Abstract
Background:
Two recent whole-exome sequencing researches identifying somatic mutations in the ubiquitin-specific protease 8 (USP8) gene in pituitary corticotroph adenomas provide exciting advances in this field. These mutations drive increased epidermal growth factor receptor (EGFR) signaling and promote adrenocorticotropic hormone (ACTH) production. This study was to investigate whether the inhibition of USP8 activity could be a strategy for the treatment of Cushing's disease (CD).
Methods:
The anticancer effect of USP8 inhibitor was determined by testing cell viability, colony formation, apoptosis, and ACTH secretion. The immunoblotting and quantitative reverse transcription polymerase chain reaction were conducted to explore the signaling pathway by USP8 inhibition.
Results:
Inhibition of USP8-induced degradation of receptor tyrosine kinases including EGFR, EGFR-2 (ERBB2), and Met leading to a suppression of AtT20 cell growth and ACTH secretion. Moreover, treatment with USP8 inhibitor markedly induced AtT20 cells apoptosis.
Conclusions:
Inhibition of USP8 activity could be an effective strategy for CD. It might provide a novel pharmacological approach for the treatment of CD.
Keywords: Adrenocorticotropic Hormone Secretion, Cell Viability, Cushing's Disease, Ubiquitin-specific Protease 8 Inhibitor
INTRODUCTION
Cushing's disease (CD), or pituitary-dependent Cushing's syndrome, is the most common cause of endogenous Cushing's syndrome accounting for about 70% of the chronic endogenous hypercortisolism.[1] It induced a series of several comorbidities and clinical complications, mainly including hypertension, diabetes mellitus, dyslipidemia, osteoporosis, cardiovascular disease, infection, and mental disorders, which associated with increased morbidity and mortality if not appropriately treated.[2] Until recently, no available medical treatment was licensed for CD although several drugs had demonstrated efficacy in lowering excess cortisol.[3,4]
Using next-generation sequencing approach, Reincke et al. have identified recurrent somatic mutations in the gene encoding USP8 in four in an initial set of ten corticotroph tumors. These mutations were validated in a small group of seven Cushing's patients, with a final prevalence of 35%.[5] Subsequently, two different retrospective studies analyzed the prevalence of ubiquitin-specific protease 8 (USP8) mutations in two large cohorts of CD patients, identifying a prevalence of 36% and 62%, respectively.[6,7] It is noteworthy that somatic mutations in USP8 gene show a remarkable specificity for CD, with no mutations found in other type of pituitary adenoma and only rare somatic mutations reported in other tumors.[5,6,7] All of the mutations were located in exon 14, defining a hotspot region that overlaps with the sequence coding for the 14-3-3 binding motif, highly conserved between different species. These mutations inhibited 14-3-3 protein binding to USP8 and resulted in a higher deubiquitinating enzyme (DUB) activation. The consequence of this hyperactivation is increased epidermal growth factor receptor (EGFR) deubiquitination and a longer retention of EGFR at the plasma membrane which leads to inhibition of degradation, thereby preventing downregulation of ligand-activated EGFR and promotes and enhances adrenocorticotropic hormone (ACTH) production.
The identification of USP8 mutations as specific contributors to the pathogenesis of ACTH-secreting pituitary adenomas represents an exciting advance in our understanding of CD. The aim of our study was to investigate the anticancer efficacy of USP8 inhibitor in CD. Here, we demonstrate that treatment with USP8 inhibitor, 9-ehtyloxyimino9H-indeno [1,2-b] pyrazine-2,3-dicarbonitrile, suppresses ACTH secretion, cell viability, and promotes cell apoptosis in AtT20 cells suggesting that UPS8 inhibitor could be a new therapeutic candidate for CD.
METHODS
Cell culture and reagents
All of the cell lines were obtained from the American Tissue Type Collection (ATCC, Manassas, VA, USA). The mouse AtT20 pituitary corticotroph cell line and hepatocellular carcinoma cell line Hepa 1-6 were maintained in Dulbecco's modified Eagle's medium (DMEM) (GIBCO, New York, USA) containing 10% fetal bovine serum (FBS) (GIBCO, New York, USA) and 2 mmol/L L-glutamine and 100 IU/ml penicillin and 100 µg/ml streptomycin (GIBCO, New York, USA) at 37°C in a humidified incubator with 5% CO2. The cells were starved with DMEM supplemented with 2% FBS for 16 h prior to each experiment. The 9-ehtyloxyimino9H-indeno [1,2-b] pyrazine-2,3-dicarbonitrile was obtained from Melone Pharmaceutical, Dalian, China.
Cell proliferation assay and colony formation assay
The AtT20 cells were seeded at 2 × 103 cells per well in 96-well plates and left them to attach for 24 h. After that we changed the medium to cell culture medium with 2% FBS with indicated concentrations of USP8 inhibitor for 24 and 48 h. Viable cells were measured using a Cell Counting Kit-8 (CCK-8, Dojindo, Japan) according to the manufacturer's instructions.
AtT20 cells were plated at a density of 103 cells per well in a 6-well plate in DMEM culture medium containing 10% FBS. The medium with indicated concentrations of USP8 inhibitor was replaced every 3 or 4 days. After 15 days, colonies were fixed with 4% paraformaldehyde (Sigma, St. Louis, USA) for 20 min and stained with 0.05% crystal violet at room temperature for 20 min. Then, the cells were washed three times with phosphate-buffered saline (PBS) for 5 min. Colonies containing more than 50 cells were counted using a light microscope.
Western blotting
Total cell lysate was prepared with radioimmunoprecipitation assay buffer containing Protease Inhibitor Cocktail. Protein concentrations were measured by DC protein assay reagent (Bio-Rad, CA, USA) and extracts resolved by SDS/PAGE on 8% gels. Membranes were blocked for 2 h at room temperature in tris buffered saline-tween-20 containing 5% nonfat dried milk (Bio-Rad), washed, and then incubated with primary antibodies (anti-EGFR from Santa Cruz, CA, USA; anti-pErbB2, anti-Met, anti-Akt, anti-pAkt, anti-GAPDH, anti-p27/kip1, anti-cleaved-caspase 3, anti-bax, and anti-bcl-2 from Cell Signaling Technology, Boston, USA) at 4°C overnight. After washing, membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (Cell Signaling Technology, Boston, USA). The signal was detected using enhanced chemiluminescence (PerkinElmer, Waltham, MA, USA).
Adrenocorticotropic hormone assay
The cells were incubated for 4 and 24 h with the indicated concentrations of USP8 inhibitor. The medium was then aspirated, and the ACTH levels in the supernatants were measured using an ACTH enzyme-linked immunosorbent assay kit (Phoenix, Milpitas, USA).
Apoptosis assay
Cell apoptosis was measured using the fluorescein isothiocyanate (FITC) Annexin V Apoptosis detection Kit I (BD Biosciences Pharmingen, San Jose, CA, USA) according to the manufacturer's instructions. AtT20 cells were plated in 6-well plates. After exposure to USP8 inhibitor for 24 and 48 h, cells were detached and then washed once with cold PBS, suspended in 1 × binding buffer at a concentration of 5 × 105 cells/ml. And then FITC Annexin V and propidium iodide were added. After incubating for 15 min at room temperature in the dark, 200 µl of 1 × binding buffer were added to each tube. The cells were analyzed with a BD FACSVerse flow cytometer (BD Biosciences Pharmingen) and BD FACSuite Software (BD Biosciences Pharmingen). The fraction of the cell population in different quadrants was analyzed using quadrant statistics.
Statistical analysis
The statistical analysis was performed using the SPSS version 16.0 (SPSS Inc., USA). Values are described as a mean ± standard deviation (SD). Significant differences were analyzed using two-tail unpaired Student's t-test and one-way ANOVA. A value of P < 0.05 was considered statistically significant.
RESULTS
Ubiquitin-specific protease 8 inhibitor inhibit cell viability by downregulating oncogenic receptor tyrosine kinases
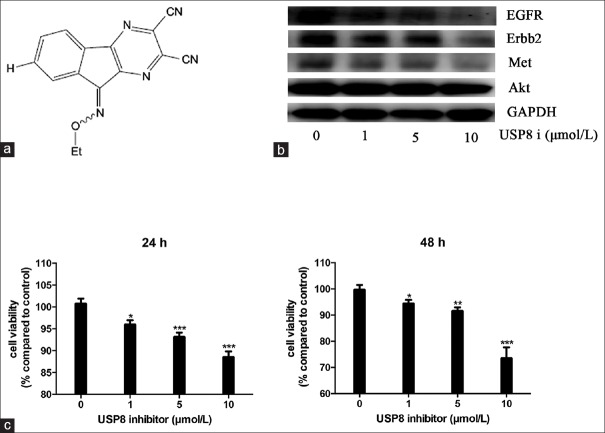
To investigate that targeting USP8 with its specific inhibitor might exhibit an anticancer effect in the corticotroph adenomas, we first examined the effect of USP8 inhibitor on downstream protein levels including EGFR, ERBB2, and Met. AtT20 cells were treated with a recently synthesized USP8 inhibitor, 9-ehtyloxyimino9H-indeno [1,2-b] pyrazine-2,3-dicarbonitrile [Figure 1a].[8,9] Our data revealed that treatment with USP8 inhibitor could effectively downregulate the expression levels of EGFR, ERBB2, and Met in AtT20 cells in a dose-dependent manner [Figure 1b], demonstrating the inhibition potency of this small molecule for USP8 in AtT20 cells. The treatment of USP8 inhibitor for 24 and 48 h induced an inhibition of cell viability from concentration of 1 µmol/L (4.1%, 4.7%; P < 0.05) and the maximum inhibition was obtained with 10 µmol/L (12.4%, 27.8%; P < 0.001) [Figure 1c]. Moreover, treatment with USP8 inhibitor for 36 h also could inhibit cell growth, while it had no effect on cell growth after 12 h treatment (data not shown).
Figure 1.
Ubiquitin-specific protease 8 inhibitor suppresses AtT20 cell growth by downregulation of oncogenic receptor tyrosine kinases. (a) Chemical structure of ubiquitin-specific protease 8 inhibitor. (b) Effect of ubiquitin-specific protease 8 inhibitor on receptor tyrosine kinases, epidermal growth factor receptor, ERBB2, Met, and Akt. (c) Effects of ubiquitin-specific protease 8 inhibitor on cell viability. * P<0.05, ** P<0.01, ***P<0.001.
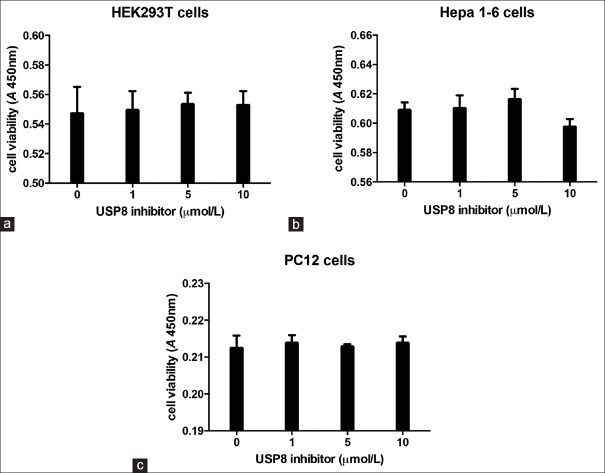
Effects of ubiquitin-specific protease 8 inhibitor on cell viability of renal, adrenal, and liver cells
To determine the specificity of USP8 inhibitor effects, cell viability was assessed in Hepa 1-6, HEK293T, and PC12 cell lines after 24 h treatment without or with increasing concentration of USP8 inhibitor (1–10 µmol/L). As shown in Figure 2a–2c, USP8 inhibitor did not significantly modify the viability of any investigated cell line.
Figure 2.
Effects of ubiquitin-specific protease 8 inhibitor on cell viability of liver, renal, and adrenal cells. Cells were incubated for 24 h with 1–10 μmol/L ubiquitin-specific protease 8 inhibitor; control cells were treated with vehicle solution. HEK 293T (a), Hepa 1-6 (b), PC12 (c), cell viability was assessed in at least three independent experiments with six replicates.
Ubiquitin-specific protease 8 inhibitor inhibits the clonogenic ability of AtT20 cells
Next, we explore whether USP8 inhibitor would have an effect on the clonogenic ability of AtT20 cells [Figure 3a and 3b]. AtT20 cells were seeded in complete growth medium and allowed to adhere for 24 h. The medium was then replaced with complete growth medium containing the indicated concentrations of USP8 inhibitor, and the ability of AtT20 cells to form colonies was monitored over the next 15 days. Our data showed that significant inhibition (9.4%; P < 0.05) of colony formation was detected with 1 µmol/L USP8 inhibitor and maximum reduction (94%; P < 0.001) of clonogenic ability was obtained when 10 µmol/L USP8 inhibitor were used.
Figure 3.
Formation of AtT20 cells colonies. The number of AtT20 cell colonies was determined after 14 days of culture in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum contain ubiquitin-specific protease 8 inhibitor at concentrations of 1–20 μmol/L. Phase contrast microscopy of AtT20 cell colony was observed on 6-well culture plates (a) and quantitative representation of the colonies formed (b). * P<0.05, *** P<0.001.
Ubiquitin-specific protease 8 inhibitor induces apoptosis in AtT20 cells
To investigate whether USP8 inhibitor reduces cell viability by inducing apoptosis, flow cytometry analysis and apoptosis-related proteins analysis were performed. The results showed that dose-dependent treatment with 1–10 µmol/L USP8 inhibitor for 24 and 48 h markedly induced early apoptosis at a level of 11.1% and 29.2%, 12.3%, and 31.6%, respectively [Figure 4a]. However, gefitinib treatment induced early apoptosis at a level of 14.9%. Moreover, the pro-apoptotic effect of USP8 inhibitor was accompanied by the induction of activated caspase-3 and Bax expression and the suppression of Bcl-2 expression [Figure 4b].
Figure 4.
Ubiquitin-specific protease 8 inhibitor-induced apoptosis in AtT20 cells. (a) AtT20 cells were treated with indicated concentration of ubiquitin-specific protease 8 inhibitor for 24 and 48 h. Cells were washed and labeled with annexin V fluorescein isothiocyanate and propidium iodide and analyzed by flow cytometry. Data are presented as % of gated cells. (b) The apoptosis-related protein levels of Bcl-2, Bax, P27, and cleaved caspase-3 were analyzed by Western blot in AtT20 cells treated with ubiquitin-specific protease 8 inhibitor.
Ubiquitin-specific protease 8 inhibitor suppressed proopiomelanocortin gene expression and adrenocorticotropic hormone secretion in AtT20 cells
AtT20 cells were incubated with USP8 inhibitor for 4 and 24 h to determine its effects on proopiomelanocortin (POMC) mRNA levels. As shown, POMC mRNA levels decreased in a dose-dependent manner, with significant effects observed from 5 µmol/L (32.1%; P < 0.05) [Figure 5a and 5b]. To determine the effects of USP8 inhibitor on ACTH secretion, ACTH levels were assessed in conditioned medium in AtT20 cells treatment for 4 and 24 h. As we can see, USP8 inhibitor significantly reduced ACTH secretion after 4 h treatment at both 5 and 10 µmol/L (26.1% and 30.1%, respectively; P < 0.01). After 24 h, USP8 inhibitor significantly reduced ACTH secretion at ≥5 µmol/L (from 16.7% to 40.5%; P < 0.001) [Figure 5c and 5d]. In addition, USP8 inhibitor could enhance the effects of dexamethasone on endogenous ACTH secretion.
Figure 5.
Effect of ubiquitin-specific protease 8 inhibitor on proopiomelanocortin expression and adrenocorticotropic hormone secretion. AtT20 cells were incubated for 4 and 24 h with 1–20 μmol/L ubiquitin-specific protease 8 inhibitor; proopiomelanocortin mRNA expression was assessed by quantitative reverse transcription polymerase chain reaction (a and b). Adrenocorticotropic hormone levels were measured in conditioned medium by enzyme-linked immunosorbent assay in six independent experiments in three replicates (c and d). * P<0.05, **P <0.01, *** P<0.001.
DISCUSSION
Posttranslational modification of proteins by ubiquitination represents a central mechanism for modulating a wide range of cellular functions, such as protein stability, intracellular transport, protein interactions, and transcriptional activity.[10] Analogous to other posttranslational modifications, ubiquitination is a reversible process counteracted by DUBs.[11] Due to the key role for deubiquitination in regulating the stability and activity of a variety of proteins crucial to cell cycle progression, apoptosis and DNA damage repair including p53,[12] MDM2,[13] histones,[14] Norch,[15] β-catenin,[16] and more DUBs have been considered as good targets for cancer treatment. USPs, with more than sixty members, comprise the largest class of DUBs. More and more researches have focused on assessing their function, substrates, and role in specific diseases, especially cancer. USP8, also designated as Ub-specific protease Y, is an ubiquitin isopeptidase that belongs to the USP family of cysteine proteases. USP8 was originally identified to enhance cell growth as its expression increases upon serum stimulation in cancer cells.[17] In addition, it has been reported that USP8 interacts with a number of clinically relevant cancer targets including Cdc25,[18] Erbb2,[19] EGFR,[20] and Nrdp1[21] and implying a crucial role of USP8 in cancers.
Two recent studies demonstrated that the USP8 gene is frequently mutated in corticotroph adenomas.[5,7] ACTH adenomas harboring USP8 mutant had higher EGFR levels, expressed more POMC mRNAs, and had higher ACTH production than those with wild-type USP8. USP8 knockdown in primary ACTH-secreting tumor cells, however, reduced ACTH secretion and EGFR levels, suggesting that inhibition of USP8 activity may be an effective treatment strategy for CD.[7]
Recently, it was also demonstrated that USP8 inhibitor impairs the growth of gefitinib-resistant and -sensitive nonsmall cell lung cancer cells by decreasing receptor tyrosine kinase (RTK) expression.[8,22] Here, we explored the effect of the novel specific USP8 inhibitor, 9-ehtyloxyimino9H-indeno [1,2-b] pyrazine-2,3-dicarbonitrile, on murine AtT20 pituitary adenoma cells. First, we assessed the potency of USP8 inhibitor in AtT20 cells. Inhibition of USP8 results in a dramatic decrease in the total protein levels of RTKs including EGFR, ERBB2, and Met, while has no effect on Akt protein level. Moreover, USP8 inhibitor has an inhibitory effect on the cell viability of AtT20 cells in a concentration-dependent manner, while it does not affect cell viability of the endocrine cell PC-12, indicating that USP8 inhibitor cytotoxic effects are not generalized to endocrine cells. In addition, the viability of nonendocrine cells, such as Hepa 1-6 cell lines and HEK 293T cells, is not influenced by the drug, supporting the hypothesis that USP8 inhibitor acts rapidly with a specific effect at the pituitary level.
Moreover, we observed that the inhibitory effects of USP8 inhibitor on mouse ACTH-secreting pituitary adenoma cell viability are, at least in part, due to apoptosis induction, accompanied by increased cleaved caspase 3 and Bax and decreased Bcl-2 as previously reported in other experimental models.[8,22] Arrest of tumor growth is often associated with induction of cell apoptosis, and USP8 inhibitor has been shown to induce apoptosis in tumor cells, in parallel with growth inhibition. In HCC827 GR cells, treatment with 0.1–10 µmol/L USP8 inhibitor markedly induced early apoptosis at a level of 29.7% and 40.8%.
Excess ACTH production and hypercortisolemia are associated with the progression of CD, decreasing hormone levels, therefore, is a therapeutic goal. We finally investigated whether USP8 inhibitor had any effect on ACTH secretion in AtT20 cells. Our data showed that USP8 inhibitor could decrease ACTH secretion from 5 µmol/L, which clearly demonstrate the potential of USP8 inhibitor to achieve the therapeutic purpose of lowering hormone secretion.
In conclusion, USP8 inhibitor could inhibit proliferation, abolishes clonogenic ability, and induces apoptosis in pituitary corticotroph tumor cell-AtT20 cells. We also demonstrate that USP8 inhibitor suppresses POMC mRNA levels and ACTH secretion. We therefore propose small molecular USP8 inhibitor as pharmacotherapy against CD with dual effects of suppressing ACTH overproduction to alleviate hypercortisolemia and metabolic complications, while also achieving control or shrinkage of pituitary corticotroph tumor growth. Collectively, our findings suggest that USP8 could be a new therapeutic target for CD.
Financial support and sponsorship
This work was supported by grants to Qing-Fang Sun from National Natural Science Foundation of China (No. 81270856) and National High-tech R&D Program (863 program) (No. 2014AA020611).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yi Cui
REFERENCES
- 1.Pivonello R, De Martino MC, De Leo M, Lombardi G, Colao A. Cushing's syndrome. Endocrinol Metab Clin North Am. 2008;37:135–49. doi: 10.1016/j.ecl.2007.10.010. ix. doi: 10.1016/j.ecl.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Newell-Price J, Bertagna X, Grossman AB, Nieman LK. Cushing's syndrome. Lancet. 2006;367:1605–17. doi: 10.1016/S0140-6736(06)68699-6. doi: 10.1016/s0140-6736(06) 68699-6. [DOI] [PubMed] [Google Scholar]
- 3.Pivonello R, De Leo M, Cozzolino A, Colao A. The treatment of Cushing's disease. Endocr Rev. 2015;36:385–486. doi: 10.1210/er.2013-1048. doi: 10.1210/er.2013-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feelders RA, de Bruin C, Pereira AM, Romijn JA, Netea-Maier RT, Hermus AR, et al. Pasireotide alone or with cabergoline and ketoconazole in Cushing's disease. N Engl J Med. 2010;362:1846–8. doi: 10.1056/NEJMc1000094. doi: 10.1056/NEJMc1000094. [DOI] [PubMed] [Google Scholar]
- 5.Reincke M, Sbiera S, Hayakawa A, Theodoropoulou M, Osswald A, Beuschlein F, et al. Mutations in the deubiquitinase gene USP8 cause Cushing's disease. Nat Genet. 2015;47:31–8. doi: 10.1038/ng.3166. doi: 10.1038/ng.3166. [DOI] [PubMed] [Google Scholar]
- 6.Perez-Rivas LG, Theodoropoulou M, Ferraù F, Nusser C, Kawaguchi K, Stratakis CA, et al. The gene of the ubiquitin-specific protease 8 Is frequently mutated in adenomas causing Cushing's disease. J Clin Endocrinol Metab. 2015;100:E997–1004. doi: 10.1210/jc.2015-1453. doi: 10.1210/jc.2015-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma ZY, Song ZJ, Chen JH, Wang YF, Li SQ, Zhou LF, et al. Recurrent gain-of-function USP8 mutations in Cushing's disease. Cell Res. 2015;25:306–17. doi: 10.1038/cr.2015.20. doi: 10.1038/cr.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeong CH. Inhibition of ubiquitin-specific peptidase 8 suppresses growth of gefitinib-resistant non-small cell lung cancer cells by inducing apoptosis. J Cancer Prev. 2015;20:57–63. doi: 10.15430/JCP.2015.20.1.57. doi: 10.15430/jcp.2015.20.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colombo M, Vallese S, Peretto I, Jacq X, Rain JC, Colland F, et al. Synthesis and biological evaluation of 9-oxo-9H-indeno[1,2-b] pyrazine-2,3-dicarbonitrile analogues as potential inhibitors of deubiquitinating enzymes. ChemMedChem. 2010;5:552–8. doi: 10.1002/cmdc.200900409. doi: 10.1002/cmdc.200900409. [DOI] [PubMed] [Google Scholar]
- 10.Popovic D, Vucic D, Dikic I. Ubiquitination in disease pathogenesis and treatment. Nat Med. 2014;20:1242–53. doi: 10.1038/nm.3739. doi: 10.1038/nm.3739. [DOI] [PubMed] [Google Scholar]
- 11.Sowa ME, Bennett EJ, Gygi SP, Harper JW. Defining the human deubiquitinating enzyme interaction landscape. Cell. 2009;138:389–403. doi: 10.1016/j.cell.2009.04.042. doi: 10.1016/j.cell.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li M, Chen D, Shiloh A, Luo J, Nikolaev AY, Qin J, et al. Deubiquitination of p53 by HAUSP is an important pathway for p53 stabilization. Nature. 2002;416:648–53. doi: 10.1038/nature737. doi: 10.1038/natur.e737. [DOI] [PubMed] [Google Scholar]
- 13.Li M, Brooks CL, Kon N, Gu W. A dynamic role of HAUSP in the p53-Mdm2 pathway. Mol Cell. 2004;13:879–86. doi: 10.1016/s1097-2765(04)00157-1. doi: 10.1016/S1097-2765(04)00157-1. [DOI] [PubMed] [Google Scholar]
- 14.Joo HY, Jones A, Yang C, Zhai L, Smith AD, 4th, Zhang Z, et al. Regulation of histone H2A and H2B deubiquitination and Xenopus development by USP12 and USP46. J Biol Chem. 2011;286:7190–201. doi: 10.1074/jbc.M110.158311. doi: 10.1074/jbc.M110.158311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moretti J, Chastagner P, Liang CC, Cohn MA, Israël A, Brou C. The ubiquitin-specific protease 12 (USP12) is a negative regulator of notch signaling acting on notch receptor trafficking toward degradation. J Biol Chem. 2012;287:29429–41. doi: 10.1074/jbc.M112.366807. doi: 10.1074/jbc.M112.366807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao B, Schlesiger C, Masucci MG, Lindsten K. The ubiquitin specific protease 4 (USP4) is a new player in the Wnt signalling pathway. J Cell Mol Med. 2009;13:1886–95. doi: 10.1111/j.1582-4934.2008.00682.x. doi: 10.1111/j1582-4934.2009.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mizuno E, Iura T, Mukai A, Yoshimori T, Kitamura N, Komada M. Regulation of epidermal growth factor receptor down-regulation by UBPY-mediated deubiquitination at endosomes. Mol Biol Cell. 2005;16:5163–74. doi: 10.1091/mbc.E05-06-0560. doi: 10.1091/mbc.E05-06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gnesutta N, Ceriani M, Innocenti M, Mauri I, Zippel R, Sturani E, et al. Cloning and characterization of mouse UBPy, a deubiquitinating enzyme that interacts with the ras guanine nucleotide exchange factor CDC25(Mm)/Ras-GRF1. J Biol Chem. 2001;276:39448–54. doi: 10.1074/jbc.M103454200. doi: 10.1074/jbc.M103454200. [DOI] [PubMed] [Google Scholar]
- 19.Meijer IM, van Leeuwen JE. ERBB2 is a target for USP8-mediated deubiquitination. Cell Signal. 2011;23:458–67. doi: 10.1016/j.cellsig.2010.10.023. doi: 10.1016/j.cellsig-2010.10.023. [DOI] [PubMed] [Google Scholar]
- 20.Alwan HA, van Leeuwen JE. UBPY-mediated epidermal growth factor receptor (EGFR) de-ubiquitination promotes EGFR degradation. J Biol Chem. 2007;282:1658–69. doi: 10.1074/jbc.M604711200. doi: 10.1074/jbc.M604711200. [DOI] [PubMed] [Google Scholar]
- 21.Avvakumov GV, Walker JR, Xue S, Finerty PJ, Jr, Mackenzie F, Newman EM, et al. Amino-terminal dimerization, NRDP1-rhodanese interaction, and inhibited catalytic domain conformation of the ubiquitin-specific protease 8 (USP8) J Biol Chem. 2006;281:38061–70. doi: 10.1074/jbc.M606704200. doi: 10.1074/jbc.M606704200. [DOI] [PubMed] [Google Scholar]
- 22.Byun S, Lee SY, Lee J, Jeong CH, Farrand L, Lim S, et al. USP8 is a novel target for overcoming gefitinib resistance in lung cancer. Clin Cancer Res. 2013;19:3894–904. doi: 10.1158/1078-0432.CCR-12-3696. doi: 10.1158/1078-0432.ccr-12-3696. [DOI] [PMC free article] [PubMed] [Google Scholar]