At the end of March 2013, the first case of human infection with avian influenza A(H7N9) virus was confirmed in Shanghai. From April to May 2013, 18 patients with avian influenza A(H7N9) virus infection were hospitalized in Shanghai Public Health Clinical Center, and finally, 12 of them survived. The short-term prognosis of these patients had been described previously,[1] but the long-term prognosis remained unclear.
From September to November 2013, 5 survivors with avian influenza A(H7N9) virus infection discharged from Shanghai Public Health Clinical Center were admitted to Zhongshan Hospital, Fudan University. Data of demographic characteristics, diagnosis, and underlying comorbidities were obtained from the patients. Then, the patients had undergone thoracic high-resolution computed tomography (HRCT) and pulmonary function tests (PFT). The HRCT was evaluated by a radiologist and a pulmonologist independently and then reached consensus. The presence and distribution of the following radiologic abnormalities, namely, consolidations, ground-glass opacities (GGOs), air bronchograms, centrilobular nodules, interlobular septal thickening, reticulations, subpleural linear opacities, cystic changes, and pleural effusion, were assessed for each patient. The following indices of PFT, namely, forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), FEV1/FVC, total lung capacity (TLC), and the diffusion capacity for carbon monoxide of lung (DLCO), were assessed and expressed as percentages of values predicted. Bronchial provocation and dilation test were not included in the protocol. This study was approved by the Institutional Review Board of Zhongshan Hospital, Fudan University.
The characteristics of these 5 survivors are shown in Table 1. All the 5 patients were older than 60 years, and 3 were male. After discharge, only case 4 had mild respiratory tract infection symptoms while the others had no physical discomfort.
Table 1.
Clinical characteristics of 5 survivors after the first outbreak of human infections with avian influenza A(H7N9) virus in Shanghai
| Case number | Age (years) | Sex | Date of diagnosis by virus examination | Underlying diseases | PFT results | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| FVC (% predicted) | FEV1 (% predicted) | FEV1/FVC (%) | TLC (% predicted) | DLCO (% predicted) | ||||||
| 1 | 67 | Male | April 6, 2013 | None | ||||||
| 2 | 74 | Female | April 19, 2013 | Hypertension Diabetes Cardiovascular diseases Cholecystolithiasis |
63.5 | 76.7 | 82.8 | 71.6 | 56.9 | |
| 3 | 62 | Male | April 9, 2013 | Hypertension | 55.4 | 61.5 | 86.9 | 53.2 | 44.1 | |
| 4 | 75 | Female | April 9, 2013 | Hypertension Diabetes Cardiovascular diseases Breast cancer |
||||||
| 5 | 67 | Male | April 20, 2013 | Diabetes Benign prostate hyperplasia |
88.1 | 81.8 | 72.3 | 86.5 | 78.1 | |
| Case number | HRCT findings | |||||||||
| Consolidation | GGO | Air bronchogram | Centrilobular nodules | Interlobular septal thickening | Reticulation | Subpleural linear opacity | Cystic change | Pleural effusion | Distribution | |
| 1 | − | + | + | − | + | + | + | + | − | LU, RL, LL |
| 2 | − | + | − | + | − | − | − | − | − | RU, RL, LU, LL |
| 3 | − | + | − | − | + | + | + | + | − | RU, RM, RL, LU, LL |
| 4 | − | + | + | − | + | − | + | + | − | RU, RL, LL |
| 5 | − | + | − | − | + | + | + | + | − | RU, RM, RL, LU, LL |
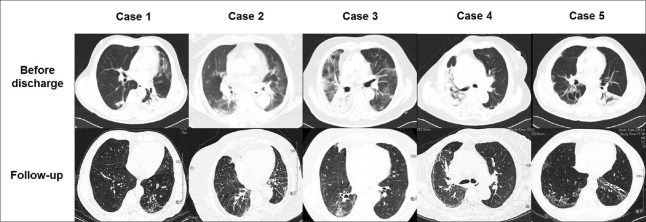
Compare with the thoracic CT before discharge, most of the lesions had been resolved, but all survivors still had bilateral lesions on HRCT. The lesions could be detected in at least 3 lobes and were frequently observed in the right lower (RL) and left lower (LL) lobes. GGOs were observed in all survivors. Interlobular septal thickening, subpleural linear opacities, and cystic changes were the second most common abnormalities. Consolidations and pleural effusion were not detected in any survivor [Figure 1].
Figure 1.
Compared with the computed tomography image before discharge, most of the lesions have been resolved at follow-up. Thoracic high-resolution computed tomography image of these 5 survivors showed that after approximately 6 months of discharge, the main imaging findings were ground-glass opacities, interlobular septal thickening, subpleural linear opacities, and cystic changes.
PFT was carried out for 3 survivors. The other 2 (case 1 and 4) were not qualified due to poor compliance. The values of FEV1, FVC, TLC, and DLCO were lower than predicted (<80%) for case 2 and 3 while their FEV1/FVC remained normal. The PFT result of case 5 was almost in normal range.
A previous study had revealed that the main imaging findings of HRCT during the initial stage of avian influenza A(H7N9) virus infection were GGOs, consolidations, air bronchograms, and interlobular septal thickening. The lesions were distributed in at least 3 lobes and were mostly involved in the right lower (RL), right upper (RU), and LL lobes.[2] From this study, GGOs and interlobular septal thickening could still be frequently detected at convalescent stage, but consolidations and air bronchograms could be gradually resolved after discharge. Subpleural opacities and cystic changes could only be observed at convalescent stage. Although 4 of 5 survivors in our study had no physical discomfort at follow-up, the results of HRCT indicated that the resolving of lesions was slow, which was similar to patients with avian influenza A(H5N1) virus infection. Lu et al. had followed up two patients with avian influenza A(H5N1) virus infection for 12 and 24 months, respectively, and revealed that the resolving of lesions was slow. In one case, fibrotic reticular opacities, fibrotic linear opacities, and small patchy opacities were observed on CT at the 7th month of follow-up. However, the results at the end of follow-up revealed that most of the lesions could be gradually resolved.[3] Therefore, more studies were needed to clarify whether the lesions of patients with avian influenza A(H7N9) virus infection could be resolved or not.
None of the previous studies have mentioned the PFT changes of these patients at initial stage because they were in poor condition during hospitalization, which made them unqualified for PFT. In this study, 3 survivors had undergone PFT, and the results revealed that after approximately 6 months of discharge, FEV1/FVC was in normal range, while TLC and DLCO were obviously lower than predicted in 2 of 3 survivors, which indicated restrictive ventilation and diffusion dysfunction. Liu et al. had carried out a 1-year follow-up for 48 patients with mild avian influenza A(H1N1) virus infection. Among these patients, 26 had abnormal pulmonary function, including 16 patients with diffusion disorder (decreased DLCO). However, there was no simple limitation (decreased TLC) among these patients, besides, 16 patients were suffered from small airway function disorder.[4] Since there were only the PFT results of 1 year after discharge in their study, it was not easy to make a comparison of the PFT results between avian influenza A(H1N1) and avian influenza A(H7N9) virus infection.
Compared with the previous pandemic avian influenza A(H1N1) and avian influenza A(H5N1) virus infection, the median age of the patients with avian influenza A(H7N9) virus infection was much greater, and the male patients were the major component.[5] Therefore, the coexistence of avian influenza A(H7N9) virus infection and other chronic disease was common. However, in this study, none of the patients had a history of lung disease. It was consistent with the study by Wang et al., in which only 10 of 105 patients with avian influenza A(H7N9) virus infection had a history of chronic lung disease.[5] Thus, it was plausible that the lesions of the lung were primarily caused by avian influenza A(H7N9) virus infection rather than other diseases.
In conclusion, our report revealed that the radiological lesions and the damage of pulmonary function were not consistent with clinical symptoms, and the lesions of avian influenza A(H7N9) virus infection in lung may last for a long time. More studies were needed to clarify whether the lesions could be completely resolved or not.
Financial support and sponsorship
This research was supported by the grant from the National Natural Science Foundation of China (No. 81500026).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yuan-Yuan Ji
REFERENCES
- 1.Lu S, Li T, Xi X, Chen Q, Liu X, Zhang B, et al. Prognosis of 18 H7N9 avian influenza patients in Shanghai. PLoS One. 2014;9:e88728. doi: 10.1371/journal.pone.0088728. doi: 10.1371/journal.pone.0088728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Q, Zhang Z, Shi Y, Jiang Y. Emerging H7N9 influenza A (novel reassortant avian-origin) pneumonia: Radiologic findings. Radiology. 2013;268:882–9. doi: 10.1148/radiol.13130988. doi: 10.1148/radiol.13130988. [DOI] [PubMed] [Google Scholar]
- 3.Lu PX, Wang YX, Zhou BP, Ge Y, Zhu WK, Chen XC, et al. Radiological features of lung changes caused by avian influenza subtype A H5N1 virus: Report of two severe adult cases with regular follow-up. Chin Med J. 2010;123:100–4. doi: 10.3760/cma.j.issn.0366-6999.2010.01.018. [PubMed] [Google Scholar]
- 4.Liu W, Peng L, Liu H, Hua S. Pulmonary function and clinical manifestations of patients infected with mild influenza A virus subtype H1N1: A one-year follow-up. PLoS One. 2015;10:e0133698. doi: 10.1371/journal.pone.0133698. doi: 10.1371/journal.pone.0133698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang C, Yu H, Horby PW, Cao B, Wu P, Yang S, et al. Comparison of patients hospitalized with influenza A subtypes H7N9, H5N1, and 2009 pandemic H1N1. Clin Infect Dis. 2014;58:1095–103. doi: 10.1093/cid/ciu053. doi: 10.1093/cid/ciu053. [DOI] [PMC free article] [PubMed] [Google Scholar]