Abstract
We previously showed that elevated intracellular Ca2+ ([Ca2+]i) in the molecular layer and granule cells in cerebellar slices is responsible for the initial increases in frequency of spontaneous or miniature inhibitory postsynaptic currents (sIPSCs or mIPSCs) of Purkinje cells following methylmercury (MeHg) treatment. To identify the contribution of different Ca2+ sources to MeHg-induced stimulation of spontaneous GABA release, we examined sIPSC or mIPSC frequency of Purkinje cells in acutely prepared cerebellar slices using whole-cell patch-clamp recording techniques under conditions of lowered [Ca2+]o, pretreatment with caffeine, cyclopiazonic acid (CPA), thapsigargin or ruthenium red (RR) to deplete ryanodine-sensitive and insensitive intracellular Ca2+ stores or mitochondria, or a combination of lowering [Ca2+]o and increased BAPTA buffering. Lowering [Ca2+]o significantly reduced sIPSC or mIPSC frequency and amplitudes, but failed to completely prevent MeHg-induced increase in these events frequency. Caffeine, CPA, or thapisgargin also minimized MeHg-induced increase in sIPSC frequency, yet none of them completely blocked MeHg-induced increase in sIPSC frequency. Similarly, the mitochondrial Ca2+ transport inhibitor RR, or a combination of lowering [Ca2+]o and BAPTA buffering reduced but did not prevent MeHg-induced changes in mIPSC frequency. Consistently, confocal Ca2+ imaging under low [Ca2+]o conditions or in the presence of caffeine or CPA exhibited a marked reduction of MeHg-induced increases in [Ca2+]i in both molecular and granule layers. Thus, these results verify that a combination of extracellular Ca2+ influx and Ca2+ release from different intracellular Ca2+ pools all contribute to MeHg-induced increase in [Ca2+]i and spontaneous GABA release, although extracellular Ca2+ appears to be the primary contributor.
Keywords: methylmercury, intracellular Ca2+, synaptic transmission, cerebellar slice
One of the most consistent observations of in vitro effects of methylmercury (MeHg), a potent environmental neurotoxicant, on synaptic transmission is that acute application of MeHg always initially stimulates spontaneous neurotransmitter release (Atchison, 1986, 1987; Atchison and Narahashi, 1982; Juang, 1976; Juang and Yonemura, 1975). In the central nervous system, this is expressed as increased frequency of spontaneous or miniature excitatory or inhibitory postsynaptic currents (sEPSCs and sIPSCs or mIPSCs) recorded from cerebellar neurons in rat cerebellar slices (Yuan and Atchison, 2003, 2005, 2007; Yuan et al., 2005). Using a combination of confocal imaging of intracellular Ca2+ concentration ([Ca2+]i) and whole-cell patch clamp recordings of sIPSCs and sEPSCs in Purkinje cells in freshly isolated cerebellar slices of rat, we previously showed that in vitro exposure of rat cerebellar slices to MeHg increased [Ca2+]i throughout the molecular layer (including parallel- and climbing fibers, dendrites and the subplasmalemmal shell of Purkinje cells) and granule cells (Yuan and Atchison, 2007). The time courses of onset of increases in [Ca2+]i in the molecular layer and granule cells coincide with the onset of increases in frequency of sEPSCs or sIPSCs. The temporal and spatial changes in [Ca2+]i in the molecular layer are correlated with the early stimulatory effects of MeHg on spontaneous postsynaptic responses. MeHg-induced increase in frequency of sIPSCs was reliably and significantly suppressed, although not prevented completely in the presence of the membrane permeable [Ca2+]i chelator BAPTA-AM (Yuan and Atchison, 2007).
It is well established that both influx of extracellular Ca2+ and release of Ca2+ from intracellular Ca2+ stores in presynaptic terminals contribute to neurotransmitter release (Bardo et al., 2002; Collin et al., 2005; De Koninck and Mody, 1996; Martínez-Serrano and Satrústegui, 1992; Savi and Sciancalepore, 1998; Simkus and Stricker, 2002) and help shape synaptic plasticity. However, the sources of Ca2+ contributing to MeHg-induced increases in [Ca2+]i in the presynaptic terminals and leading to its early stimulation of spontaneous synaptic transmission remain to be characterized. Based on the consistent effects of MeHg on [Ca2+]i homeostasis in a variety of cell types (Edwards et al., 2005; Hare and Atchison, 1995; Hare et al., 1993; Kauppinen et al., 1989; Komulainen and Bondy, 1987; Limke and Atchison, 2002; Limke et al., 2003; Marty and Atchison, 1997, 1998; Roos et al., 2012), we hypothesized that both increased influx of extracellular Ca2+ and release of Ca2+ from different intracellular stores contribute to the early stimulatory effects on spontaneous transmitter release. To determine this, we examined effects of MeHg on spontaneous synaptic transmission by lowering extracellular Ca2+ concentration ([Ca2+]o) or in the presence of different intracellular store inhibitors, or a combination of both. We sought to determine specifically: (1) if reducing extracellular Ca2+; (2) depletion of release of Ca2+ from ryanodines-sensitive and -insensitive intracellular Ca2+ stores; or (3) altering mitochondrial Ca2+ stores could prevent or reduce MeHg-induced initial stimulation of spontaneous synaptic transmission. However, we did not intend to identify exactly how much each Ca2+ source contributes to the total increase in [Ca2+]i in neurons and spontaneous release of transmitter at this time because of the complex relationships and interactions among extracellular Ca2+ influx, different intracellular Ca2+ stores, and other intracellular Ca2+ buffers (Amaral and Pozzo-Miller, 2012; Bardo et al., 2002; Garaschuk et al., 1997; Roos et al., 2012; Rusakov, 2006; Savi and Sciancalepore, 1998; Smyth et al., 2010). For instance, refilling of intracellular stores requires the presence of extracellular Ca2+ (Garaschuk et al., 1997; Savi and Sciancalepore, 1998), reducing or removing extracellular Ca2+ will affect the refilling of intracellular stores.
MATERIALS AND METHODS
Preparation of Cerebellar Slices
All animal procedures complied with the National Institutes of Health of the USA guidelines on animal care and were approved by Michigan State University Institutional Animal Use and Care Committee. Sagittal cerebellar slices (200 µm) were prepared as described previously (Yuan and Atchison, 1999, 2003, 2007), using Sprague-Dawley rats (14–21 days postnatal, either gender) (Harlan Industries, Verona, WI). The “slicing” solution contained (in mM): 125, NaCl; 2.5, KCl; 4, MgCl2; 1.25, KH2PO4; 26, NaHCO3; 1, CaCl2 and 25, D-glucose (pH 7.35–7.4 when saturated with 95% O2 /5% CO2 at room temperature of 22–25°C). Artificial cerebrospinal fluid (ACSF), in which all experiments were conducted, consisted of (in mM): 125, NaCl; 2.5, KCl; 1, MgCl2; 1.25, KH2PO4; 26, NaHCO3; 2, CaCl2 and 25, D-glucose (pH 7.35–7.4 saturated with 95% O2/5% CO2 at room temperature). In experiments in which low Ca2+-containing ACSF was used, no CaCl2 was added, and the Ca2+ was replaced by equimolar Mg2+. No EGTA was added to the extracellular solution because it will make cell membranes very weak and patch clamp recordings unstable. For this reason, we defined this solution as “low” Ca2+-containing, not Ca2+-free ACSF.
Whole-Cell Recording in Cerebellar Slices
Although MeHg causes initial stimulatory effects on both sEPSCs and sIPSCs in cerebellar granule and Purkinje cells (Yuan and Atchison, 2003, 2005, 2007), this study focused only on mIPSCs or sIPSCs in Purkinje cells, because, (1) the pattern of MeHg effects on sEPSCs and sIPSCs in granule and Purkinje cells is basically similar; (2) under symmetrical Cl- conditions, the frequency and amplitudes of mIPSCs or sIPSCs are higher and larger than those of sEPSCs in both granule and Purkinje cells. In addition, the frequency and amplitudes of sIPSCs recorded from Purkinje cells are also higher and larger than those recorded from granule cells. These characteristics of sIPSCs or mIPSCs in Purkinje cells would make it much easier for distinguishing of a synaptic response from the baseline noise and data analysis (Bardo et al., 2002); (3) the axons (parallel fibers) of granule cells synapse on both Purkinje cells and GABAergic interneurons, which in turn make synaptic contacts with the dendrites and soma of Purkinje cells. Importantly, the axons of Purkinje cells are the only output from cerebellar cortex. Thus, any cerebellar cortical neuronal (including granule cells) dysfunction will be ultimately expressed in or through Purkinje cells.
Whole-cell patch clamp recording methods were detailed in Yuan and Atchison (2003, 2005, 2007). Purkinje cells in slices were identified based on their characteristic electrophysiological properties and visually by their size, shape, and location using a Nikon E600FN upright microscope (Nikon Optics, Tokyo, Japan) equipped with Nomarski optics (x 40 water immersion objective) and Sony IR-1000 infrared CCD video camera system (DAGE MTI, Michigan City, IN). Recording electrodes were fire polished and had a resistance of 2–4 MΩ when filled with the following pipette solution. The pipette solution consisted of (in mM) 140, CsCl; 4, NaCl; 0.5, CaCl2; 10, HEPES; 5, EGTA, 2, Mg-ATP and 0.4, GTP (pH 7.3 adjusted with CsOH) for recording sIPSCs. The holding potential was −60 mV for recording of sIPSCs. 6-Cyano-7-nitroquinoxaline-2,3-dione (CNQX, 10 µM) and amino-5-phosphonopentanoic acid (APV, 50–100 µM) were added to the external solution to block glutamate receptor-mediated sEPSCs. For recordings of miniature IPSCs (mIPSCs), 0.5–1 μM tetrodotoxin (TTX) was added subsequently to the external solution in addition to CNQX and APV. In experiments involving using “low” Ca2+-containing ACSF solution, TTX were sometimes added simultaneously with “low” Ca2+ solution to shorten the time duration required for pre-MeHg treatment because maintaining long-duration stable recordings was difficult under “low” Ca2+ conditions. Data acquisition was as described in Yuan and Atchison (2003, 2005, 2007). Whole cell currents were filtered at 2–5 kHz with an 8-pole low-pass Bessel filter and digitized at 10–20 kHz for later off-line analysis using pClamp 9.0 program (Molecular Devices, Inc., Sunnyvale, CA). All experiments were carried out at room temperature of 22–25°C. Only 1 slice per rat was used for any given experiment (if more than 1 slice was examined from a given animal, data were averaged and counted as one) and only 1 concentration of MeHg was applied per slice.
Data analysis was as described previously in Yuan and Atchison (2003, 2005, 2007). In brief, spontaneous synaptic currents were first screened automatically using MiniAnalysis software (Synaptosoft Inc., Decatur, GA) with a set of the pre-specified parameters and then accepted or rejected manually with an event detection amplitude threshold at 10 pA for sIPSCs or mIPSCs and based on the kinetic properties (fast rising phase and slow decay phase) of the spontaneous events. Unless specified otherwise, synaptic events per cell over a 2-min period were averaged to calculate the frequency and amplitude of spontaneous synaptic currents. Amplitudes of currents were measured after subtraction of the baseline noise. Because the changes in frequency and mean amplitude of sIPSCs or mIPSCs do not appear to be MeHg concentration dependent (Yuan and Atchison, 2003, 2005, 2007), data collected from 20 or 100 μM MeHg were pooled for statistical analysis.
Confocal Fluorescence Microscopy
Cerebellar slices were loaded for 40–60 min at room temperature with 4 μM Fluo-4 acetoxymethyl ester in the presence of 0.02% (w v−1) pluronic F-127. Slices were then washed thoroughly with ACSF. Confocal images of Fluo-4 fluorescence ([Ca2+]i) in slices were obtained using a Leica TCS SL laser scanning microscope system (Leica Microsystem, Heidelberg GmbH., Germany) equipped with Nomarski optics (x 40 water immersion objective, n.a, 0.75). Fluo-4 fluorescence was excited by 488 nm light from an argon laser attenuated to 20% with neutral density. Emitted fluorescence was collected at >515 nm. Images (512 x 512 pixels, xyz or xyt scan mode) were collected before and during exposure to 20 or 100 µM MeHg. To avoid photobleaching of Fluo-4, sensitivity was adjusted to a minimal level. Changes in Fluo-4 fluorescence intensity in a given cerebellar cortical layer were analyzed using Leica software. Data from several regions of interest defined within this layer were averaged. The background Fluo-4 fluorescence was subtracted from all regions of interest before averaging.
Statistical Analysis
Data were analyzed statistically using Student’s paired t-test for MeHg-induced changes in spontaneous synaptic transmission, unless otherwise specified. The two-sample Kolmogorov–Smirnov test was used to compare the differences between 2 cumulative amplitude or inter-event distribution curves obtained before and after MeHg treatment at a given experiment. Values were considered statistically significant at P ≤ .05. The data are presented as mean ± standard error of the mean (SEM). Each experiment was replicated a minimum of 4 times; the actual number of replicates for each experiment is listed in the corresponding figure legend.
Chemicals
Methylmercuric chloride, purchased from ICN Biomedical, Inc. (Costa, CA), was dissolved in deionized water to a final concentration of 10 mM to serve as a stock solution. The applied solutions were diluted with ACSF just before perfusion. Two different MeHg concentrations (20 μM and 100 µM) were used in this study. The reasons for use of these relatively higher concentrations of MeHg have been explained elsewhere (Yuan and Atchison, 2003, 2005) and will not be restated here. In this study, all experiments involved modulations of [Ca2+]o and [Ca2+]i in neurons, which often made the electrophysiological recordings unstable, especially for experiments with low [Ca2+]o or chemicals that interfere with mitochondrial function such as ruthenium red (RR) (Korge and Weiss, 1999; Tapia and Velasco, 1997; Velasco and Tapia, 2000). We have previously shown that the pattern of effects of MeHg at 4–500 μM on central synaptic transmission is generally similar, but the times to onset of MeHg effects are very different (Yuan and Atchison, 1993, 1997, 2003). It took 150–240 min for 4 μM MeHg or 40–60 min for 10 μM MeHg to cause a similar initial stimulatory effect as those were caused by 100–500 μM within 1–5 min (Yuan and Atchison, 1993, 2003). It is almost impossible to maintain a long duration of stable recording in a reduced [Ca2+]o environment to see the stimulatory effects of lower MeHg concentrations. Thus, a higher concentration (100 μM) MeHg was often used to shorten the time course of MeHg effect in most experiments in this study. Fluo-4/AM was purchased from Invitrogen (Carlsbad, CA). 1,2 bis(2-aminophenoxy)ethane-N,N,N’,N’-tetra-acetic acid acetoxymethyl ester (BAPTA/AM), pluronic F-127, TTX, CNQX, APV, (-)-bicuculline methobromide, caffeine, cyclopiazonic acid (CPA), thapsigargin, and RR were all purchased from Sigma Chemical Co. (St Louis, MO).
RESULTS
Reducing [Ca2+]o in the External Solution Reduced, but Failed to Prevent, the MeHg-Induced Initial Increases in Frequency of sIPSCs or mIPSCs
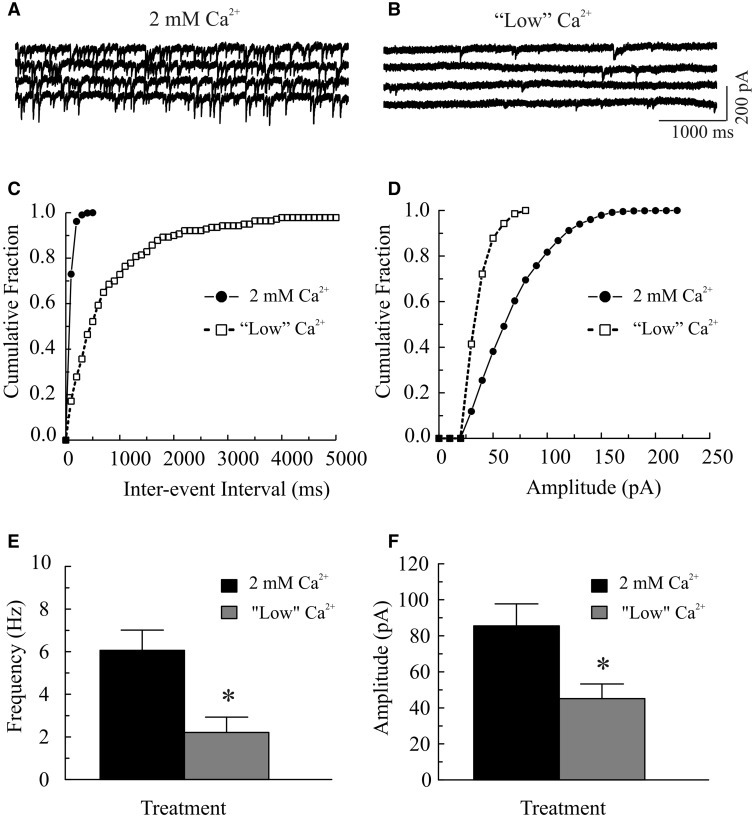
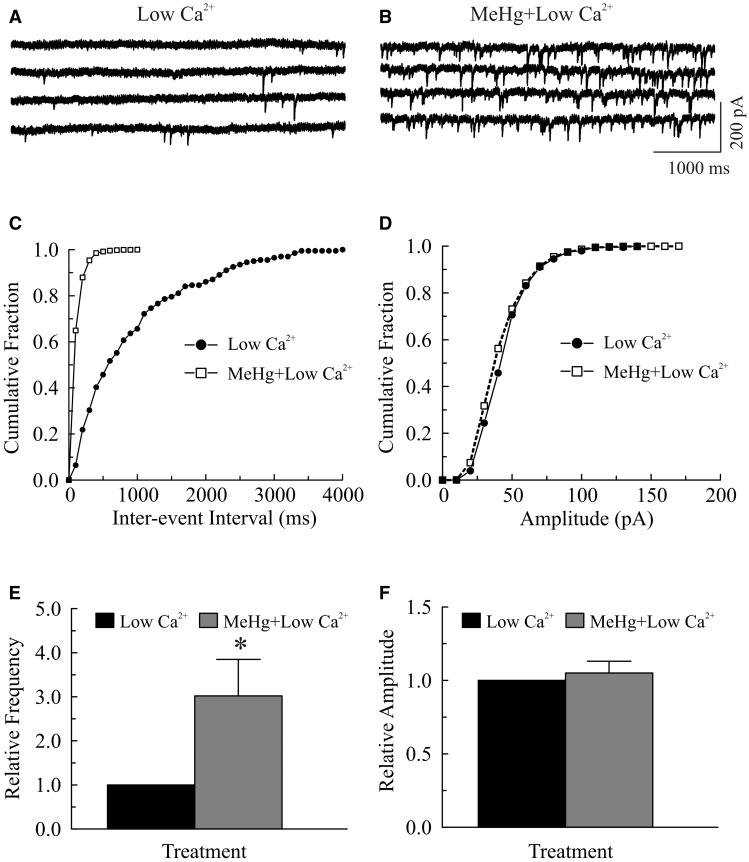
Increased influx of extracellular Ca2+ contributes to the MeHg-induced increase in [Ca2+]i (Edwards et al., 2005; Hare and Atchison, 1995; Hare et al., 1993; Limke and Atchison, 2002; Limke et al., 2003; Marty and Atchison, 1997, 1998). Consequently, we first examined the effect of reducing [Ca2+]o on MeHg-induced changes in the frequency of sIPSCs or mIPSCs. As expected, reducing [Ca2+]o from 2 mM to a low Ca2+-containing (no Ca2+ was added) ACSF significantly reduced the frequency and amplitude of sIPSCs (Fig. 1). Figures 1A and 1B show a representative recording of sIPSCs from a Purkinje cell before and after changing extracellular Ca2+ concentration, respectively. The mean frequency of sIPSCs was reduced from 6.1 ± 0.8 Hz in 2 mM Ca2+-containing ACSF to 2.2 ± 0.7 Hz in “low” Ca2+-containing ACSF (P < .05, n = 9), whereas the mean amplitude was reduced from 85.5 ± 12.2 pA to 45.5 ± 8.2 pA (P < .05, n = 9). Consistently, the cumulative inter-event interval distribution curve was shifted to the right (Fig. 1C), indicating a reduction of sIPSC frequency; whereas the cumulative amplitude distribution curve was shifted to left (Fig. 1D, suggesting a reduction of sIPSC amplitude. Adding TTX (0.5–1 μM) further reduced both their frequency and amplitude (data not shown). However, application of MeHg (100 μM) still significantly increased mIPSC frequency. In the representative example shown in Figure 2, the frequency and mean amplitude of mIPSCs were 1.1 Hz and 45.2 pA (averaged from 201 events over a 3 min period), respectively, prior to MeHg treatment (Fig. 2A). After 4 min exposure to 100 μM MeHg, the frequency of mIPSCs was increased to 9.9 Hz, whereas the mean amplitude of mIPSCs was 42.3 pA (averaged from 1788 mIPSCs over a 3-min period) (Fig. 2B). The cumulative inter-event interval distribution curve was shifted to the left (Fig. 2C), suggesting an increased mIPSC frequency. In contrast, the cumulative amplitude distribution curve was virtually unchanged (Fig. 2D), suggesting an increased number of mIPSCs without changes in amplitudes. These changes in mIPSCs were observed in 10 slices from 8 animals. On average, the mean mIPSC frequency increased 302% (from 2.2 ± 0.7 Hz to 7.0 ± 2.5 Hz, n = 8, P < .05, Fig. 2E), whereas mean amplitude was unchanged (45.1 ± 8.2 pA vs. 48.7 ± 10.7 pA, Fig. 2F) following MeHg treatment.
FIG. 1.
Decreasing extracellular Ca2+ significantly reduces the frequency and amplitudes of spontaneous inhibitory postsynaptic current (sIPSCs) in Purkinje cells in cerebellar slices. A, sIPSCs were recorded from a Purkinje cell in a sagittal cerebellar slice at a holding potential of −60 mV in the presence of 10 µM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and 50 µM amino-5-phosphonopentanoic acid (APV) in regular artificial cerebrospinal fluid (ACSF) (2 mM Ca2+). B, The slice was then incubated in a low [Ca2+]o (no CaCl2 was added to the ACSF) for 5 min. C, Effect of low Ca2+ on cumulative fraction of inter-event intervals of miniature inhibitory postsynaptic currents (mIPSCs) from the same cell shown in (A–B); bin size: 100 ms. D, Effect of low Ca2+ on amplitude distribution of mIPSCs from the same cell in (A–B), bin size: 10 pA. E–D, Changes in mean frequency and amplitude of sIPSCs by reducing extracellular Ca2+. Each trace is a representative depiction of 9 individual experiments. The asterisk indicates a statistically significant difference between the 2 groups (P < .05).
FIG. 2.
Removing extracellular Ca2+ failed to block the methylmercury (MeHg)-induced initial increase in the frequency of miniature inhibitory postsynaptic currents (mIPSCs) in Purkinje cells in cerebellar slices. A–B, mIPSCs were recorded from a Purkinje cell in a sagittal cerebellar slice at a holding potential of −60 mV in the presence of 10 µM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), 50 µM amino-5-phosphonopentanoic acid (APV) and 1.0 µM TTX in a low [Ca2+]o artificial cerebrospinal fluid (ACSF) (no CaCl2 was added to the ACSF) for 10 min (A) and then exposed to 100 μM methylmercury (MeHg) in this low [Ca2+]o bath solution for 4 min (B). C, Effect of MeHg on cumulative fraction of inter-event intervals of mIPSCs from the same cell shown in (A); bin size: 100 ms. D, Effect of MeHg on amplitude distribution of mIPSCs from the same cell in (A), bin size: 10 pA. E–F, MeHg-induced changes in relative frequency or amplitude of mIPSCs under low Ca2+ conditions. Each trace is a representative depiction of 8 individual experiments. The asterisk indicates a statistically significant difference between the 2 groups (P < .05).
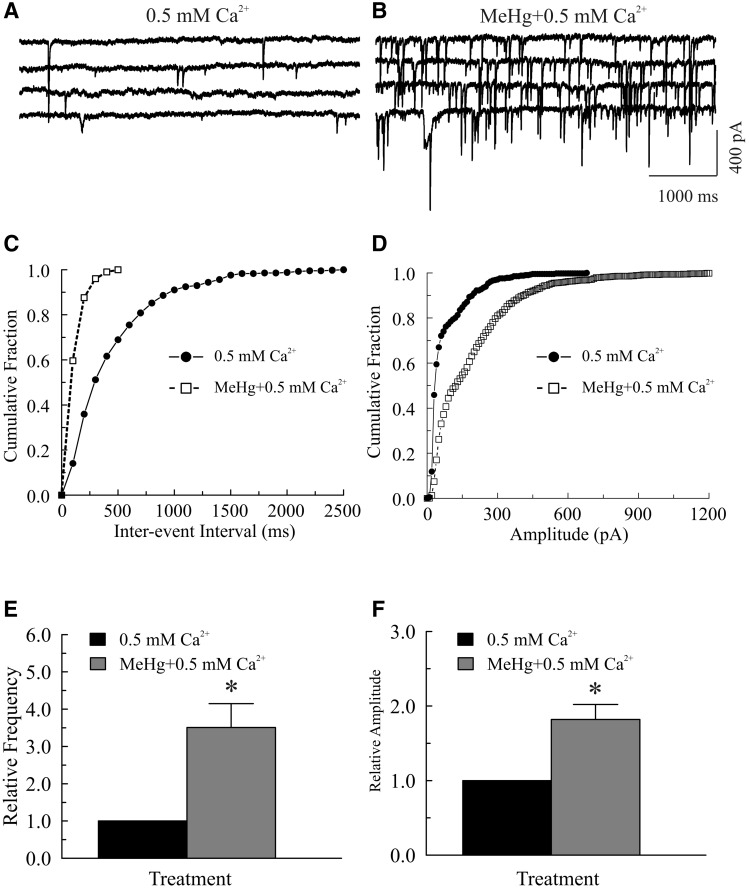
Next, we tested whether the presence of a small amount of added external Ca2+ would change the effect of MeHg on sIPSC amplitudes. Decreasing [Ca2+]o from 2 to 0.5 mM also significantly reduced the frequency of sIPSCs or mIPSCs (data not shown). Again, MeHg exposure not only increased the frequency, but also increased mean amplitude of sIPSCs or mIPSCs (Fig. 3). On average, the maximum frequency and mean amplitude (12.2 ± 3.1 Hz and 75.5 ± 24.1 pA) were >351% or >182% of those prior to MeHg treatment (3.2 ± 0.9 Hz and 40.2 ± 9.7 pA, P < .05, n = 6, Figs. 3E and 3F). Thus, extracellular Ca2+ appears to be a primary contributor to MeHg-induced increase in sIPSC or mIPSC frequency and amplitudes, but simply reducing [Ca2+]o was not sufficient to prevent the MeHg-induced initial stimulation of spontaneous transmitter release.
FIG. 3.
The presence of extracellular Ca2+ is a critical factor in determining the pattern of methylmercury (MeHg)-induced initial increase in miniature inhibitory postsynaptic currents (mIPSCs) in Purkinje Cells in cerebellar slices. A, mIPSCs were recorded from a Purkinje cell in a sagittal cerebellar slice under similar conditions as described in Figure 2A, but the slice was first incubated with artificial cerebrospinal fluid (ACSF) containing 0.5 mM [Ca2+]o for 30 min and then exposed to 20 μM MeHg in 0.5 mM [Ca2+]o bath solution for the indicated time periods (B). C, Effect of MeHg on cumulative fraction of inter-event intervals of mIPSCs from the same cell shown in (A). Bin size: 100 ms. D, Effect of MeHg on amplitude distribution of mIPSCs from the same cell in (A). Bin size: 10 pA. E–F, MeHg-induced changes in relative frequency or amplitude of mIPSCs in 0.5 mM Ca2+-containing ACSF. Each trace is a representative depiction of 6 individual experiments, each from a separate rat. The asterisk indicates a statistical significance in difference between the 2 groups (P < .05).
Both Ryanodine-Sensitive and -Insensitive Intracellular Ca2+ Pools Contribute to MeHg-Induced Stimulation of Spontaneous Inhibitory Synaptic Currents
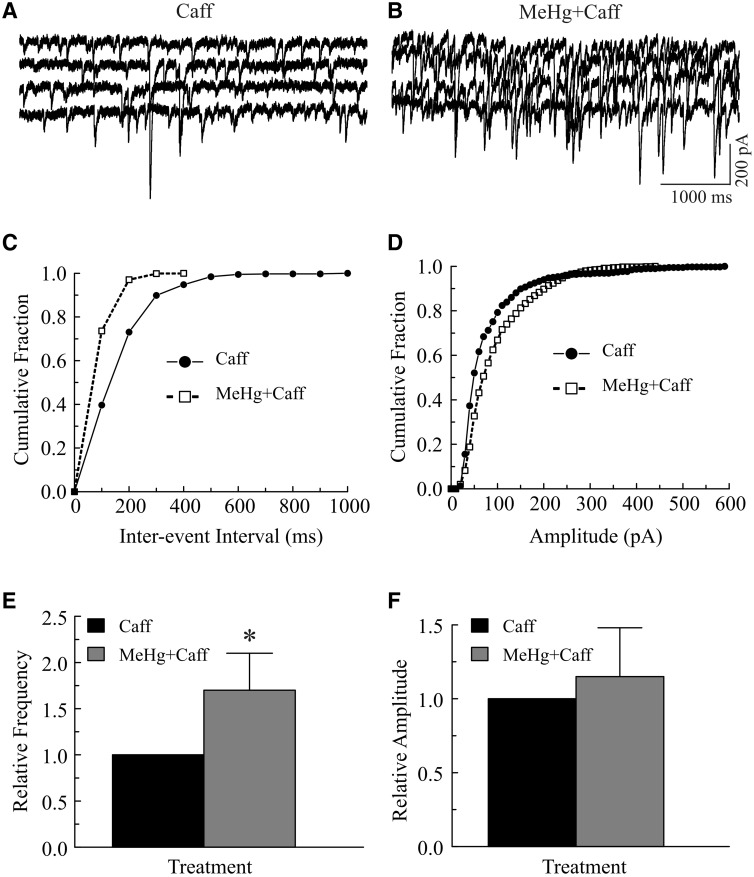
Failure in complete prevention of the MeHg-induced increases in sIPSC or mIPSC frequency in the reduced [Ca2+]o environment implies that other sources of Ca2+ such as release of Ca2+ from different intracellular stores also contribute to the initial stimulatory effects. To test this, slices were pretreated with 10 mM caffeine, a ryanodine receptor activator (Barry and Cheek, 1994; McPherson et al., 1991), or 25 μM CPA, an inhibitor of smooth endoplasmic reticulum Ca2+-ATPase (SERCA) (Simkus and Stricker, 2002), in regular ACSF before exposure to MeHg. Because most mIPSCs were too small to be detected in the presence of 10 mM caffeine in the bath (De Koninck and Mody, 1996; Savi and Sciancalepore, 1998), only sIPSCs were examined in this set of experiments. Consistent with previous reports that bath application of 10 mM caffeine suppressed GABAA receptor-mediated currents (Akopian et al., 1998; De Koninck and Mody, 1996; Savi and Sciancalepore, 1998), bath application of 10 mM caffeine for 10–15 min significantly reduced the frequency and amplitudes of sIPSCs (from 10.8 ± 2.6 Hz to 5.8 ± 2.4 Hz and 104.4 ± 26.9 pA to 58.4 ± 11.0 pA, respectively, P < .05, n = 7). However, application of 20 or 100 μM MeHg still caused a significant increase in sIPSC frequency (Fig. 4). Figures 4A–D depict a representative example of effects of pretreatment of slices with caffeine on MeHg-induced changes in sIPSCs frequency and amplitude. Continuous combined exposure of the slice to MeHg plus caffeine for 15 min following 10 min treatment with caffeine markedly increased sIPSC frequency and shifted the inter-event-interval curve to the left (increased frequency, Fig. 4C). However, the cumulative amplitude distribution curve was virtually unchanged (Fig. 4D), suggesting no change in sIPSC amplitudes. On average, the frequency was increased from 6.4 ± 2.2 Hz in the presence of caffeine alone to 9.9 ± 2.4 Hz in the presence of both caffeine and MeHg (P < .05, n = 7), about a 155% increase (Fig. 4E). In contrast, the mean amplitude of sIPSCs was virtually unchanged (54.2 ± 11.0 pA vs. 66.8 ±17.8 pA, P > 0.05, n = 7, Fig. 4F). These results indicate that caffeine pretreatment alone did not prevent MeHg-induced increase in frequency of sIPSCs.
FIG. 4.
Pretreatment of slices with 10 mM caffeine did not prevent the methylmercury (MeHg)-induced initial increase in frequency of spontaneous inhibitory postsynaptic currents (sIPSCs) in Purkinje cells. A–B, sIPSCs were recorded from a representative Purkinje cell in a slice at a holding potential of −60 mV in the presence of 10 µM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and 50 µM amino-5-phosphonopentanoic acid (APV). The slice was first incubated with 10 mM caffeine (Caff) in the bath for 15 min and then exposed to 20 μM MeHg plus 10 mM caffeine (MeHg+Caff) in 2 mM Ca2+-containing artificial cerebrospinal fluid (ACSF) for 5 min. C, Effect of MeHg on cumulative fraction of inter-event intervals of sIPSCs from the same cell shown in (A). Bin size: 100 ms. D, Effect of MeHg on amplitude distribution of sIPSCs from the same cell in (A). Bin size: 10 pA. E–F, Comparisons of MeHg effects on relative frequency or amplitude of sIPSCs in the presence of caffeine in regular ACSF. Each trace is a representative depiction of 6 individual experiments. The asterisk indicates a statistically significant difference between the 2 groups (P < .05).
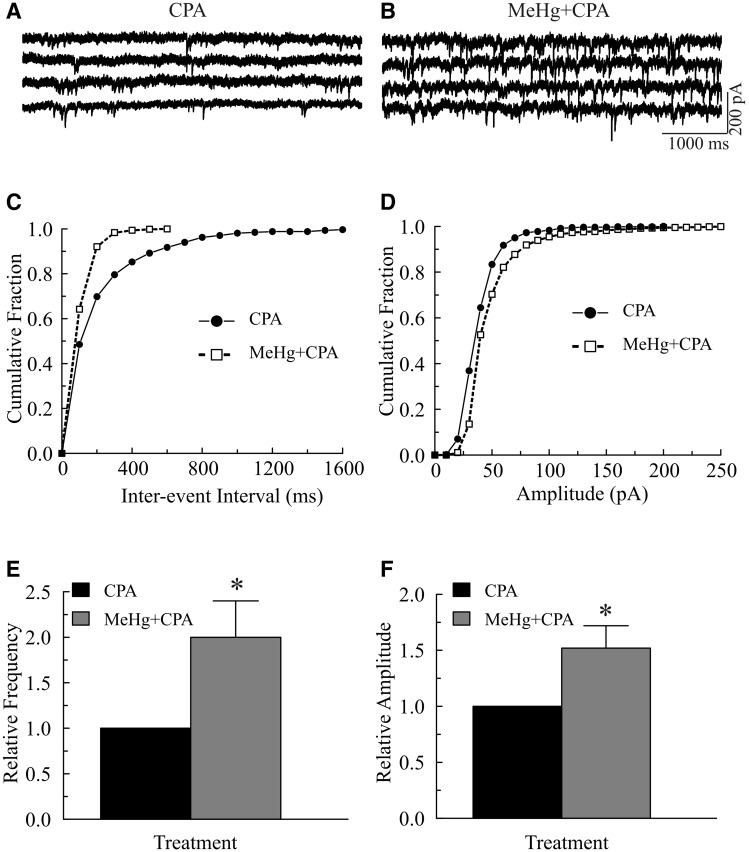
Similarly, in 6 of 8 slices from 7 animals, CPA (25 μM, 10–15 min) reduced both frequency and amplitudes of sIPSCs. The other 2 slices showed a slight increase in frequency and amplitude of sIPSCs (data not shown). Subsequent application of 20 or 100 μM MeHg still caused a pronounced increase in frequency and mean amplitude of sIPSCs in all 8 slices examined. Figures 5A–D show a representative example of effects of CPA pretreament on sIPSC frequency and amplitude, respectively. MeHg (100 μM, 4 min) shifted the cumulative inter-event interval curves to the left (Fig. 5C), but the cumulative amplitude distribution curves were shifted slightly to the right (Fig. 5D). On average, MeHg increased the frequency to 215 ± 46% (from 4.6 ± 1.6 Hz in the presence of CPA to 9.9 ± 2.1 Hz in the presence of both MeHg and CPA, P < 0.05, n = 7, Fig. 5E), whereas MeHg increased mean amplitude of sIPSCs from 61.4 ± 12.6 pA to 92.9 ± 18.7 pA (P < 0.05, n = 7), about 152 ± 20% (Fig. 5F). Pretreatment of slices for 10–15 min with 1 μM thapsigargin, another SERCA inhibitor which prevents refilling of IP3-sensitive Ca2+ pool (for review see Denmeade and Isaacs, 2005; Sabala et al., 1993), also failed to prevent MeHg-induced changes in spontaneous release of neurotransmitter (data not shown). Thus, depletion of intracellular Ca2+ stores by release of Ca2+ from ryanodine-sensitive stores or by block of refilling of Ca2+ stores reduced, but did not prevent, MeHg-induced changes in the frequency of sIPSCs.
FIG. 5.
Effect of cyclopiazonic acid (CPA) pretreatment on the methylmercury (MeHg)-induced initial increase in spontaneous inhibitory postsynaptic currents (sIPSCs) in Purkinje cells. A–B, sIPSCs were recorded from a representative Purkinje cell in a sagittal cerebellar slice under similar conditions as described in Figure 4, but the slice was first incubated with 25 μM CPA for 15 min and then exposed to 20 μM MeHg plus 25 μM CPA (MeHg+CPA) in artificial cerebrospinal fluid (ACSF) for 4 min. C, Effect of MeHg plus CPA on cumulative fraction of inter-event intervals of sIPSCs from the same cell shown in (A). Bin size: 100 ms. D, Effect of MeHg plus CPA on amplitude distribution of sIPSCs from the same cell in (A). Bin size: 10 pA. E–F, Comparisons of MeHg effect on relative frequency or amplitude of sIPSCs in in the presence of CPA in regular ACSF. Each trace is a representative depiction of 7 individual experiments. The asterisk indicates a statistically significant difference between the 2 groups (P < .05).
Inhibition of Mitochondrial Ca2+ Transport also Failed to Prevent the MeHg-Induced Early Increases in Frequency and Amplitude mIPSCs
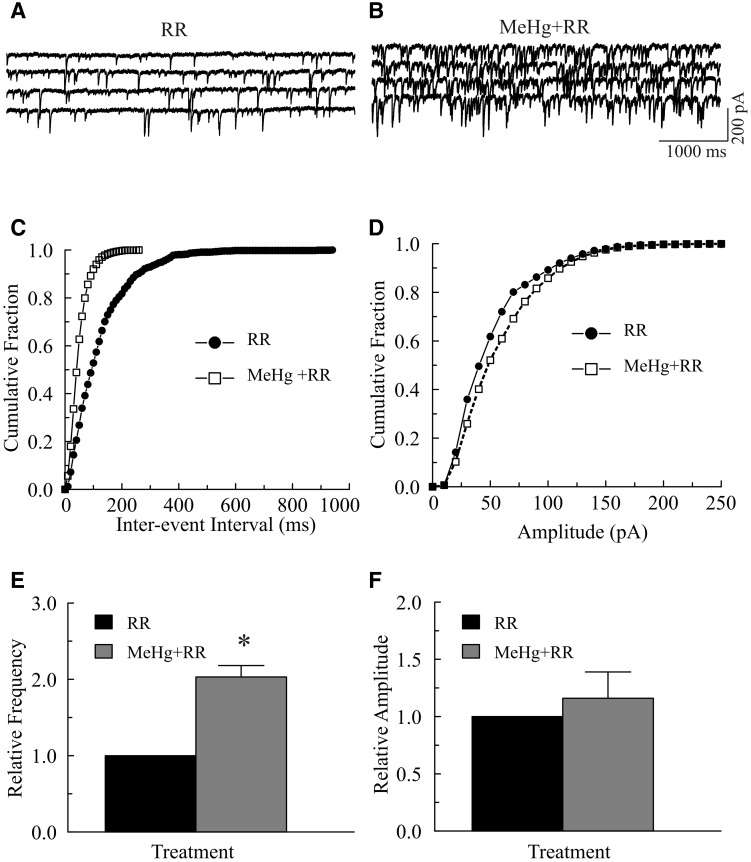
Mitochondria play an important role in regulation of [Ca2+]i homeostasis (Martínez-Serrano and Satrústegui, 1992; Nicholls, 2005; Scott, 2007). To determine any potential involvement of mitochondria in MeHg-induced initial stimulatory effect, we tested whether RR, an inhibitor of mitochondrial Ca2+ transport, could alter the MeHg-induced initial increase in mIPSCs. In all slices examined, incubation of slices with 20 μM RR in regular ACSF always caused a marked increase in mIPSC frequency and amplitude. However, continuous exposure of slices to 20 or 100 μM MeHg induced a further increase in mIPSC frequency (Fig. 6). In the case shown in Figures 6A–D, exposure of the slice to 100 μM MeHg for 4 min in the presence of 20 μM RR increased mIPSC frequency (shifted the inter-event-interval curve to the left). MeHg increased the mIPSC amplitude (the cumulative amplitude distribution curve is shifted to the right). On average, MeHg increased the frequency from 12.0 ± 1.6 Hz in the presence of RR alone to 24.2 ± 3.7 Hz in the presence of both MeHg and RR (203 ± 15%, P < .05, n = 5, Fig. 6E). In contrast, MeHg did not cause a significant increase in mean mIPSC amplitude (68.3 ± 14.3 pA in the presence of RR alone vs. 88.5 ± 35.4 pA in the presence of both RR and MeHg, P > .05, n = 5). Thus, pretreatment of slices with RR also minimized, but did not prevent MeHg-induced stimulatory effects on spontaneous synaptic responses. Taken together, these results confirm that multiple sources of Ca2+ can contribute to MeHg-induced increase in [Ca2+]i and the frequency of sIPSCs or mIPSCs.
FIG. 6.
Effects of ruthenium red (RR) pretreatment on the methylmercury (MeHg)-induced initial stimulatory effects on miniature inhibitory postsynaptic currents (mIPSCs). A–B, mIPSCs were recorded from a Purkinje cell in a slice at a holding potential of −60 mV in the presence of 10 µM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), 50 µM amino-5-phosphonopentanoic acid (APV) and 1.0 µM TTX. The slice was first incubated with 20 μM RR in regular artificial cerebrospinal fluid (ACSF) for 15 min (A) and then exposed to 100 μM MeHg plus 20 μM RR (MeHg+RR) in regular ACSF for 6 min (B). C, Effect of MeHg plus RR on cumulative fraction of inter-event intervals of mIPSCs from the same cell shown in (A). Bin size: 100 ms. D, Effect of MeHg on amplitude distribution of mIPSCs from the same cell in (A). Bin size: 10 pA. E–F, Comparisons of MeHg-induced changes in relative frequency or amplitude of mIPSCs in in the presence of RR in regular ACSF. Each trace is a representative depiction of 5 individual experiments. The asterisk indicates a statistically significant difference between the 2 groups (P < .05).
Combination of Reduced [Ca2+]o and Intracellular Ca2+ Buffering with BAPTA-AM Also Failed to Prevent Completely MeHg-Induced Increase in sIPSC or mIPSC Frequency
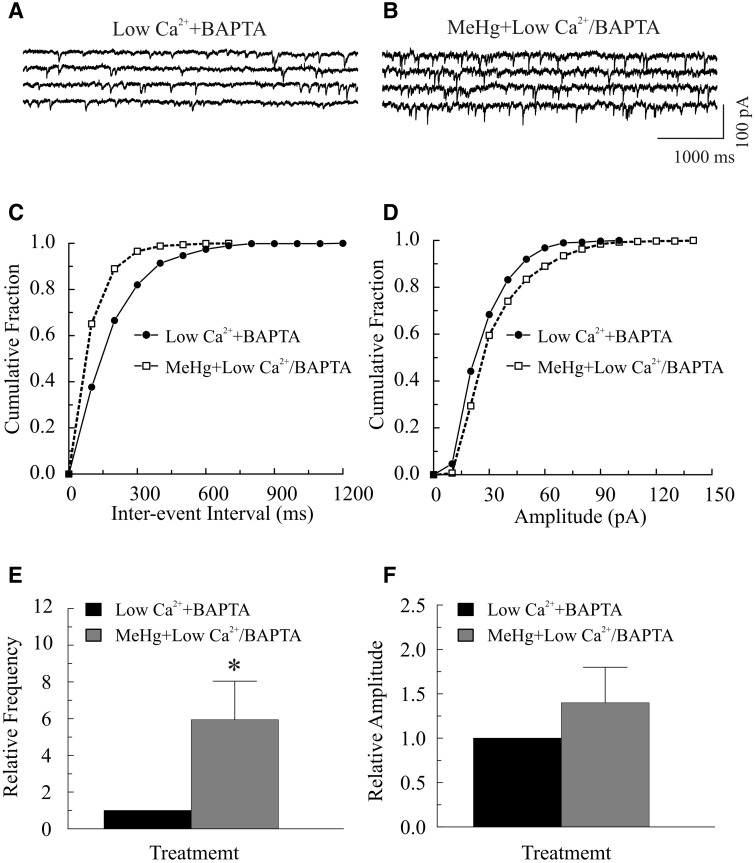
Next, we tested whether a combination of increasing intracellular Ca2+ buffering by BAPTA and reducing [Ca2+]o could eliminate the MeHg-induced increase in mIPSC frequency. Incubation of slices with 50 μM BAPTA in regular ACSF for 30 min significantly reduced the frequency and amplitude of sIPSCs/mIPSCs (data not shown). Reducing [Ca2+]o in the presence of BAPTA caused a further decrease in mIPSC frequency. However, MeHg exposure still caused a typical increase in frequency of mIPSCs (Fig. 7). Figures 7A–D show a representative example of exposure of slices to 20 μM MeHg in the low [Ca2+]o solution and the presence of BAPTA on mIPSCs. On average, MeHg increased mIPSC frequency from 1.1 ± 0.6 Hz to 6.7 ± 2.4 Hz (P < .05, n = 7) and amplitude from 30.3 ± 8.6 pA to 42.8 ± 12.5 pA (P > .05, n = 7), respectively.
FIG. 7.
Combined treatment of lowering [Ca2+]o and BAPTA-AM suppressed, but did not completely block, methylmercury (MeHg)-induced initial increase in spontaneous inhibitory postsynaptic current (sIPSC) or miniature inhibitory postsynaptic current (mIPSC) frequency of Purkinje cells in cerebellar slices. A–B, mIPSCs were recorded from a Purkinje cell in a sagittal cerebellar slice at a holding potential of −60 mV in the presence of 10 µM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), 50 µM amino-5-phosphonopentanoic acid (APV) and 1.0 µM TTX. The slice was first incubated with 50 μM BAPTA-AM for 30 min (A), then in a low [Ca2+]o artificial cerebrospinal fluid (ACSF) (no CaCl2 was added to ACSF) plus BAPTA (Low Ca2++BAPTA) for an additional 10 min prior to exposure to 20 μM MeHg plus 50 μM BAPTA-AM in this low [Ca2+]o bath solution (MeHg+Low Ca2+/BAPTA) for 35 min (B). C, Effect of MeHg plus BAPTA on cumulative fraction of inter-event intervals of mIPSCs from the same cell shown in (A). Bin size: 100 ms. D, Effect of MeHg plus BAPTA on amplitude distribution of mIPSCs from the same cell in (A). Bin size: 10 pA. E–F, Comparisons of MeHg-induced changes in relative frequency or amplitude of mIPSCs in in the presence of BAPTA in regular ACSF. Each trace is a representative depiction of 7 individual experiments. The asterisk indicates a statistically significant difference between the 2 groups (P < .05).
Reducing [Ca2+]o or Depletion of Intracellular Ca2+ Stores Suppresses MeHg-Induced Changes in Fluo-4 Fluorescence Intensity
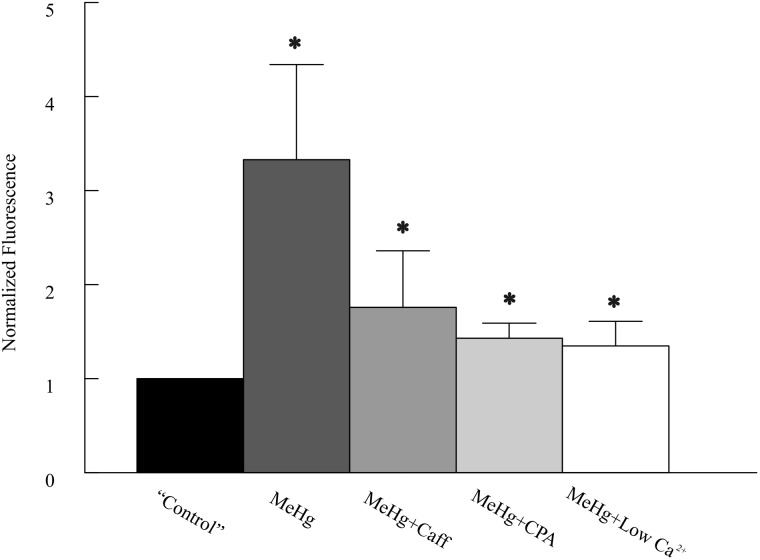
To verify if the reduction of [Ca2+]o alters MeHg-induced changes in [Ca2+]i in cerebellar neurons, we next examined temporal and spatial changes in [Ca2+]i simultaneously in the molecular (ML) and granule cell layers (GCL) of cerebellar slices preloaded with the Ca2+-selective indicator Fluo-4 using fast confocal laser-scanning microscopy. In the absence of extracellular Ca2+, 20 or 100 μM MeHg still induced 120–130% increases in peak Fluo-4 fluorescence in both layers of cerebellar slices (only ML data are shown in Fig. 8). However, the changes in fluorescence intensity induced by MeHg in the absence of extracellular Ca2+ were much smaller compared with those seen in the presence of 2 mM [Ca2+]o, in which 20 or 100 μM MeHg caused 190–333% increases in Fluo-4 florescence in the ML and GCLs (P < 0.05, n = 4–7). Apparently, removing extracellular Ca2+ suppressed the MeHg-induced increase in [Ca2+]i in both ML and GCL. Thus, these data suggest that influx of extracellular Ca2+ is a major contributor to MeHg-induced increases in [Ca2+]i and associated increases in spontaneous release of transmitter.
FIG. 8.
Comparison of effects of methylmercury (MeHg) on fluo-4 fluorescence intensity in the ML of cerebellar slices with or without pretreatment with low [Ca2+]o, caffeine or cyclopiazonic acid (CPA). Slices were treated with MeHg alone, or in combination with application of low [Ca2+]o, 10 mM caffeine or 25 μM CPA, respectively. Data were normalized to that of prior to MeHg treatment (“Control”). Note the labels: the control actually represents pretreatment with low Ca2+, caffeine, or CPA; MeHg, MeHg treatment alone in artificial cerebrospinal fluid (ACSF), MeHg+Caff, MeHg plus caffeine treatment; MeHg+CPA, MeHg plus CPA treatment; and MeHg+Low Ca2+, MeHg in low Ca2+-containing ACSF. The asterisks indicate significant differences between control and MeHg treatment in the absence or presence of caffeine, CPA or low [Ca2+]o (P < 0.05, Student’s paired t test). The values are mean ± SEM of 4–7 individual experiments
Confocal Ca2+ imaging consistently showed that caffeine or CPA pretreatment also failed to prevent MeHg-induced increases in Fluo-4 fluorescence, although changes in Fluo-4 fluorescence induced by MeHg were not as intense as those obtained in the absence of these agents (Fig. 8). Thus, these data suggest that release of Ca2+ from intracellular Ca2+ stores should at least partially contributes to a MeHg-induced increase in [Ca2+]i and spontaneous release of neurotransmitter. Because RR itself caused a significant reduction of Fluo-4 fluorescence intensity, the effect of RR pretreatment on MeHg-induced changes in Fluo-4 fluorescence intensity was not examined. Interestingly, confocal Ca2+ imaging in the presence of BAPTA plus reduced [Ca2+]o in 2 slices showed that MeHg did not cause any significant further increase in Fluo-4 fluorescence intensity (unpublished observation). Thus, these data suggest that changes in [Ca2+]i may be not the only factor for the MeHg-induced early stimulatory effect.
DISCUSSION
The primary goal of this study was to determine qualitatively the sources of Ca2+ that contribute to MeHg-induced initial stimulation of spontaneous synaptic transmission. We found that reducing [Ca2+]o, pretreatments of slices with the ryanodine receptor activator, the SERCA, or the mitochondrial Ca2+ transport inhibitors each reduced but none completely blocked MeHg-induced initial increase in spontaneous synaptic transmission. Moreover, a combination of reducing [Ca2+]o and intracellular Ca2+ buffering by BAPTA effectively suppressed but again failed to block completely MeHg-induced initial increases in spontaneous synaptic transmission. One of the most common neurotoxic effects of MeHg in vitro is disruption of [Ca2+]i homeostasis in a variety of types of cells (Edwards et al., 2005 and Roos et al., 2012 for review; Hare and Atchison, 1995; Hare et al., 1993; Limke and Atchison, 2002; Limke et al., 2003; Marty and Atchison, 1997, 1998). In NG108-15 neuroblastoma cells, MeHg caused a biphasic increase in [Ca2+]i, ie, an initial mild increase phase (1st phase) and followed by a rapid and large increase phase (2nd phase) (Hare and Atchison, 1995; Hare et al., 1993). The first phase was sustained and of low amplitude; time to onset of this phase was MeHg concentration-dependent and extracellular [Ca2+]o-independent, because removing or lowering [Ca2+]o did not block the first phase increase in [Ca2+]i. Thus, this phase is thought to be due primarily to release of Ca2+ from intracellular stores. In contrast, the MeHg-induced 2nd phase increase in [Ca2+]i in NG108-15 cells was much more pronounced; time to onset of this phase was inversely MeHg concentration dependent. That is, the higher the [MeHg], the more rapidly the effect ensued (Hare and Atchison, 1995; Hare et al., 1993). Removal of extracellular Ca2+ eliminated the 2nd phase increase in [Ca2+]i, suggesting that it resulted primarily from entry of a large amount of extracellular Ca2. Cerebellar granule cells in primary culture respond in a similar manner to MeHg, exhibiting the biphasic increases in [Ca2+]i (Marty and Atchison, 1997, 1998).
Consistently, MeHg always initially stimulates spontaneous release of neurotransmitter at peripheral and central synapses (Atchison, 1986; Atchison and Narahashi, 1982; Juang and Yonemura, 1975; Yuan and Atchison, 2003, 2005, 2007). Using simultaneous recordings of changes in sIPSCs and [Ca2+]i in cerebellar slices (Yuan and Atchison, 2007), we previously showed that time to onset of MeHg-induced increase in [Ca2+]i in the granule cell and molecular layers correlated with that of increased sIPSC frequency in Purkinje cells. Thus, a MeHg-induced increase in presynaptic [Ca2+]i must be responsible for the initial stimulation of spontaneous transmitter release. The question is, which source, extracellular or intracellular Ca2+ or both, contributes to MeHg-induced increase in spontaneous neurotransmitter release. To address this, we examined the effect of lowering [Ca2+]o on MeHg-induced stimulation of spontaneous GABA release. As expected, lowering [Ca2+]o significantly reduced MeHg-induced increases in sIPSC or mIPSC frequency, suggesting that MeHg-induced influx of extracellular Ca2+ did contribute to MeHg-induced stimulation of GABA release. Interestingly, MeHg virtually had no effect on mean amplitudes of mIPSCs in the absence of extracellular Ca2+, suggesting that extracellular Ca2+ influx is an important factor in determining MeHg-induced changes in sIPSC or mIPSC amplitudes. Both pre- and/or postsynaptic actions could possibly be involved.
However, failure of lowering [Ca2+]o to completely block MeHg-induced increases in sIPSC or mIPSC frequency clearly suggests an involvement of other sources of Ca2+, most likely contributions of release of Ca2+ from intracellular stores, ie, the MeHg-induced first phase elevation of [Ca2+]i. Both ryanodine-sensitive and inositol 1,4,5-trisphosphate (IP3)-sensitive intracellular Ca2+ stores can release Ca2+ into the cytosol leading to an increase in [Ca2+]i and modulate GABAergic synaptic transmission (Bardo et al., 2002; Llano et al., 2000; Savi and Sciancalepore, 1998; Warrier et al., 2005). In fact, treatment of NG108-15 cells with thapsigargin significantly reduced the MeHg-induced first phase [Ca2+]i increase. In addition, pretreating NG108-15 cells with bradykinin, which stimulates generation of IP3 and subsequent release of Ca2+ from IP3-sensitive stores to deplete this intracellular Ca2+ pool, also significantly reduced MeHg-induced first phase Ca2+ increase. Similarly, depletion of ryanodine-sensitive intracellular Ca2+ stores with caffeine significantly reduced MeHg-induced first phase increase in [Ca2+]i (Hare and Atchison, 1995). Pretreatment of slices with each of these agents all significantly reduced, but did not completely block, the MeHg-induced stimulation of spontaneous GABA release in this study. Thus, release of Ca2+ from both ryanodine-sensitive and -insensitive Ca2+ stores plays a role in MeHg-induced increase in sIPSC or mIPSC frequency.
In addition to release of Ca2+ from ryanodine-sensitive and -insensitive Ca2+ stores, release or uptake of Ca2+ by mitochondria also plays an important role in regulation of [Ca2+]i homeostasis. In fact, the MeHg-induced first phase of [Ca2+]i increase appears to result primarily from release of mitochondrial Ca2+ in cerebellar granule cells in culture (Limke and Atchison, 2002; Limke et al., 2003, 2004). However, pre-depletion of mitochondrial Ca2+ did not completely abolish the MeHg-induced first phase [Ca2+]i increase, which is consistent with the involvement of release of Ca2+ from other intracellular Ca2+ stores. Demonstration that this pool of Ca2+i contributes measurably to the increased spontaneous release of neurotransmitter evoked by MeHg is shown by the ability of RR to suppress significantly MeHg-induced increase in frequency of miniature end plate potentials and release of acetylcholine (Levesque and Atchison, 1987; Levesque et al., 1992). In this study, RR pretreatment also minimized MeHg-caused increase in frequency of sIPSCs or mIPSCs. Again, RR was not sufficient to block completely MeHg effects on spontaneous GABA release. Thus, our results confirm that mitochondrial Ca2+ is one of the intracellular Ca2+ pools that contributes to MeHg-induced stimulation of spontaneous release of neurotransmitter.
One interesting observation is that bath application of caffeine or CPA decreased both frequency and amplitudes of sIPSCs. It has been previously shown that brief “pulse” application of caffeine increased the frequency of spontaneous synaptic responses (Martin and Buño, 2003; Savi and Sciancalepore, 1998), whereas bath application of 10 mM caffeine reduced the frequency and amplitude of mIPSCs (De Koninck and Mody, 1996; Savi and Sciancalepore, 1998). The mechanism for the dual effects (potentiation-inhibition) of caffeine remains unclear, but the inhibition is thought to be due to a postsynaptic mechanism because bath application of caffeine inhibited currents evoked by direct application of GABA onto the postsynaptic cells (Akopian et al., 1998; Savi and Sciancalepore, 1998). Because of this effect, the reduction in mIPSC frequency presumably results from less detectable events due to a reduced amplitude of mIPSCs postsynaptically (De Koninck and Mody, 1996; Savi and Sciancalepore, 1998). Interestingly, the caffeine-induced depression of GABAA receptor-mediated currents in ganglion cells of the turtle retina could be prevented by introducing the intracellular Ca2+ chelator BAPTA into the cells, implying that elevation of intracellular Ca2+ is responsible for reduction of GABAA receptor-mediated currents. In addition, caffeine-induced effects on GABAA receptor-mediated currents could also be markedly attenuated by pre-depleting intracellular Ca2+ stores with pretreatment of thapisgargin or ryanodine (Akopian et al., 1998), suggesting that caffeine-induced Ca2+ release from intracellular stores inhibits GABAA receptor-mediated currents. However, evidence suggested that whether caffeine stimulates or suppresses GABAA receptor-mediated responses depends on the levels of increase in free [Ca2+]i: a large (>1 μM) rise in [Ca2+]i causes a depression of mIPSCs, whereas a small rise in [Ca2+]i induces potentiation (De Koninck and Mody, 1996). CPA treatment also reduced the frequency and amplitudes of sIPSCs in most slices examined. These results are generally in agreement with the effects of CPA on mEPSCs in layer II neurons of rat barrel cortex (Simkus and Stricker, 2002), in which CPA significantly reduced the mEPSC frequency and slightly reduced mEPSC amplitudes. However, our results are inconsistent with those of Bardo et al. (2002), in which application of 25 μM CPA for 10–15 min caused a significant increase in mIPSC frequency in 8 of 10 Purkinje cells tested in cerebellar slices. In contrast, 6 of 8 cerebellar slices from 7 rats in our study showed a reduction in both frequency and amplitudes of sIPSCs; only 2 slices showed a slight increase in sIPSC frequency.
We previously showed that pretreatment of cerebellar slices with BAPTA/AM significantly reduced both frequency and amplitude of sIPSCs in Purkinje cells, but BAPTA treatment did not completely prevent MeHg-induced initial increase in sIPSC frequency (Yuan and Atchison, 2007). One possibility is that MeHg-induced continuous influx of extracellular Ca2+ may saturate the buffering capacity of BAPTA. To test this, a combination of lowering [Ca2+]o and BAPTA treatment was used in this study to determine if this combination could prevent the MeHg-induced initial increase in mIPSC frequency. Surprisingly, this combination also failed to block the MeHg-induced initial increase in mIPSC frequency, although it did markedly reduce the extent of MeHg-induced increases. However, the question is whether all the treatments described above effectively modulated MeHg-induced changes in [Ca2+]i in both molecular and granule layers in slices. To address this, we selectively measured MeHg-induced changes in [Ca2+]i in the molecular and GCLs in the presence of caffeine, CPA or under low [Ca2+]i conditions. These treatments minimized although they did not completely block MeHg-induced increases in [Ca2+]i in the molecular and GCLs, which is consistent with the assumption that multiple sources of Ca2+ contributed to MeHg-induced increase in [Ca2+]i and frequency of sIPSCs or mIPScs. Interestingly, no MeHg-induced increase in [Ca2+]i was seen in 2 slices following a combined treatment of slices with BAPTA and lowering [Ca2+]o. However, this combination did not completely prevent MeHg-induced initial increase in frequency of sIPSCs or mIPSCs either. Thus, other unknown factors may be also involved in stimulation of spontaneous transmitter release. The possibility that MeHg itself may behave like Ca2+ to stimulate transmitter release cannot be ruled out.
In conclusion, pretreatment of cerebellar slices with reduced [Ca2+]o, depletion of ryanodine-sensitive or -insensitive intracellular Ca2+ stores, or mitochondria or increased intracellular buffering by BAPTA all significantly reduced, but none completely, prevented MeHg-induced stimulation of spontaneous neurotransmitter release. These data suggest that multiple Ca2+ sources and possibly other unknown factors are involved in MeHg-induced early stimulatory effects on spontaneous synaptic transmission.
ACKNOWLEDGMENT
The authors gratefully acknowledge the expert word processing and graphic assistance of Elizabeth Hill and Jessica Hauptman Gevers, respectively.
FUNDING
The Institute of Environmental Health Science at National Institute of Health [Grants R01-ES03299, R01-ES11662, and R01-ES24064].
REFERENCES
- Akopian A., Gabriel R., Witkovsky P. (1998). Calcium release from intracellular stores inhibits GABAA-mediated currents in ganglion cells of the turtle retina. J. Neurophysiol. 80, 1105–1115. [DOI] [PubMed] [Google Scholar]
- Amaral M. D., Pozzo-Miller L. (2012). Intracellular Ca2+ stores and Ca2+ influx are both required for BDNF to rapidly increase quantal vesicular transmitter release. Neural Plast. 2012, 203536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atchison W. D. (1986). Extracellular calcium-dependent and independent effects of methylmercury on spontaneous and potassium-evoked release of acetylcholine at the neuromuscular junction. J. Pharmacol. Exp. Ther. 237, 672–680. [PubMed] [Google Scholar]
- Atchison W. D. (1987). Effects of activation of sodium and calcium entry on spontaneous release of acetylcholine induced by methylmercury. J. Pharmacol. Exp. Ther. 241, 131–139. [PubMed] [Google Scholar]
- Atchison W. D., Narahashi T. (1982). Methylmercury-induced depression of neuromuscular transmission in the rat. Neurotoxicology 3, 37–50. [PubMed] [Google Scholar]
- Bardo S., Robertson B., Stephen G. J. (2002). Presynaptic internal Ca stores contribute to inhibitory neurotransmitter release onto mouse cerebellar Purkinje cells. Br. J. Pharmacol. 137, 529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry V. A., Cheek T. R. (1994). A caffeine- and ryanodine-sensitive intracellular Ca2+ store can act as a Ca2+ source and a Ca2+ sink in PC12 cells. Biochem. J. 300, 589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin T., Marty A., Lláno I. (2005). Presynaptic calcium stores and synaptic transmission. Curr. Opin. Neurobiol. 15, 275–281. [DOI] [PubMed] [Google Scholar]
- De Koninck Y., Mody I. (1996). The effects of raising intracellular calcium on synaptic GABAA receptor-channels. Neuropharmacology 35, 1365–1374. [DOI] [PubMed] [Google Scholar]
- Denmeade S., Isaacs J. T. (2005). The SERCA pump as a therapeutic target: Making a “smart bomb” for prostate cancer. Cancer Biol. Ther. 4, 14–22. [DOI] [PubMed] [Google Scholar]
- Edwards J. R., Marty M. S., Atchison W. D. (2005). Comparative sensitivity of rat cerebellar neurons to dysregulation of divalent cation homeostasis and cytotoxicity caused by methylmercury. Toxicol. Appl. Pharmacol. 208, 222–232. [DOI] [PubMed] [Google Scholar]
- Garaschuk O., Yaari Y., Konnerth A. (1997). Release and sequestration of calcium by ryanodine-sensitive stores in rat hippocampal neurons. J. Physiol. 502, 13–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare M. F., Atchison W. D. (1995). Methylmercury mobilizes Ca++ from intracellular stores sensitive to inositol 1,4,5-trisphosphate in NG108-15 cells. J. Pharmacol. Exp. Ther. 272, 1016–1023. [PubMed] [Google Scholar]
- Hare M. F., McGinnis K. M., Atchison W. D. (1993). Methylmercury increases intracellular concentrations of Ca++ and heavy metals in NG108-15 cells. J. Pharmacol. Exp. Ther. 266, 1626–1635. [PubMed] [Google Scholar]
- Juang M. S. (1976). An electrophysiological study of the action of methylmercuric chloride and mercuric chloride on the sciatic nerve-sartorius muscle preparation of the frog. Toxicol. Appl. Pharmacol. 37, 339–348. [DOI] [PubMed] [Google Scholar]
- Juang M. S., Yonemura K. (1975). Increased spontaneous transmitter release from presynaptic nerve terminal by methylmercuric chloride. Nature 256, 211–213. [DOI] [PubMed] [Google Scholar]
- Kauppinen R. A., Komulainen H., Taipale H. (1989). Cellular mechanisms underlying the increase in cytosolic free calcium concentration induced by methylmercury in cerebrocortical synaptosomes from guinea pig. J. Pharmacol. Exp. Ther. 248, 1248–1254. [PubMed] [Google Scholar]
- Komulainen H., Bondy S. C. (1987). Increased free intrasynaptosomal Ca2+ by neurotoxic organometals: Distinctive mechanisms. Toxicol. Appl. Pharmacol. 88, 77–86. [DOI] [PubMed] [Google Scholar]
- Korge P., Weiss J. N. (1999). Thapsigargin directly induces the mitochondrial permeability transition. Eur. J. Biochem. 265, 273–280. [DOI] [PubMed] [Google Scholar]
- Levesque P. C., Atchison W. D. (1987). Interaction of mitochondrial inhibitors with methylmercury on spontaneous quantal release of acetylcholine. Toxicol. Appl. Pharmacol. 87, 315–324. [DOI] [PubMed] [Google Scholar]
- Levesque P. C., Hare M. F., Atchison W. D. (1992). Inhibition of mitochondrial Ca2+ release diminishes the effectiveness of methyl mercury to release acetylcholine from synaptosomes. Toxicol. Appl. Pharmacol. 115, 11–20. [DOI] [PubMed] [Google Scholar]
- Limke T. L., Atchison W. D. (2002). Acute exposure to methylmercury opens the mitochondrial permeability transition pore in rat cerebellar granule cells. Toxicol. Appl. Pharmacol. 178, 52–61. [DOI] [PubMed] [Google Scholar]
- Limke T. L., Bearss J. J., Atchison W. D. (2004). Acute exposure to methylmercury causes Ca2+ dysregulation and neuronal death in rat cerebellar granule cells through an M3 muscarinic receptor-linked pathway. Toxicol. Sci. 80, 60–68. [DOI] [PubMed] [Google Scholar]
- Limke T. L., Otero-Montañãnez J. K., Atchison W. D. (2003). Evidence for interactions between intracellular stores during methylmercury-induced intracellular calcium dysregulation in rat cerebellar granule neurons. J. Pharmacol. Exp. Ther. 304, 948–958. [DOI] [PubMed] [Google Scholar]
- Lláno I., Gonzalez J., Caputo C., Lai F. A., Blayney L. M., Tan Y. P., Marty A. (2000). Presynaptic calcium stores underlie large-amplitude miniature IPSCs and spontaneous calcium transients. Nat. Neurosci. 3, 1256–1265. [DOI] [PubMed] [Google Scholar]
- Martín E. R., Buño W. (2003). Caffeine-mediated presynaptic long-term potentiation in hippocampal CA1 pyramidal neurons. J. Neurophysiol. 89, 3029–3038. [DOI] [PubMed] [Google Scholar]
- Martínez-Serrano A., Satrústegui J. (1992). Regulation of cytosolic free calcium concentration by intrasynaptic mitochondria. Mol. Biol. Cell 3, 235–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty M. S., Atchison W. D. (1997). Pathways mediating Ca2+ entry in rat cerebellar granule cells following in vitro exposure to methylmercury. Toxicol. Appl. Pharmacol. 147, 319–330. [DOI] [PubMed] [Google Scholar]
- Marty M. S., Atchison W. D. (1998). Elevation of intracellular Ca2+ as a probable contributor to decreased viability in cerebellar granule cells following acute to methylmercury. Toxicol. Appl. Pharmacol. 150, 98–105. [DOI] [PubMed] [Google Scholar]
- McPherson P. S., Kim Y.-K., Valdivia H., Knudson C. M., Takekura H., Franzini-Amstrong C., Coranado R., Campbell K. P. (1991). The brain ryanodine receptor: A caffeine-sensitive calcium release channel. Neuron 7, 17–25. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G. (2005). Mitochondria and calcium signaling. Cell Calcium 38, 311–317. [DOI] [PubMed] [Google Scholar]
- Roos D., Seeger R., Puntel R., Vargas Barbosa N. (2012). Role of calcium and mitochondria in MeHg-mediated cytotoxicity. J. Biomed. Biotechnol. 2012, 248764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusakov D. A. (2006). Ca2+-dependent mechanisms of presynaptic control at central synapses. Neuroscientist 12, 317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabala P., Czarny M., Woranczak J. P., Barańska J. (1993). Thapisgargin: Potent inhibitor of Ca2+ transporter ATP-ase of endoplasmic and sarcoplasmic reticulum. Acta Biochim. Pol. 40, 309–319. [PubMed] [Google Scholar]
- Savi N., Sciancalepore M. (1998). Intracellular calcium stores modulate miniature GABA-mediated synaptic currents in neonatal rat hippocampal neurons. Eur. J. Neurosci. 10, 3379–3386. [DOI] [PubMed] [Google Scholar]
- Scott R. (2007). Use-dependent control of presynaptic calcium signalling at central synapses. J. Anat. 210, 642–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkus C. R. L., Stricker C. (2002). The contribution of intracellular calcium stores to mEPSCs recorded in layer II neurones of rat barrel cortex. J. Physiol. 545, 521–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth J. T., Hwang S.Y., Tomita T., DeHaven W. I., Mercer J. C., Putney J. W. (2010). Activation and regulation of store-operated calcium entry. J. Cell. Mol. Med. 14, 2337–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia R., Velasco I. (1997). Ruthenium red as a tool to study calcium channels, neuronal death and the function of neural pathways. Neurochem. Int. 30, 137–147. [DOI] [PubMed] [Google Scholar]
- Velasco I., Tapia R. (2000). Alteration of intracellular homeostasis and mitochondrial function are involved in ruthenium red neurotoxicity in primary cortical cultures. J. Neurosci. Res. 60, 543–551. [DOI] [PubMed] [Google Scholar]
- Warrier A., Borges S., Dalcino D., Walters C., Wilson M. (2005). Calcium from internal stores triggers GABA release from retinal amacrine cells. J. Neurophysiol. 94, 4196–4208. [DOI] [PubMed] [Google Scholar]
- Yuan Y., Atchison W. D. (1993). Disruption of by methylmercury of membrane excitability and synaptic transmission of CA1 neurons in hippocampal slices of the rat. Toxicol. Appl. Pharmacol. 120, 203–215. [DOI] [PubMed] [Google Scholar]
- Yuan Y., Atchison W. D. (1997). Action of methylmercury on GABAA receptor-mediated inhibition is primarily responsible for its early stimulatory effects on hippocampal CA1 synaptic transmission. J. Pharmacol. Exp. Ther. 282, 64–73. [PubMed] [Google Scholar]
- Yuan Y., Atchison W. D. (1999). Comparative effects of methylmercury on parallel-fiber and climbing-fiber response of rat cerebellar slices. J. Pharmacol. Exp. Ther. 288, 1015–1025. [PubMed] [Google Scholar]
- Yuan Y., Atchison W. D. (2003). Methylmercury differentially affects GABAA receptor-mediated spontaneous IPSCs in Purkinje and granule cells of rat cerebellar slices. J. Physiol. 550, 191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y., Atchison W. D. (2005). Methylmercury induces a spontaneous, transient slow inward chloride current in Purkinje cells of rat cerebellar slices. J. Pharmacol. Exp. Ther. 313, 751–764. [DOI] [PubMed] [Google Scholar]
- Yuan Y., Atchison W. D. (2007). Methylmercury-induced increase of intracellular Ca2+ concentration in presynaptic fibers causes increased frequency of spontaneous synaptic responses in cerebellar slice of rat. Mol. Pharmacol. 71, 1109–1121. [DOI] [PubMed] [Google Scholar]
- Yuan Y., Otero-Montañez J. K., Yao A., Herden C. J., Sirois J. E., Atchison W. D. (2005). Inwardly rectifying and voltage-gated outward potassium channels exhibit low sensitivity to methylmercury. Neurotoxicology 26, 439–454. [DOI] [PubMed] [Google Scholar]