Abstract
PCB126 (3,3′,4,4′,5-pentachlorobiphenyl) is a potent aryl hydrocarbon receptor agonist and induces oxidative stress. Because liver manganese (Mn) levels decrease in response to PCB126, a Mn dietary study was designed to investigate the role of Mn in PCB126 toxicity. Male Sprague Dawley rats received diets containing 0, 10, or 150 ppm added Mn for 3 weeks, followed by a single ip injection of corn oil or PCB126 (5 µmol/kg body weight). After 2 weeks, Mn, Cu, Zn, and Fe levels in the heart, liver, and liver mitochondria, and Mn-containing superoxide dismutase (MnSOD) and metallothionein mRNA, MnSOD protein, and MnSOD activity were determined. Mn levels in liver, heart, and liver mitochondria were strongly decreased by the Mn-deficient diet. Small effects on Fe levels and a stepwise increase in MnSOD activity with dietary Mn were also visible. PCB126 caused profound changes in Cu (up), Zn, Fe, and Mn (down) in liver, but not in heart, and differing effects (Cu, Zn, and Fe up, Mn down) in liver mitochondria. Liver MnSOD and metallothionein mRNA levels and MnSOD protein were increased but MnSOD activity was decreased by PCB126. PCB126-induced liver enlargement was dose-dependently reduced with increasing dietary Mn. These changes in metals homeostasis and MnSOD activity in liver but not heart may be a/the mechanism of PCB126 liver-specific toxicity. Specifically, transport of Fenton metals (Cu, Fe) into and Mn out of the mitochondria, a probable mechanism for lower MnSOD activity, may be a/the cause of PCB126-induced oxidative stress. The role of metallothioneins needs further evaluation. Dietary Mn slightly alleviated PCB126-induced toxicities.
Keywords: MnSOD, PCB126, manganese, aryl hydrocarbon receptor, diet, metallothionein
Manganese (Mn) is an essential trace element for animals and humans. It is required for protein and fat metabolism, immune and nervous system function, bone health and blood sugar regulation. It is a critical component for enzymes involved in energy production and antioxidant response (Aschner et al., 1999; Michalke, 2004). In animal models, Mn deficiency caused skeletal abnormalities, impaired reproductive function, altered lipid metabolism, and other health problems (Baly et al., 1990; Davis et al., 1990). Although Mn is readily available from a variety of foods, several cases of Mn deficiency have been reported with symptoms such as dermatitis, hypocholesterolemia, and short stature, if the Mn deficiency happened early in life (Finley and Davis, 1999; Friedman et al., 1987). In addition, Mn metabolism can be disturbed as the result of other disease conditions or the disruption of the homeostasis of other metals. Several diseases have been linked to changes in Mn homeostasis such as epilepsy, maple syrup urine disease, phenylketonuria, amyotrophic lateral sclerosis, and acromegaly (Bowman et al., 2011; Finley and Davis, 1999). The liver is key for maintaining Mn homeostasis (Finley, 1998; Schramm and Brandt, 1986). Liver is among the organs that have the highest Mn levels (Dorman et al., 2006), it produces 2 of the main plasma transport proteins of Mn—albumin and transferrin (Crossgrove and Zheng, 2004), and hepatobiliary excretion is the major route of elimination for Mn (Dorman et al., 2006). Therefore, anything that disrupts normal liver function can potentially affect Mn levels in the liver and other organs.
The Mn-containing superoxide dismutase (MnSOD) converts superoxide radical to hydrogen peroxide and hence serves as an essential antioxidant in mitochondria. Increased levels of MnSOD are protective against cancer (Oberley and Oberley, 1997; Petkau et al., 1975; St Clair et al., 1992). The MnSOD protein is a homotetramer with 1 prosthetic manganese atom at the active site of each subunit. MnSOD is encoded by the nuclear sod2 gene and acquires the manganese atom after translation during the mitochondrial localization (Culotta et al., 2006). As a result, the availability of this essential element plays an important role in the regulation of the enzyme. Severe Mn deficiency led to decreased MnSOD activity in the heart and/or liver (Li et al., 2011; Malecki and Greger, 1996; Zidenberg-Cherr et al., 1985). Such depression of MnSOD activity has been correlated with excess lipid peroxidation in hepatic mitochondria (Malecki and Greger, 1996; Zidenberg-Cherr et al., 1983) and may render animals and humans more susceptible to cancer.
Metallothionein (MT), a family of cysteine-rich, low molecular weight proteins, functions in the transport of metals, and in the defense against oxidative stress. MT consists of 4 isoforms with MT1 and MT2 being expressed in almost every tissue. Dietary Mn supplementation has been shown to increase the expression of MT, and to increase antioxidant defenses (Kobayashi et al., 2007). In addition, MT is transported into the mitochondria, thus affecting the intracellular metal distribution (Lindeque et al., 2010).
Environmental contaminants, like polychlorinated biphenyls (PCBs) and related persistent pollutants alter metals homeostasis in rodent liver (Al-Bayati et al., 1987; Elsenhans et al., 1991; Wahba et al., 1988). This is particularly true for compounds like 2,3,7,8-tetrachlorodibenzo-dioxin (TCDD), that are ligands of the aryl hydrocarbon receptor (AhR). PCB126 (3,3′,4,4′,5-pentachlorobiphenyl) is a dioxin-like member of the PCB family which exerts its toxicity, comparable to that of TCDD, mainly through the AhR (Bandiera et al., 1982; Safe, 1990; Van den Berg et al., 2006). Studies found that PCB126 caused a number of hepatocellular alterations such as liver hypertrophy, fatty change, necrosis, and micronutrient dysregulation in rats (Lai et al., 2010, 2011, 2013). The incidence of liver cancer was also increased in PCB126-treated rodents (Hailey et al., 2005; NTP, 2006; Silberhorn et al., 1990; Yoshizawa et al., 2007). The consistent finding of PCBs as animal carcinogens and increasing epidemiologic evidence for PCBs as human carcinogens have led the International Agency for Research on Cancer to recently upgrade PCBs to Group I Human Carcinogens (Lauby-Secretain et al., 2013). One possible common mechanism leading to these toxic manifestations of dioxin-like compounds is through increased oxidative stress associated with the activation of the AhR signaling pathway. Mitochondria, as a major user of molecular oxygen, represent a potential source of reactive oxygen species (Chen, 2010; Coteur et al., 2001; Hassoun et al., 2002).
Given our previous studies showing that Mn levels in the rat liver were decreased following PCB126 treatment (Lai et al., 2011), we were particularly interested in the influence of Mn in the toxicity of this PCB, especially related to oxidative stress, the hepatic antioxidant defenses, and the expression of mitochondrial MnSOD. The purpose of this study therefore was to examine the effect of dietary Mn on the toxicity of PCB126 through modification of antioxidant proteins and alteration of micronutrients. The ultimate goal is to evaluate the feasibility to mitigate PCB toxicity through dietary Mn supplementation.
MATERIALS AND METHODS
Animals, treatment, and organ preparation
The experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Iowa. Thirty-eight 3- to 4-week-old male Sprague Dawley rats from Harlan Laboratories (Indianapolis, Indiana) were individually housed in metal wire cages and randomly divided into 3 dietary groups. Each group was fed 1 of the 3 AIN-93-based diets obtained from Harlan Teklad (Madison, Wisconsin): deficient manganese diet (with no added Mn, 13 rats), adequate manganese diet (standard AIN-93 diet containing 10 µg/kg added Mn, 13 rats), and supplemented manganese diet (150 µg/kg added Mn, 12 rats). Mn supplement was in the form of inorganic manganese carbonate. The information on the detailed manganese content of each diet is listed in Table 1. Rats were allowed to acclimatize to the diets for 3 weeks before receiving a single ip injection of either corn oil (5 ml/kg b.w., 6 animals per group per diet) or PCB126 (5 µmol/kg b.w., 6 or 7 animals per group per diet) dissolved in corn oil. The synthesis and characterization of PCB126 were described previously (Lai et al., 2010). The average body weight at time of injection was 246 ± 17 g. Feed consumption and body weight of the animals were monitored twice a week. All animals were sacrificed 2 weeks after the injection by carbon dioxide asphyxiation followed by cervical dislocation. Liver, heart, lung, and thymus tissues were excised immediately after euthanasia, weighed, flash frozen in liquid nitrogen, and stored at −80°C for later use.
TABLE 1.
Composition of Diets
| Diet | Mn Content | Mn Form |
|---|---|---|
| Deficient Mn | < 2 µg/g (stated to be 0.4 µg/g by supplier) | NA |
| Adequate Mn | 11 µg/g (stated to be 10.4 µg/g by supplier) | Manganese carbonate |
| Supplemental Mn | 150 µg/g | Manganese carbonate |
NA, not applicable. The Mn content was determined by ICP-MS.
Tissue total RNA extraction
Total RNA from frozen tissue samples was isolated using the RNeasy Mini Kit from Qiagen Inc (Valencia, California) according to the manufacturer’s instructions. The quantity and quality of RNA were determined spectrophotometrically by the absorbance at 260 nm (A260) and the ratio of A260 and A280 in 10 mM Tris buffer at neutral pH.
Reverse transcription and real time PCR (qPCR)
In total, 0.4 µg (heart) or 1 µg (liver) of total RNA was reverse-transcribed into cDNAs in a 20-µl reaction volume using the High Capacity RT Kit from Applied Biosystems by Life Technologies Co (Carlsbad, California) following the manufacturer’s protocol. qPCR was carried out using 1–5 ng cDNA and the Power SYBR Green Master Mix from Applied Biosystems in each 20-µl reaction and an Eppendorf Realplex2 Master Cycler (Hamburg, Germany). The primer sequences and cycling conditions for MnSOD, MT1, and the house keeping genes GAPDH and β-actin were adopted from literature (Lai et al., 2013; Pardo et al., 2008; Rubiolo et al., 2008). All primers were synthesized by IDT (Coralville, Iowa) and the sequences can be found in Supplementary Table 1. The ΔΔCt or Pfaffl method was used to calculate the relative mRNA expression of each gene. The relative expression of GAPDH and β-actin mRNA was found to be similar across all groups.
Western blotting
Approximately 0.5 g of fresh liver was homogenized in 1.34 mM DETAPAC (diethylenetriaminepentaacetic acid) in 0.05 M potassium phosphate buffer, pH 7.8. The protein concentration of the whole liver homogenate was measured using the Bradford assay (Bradford, 1976). Five µg of protein from each sample was separated by 12.5% SDS PAGE in the NuPAGE LDS Sample Buffer (Invitrogen, Carlsbad, California) at 100 V for 100 min. Proteins were then transferred from the gel to a PVDF membrane in tris-glycine buffer containing 15% methanol at 100 V for 1 h. The blot was cut and the top half was incubated overnight at 4°C with primary antibody against MnSOD at 1:5000 dilution (No. 06-984, Upstate Millipore, Billerica, Massachusetts) followed by a 1-h incubation at room temperature with HRP-conjugated anti-rabbit secondary antibody (sc-2004 Santa Cruz Biotech, Santa Cruz, California) at 1:3000 dilution. The bottom half of the blot was incubated for 1 h at room temperature with HRP-conjugated anti-GAPDH antibody (sc-25778, Santa Cruz Biotech) as loading control for the lanes. Chemiluminescence was developed using SuperSignal West Femto Chemiluminescent Substrate from Thermo Scientific Pierce (Rockford, Illinois) and visualized with the typhoon imaging system from GE Healthcare (Piscataway, New Jersey). Densitometry was performed using the GE Healthcare’s ImageQuant 5.2 software and the density of MnSOD was normalized to that of GAPDH on each blot. The expression of GAPDH protein was found to be similar across all groups. This Western blot protocol is routinely performed in the laboratory with established linearity range of protein loading.
MnSOD activity determination
MnSOD activity was measured using the competitive inhibition assay developed by Spitz and Oberley (1989). These experiments were conducted in the Radiation and Free Radical Research Core laboratory of the Holden Comprehensive Cancer Center at the University of Iowa with a standardized protocol and established linearity range. Briefly, reduction of nitroblue tetrazolium (NBT) by superoxide radical generated from xanthine and xanthine oxidase is reduced by the radical scavenger SOD. The rate of inhibition correlates with the activity of SOD in the reaction mixture. In the presence of cyanide, the activity of CuZnSOD is inhibited and the spectral change of NBT is solely dependent on the activity of MnSOD. One unit of activity is defined as the amount of protein that inhibits NBT reduction by 50% and the MnSOD activity is reported in unit/mg protein.
Mitochondria purification
Liver mitochondria were isolated employing previously published methods (Frezza et al., 2007; Wieckowski et al., 2009). Briefly, approximately 2 grams of fresh liver tissue were homogenized in a Dounce tissue homogenizer in IBc buffer containing 10 mM Tris-MOPS, 1 mM EGTA-Tris, and 200 mM sucrose (pH 7.4). The homogenate was washed several times by centrifugation at 7000 × g. The pellet (ie crude mitochondria extract) was resuspended in MRB buffer containing 250 mM mannitol, 5 mM Hepes, and 0.5 mM EGTA (pH 7.4). The crude extract was then layered on top of Percoll medium containing 225 mM mannitol, 25 mM Hepes (pH 7.4), 1 mM EGTA, and 30% Percoll from GE Healthcare and centrifuged at 95 000 × g in a Beckman Coulter Ultracentrifuge (Fullerton, California). Pure mitochondrial fraction was collected and the protein concentration determined by the Bradford assay. The purity was checked by Western blot. No MnSOD could be detected in the supernatant fractions while no cytosolic GAPDH was detected in the mitochondrial fraction.
Metal measurements
Approximately 0.5 g liver and heart tissue and the mitochondrial extracts were used to determine manganese, copper, zinc, and iron levels using a previously described method (Lai et al., 2011). Briefly, samples were pretreated with microwave-assisted closed-vessel acid (HNO3) digestion prior to instrument measurement. Metal concentrations were quantitatively determined with an elemental mass spectrometer by inductively coupled plasma—mass spectrometry (ICP-MS) using a Perkin-Elmer ELAN II ICP-MS equipped with a SC-2 auto sampler (Elemental Scientific, Omaha). Results were normalized to tissue weights or protein concentrations (mitochondria extract). For statistical analysis results from the medium and supplemented Mn groups were compared with the corresponding controls in the low Mn diet group.
Histology
Tissue sections were fixed in 10% neutral buffered formalin. Fixed tissues were then routinely processed, paraffin embedded, and sectioned at 4 µm. Sections were stained with hematoxylin and eosin and coverslipped.
Statistics
Data are reported as means ± SE. The relative growth of animals was calculated by subtracting the body weight at injection from the final b.w. Organ weights were calculated as percent (liver) or per thousand (heart, thymus, lung) of body weight of the animal. The ANOVA analysis was performed by using the SAS procedure PROC GLM (SAS version 9.2 Cart, North Carolina) to determine the statistical significance of diet and treatment. The LSMEANS procedure was applied to obtain P values for pairwise comparisons. A P value < .05 was considered statistically significant. Normality was confirmed using the UNIVARIATE function of SAS followed by the NPAR1WAY function, as necessary.
RESULTS
Body and Organ Weights
Consistent with previous findings, PCB126 significantly slowed body weight gain in rats regardless of the diets (Table 2). This happened even though the feed intake was nearly the same in all groups, regardless which injection the animals received, except the supplemented Mn control group (Supplementary Figure 1). This group had a higher food intake compared with the other control groups and the corresponding PCB group on supplemented Mn. Based on food consumption and body weight, the mean Mn uptake was approximately 0.028 and 0.026 mg/kg-bw/day in corn oil and PCB126 treatment groups, respectively, at the lowest dietary Mn level; 0.79 and 0.72 mg/kg-bw/day in corn oil and PCB126 treatment groups, respectively, at the adequate dietary Mn level; and 11 and 9.9 mg/kg-bw/day in corn oil and PCB126 treatment groups, respectively, at the highest dietary Mn level. Dietary manganese appeared to cause slightly reduced liver weights in the control animals, although the effect did not rise to statistical significance. PCB126 significantly increased the liver weights of the rats on all 3 diets; however, the degree of liver hypertrophy was dose-dependently decreased with increasing levels of Mn in the diet, and this was statistically significant in the supplemented Mn group and for diet overall. Mn did not affect the weights of heart, thymus, and lung in any treatment group. PCB126 on the other hand caused a significant decrease in absolute (data not shown) and relative thymus weights due to a dramatic thymus involution and an increase in relative lung weight (Table 2).
TABLE 2.
Body and Organ Weights in Response to PCB126 and Dietary Mn Levels
| Dietary Mn Level (Added Amount of Mn) | Deficient (0 µg/kg Diet) | Adequate (10 µg/kg) | Supplemented (150 µg/kg) | Overall Diet | |
|---|---|---|---|---|---|
| Growth (%) | Corn oil | 22.0 ± 1.5 | 25.0 ± 1.6 | 24.0 ± 0.5 | — |
| PCB126 | 12.8 ± 1.5a | 12.9 ± 3.3a | 9.9 ± 2.1a | — | |
| Relative liver weight (%) | Corn oil | 4.49 ± 0.09 | 4.40 ± 0.05 | 4.34 ± 0.09 | — |
| PCB126 | 8.03 ± 0.15a | 7.40 ± 0.27a | 7.02 ± 0.27a,b | * | |
| Relative heart weight (‰) | Corn oil | 3.82 ± 0.09 | 3.68 ± 0.04 | 3.73 ± 0.07 | — |
| PCB126 | 3.57 ± 0.09 | 3.49 ± 0.05a | 3.58 ± 0.04 | — | |
| Relative thymus weight (‰) | Corn oil | 2.28 ± 0.13 | 2.37 ± 0.14 | 2.22 ± 0.09 | — |
| PCB126 | 0.71 ± 0.11a | 0.49 ± 0.02a | 0.55 ± 0.03a | — | |
| Relative lung weight (‰) | Corn oil | 4.72 ± 0.29 | 4.72 ± 0.28 | 4.86 ± 0.32 | — |
| PCB126 | 7.22 ± 0.57a | 6.36 ± 0.33a | 6.49 ± 0.53a | — | |
aStatistically significant compared with the corn oil-treated animals on the same diet (PCB effect).
bStatistically significant compared with those on low Mn diet and the same treatment (diet effect).
*Overall significant effect of the diet. P < .05; n = 6 or 7.
After 3 weeks on diets containing 0, 10, or 150 µg/kg added manganese carbonate, male Sprague Dawley rats received a single ip injection of corn oil or PCB126 (5 µmol/kg b.w.) and were sacrificed 2 weeks later. Growth %: ratio between weight gain after injection and b.w. at injection time. Relative organ weights: ratios between organ weights and final b.w. Data are presented as mean ± SE.
Histology
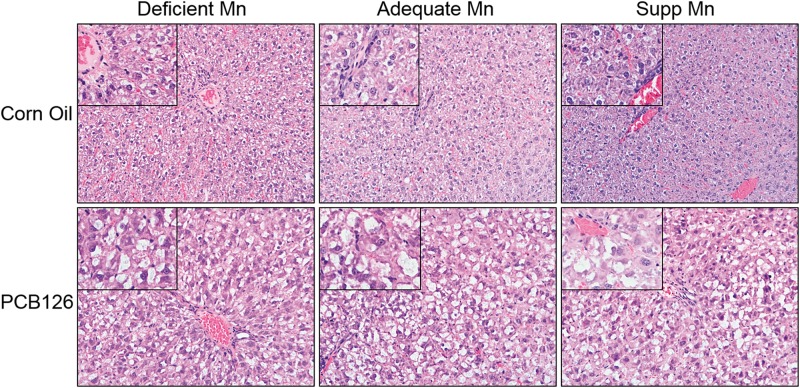
Microscopic examination of liver tissues revealed hepatocytes with large areas of cytoplasmic pallor (hydropic degeneration) with occasional peripheralization of nuclei and small to moderate circular vacuoles (lipid) (Figure 1; Supplementary Table 2). The hydropic degeneration was the most severe change noted. The changes in the PCB treated livers were consistent with previously documented PCB liver changes including hepatocellular enlargement due to hydropic degeneration and hepatocellular lipid accumulation. Other histologic changes that were present in control and PCB treated livers included mild lymphocytic portal inflammation and foci of extramedullary hematopoiesis. There were no significant changes noted in the control Mn groups (low, medium, and supplemental Mn). In the PCB treated groups, there was variation in the severity of vacuolar change within each group that did not appear to be related to the Mn diet; however, this possibility cannot be totally ruled out. The number of livers with less significant vacuolar change in each Mn PCB group was 2/6 (low Mn), 3/6 (med Mn), and 3/6 (supplemented Mn).
FIG. 1.
Histopathology of livers following PCB126 (3,3′,4,4′,5-pentachlorobiphenyl) exposure with varying levels of dietary manganese (Mn). No alterations were seen between the dietary groups, however, cytoplasmic pallor with occasional peripheralization of nuclei and small to moderate circular vacuoles were visible in the PCB126 treated groups. These images are representative images of the various groups. Main images are ×20 magnification with ×40 magnification in inset.
Gene Expression
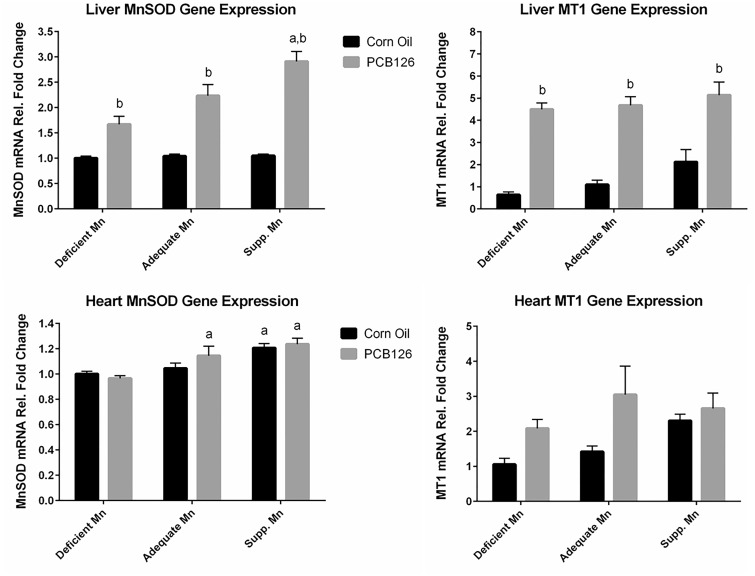
Dietary Mn levels did not affect the basal levels of MnSOD mRNA in the liver as visible in the control animals shown in Figure 2 (top left) but PCB126 significantly induced MnSOD and MT expression in all dietary groups. The extent of MnSOD induction was dependent on dietary Mn levels, ranging from 1.5-fold in the deficient Mn group to almost 3-fold in the supplemented Mn diet group. Very different effects were seen in the heart (Figure 2, bottom left), where basal levels of MnSOD mRNA were positively correlated with dietary Mn, while PCB126 did not produce any significant effect in any diet group. MT basal expression seemed to increase with dietary Mn, most visible in the supplemented Mn group, and a PCB126 mediated induction was most noticeable in the adequate Mn group, but none of these effects reached statistical significance (Figure 2, top and bottom right).
FIG. 2.
Mn-containing superoxide dismutase (MnSOD) and metallothionein (MT1) mRNA levels in liver (top) and heart (bottom) in response to PCB126 and dietary Mn. Male Sprague Dawley rats received diets containing 0, 10.5, or 150 µg/kg manganese carbonate for 5 weeks and 1 single ip injection of corn oil or PCB126 at 5 µmol/kg b.w. after 3 weeks on the diet before being sacrificed. The MnSOD and MT1 mRNA quantity was first normalized to the quantity of the housekeeping gene GAPDH and then all the results were normalized to the low Mn corn oil control level. GLM with LSMEANS procedure of ANOVA was used to determine overall significance of diet or treatment. P values for pairwise comparisons were calculated by LSMEANS. aStatistically significant compared with low Mn diet and same treatment (diet effect), bStatistically significant compared with the corn oil-treated animals on the same diet (polychlorinated biphenyl effect), with P < .05 and n = 6 or 7.
MnSOD Protein Levels
As shown in Supplementary Figure 2 (top), PCB126 increased liver MnSOD protein levels in all 3 dietary groups, which is consistent with mRNA expression. Dietary Mn levels did not influence the extent of this increase. In contrast, PCB126 or diet did not significantly affect heart MnSOD protein levels (Supplementary Figure 2, bottom). Although MnSOD protein levels of the medium Mn group were higher than those of the low Mn group, this effect was not statistically significant and no further increase in MnSOD protein levels were seen in the Mn supplemented group.
Enzymatic Activity of MnSOD
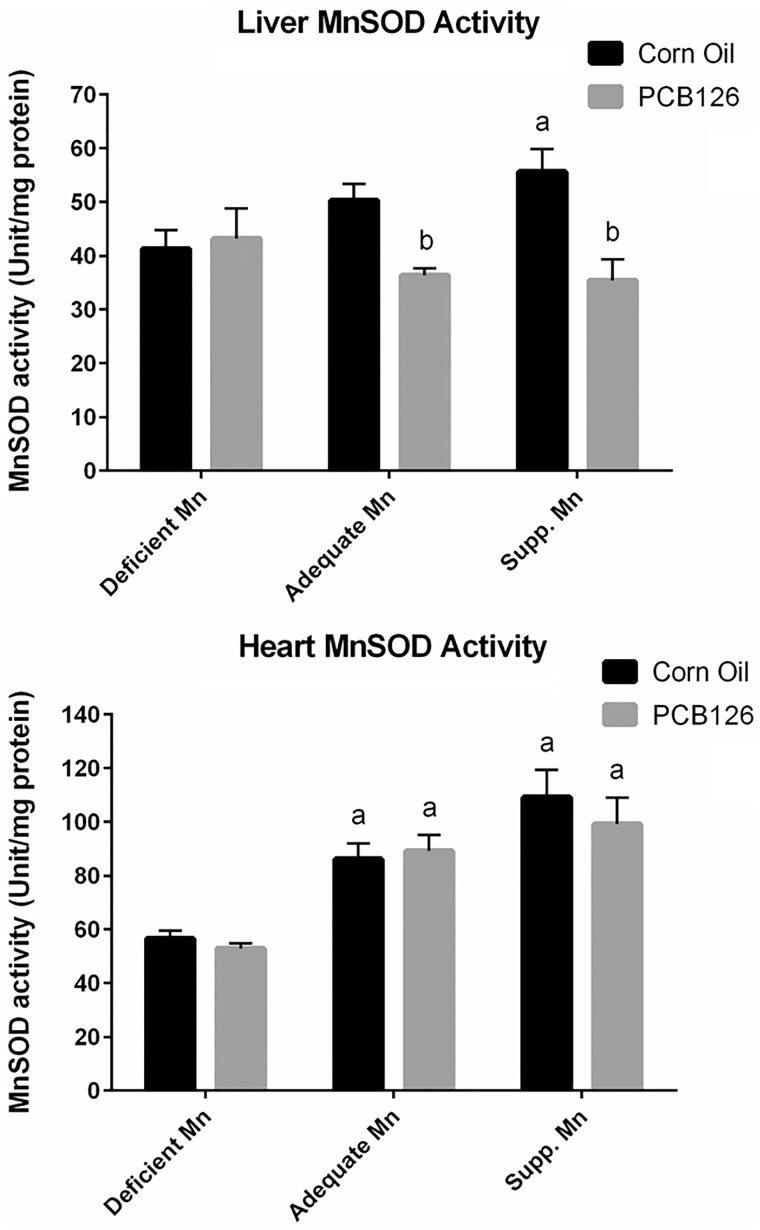
The basal level of liver MnSOD activity was dose-dependently increased with increasing dietary Mn levels (Figure 3, top). However, PCB126 treatment overrode this increase, resulting in significantly lower MnSOD activities in the adequate and supplemented Mn diet group compared with the corn oil control on the same diet. PCB126 exposure had no effect on MnSOD activity in the deficient Mn diet group. Heart MnSOD activity levels were consistent with the corresponding mRNA expression levels (Figure 3, bottom), ie significantly increased with increasing levels of Mn in the diet. However, the extent of this diet-dependent increase in heart MnSOD activity (up to 2-fold) was much more dramatic than the mRNA increase (1.2-fold). PCB126, on the other hand, had no effect on MnSOD activity in any dietary group.
FIG. 3.
MnSOD activity in response to PCB126 and dietary Mn. A, MnSOD activity in the liver. B, MnSOD activity in the heart. Experimental details and statistics are described in Figure 2.
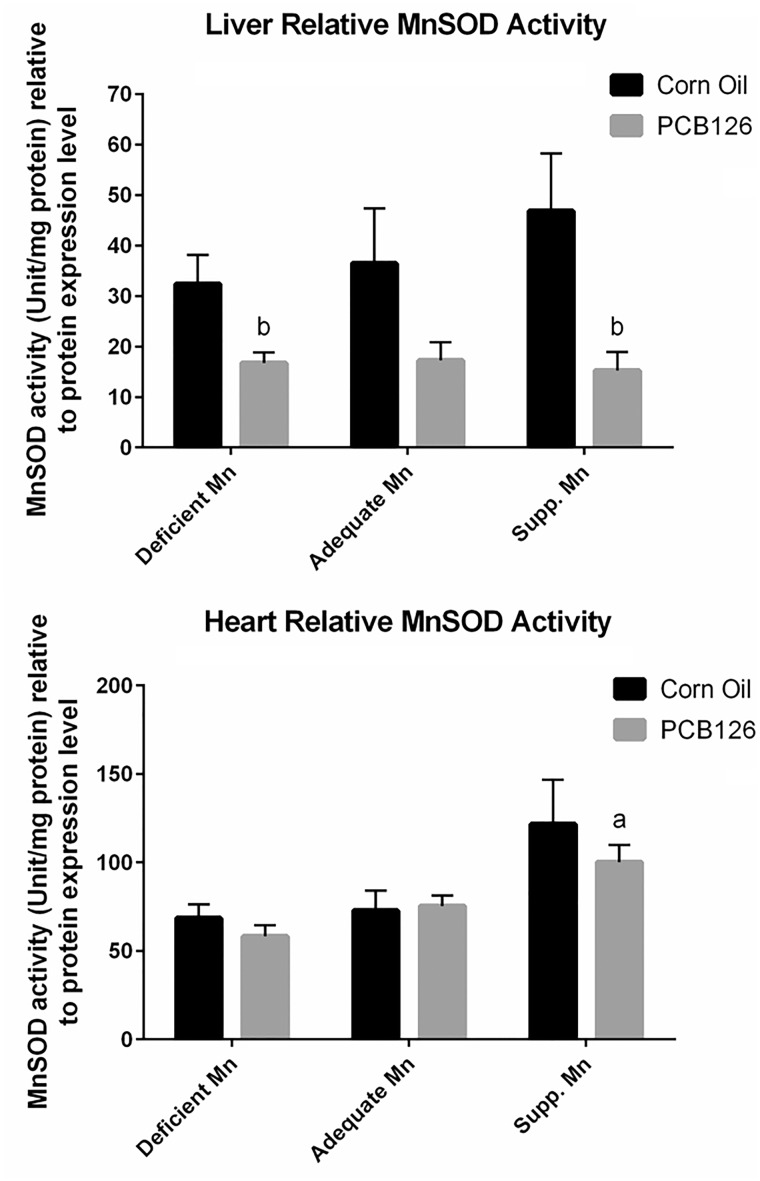
When expressed as activity relative to protein expression, similar trends were found (Figure 4). In the liver, the relative MnSOD activity levels appear to increase with dietary Mn levels in a dose-dependent manner in the corn oil controls groups, while relative activity levels remained unchanged across diet groups when treated with PCB126. The decrease of MnSOD activity in response to PCB126 treatment appears to be more dramatic when the activity is normalized to MnSOD protein levels. A drop in relative activity level was observed in the Mn deficient group with PCB126 treatment, while the absolute activity level was similar between treatments on this diet (Figure 4, top). In the heart, relative MnSOD activity also increased with dietary Mn level, whereas PCB126 treatment did not significantly affect this parameter (Figure 4, bottom).
FIG. 4.
Relative MnSOD activity (to protein expression of MnSOD) in response to PCB126 and dietary Mn. A, Relative MnSOD activity in the liver. B, Relative MnSOD activity in the heart. Experimental details and statistics are described in Figure 2.
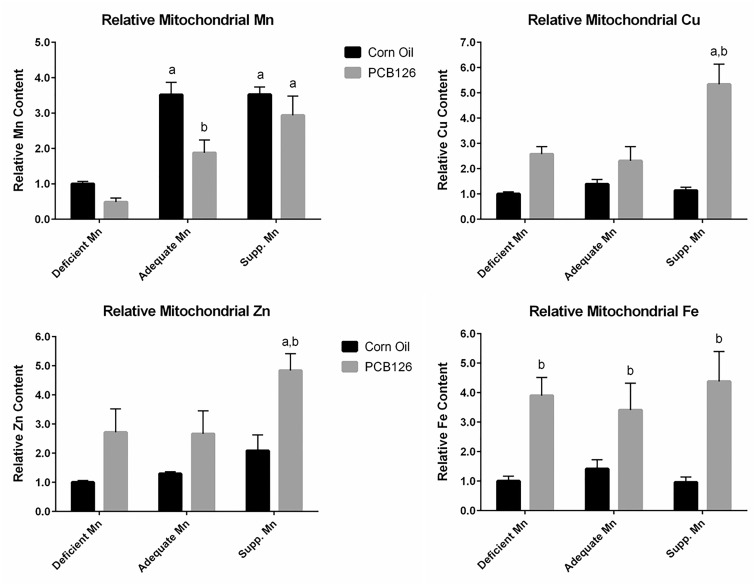
Mitochondrial Metals Levels
We isolated liver mitochondria in an attempt to determine metal levels in these organelles. Unfortunately, raw values for Mn from most PCB126-treated samples were below the quantitation limit of the method. This means that although Mn levels in these samples were above the detection limit, they were too low to be accurately quantified. As an alternative qualitative approach, we normalized the raw data to the protein concentration of each sample, and interpreted these data qualitatively. The raw Mn data and protein levels are given in the Supplemenary Table 3, while Figure 5 (top, left) depicts the calculated relative Mn levels in each group after arbitrarily setting the value of the low Mn control animals as 1. In corn oil control animals, the Mn level in liver mitochondria from animals in the deficient Mn group was much lower than that in the other dietary groups; the supplemented Mn diet did not increase Mn levels in liver mitochondria beyond the level in the normal (adequate Mn) diet. In the PCB126-treated animals, the mitochondrial Mn level increased with increasing dietary Mn levels. However, compared with the controls on the same diet, PCB126 decreased mitochondrial Mn content. This effect was apparent in all dietary groups.
FIG. 5.
Mitochondrial metal levels in the liver in response to PCB126 and dietary Mn. Exposure is described in Figure 1. The values were first normalized to protein concentrations and then to the value of corn oil-treated rats on low Mn diet.
Mitochondrial Cu and Fe were within quantitation range. In contrast, many values of Zn were not, because the detection limit of our method was 5 times higher than for the other metals. Raw values for Zn in the mitochondria are shown in the Supplementary Table 3. Cu and Zn levels were similar to each other and were around 10 times higher than those of Mn and 10 times lower than those of Fe. A similar normalization procedure as described for Mn was applied to all other metals and the resulting data are depicted in Figure 5. PCB126 treatment increased the mitochondrial level of all of these metals (Cu, Zn, and Fe) in all dietary groups. The PCB126-effect for mitochondrial Cu and Zn levels was highest in the supplemented Mn diet groups and statistically significant for Cu, where the increase was nearly 5-fold. The mitochondrial Cu and Zn levels in the deficient and adequate Mn diet groups were similar, more than doubled for Cu, and almost 2-fold increased for Zn in PCB126 animals compared with the control animals. Mitochondrial Fe levels were approximately 4-fold higher in PCB126-exposed rats compared with the corn oil controls while dietary Mn did not seem to have an influence.
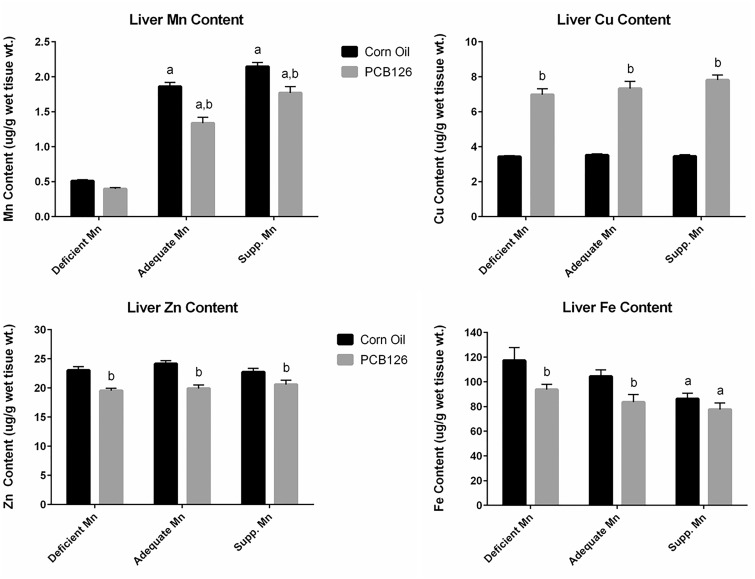
Tissue Metal Levels
In the measurement of tissue metal levels, all the values are well above the quantitation limit of the method. Basal levels of Mn, Zn, Cu, and Fe differ greatly in the liver and heart in the adequate Mn dietary control groups: liver contains higher Mn (1.9 vs 0.4 µg/g wet tissue), Zn (24 vs 15 µg/g wet tissue) and iron (104 vs 74 µg/g wet tissue), and less copper (3.5 vs 5.1 µg/g wet tissue) compared with the heart. Liver Mn tissue levels varied similarly to liver mitochondrial levels (Figure 6, top left; data in the Supplemenary Table 4). They were significantly and dose-dependently increased with dietary Mn levels, particularly between the deficient and adequate Mn diets (3.7-fold), less so from adequate to supplemented Mn diet (1.2-fold). PCB126, on the other hand, decreased Mn in rat livers significantly in the adequate and supplemented Mn diet groups.
FIG. 6.
Liver metal levels in response to PCB126 and dietary Mn. For experimental details and statistics see Figure 2. Liver total metals contents was normalized to the tissue weight used to determine metal levels by inductively coupled plasma—mass spectrometry.
Liver Cu was significantly increased by PCB126 treatment to more than 2-fold in all diet groups, and the extent of increase was slightly but significantly higher with supplemented Mn compared with lower dietary Mn levels (Figure 6, top right). Unlike mitochondrial Zn and Fe, which were increased by PCB126, liver Zn levels were significantly reduced (approximately 17%) by PCB126 treatment (Figure 6, bottom left). Liver Fe was also reduced by PCB126, which was statistically significant (15%–20%) in the rats on deficient and adequate Mn diets (Figure 6, bottom right). In addition, there was a dose-dependent decrease of liver Fe with increasing Mn levels in the diet in both corn oil and PCB126-treated rats.
Metal levels in the heart (Supplementary Figure 3 and Table 5) were generally not affected by PCB126 treatment or dietary Mn levels, except Mn (Supplementary Figure 3, top left), which was significantly higher in all adequate and supplemented Mn dietary groups. Here also, the level of increase in tissue Mn was most pronounced between deficient and adequate Mn diets, less so between the adequate and supplemented Mn diets.
DISCUSSION
Our previous results indicated that TCDD-treatment significantly reduced the activity of the critical antioxidant enzyme MnSOD in rat liver (Thampi, 1994). This reduction could potentially aggravate the oxidative stress associated with the activation of the Ah receptor by this ligand. We also observed that PCB126, another potent Ah receptor agonist, significantly decreased hepatic Mn levels (Lai et al., 2011). In addition, rats fed a standard Teklad AIN-93 diet versus the widely used Harlan 7013 diet (NIH-31 modified 6% mouse/rat diet) demonstrated different MnSOD activity changes in response to PCB126 exposure (unpublished data). These effects could be due to the differing concentrations of Mn in these 2 standard diets. In this study, we fed differing dietary Mn levels to elucidate the effect of Mn on the expression of MnSOD in response to PCB126 and to determine if Mn supplementation is protective against PCB126-induced liver toxicity and if Mn supplementation can modulate MnSOD expression in response to PCB126. The deficient Mn diet was Teklad AIN-93 M with no added Mn. Mn was only present as impurities from other dietary ingredients, reaching approximately 0.4 µg/g as determined by the Teklad Company. The adequate Mn diet was the standard Teklad AIN-93 M which contains 10 µg/g added Mn in the form of manganese carbonate. The supplemented Mn diet contained 150 µg/g added Mn as manganese carbonate, which is consistent with the amount of Mn in the Harlan 7013 diet and known to be nontoxic. Excess Mn could cause neurotoxicity, and therefore it was critical to ensure that the Mn levels in the diet would not cause adverse effects commonly associated with Mn intoxication. According to the manufacturer’s data sheets, a basal Teklad AIN-93 M contains different levels of metals compared with Harlan 7013 diet, a common laboratory diet in rat studies. As shown in Supplementary Table 6, AIM-93M diet contains less iron (48 vs 336 mg/kg), less zinc (30 vs 48 mg/kg), less manganese (11 vs 156 mg/kg), and less copper (6 vs 13 mg/kg) compared with the 7013 diet.
Dietary Mn levels did not affect the growth before or after PCB126 exposure. Although added Mn did not alleviate effects caused by PCB126, such as a trend in heart weight reduction, thymic involution and increased lung weight, the supplemented Mn diet did significantly reduce the PCB-induced liver enlargement in these animals. Hepatomegaly is a common manifestation of TCDD and PCB toxicity and has been shown to be the result of hepatocellular hypertrophy associated with increased hepatic protein concentration and enhanced accumulation of glycogen and lipid (Christian et al., 1986; NTP, 2006). Rodent dietary Mn toxicity studies by the National Toxicology Program (NTP, 1993) showed a decrease of liver weight by middle- and long-term Mn sulfate supplementation. In hamsters, dietary Mn sulfate slightly reduced aflatoxin-induced bile duct cell hyperplasia and significantly reduced enlarged nuclei and liver glycogen levels (Hastings and Llewellyn, 1987; Katzen and Llewellyn, 1987). The authors proposed Mn-caused changes in membrane chemistry as mechanism for these protective effects. In rats on a high polyunsaturated fatty acid diet, dietary Mn protected against oxidation of heart mitochondrial membranes (Malecki and Greger, 1996). The protective effect of supplemented Mn against PCB126-induced liver enlargement could be due to similar mechanisms and we propose that increased MnSOD activity may be the cause for this protection of mitochondrial membranes against oxidative stress. It should be noted, though, that the degree of this protection was small, in contrast to the hypertrophy caused by PCB126.
In the heart, MnSOD mRNA, protein and activity were all increased by Mn supplementation, which is consistent with other studies (Li et al., 2011), and PCB126 had no additional effect. In contrast, the liver reacted differently to PCB126, which became apparent when we analyzed all 3 levels of MnSOD expression. Previous studies measured only MnSOD mRNA or MnSOD protein or activity changes in response to AhR ligands (Cheng et al., 2009; Jin et al., 2010; Kono et al., 2002; Murugesan et al., 2008; Shimizu et al., 2003). Our data suggest that MnSOD is regulated by PCB126 at least at 2 levels, transcriptionally and posttranslationally. The transcriptional and translational inductions of MnSOD expression could be the result of increased oxidative stress imposed by PCB126, an AhR agonist. Chemicals such as co-planar PCBs and TCDD are known to induce oxidative stress (Chen, 2010; Hassoun et al., 2002). An in-depth analysis of possible mechanisms of this regulation is discussed elsewhere (Wang et al., unpublished data). The differences of MnSOD response between the heart and liver could be the result of different bioavailability of PCB126, as liver is the major organ for xenobiotic response and the major organ affected by the intraperitoneal exposure. The amount of PCB126 that reached the heart after liver accumulation and redistribution to the adipose tissues may be minimal.
In terms of MnSOD expression in response to dietary Mn levels, we found that both MnSOD mRNA and activity levels were increased in the liver and heart in the corn oil control groups with increasing dietary Mn but MnSOD protein levels remained unchanged. The increased MnSOD mRNA levels with increasing dietary Mn levels indicate a transcriptional induction both in the liver and in the heart, while the increased MnSOD activity relative to MnSOD protein expression suggests increased posttranslational activation with increased dietary Mn. These findings are consistent with those reported by others. Lee et al. (2013) reported increased MnSOD activity, unchanged apoprotein, and increased metallation of the enzyme in mice receiving ip injections of 4 mg/kg-bw Mn chloride in addition to dietary levels of 80 or 100 µg Mn/g-diet. Similarly, Jouihan et al. (2008) found ip injections of Mn chloride reversed mitochondrial Mn deficiency and increased MnSOD activity in Hfe−/− mice.
MT is an important protein involved in antioxidant defense and metal homeostasis. In the liver, MT1 expression was significantly upregulated by PCB126 in all the different dietary groups. In the heart, Mt1 gene expression was nonsignificantly increased and hardly higher in the supplemental Mn group compared with the corresponding control, which may be the result of the increased basal level of expression seen in the supplemental Mn group. MT is known to traffic throughout the cell, in particular in the mitochondria, where it can disrupt respiration (Lindeque et al., 2010; Ye et al., 2001). Movement of MT into the mitochondria following PCB126 exposure may partially explain the changes seen in the mitochondrial metal levels. Additional studies are planned to investigate this possibility.
Metal levels in the heart were stable. PCB126 did not influence Mn, Cu, Fe, or Zn, indicating resilience of this tissue to xenobiotic influence. The only diet-related effect was that Mn in the adequate and supplemented groups was twice as high as in the deficient Mn diet group. This suggests that the deficient (0.4 mg/kg) Mn diet may be insufficient to achieve the maximum heart level. It should be pointed out, however, that the basal level of Mn in the heart with Mn adequate or supplemented diet was much lower than the corresponding levels in the liver (approximately 0.4 and 1.7 µg/kg for heart and liver, respectively). Therefore, whether this lower level in the heart is suboptimal and causing health problems remains to be determined. The heart MnSOD activity correlated very well with the heart Mn level but quantitatively not so well with sod2 mRNA levels, suggesting that Mn may be the determining, rate-limiting factor for MnSOD activity.
In the liver, dietary Mn levels modulated tissue levels of Mn and Fe but in opposite directions. Finley and Davis (1999) summarized that high dietary Mn interferes with Fe homeostasis. This may explain the significantly reduced Fe levels in the livers of the supplemented Mn diet group. In addition, tissue levels of all 4 tested metals were significantly changed by PCB126, ie increased Cu and decreased Mn, Zn, and Fe, consistent with our previous findings (Lai et al., 2011). Many metal transporters can transport several different metals. It was hypothesized that Fe transporters may interact with Mn (Finley and Davis, 1999). The zinc transporters ZIP8 (Slc39a8) and ZIP14 (Slc39a14) also transport Mn and Cd, and ZIP14 is capable of transporting Fe. Thus, any factor that is interfering with one of these transporters may decrease the absorption of other metals. Because liver Fe, Mn, and Zn concentrations were all decreased to a relatively similar extent in PCB126-treated rats, changes in the activity of 1 or more metal transporters could be responsible. Another candidate is the divalent metal transporter (DMT1) (Slc11a2) (Himeno et al., 2009), which is known to transport Fe as well as Mn. DMT1 and the iron storage and iron acquisition proteins ferritin and transferring receptor 1 (Trf-1), respectively, are regulated by iron regulating proteins (IRP1 and IRP2) (Hentze et al., 2004). TCDD altered ferritin, transferrin receptor, and IRP1 or IRP2 (Santamaria et al., 2011). Whether PCB-induced decrease of liver Fe and Mn and/or Zn involves these proteins needs to be determined. In contrast, Cu levels in the liver were increased due to PCB126 exposure. It was suggested that increased Cu levels in the liver might result from impaired biliary excretion of this metal (Elsenhans et al., 1991). However, Mn is also excreted into the bile and was decreased in the liver of PCB126-exposed animals, making this option less attractive. Alternatively, changes in storage and sequestration may be involved in Cu increases in the liver. MTs are cysteine-rich proteins that are known for their function as antioxidants and for the storage and transport of Zn, Cu, Cd, and Hg (Vallee, 1995). MTs were increased after PCB126 exposure, which may partially explain the increases in Cu. MTs have a greater binding affinity for Cu than for Zn, which could explain the different effects of PCB126 treatment on these 2 metals. Nishimura et al. (2001) reported a similar increase in MT transcription and proteins and hepatic Cu levels after exposure of rats to TCDD. However, they also saw an increase in hepatic Zn and Fe levels. This discrepancy with our findings may have been caused by the fact that they used female rats, low doses of TCDD, and sacrificed the animals 1 week after exposure. Gender-specific responses have been reported and there are some indications that PCB126 and TCDD do not always have the identical effects on gene regulation (Vezina et al., 2004; Wahba et al., 1988). Another likely reason for these different responses may be their choice of diet (not described), which may have contained much higher levels of Fe and Zn than our AIN-93 diet.
As for mitochondrial metal levels in the liver, we followed the methods described by Frezza et al. (2007) and Wieckowski et al. (2009) to isolate liver mitochondria. Of the 4 metals examined, only some measurements of Mn and Zn were below the limits of quantification, which occurred only in PCB126-treated animals. We were able to reliably quantify mitochondrial metals in the control groups on each diet for all metals examined. Lee et al. (2013) were able to quantify mitochondrial Mn level in the liver in mice, but much higher Mn intake levels were used. Mice received ip injections of soluble MnCl2 at 12 mg/kg/day, in addition to the basal diet containing Mn at 80 or 100 mg/kg-diet. The mean Mn intake in our study was 0.026–11 mg/kg/day, and the insoluble Mn carbonate was used in the diet. The oral absorption of Mn is only approximately 1%–5% (Roth and Garrick, 2003). The AIN-93 M diet used in this experiment contains less Mn (11 mg/kg in the adequate Mn dietary group vs 156 mg/kg) and Zn (30 vs 48 mg/kg) compared with the regular 7013 rodent diet used routinely for nondietary studies. This could have led to the very low levels of these metals in our experiment. In addition, The AIN-93 M diet contains much more Ca (4998 vs 1.19 mg/kg) and Mg (510.3 vs 0.2 mg/kg) compared with the 7013 diet (Supplementary Table 6). This can significantly affect the absorption of Zn and Mn—the combination of Ca and Mg levels is inhibitory to Mn absorption (Maas et al., 1969), and Ca inhibits Zn absorption (Lonnerdal, 2000). All these factors may have contributed to unusually low Zn and Mn levels in the mitochondria extract in this study, making it more challenging to detect these metals. Even though we were able to quantify metal levels in the mitochondria in the control groups, PCB126 treatment significantly decreased mitochondrial Mn and Zn levels as well as total mitochondrial protein levels, making it even more challenging to quantify the metals. MnSOD is a mitochondrial enzyme that contains Mn. Its relative activity (normalized to protein expression) was reduced in response to PCB126 treatment, and increased in response to dietary Mn levels (Figure 4). These trends coincide with the trends of the measured of mitochondrial Mn levels in the liver, and suggest that the measurements of mitochondrial Mn can actually be used qualitatively and reflect the mitochondrial response to PCB126 treatment.
The mitochondrial levels of Mn and Cu correlated well with total liver levels, even though some measurements of Mn fell below the quantitation limit of the method. A noteworthy exception is the supplemented dietary Mn group, where PCB126 increased mitochondrial Cu concentration to a much higher extent than in the other 2 dietary groups. Because this supplementary level of Mn is the “normal” level of other popular rat diets, such a profound influx of the strong Fenton catalyst Cu into mitochondria after exposure to AhR ligands could potentially be quite common.
Interestingly, mitochondrial Fe and Zn levels were significantly elevated in PCB126-treated rats while total tissue levels were significantly reduced. In a hereditary hemochromatosis mouse, the increased Fe in the mitochondria, especially free Fe, lead to an increase of oxidative stress in the mitochondria and reduced mitochondrial uptake of Mn, Zn, and Cu (Jouihan et al., 2008). However, PCB126 increased, not decreased mitochondrial Cu and Zn and reduced only mitochondrial Mn, suggesting that the effect of PCB126 is mediated through another, more specific mechanisms. With TCDD a similar increase in liver mitochondrial Fe and Cu levels was observed, accompanied by an increase in hepatic Fe and Cu levels, decrease in microsomal Fe, and no changes in Zn levels (Al-Bayati et al., 1987; Wahba et al., 1988, 1990). Differences for male and female rats were observed, like much higher hepatic Fe levels in females compared with males. The authors proposed that mitochondria may function as a compensatory deposit for excess iron in the cell. However, hepatic Fe levels were lower in rats exposed to PCB126, which suggests a more specific, possibly transporter-related explanation. These differences in the findings with 2 different compounds that act through the same mechanism, binding to the AhR, emphasizes the need to control for possible confounding factors, particularly diet and gender, which may strongly influence the magnitude and even direction of the effects of xenobiotics on metal homeostasis. Independent of these differences, an increase of Fe and Cu, 2 very efficient Fenton reagents in liver mitochondria, may result in the increased formation of highly reactive hydroxyl radicals, damage to DNA, lipids, and proteins, disturbance of the redox functions of mitochondria, and may possibly be an underlying mechanism of the toxicity of AhR agonists.
It should be noted that although Mn is an essential metal, chronic excess exposure through inhalation and ingestion can affect the release of dopamine from dopaminergic neurons in the brain, which is known as manganism (Guilarte, 2010). We carefully selected the dietary Mn levels to be tested in this study to ensure that no neurologic effects would occur. In humans, the U.S. Food and Nutrition Board/Institute of Medicine established a Tolerable upper level intake (UL) of 11 mg Mn/day for adults (Trumbo et al., 2001). Should Mn supplementation be further considered as a remediation for PCB126 toxicity, the total levels ingested per day cannot exceed the UL in humans.
In summary, Mn supplementation significantly reduced the PCB126-induced hepatomegaly. It also significantly increased the mRNA, protein and activity levels of MnSOD in the heart in a dose-dependent manner. In the liver, however, Mn supplementation failed to fully reverse the reduction of MnSOD activity by PCB126. Similarly, MT expression in the heart was relatively unaffected by diet or PCB126, whereas the liver showed a marked increase in expression following PCB126 exposure. MT or/and other factors may be the reason for the disturbed metal homeostasis in liver mitochondria, where PCB126 exposure caused a large increase in Cu, Fe, and Zn, which may increase oxidative stress, and a decrease in mitochondrial Mn, which is a possible cause for the reduced MnSOD activity and general toxicity. Overall, dietary Mn supplementation had partial protective effect against PCB126 toxicities.
FUNDING
This work was supported by the National Institute of Environmental Health Sciences (ES 013661, ES 05605) and the National Cancer Institute (P30 CA 086862) of the National Institutes of Health. The opinions expressed are solely those of the authors and do not reflect an official policy of the granting agencies. We gratefully acknowledge support from the Iowa Superfund Research Program (P42 ES 013661) Training Core (B.W. and W.D.K.) and a University of Iowa Graduate Presidential Fellowship (B.W.).
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
ACKNOWLEDGMENTS
The authors thankfully recognize Dr Gregor Luthe for the synthesis of PCB126, and Susanne Flor and members of laboratory for laboratory infrastructure and help with the animal studies. Advice and consultation of the Radiation and Free Radical Research Core of the Holden Comprehensive Cancer Center regarding the MnSOD assays are gratefully acknowledged.
REFERENCES
- Al-Bayati Z. A., Stohs S. J., Al-Turk W. A. 1987. TCDD, dietary iron and hepatic iron distribution in female rats. Bull. Environ. Contam. Toxicol. 38, 300–307. [DOI] [PubMed] [Google Scholar]
- Aschner M., Vrana K. E., Zheng W. 1999. Manganese uptake and distribution in the central nervous system (CNS). Neurotoxicology 20, 173–180. [PubMed] [Google Scholar]
- Baly D. L., Schneiderman J. S., Garcia-Welsh A. L. 1990. Effect of manganese deficiency on insulin binding, glucose transport and metabolism in rat adipocytes. J. Nutr. 120, 1075–1079. [DOI] [PubMed] [Google Scholar]
- Bandiera S., Safe S., Okey A. B. 1982. Binding of polychlorinated biphenyls classified as either phenobarbitone-, 3-methylcholanthrene- or mixed-type inducers to cytosolic Ah receptor. Chem. Biol. Interact. 39, 259–277. [DOI] [PubMed] [Google Scholar]
- Bowman A. B., Kwakye G. F., Herrero Hernandez E., Aschner M. 2011. Role of manganese in neurodegenerative diseases. J. Trace Elem. Med. Biol. 25, 191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Chen F. 2010. Induction of oxidative stress and cytotoxicity by PCB126 in JEG-3 human choriocarcinoma cells. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 45, 932–937. [DOI] [PubMed] [Google Scholar]
- Cheng J., Yang Y., Ma J., Wang W., Liu X., Sakamoto M., Qu Y., Shi W. 2009. Assessing noxious effects of dietary exposure to methylmercury, PCBs and Se coexisting in environmentally contaminated rice in male mice. Environ. Int. 35, 619–625. [DOI] [PubMed] [Google Scholar]
- Christian B. J., Menahan L. A., Peterson R. E. 1986. Intermediary metabolism of the mature rat following 2,3,7,8-tetrachlorodibenzo-p-dioxin treatment. Toxicol. Appl. Pharmacol. 83, 360–378. [DOI] [PubMed] [Google Scholar]
- Coteur G., Danis B., Fowler S. W., Teyssie J. L., Dubois P., Warnau M. 2001. Effects of PCBs on reactive oxygen species (ROS) production by the immune cells of Paracentrotus lividus (Echinodermata). Mar. Pollut. Bull. 42, 667–672. [DOI] [PubMed] [Google Scholar]
- Crossgrove J., Zheng W. 2004. Manganese toxicity upon overexposure. NMR Biomed. 17, 544–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culotta V. C., Yang M., O’Halloran T. V. 2006. Activation of superoxide dismutases: Putting the metal to the pedal. Biochim. Biophys. Acta 1763, 747–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. D., Ney D. M., Greger J. L. 1990. Manganese, iron and lipid interactions in rats. J. Nutr. 120, 507–513. [DOI] [PubMed] [Google Scholar]
- Dorman D. C., Struve M. F., Marshall M. W., Parkinson C. U., James R. A., Wong B. A. 2006. Tissue manganese concentrations in young male rhesus monkeys following subchronic manganese sulfate inhalation. Toxicol. Sci. 92, 201–210. [DOI] [PubMed] [Google Scholar]
- Elsenhans B., Forth W., Richter E. 1991. Increased copper concentrations in rat tissues after acute intoxication with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Arch. Toxicol. 65, 429–432. [DOI] [PubMed] [Google Scholar]
- Finley J. W. 1998. Manganese uptake and release by cultured human hepato-carcinoma (Hep-G2) cells. Biol. Trace Elem. Res. 64, 101–118. [PubMed] [Google Scholar]
- Finley J. W., Davis C. D. 1999. Manganese deficiency and toxicity: Are high or low dietary amounts of manganese cause for concern? Biofactors 10, 15–24. [DOI] [PubMed] [Google Scholar]
- Frezza C., Cipolat S., Scorrano L. 2007. Organelle isolation: Functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat. Protoc. 2, 287–295. [DOI] [PubMed] [Google Scholar]
- Friedman B. J., Freeland-Graves J. H., Bales C. W., Behmardi F., Shorey-Kutschke R. L., Willis R. A., Crosby J. B., Trickett P. C., Houston S. D. 1987. Manganese balance and clinical observations in young men fed a manganese-deficient diet. J. Nutr. 117, 133–143. [DOI] [PubMed] [Google Scholar]
- Guilarte T. R. 2010. Manganese and Parkinson’s disease: A critical review and new findings. Environ. Health Perspect. 118, 1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hailey J. R., Walker N. J., Sells D. M., Brix A. E., Jokinen M. P., Nyska A. 2005. Classification of proliferative hepatocellular lesions in harlan Sprague-Dawley rats chronically exposed to dioxin-like compounds. Toxicol. Pathol. 33, 165–174. [DOI] [PubMed] [Google Scholar]
- Hassoun E. A., Wang H., Abushaban A., Stohs S. J. 2002. Induction of oxidative stress in the tissues of rats after chronic exposure to TCDD, 2,3,4,7,8-pentachlorodibenzofuran, and 3,3',4,4',5-pentachlorobiphenyl. J. Toxicol. Environ. Health A 65, 825–842. [DOI] [PubMed] [Google Scholar]
- Hastings C. E., Jr, Llewellyn G. C. 1987. Reduced aflatoxicosis in livers of hamsters fed a manganese sulfate supplement. Nutr. Cancer 10, 67–77. [DOI] [PubMed] [Google Scholar]
- Hentze M. W., Muckenthaler M. U., Andrews N. C. 2004. Balancing acts: Molecular control of mammalian iron metabolism. Cell 117, 285–297. [DOI] [PubMed] [Google Scholar]
- Himeno S., Yanagiya T., Fujishiro H. 2009. The role of zinc transporters in cadmium and manganese transport in mammalian cells. Biochimie 91, 1218–1222. [DOI] [PubMed] [Google Scholar]
- Jin M. H., Hong C. H., Lee H. Y., Kang H. J., Han S. W. 2010. Toxic effects of lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on development of male reproductive system: Involvement of antioxidants, oxidants, and p53 protein. Environ. Toxicol. 25, 1–8. [DOI] [PubMed] [Google Scholar]
- Jouihan H. A., Cobine P. A., Cooksey R. C., Hoagland E. A., Boudina S., Abel E. D., Winge D. R., McClain D. A. 2008. Iron-mediated inhibition of mitochondrial manganese uptake mediates mitochondrial dysfunction in a mouse model of hemochromatosis. Mol. Med. 14, 98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzen J. S., Llewellyn G. C. 1987. Further evidence supporting the concurrent influence of aflatoxin and manganese. Vet. Hum. Toxicol. 29, 127–132. [PubMed] [Google Scholar]
- Kobayashi K., Kuroda J., Shibata N., Hasegawa T., Seko Y., Satoh M., Tohyama C., Takano H., Imura N., Sakabe K., et al. 2007. Induction of metallothionein by manganese is completely dependent on interleukin-6 production. J. Pharmacol. Exp. Ther. 320, 721–727. [DOI] [PubMed] [Google Scholar]
- Kono Y., Okada S., Tazawa Y., Kanzaki S., Mura T., Ueta E., Nanba E., Otsuka Y. 2002. Response of anti-oxidant enzymes mRNA in the neonatal rat liver exposed to 1,2,3,4-tetrachlorodibenzo-p-dioxin via lactation. Pediatr. Int. 44, 481–487. [DOI] [PubMed] [Google Scholar]
- Lai I., Chai Y., Simmons D., Luthe G., Coleman M. C., Spitz D., Haschek W. M., Ludewig G., Robertson L. W. 2010. Acute toxicity of 3,3',4,4',5-pentachlorobiphenyl (PCB 126) in male Sprague-Dawley rats: Effects on hepatic oxidative stress, glutathione and metals status. Environ. Int. 36, 918–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai I., Klaren W. D., Li M., Wels B., Simmons D., Olivier A. K., Haschek W. M., Wang K., Ludewig G., Robertson L. W. 2013. Does dietary copper supplementation enhance or diminish PCB126 toxicity in the rodent liver? Chem. Res. Toxicol. 26, 634–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai I. K., Chai Y., Simmons D., Watson W. H., Tan R., Haschek W. M., Wang K., Wang B., Ludewig G., Robertson L. W. 2011. Dietary selenium as a modulator of PCB 126-induced hepatotoxicity in male Sprague-Dawley rats. Toxicol. Sci. 124, 202–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauby-Secretain B., Loomis D., Grosse Y., El Ghissassi F., Bouvard V., Benbrahim-Tallaa L., Guha N., Baan R., Mattock H., Straif K., et al. 2013. Carcinogenicity of polychlorinated biphenyls and polybrominated biphenyls. Lancet Oncol. 14, 287–288. [DOI] [PubMed] [Google Scholar]
- Lee S. H., Jouihan H. A., Cooksey R. C., Jones D., Kim H. J., Winge D. R., McClain D. A. 2013. Manganese supplementation protects against diet-induced diabetes in wild type mice by enhancing insulin secretion. Endocrinology 154, 1029–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Lu L., Hao S., Wang Y., Zhang L., Liu S., Liu B., Li K., Luo X. 2011. Dietary manganese modulates expression of the manganese-containing superoxide dismutase gene in chickens. J. Nutr. 141, 189–194. [DOI] [PubMed] [Google Scholar]
- Lindeque J. Z., Levanets O., Louw R., van der Westhuizen F. H. 2010. The involvement of metallothioneins in mitochondrial function and disease. Curr. Protein Pept. Sci. 11, 292–309. [DOI] [PubMed] [Google Scholar]
- Lonnerdal B. 2000. Dietary factors influencing zinc absorption. J. Nutr. 130, 1378S–1383S. [DOI] [PubMed] [Google Scholar]
- Maas E. V., Moore D. P., Mason B. J. 1969. Influence of calcium and magnesium on manganese absorption. Plant Physiol. 44, 796–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malecki E. A., Greger J. L. 1996. Manganese protects against heart mitochondrial lipid peroxidation in rats fed high levels of polyunsaturated fatty acids. J. Nutr. 126, 27–33. [DOI] [PubMed] [Google Scholar]
- Michalke B. 2004. Manganese speciation using capillary electrophoresis-ICP-mass spectrometry. J. Chromatogr. A 1050, 69–76. [DOI] [PubMed] [Google Scholar]
- Murugesan P., Muthusamy T., Balasubramanian K., Arunakaran J. 2008. Polychlorinated biphenyl (Aroclor 1254) inhibits testosterone biosynthesis and antioxidant enzymes in cultured rat Leydig cells. Reprod. Toxicol. 25, 447–454. [DOI] [PubMed] [Google Scholar]
- Nishimura N., Miyabara Y., Suzuki J. S., Sato M., Aoki Y., Satoh M., Yonemoto J., Tohyama C. 2001. Induction of metallothionein in the livers of female Sprague-Dawley rats treated with 2,3,7 ,8-tetrachlorodibenzo-p-dioxin. Life Sci. 69, 1291–1303. [DOI] [PubMed] [Google Scholar]
- NTP. 1993. NTP toxicology and carcinogenesis studies of manganese (II) sulfate monohydrate (CAS No. 10034-96-5) in F344/N rats and B6C3F1 mice (feed studies). Natl. Toxicol. Program Tech. Rep. Ser. 428, 1–275. [PubMed] [Google Scholar]
- NTP. 2006. NTP toxicology and carcinogenesis studies of 3,3',4,4',5-pentachlorobiphenyl (PCB 126) (CAS No. 57465-28-8) in female Harlan Sprague-Dawley rats (Gavage Studies). Natl. Toxicol. Program Tech. Rep. Ser. 520, 4–246. [Google Scholar]
- Oberley T. D., Oberley L. W. 1997. Antioxidant enzyme levels in cancer. Histol. Histopathol. 12, 525–535. [PubMed] [Google Scholar]
- Pardo M., Budick-Harmelin N., Tirosh B., Tirosh O. 2008. Antioxidant defense in hepatic ischemia-reperfusion injury is regulated by damage-associated molecular pattern signal molecules. Free Radic. Biol. Med. 45, 1073–1083. [DOI] [PubMed] [Google Scholar]
- Petkau A., Chelack W. S., Pleskach S. D., Meeker B. E., Brady C. M. 1975. Radioprotection of mice by superoxide dismutase. Biochem. Biophys. Res. Commun. 65, 886–893. [DOI] [PubMed] [Google Scholar]
- Roth J. A., Garrick M. D. 2003. Iron interactions and other biological reactions mediating the physiological and toxic actions of manganese. Biochem. Pharmacol. 66, 1–13. [DOI] [PubMed] [Google Scholar]
- Rubiolo J. A., Mithieux G., Vega F. V. 2008. Resveratrol protects primary rat hepatocytes against oxidative stress damage: Activation of the Nrf2 transcription factor and augmented activities of antioxidant enzymes. Eur. J. Pharmacol. 591, 66–72. [DOI] [PubMed] [Google Scholar]
- Safe S. 1990. Polychlorinated biphenyls (PCBs), dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs), and related compounds: Environmental and mechanistic considerations which support the development of toxic equivalency factors (TEFs). Crit. Rev. Toxicol. 21, 51–88. [DOI] [PubMed] [Google Scholar]
- Santamaria R., Fiorito F., Irace C., De Martino L., Maffettone C., Granato G. E., Di Pascale A., Iovane V., Pagnini U., Colonna A. 2011. 2,3,7,8-Tetrachlorodibenzo-p-dioxin impairs iron homeostasis by modulating iron-related proteins expression and increasing the labile iron pool in mammalian cells. Biochim. Biophys. Acta 1813, 704–712. [DOI] [PubMed] [Google Scholar]
- Schramm V. L., Brandt M. 1986. The manganese(II) economy of rat hepatocytes. Fed. Proc. 45, 2817–2820. [PubMed] [Google Scholar]
- Shimizu K., Ogawa F., Watanabe M., Kondo T., Katayama I. 2003. Serum antioxidant levels in Yusho victims over 30 years after the accidental poisoning of polychlorinated biphenyls in Nagasaki, Japan. Toxicol. Ind. Health 19, 37–39. [DOI] [PubMed] [Google Scholar]
- Silberhorn E. M., Glauert H.P., Robertson L. W. 1990. Carcinogenicity of polyhalogenated biphenyls: PCBs and PBBs. Crit. Rev. Toxicol. 20, 440–496. [DOI] [PubMed] [Google Scholar]
- Spitz D. R., Oberley L. W. 1989. An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal. Biochem. 179, 8–18. [DOI] [PubMed] [Google Scholar]
- St Clair D. K., Wan X. S., Oberley T. D., Muse K. E., St Clair W. H. 1992. Suppression of radiation-induced neoplastic transformation by overexpression of mitochondrial superoxide dismutase. Mol. Carcinog. 6, 238–242. [DOI] [PubMed] [Google Scholar]
- Thampi S. M. 1994. Environmental Pollutants as Regulators of Gene Expression. Graduate Center for Toxicology, PhD. Dissertation, University of Kentucky, Lexington, pp. 221. [Google Scholar]
- Trumbo P., Yates A. A., Schlicker S., Poos M. 2001. Dietary reference intakes: Vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J. Am. Diet Assoc. 101, 294–301. [DOI] [PubMed] [Google Scholar]
- Vallee B. L. 1995. The function of metallothionein. Neurochem. Int. 27, 23–33. [DOI] [PubMed] [Google Scholar]
- Van den Berg M., Birnbaum L. S., Denison M., De Vito M., Farland W., Feeley M., Fiedler H., Hakansson H., Hanberg A., Haws L., et al. 2006. The 2005 World Health Organization reevaluation of human and Mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol. Sci. 93, 223–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina C. M., Walker N. J., Olson J. R. 2004. Subchronic exposure to TCDD, PeCDF, PCB126, and PCB153: Effect on hepatic gene expression. Environ. Health Perspect. 112, 1636–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahba Z. Z., al-Bayati Z. A., Stohs S. J. 1988. Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on the hepatic distribution of iron, copper, zinc, and magnesium in rats. J. Biochem. Toxicol. 3, 121–129. [DOI] [PubMed] [Google Scholar]
- Wahba Z. Z., Murray W. J., Stohs S. J. 1990. Altered hepatic iron distribution and release in rats after exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Bull. Environ. Contam. Toxicol. 45, 436–445. [DOI] [PubMed] [Google Scholar]
- Wieckowski M. R., Giorgi C., Lebiedzinska M., Duszynski J., Pinton P. 2009. Isolation of mitochondria-associated membranes and mitochondria from animal tissues and cells. Nat. Protoc. 4, 1582–1590. [DOI] [PubMed] [Google Scholar]
- Ye B., Maret W., Vallee B. L. 2001. Zinc metallothionein imported into liver mitochondria modulates respiration. Proc. Natl. Acad. Sci. U.S.A. 98, 2317–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa K., Heatherly A., Malarkey D. E., Walker N. J., Nyska A. 2007. A critical comparison of murine pathology and epidemiological data of TCDD, PCB126, and PeCDF. Toxicol. Pathol. 35, 865–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zidenberg-Cherr S., Hurley L. S., Lonnerdal B., Keen C. L. 1985. Manganese deficiency: Effects on susceptibility to ethanol toxicity in rats. J. Nutr. 115, 460–467. [DOI] [PubMed] [Google Scholar]
- Zidenberg-Cherr S., Keen C. L., Lonnerdal B., Hurley L. S. 1983. Superoxide dismutase activity and lipid peroxidation in the rat: Developmental correlations affected by manganese deficiency. J. Nutr. 113, 2498–2504. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.