF AQP4-Ab did not improve the sensitivity or specificity of NMOSD diagnosis.[15] The most updated guideline on NMOSD diagnosing also indicate that routine CSF testing of AQP4-Ab-seronegative patients is not recommended,[5] except in cases with additional confounding serum autoantibodies leading to uninterpretable or false-positive assay results where CSF testing may be considered.
CSF-oligoclonal band (CSF-OB) frequency over 90% of MS patients has been demonstrated in western countries, while approximately 60% in Asia.[1] In our findings, CSF-OB frequency in MS patients was 59%, which was lower than western countries (90%); it is consistent with Asian patients’ characteristics (around 60%). Low positivity might involve with ethnic or race issues. CSF-OBs, regarded as a hallmark of MS, were present in 20% of our NMOSD patients. In accordant with the reports, the detection rate of CSF-OB in NMOSD patients is around 15–30%, which is thought to be transient.[1] A follow-up study is requisite to demonstrate the rate of CSF-OB changes among NMOSD patients. Thus, the presence or absence of OBs is one of the most helpful parameters in differentiating NMOSD from MS; MOG is a target of autoantibodies in animal models of inflammatory demyelinating diseases of the CNS, such as MS.[16] The role of MOG-Abs in diseases pathogenesis is unclear.[5] Kim et al. found a strong predilection of MOG-Abs for optic nerve and less for the spinal cord, unlike AQP4-Abs, which showed opposite preferences.[17] MOG-Abs were mainly present in our MS patients. Previous studies indicate MOG-Abs tended to exist in younger patients, less frequently female, and less likely to relapse in comparison to NMOSD patients with AQP4-Ab.[18,19] High-titer MOG-Abs are present in pediatric patients but rarely in adults.[16] In this study, the median age of serum MOG-IgG positive patients was 26.5 years in NMOSD group, while MS patients were elder to have serum MOG-IgG positivity (median age was 32 years).
Another striking observation is that the patients with solely MOG-IgG present in their serum have better clinical outcomes (data not shown). Further studies are needed to confirm this observation and determine whether MOG-Ab would be useful in the differential diagnosis of patients with IIDDs, especially those both negative for CSF-SOB and serum AQP4-Ab.
The brain lesions in MS are nonspecific or indistinguishable from NMOSD lesions in general, while some NMOSD abnormalities may be parallel midline AQP4-rich regions, such as the hypothalamus and periaqueductal brainstem regions surrounding the ventricular system. The presence of lateral ventricular, juxtacortical, infratentorial, and brain stem lesions was more significant in our MS than NMOSD patients; those typical lesions would attribute to the diagnosis of MS.[4] The spinal cord lesions in NMOSD are typically located centrally and extend over three or more contiguous vertebral segments, the spinal cord lesions in MS tend to be shorter (less than one vertebral segment in length). Long vertebral spinal lesions are a hallmark for diagnosis of NMOSD in consistence with updated guideline.[5]
In general, in the study, higher female ratio is found in NMOSD than MS even without significance. Patients with NMOSD may be older at symptoms onset, while MS patients may be slightly younger (the difference did not reach the significant level). Systemic autoimmune diseases are more common in NMOSD. No distinct differences were found in routine CSF testing or biochemistry testing between groups. Serum AQP4-Ab positivity and CSF-OB are remarkably different between the NMOSD and the MS. MOG-IgG positivity tends to be seen in younger patients with MS. The potential role of MOG-Abs in CSF-SOB and serum AQP4-Ab double-negative patients needs to be investigated.
There are some limitations in this study. First, the follow-up of patients is lacking. Second, as CBAs for MOG-Abs detection are still not available in our city, we had to perform ELISA to detect the antibodies against CSF and serum MOG-Abs.[16] Third, the study is a retrospective single-site descriptive study that causes a possible geographic bias even though the patients are from multiple provinces in China. A multiple-site study would be performed in the future to describe CSF findings, as well as the clinical manifestations and radiological characteristics of patients with CNS IIDDs in China.
Financial support and sponsorship
This study was supported by a grant of the Capital Public Health Project of Beijing Municipal Science and Technology Commission (No. Z131100006813020).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yuan-Yuan Ji
REFERENCES
- 1.Matas SL, Glehn FV, Fernandes GB, Soares CA. Cerebrospinal fluid analysis in the context of CNS demyelinating diseases. Arq Neuropsiquiatr. 2013;71:685–8. doi: 10.1590/0004-282X20130151. doi: 10.1590/0004-282x20130151. [DOI] [PubMed] [Google Scholar]
- 2.Kira J. Neuromyelitis optica and opticospinal multiple sclerosis: Mechanisms and pathogenesis. Pathophysiology. 2011;18:69–79. doi: 10.1016/j.pathophys.2010.04.008. doi: 10.1016/j.pathophys.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Wingerchuk DM, Hogancamp WF, O’ Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic's syndrome) Neurology. 1999;53:1107–14. doi: 10.1212/wnl.53.5.1107. doi: 10.1212/WNL.53.5.1107. [DOI] [PubMed] [Google Scholar]
- 4.Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85:177–89. doi: 10.1212/WNL.0000000000001729. doi: 10.1212/wnl.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Disanto G, Ramagopalan SV. On the sex ratio of multiple sclerosis. Mult Scler. 2013;19:3–4. doi: 10.1177/1352458512447594. doi: 10.1177/1352458512447594. [DOI] [PubMed] [Google Scholar]
- 7.Pandit L, Asgari N, Apiwattanakul M, Palace J, Paul F, Leite MI, et al. Demographic and clinical features of neuromyelitis optica: A review. Mult Scler. 2015;21:845–53. doi: 10.1177/1352458515572406. doi: 10.1177/1352458515572406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papais-Alvarenga RM, Vasconcelos CC, Carra A, de Castillo IS, Florentin S, Diaz de Bedoya FH, et al. Central nervous system idiopathic inflammatory demyelinating disorders in South Americans: A descriptive, multicenter, cross-sectional study. PLoS One. 2015;10:e0127757. doi: 10.1371/journal.pone.0127757. doi: 10.1371/journal.pone.0127757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asgari N. Epidemiological, clinical and immunological aspects of neuromyelitis optica (NMO) Dan Med J. 2013;60:B4730. [PubMed] [Google Scholar]
- 10.Quek AM, McKeon A, Lennon VA, Mandrekar JN, Iorio R, Jiao Y, et al. Effects of age and sex on aquaporin-4 autoimmunity. Arch Neurol. 2012;69:1039–43. doi: 10.1001/archneurol.2012.249. doi: 10.1001/archneurol.2012.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pittock SJ, Lucchinetti CF. Neuromyelitis optica and the evolving spectrum of autoimmune aquaporin-4 channelopathies: A decade later. Ann N Y Acad Sci. 2016;1366:20–39. doi: 10.1111/nyas.12794. doi: 10.1111/nyas.12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jurynczyk M, Craner M, Palace J. Overlapping CNS inflammatory diseases: Differentiating features of NMO and MS. J Neurol Neurosurg Psychiatry. 2015;86:20–5. doi: 10.1136/jnnp-2014-308984. doi: 10.1136/jnnp-2014-308984. [DOI] [PubMed] [Google Scholar]
- 13.Lucchinetti CF, Mandler RN, McGavern D, Bruck W, Gleich G, Ransohoff RM, et al. A role for humoral mechanisms in the pathogenesis of Devic's neuromyelitis optica. Brain. 2002;125(Pt 7):1450–61. doi: 10.1093/brain/awf151. doi: 10.1093/brain/awf151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: Guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121–7. doi: 10.1002/ana.1032. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 15.Jarius S, Franciotta D, Paul F, Ruprecht K, Bergamaschi R, Rommer PS, et al. Cerebrospinal fluid antibodies to aquaporin-4 in neuromyelitis optica and related disorders: Frequency, origin, and diagnostic relevance. J Neuroinflammation. 2010;7:52. doi: 10.1186/1742-2094-7-52. doi: 10.1186/1742-2094-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reindl M, Di Pauli F, Rostásy K, Berger T. The spectrum of MOG autoantibody-associated demyelinating diseases. Nat Rev Neurol. 2013;9:455–61. doi: 10.1038/nrneurol.2013.118. doi: 10.1038/nrneurol.2013.118. [DOI] [PubMed] [Google Scholar]
- 17.Kim SM, Woodhall MR, Kim JS, Kim SJ, Park KS, Vincent A, et al. Antibodies to MOG in adults with inflammatory demyelinating disease of the CNS. Neurol Neuroimmunol Neuroinflamm. 2015;2:e163. doi: 10.1212/NXI.0000000000000163. doi: 10.1212/nxi.0000000000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitley J, Woodhall M, Waters P, Leite MI, Devenney E, Craig J, et al. Myelin-oligodendrocyte glycoprotein antibodies in adults with a neuromyelitis optica phenotype. Neurology. 2012;79:1273–7. doi: 10.1212/WNL.0b013e31826aac4e. doi: 10.1212/WNL.0b013e31826aac4e. [DOI] [PubMed] [Google Scholar]
- 19.Kitley J, Waters P, Woodhall M, Leite MI, Murchison A, George J, et al. Neuromyelitis optica spectrum disorders with aquaporin-4 and myelin-oligodendrocyte glycoprotein antibodies: A comparative study. JAMA Neurol. 2014;71:276–83. doi: 10.1001/jamaneurol.2013.5857. doi: 10.1001/jamaneurol.2013.5857. [DOI] [PubMed] [Google Scholar]


