Abstract
Humans are ubiquitously exposed to di(2-ethylhexyl) phthalate (DEHP), which is an environmental toxicant incorporated in consumer products. Studies have shown that DEHP targets the ovary to disrupt essential processes required for reproductive and nonreproductive health. Specifically, 10-day exposure to DEHP accelerates primordial follicle recruitment and disrupts estrous cyclicity in adult mice. However, it is unknown if these effects on folliculogenesis and cyclicity following acute DEHP exposure can have permanent effects on reproductive outcomes. Further, the premature depletion of primordial follicles can cause early reproductive senescence, and it is unknown if acute DEHP exposure accelerates reproductive aging. This study tested the hypothesis that acute DEHP exposure causes infertility, disrupts estrous cyclicity, alters hormone levels, and depletes follicle numbers by inducing atresia later in life, leading to accelerated reproductive aging. Adult CD-1 mice were orally dosed with vehicle or DEHP (20 μg/kg/day–500 mg/kg/day) daily for 10 days, and reproductive outcomes were assessed at 6 and 9 months postdosing. Acute DEHP exposure significantly altered estrous cyclicity compared to controls at 6 and 9 months postdosing by increasing the percentage of days the mice were in estrus and metestrus/diestrus, respectively. DEHP also significantly decreased inhibin B levels compared to controls at 9 months postdosing. Further, DEHP significantly increased the BAX/BCL2 ratio in primordial follicles leading to a significant decrease in primordial and total follicle numbers compared to controls at 9 months postdosing. Collectively, the adverse effects present following acute DEHP exposure persist later in life and are consistent with accelerated reproductive aging.
Keywords: di(2-ethylhexyl) phthalate, folliculogenesis, ovary, reproductive aging
Di(2-ethylhexyl) phthalate (DEHP) is a widely used synthetic plasticizer found in common consumer, medical, and building products containing polyvinyl chloride (ATSDR, 2002; Heudorf et al., 2007). Because of its incorporation in a multitude of products ranging from food and beverage packaging to flooring and roofing, 300 million pounds of DEHP are produced in the United States annually (ATSDR, 2002). DEHP is noncovalently bound to the plastics to impart flexibility, meaning DEHP can frequently leach from these products into the environment and products that humans consume (Heudorf et al., 2007). Thus, the high production volumes of DEHP, frequent use of DEHP in common items, and the ability of DEHP to leach from products lead to daily human exposure via oral ingestion, inhalation, and dermal contact (Heudorf et al., 2007). In fact, the estimated range of daily human exposure is 3–30 μg/kg/day (Doull et al., 1999; Kavlock et al., 2002). Because humans are exposed to DEHP on a daily basis, DEHP has been identified as a top contaminant in human tissues, and DEHP and its metabolites are present in human blood (Hogberg et al., 2008; Kato et al., 2004), urine (Heudorf et al., 2007; Hogberg et al., 2008; Kato et al., 2004), amniotic fluid (Silva et al., 2004), umbilical cord blood (Latini et al., 2003), breast milk (Hogberg et al., 2008), and ovarian follicular fluid (Krotz et al., 2012).
Exposure to DEHP represents a public health concern because DEHP and its metabolites are known endocrine disrupting chemicals and reproductive toxicants that have recently been shown to target the ovary (ATSDR, 2002; Hannon and Flaws, 2015; Heudorf et al., 2007). This is of further concern because the ovary is an integral regulator of reproductive and nonreproductive health. The female is born with a finite number of primordial follicles, and folliculogenesis is the irreversible maturation of these dormant primordial follicles to primary follicles, then preantral follicles, and ultimately mature antral follicles for ovulation. Proper folliculogenesis maintains the appropriate timing of reproductive senescence and supplies the ovary with an adequate number of antral follicles that synthesize sex steroid hormones (Bhattacharya and Keating, 2012; Craig et al., 2011; Hoyer and Sipes, 1996; Hoyer, 2005). These sex steroid hormones can act on numerous tissues to regulate the female reproductive tract and the hypothalamus-pituitary-ovarian (HPO) axis as well as the cardiovascular, skeletal, and brain systems (Wierman, 2007).
Previous work indicates that DEHP exposure directly disrupts the essential process of folliculogenesis by decreasing primordial follicle numbers and increasing primary follicle numbers in the ovary following 10 days of dosing (Hannon et al., 2014). In addition to the defects on folliculogenesis, DEHP exposure for 10 days alters estrous cyclicity in adult mice by increasing the percentage of days the mice were in estrus (Hannon et al., 2014). However, this previous study did not investigate if the acute 10-day exposure to DEHP has permanent effects on reproduction later in life or advances the age of reproductive senescence.
Several biological changes in the HPO axis occur during the transitory period of reproductive aging. The most striking changes are a decline in fertility with age (Menken et al., 1986) and disorganization of estrous cycles characterized by cycles with varying lengths, leading to a persistent state of estrus and ultimately a persistent state of anestrus (Gee et al., 1983; Lerner et al., 1990; Nass et al., 1984). Further, the hormones that are produced by and regulate the HPO begin to fluctuate. Specifically, the ovarian-derived steroid (estradiol, progesterone, and testosterone) and peptide (inhibin B and anti-Müllerian hormone [AMH]) hormones decrease and the pituitary-derived gonadotropins (follicle-stimulating hormone [FSH] and luteinizing hormone [LH]) increase with age (Kevenaar et al., 2006; Nelson et al., 1987; Santoro et al., 2003). In fact, it is hypothesized that the decrease in the inhibin B levels produced by ovarian follicles initiate the ongoing endocrine changes in the female and promote the rise in FSH levels (Burger, 1999; MacNaughton et al., 1992). Arguably, the most important promoter of reproductive aging is the dramatic and accelerated loss of the finite primordial follicle pool with age, which is caused by an increase in apoptosis- and/or autophagy-induced atresia, or follicle death (Faddy et al., 1992; Gougeon et al., 1994; Tatone et al., 2008). Two primary facilitators of this increase in atresia are the pro-apoptotic factor BCL2-associated X protein (BAX) and the antiapoptotic and antiautophagic factor B-cell leukemia/lymphoma 2 (BCL2) (Flaws et al., 2001; Greenfeld et al., 2007; Hsu and Hsueh, 2000; Maiuri et al., 2010; Ratts et al., 1995).
Since exposure to DEHP for 10 days accelerates primordial follicle recruitment and disrupts estrous cyclicity, this study was designed to investigate if acute exposure to DEHP in adulthood causes adverse reproductive outcomes later in life and/or accelerates reproductive aging. Specifically, we tested the hypothesis that 10-day exposure to DEHP causes infertility, disrupts estrous cyclicity, alters hormone levels, and depletes follicle numbers by inducing atresia at 6 and 9 months post-DEHP exposure, leading to early reproductive senescence.
MATERIALS AND METHODS
Chemicals
DEHP (99% purity) was purchased from Sigma-Aldrich (St. Louis, MO). Stock solutions of DEHP were prepared using tocopherol-stripped corn oil (MP Biomedicals, Solon, OH) as the vehicle. The doses selected in this study were 20 µg/kg/day, 200 µg/kg/day, 20 mg/kg/day, 200 mg/kg/day, and 500 mg/kg/day. Thus, the stock concentrations were 0.0125, 0.125, 12.5, 125, and 312.5 mg/ml for each respective dose.
These doses were selected because oral exposure to DEHP at 20 µg/kg/day–750 mg/kg/day accelerates primordial follicle recruitment in adult CD-1 mice (Hannon et al., 2014). These selected doses are also environmentally relevant (Doull et al., 1999; Kavlock et al., 2002). Specifically, the estimated range of daily human exposure to DEHP is 3–30 µg/kg/day (Doull et al., 1999; Kavlock et al., 2002). Additionally, certain populations of humans are exposed to higher levels of DEHP. Occupational exposure to DEHP can reach over 200 µg/kg/day (NTP, 2000), and patients undergoing procedures with medical equipment containing DEHP can be exposed to levels up to 14 mg/kg/day (FDA, 2001).
Animals and Study Design
Adult CD-1 mice (74–76 days of age) were purchased from Charles River Laboratories (Wilmington, MA) and were allowed to acclimate to the facility prior to dosing. The mice were housed individually at the College of Veterinary Medicine Animal Facility at the University of Illinois at Urbana-Champaign and were provided food and water ad libitum. Temperature was maintained at 22 ± 1°C with 12-h light-dark cycles to provide a controlled housing environment. The mice were orally dosed with vehicle (tocopherol-stripped corn oil) or DEHP (20 µg/kg/day–500 mg/kg/day) daily for 10 days (n = 4–6/group). Dosing volumes were determined daily by corresponding mouse weight, and the dose was given orally. Reproductive capabilities were assessed following 6 months postdosing, as described below. The 6-month postdosing time-point was chosen because the mice were of adequate reproductive age. Thus, any defect in breeding is likely attributed to DEHP exposure and not any age-related defect. The mice were euthanized in diestrus, and tissues were collected 9 months postdosing to further assess reproductive capabilities. The 9-month postdosing time-point was chosen because it was slightly beyond optimal breeding age, but follicles would still be present in the ovary in the control group. Thus, any loss of follicles in the treatment groups is likely attributed to DEHP exposure and not due to the age-related decline in follicles. The Institutional Animal Use and Care Committee at the University of Illinois at Urbana-Champaign approved all procedures involving animal care, euthanasia, and tissue collection.
Breeding Trial and Fertility Analysis
Following acute exposure to DEHP for 10 days, the mice were subjected to a breeding trial at 6 months postdosing. Female mice of the same treatment group were housed with proven, untreated male mice (75 days of age) purchased from Charles River Laboratories (Wilmington, MA). Time to pregnancy was assessed as the number of days between male introduction and the presence of a copulatory plug. Once a copulatory plug was observed, the mice were housed individually for the duration of gestation, and the mice were weighed twice weekly to monitor pregnancy weight gain. Mice that gained substantial weight following a copulatory plug, but then lost the weight without giving birth were considered to be mice that lost a pregnancy. Mice with an absent copulatory plug were considered as mice that never became pregnant and were returned to individual housing after 14 days with the proven male breeder. At birth, the number of live pups, the number of dead pups, the sex of the pups (determined by physical appearance and anogenital distance), and the weight of the pups were recorded. Percentage of live pups was calculated by dividing the number of live pups in each treatment group by the total number of pups in each treatment group and multiplying that value by 100. The opposite was conducted for the percentage of dead pups. The male to female ratio was calculated by dividing the number of males by the number of females in each litter. Average pup weight was calculated by dividing the weight of each pup in a litter by the number of pups in each litter.
Estrous Cyclicity Analysis
Following acute exposure to DEHP for 10 days, estrous cyclicity was monitored at 6 months postdosing (prior to the breeding trial) and at 9 months postdosing (prior to tissue collection). The mice were subjected to vaginal lavage daily for 14 days prior to each time-point tested (n = 4–6/group). The stage of cycle was determined by vaginal cytology based on previously defined criteria (Hartman, 1944). Percentage of days in estrus was calculated by dividing the number of days in estrus by 14 and multiplying that value by 100. The same methods were conducted for the percentage of days in metestrus/diestrus.
Analysis of Sex Steroid, Gonadotropin, and Peptide Hormone Levels
Following acute exposure to DEHP for 10 days, the mice were euthanized in diestrus at 9 months postdosing. Blood was drawn from the mice immediately following euthanasia, and serum was subjected to enzyme-linked immunosorbent assays (ELISAs) or radioimmunoassays (RIAs) at the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core (n = 3–6/group). The sex steroid hormones that were measured using ELISAs were progesterone, testosterone, and estradiol. Additionally, the testosterone/estradiol ratio and estradiol/progesterone ratio (multiplied by 1000) were calculated in ng/dl and ng/ml, respectively. The gonadotropin hormones that were measured using RIAs were FSH and LH. Additionally, the FSH/LH ratio was calculated in ng/ml. The peptide hormones that were measured using ELISAs were inhibin B and AMH. Because of limited serum volume per animal, the 500 mg/kg/day group only has a sample size of two for the inhibin B ELISA. For statistical purposes, the mean for inhibin B for the 500 mg/kg/day group included the inhibin B levels from two animals in addition to the average of their inhibin B levels.
Histological Evaluation of Follicle Numbers
Following acute exposure to DEHP for 10 days, one ovary from each mouse was collected at 9 months postdosing and fixed overnight in 4% paraformaldehyde (n = 4–6 ovaries/group). The ovaries were transferred to 70% ethanol, embedded in paraffin wax, and serial sectioned (8 µm) using a microtome. Every 10th serial section was mounted on a glass slide and stained with hematoxylin and eosin for histological evaluation of follicle numbers. Stage of follicular development was assessed using previously defined criteria (Flaws et al., 1994). Briefly, primordial follicles contained an oocyte surrounded by a single layer of squamous granulosa cells, primary follicles contained an oocyte surrounded by a single layer of cuboidal granulosa cells, preantral follicles contained an oocyte surrounded by at least two layers of cuboidal granulosa cells and theca cells, antral follicles contained an oocyte surrounded by multiple layers of cuboidal granulosa cells with a fluid filled antral space and theca cells, atretic antral follicles contained apoptotic bodies encompassing over 10% of the antral follicle, and corpora lutea were much larger structures containing large and small luteal cells. All primordial and primary follicles were counted in each section regardless of nuclear material in the oocyte, whereas only preantral and antral follicles with nuclear material in the oocyte were counted to avoid the risk of double counting the larger follicle types that can span multiple sections. Follicles transitioning between stages were counted as follicles within the more immature stage of the two stages. The total numbers of all follicles, total numbers of each type of follicle, percentages of each follicle type, percentages of atretic antral follicles, and corpora lutea numbers per number of sections counted were recorded.
Immunohistochemistry
Following acute exposure to DEHP for 10 days, some ovaries were collected at 9 months postdosing, fixed overnight in 4% paraformaldehyde, and transferred to 70% ethanol for immunohistochemical analysis of BAX and BCL2 as described previously (n = 3 ovaries/group) (Hannon et al., 2014, 2015a). Briefly, the ovaries were embedded in paraffin, sectioned at 8 µm, and five representative sections spanning the entire length of the ovary were mounted on glass slides. The ovarian tissues were incubated for one hour with the primary antibodies, BAX (1:250; Abcam, Inc., Cambridge, MA) or BCL2 (1:100; Abcam, Inc., Cambridge, MA), 20 min with a secondary biotinylated goat antirabbit antibody (Vector Laboratories, Inc., Burlingame, CA), followed by 20 minutes with an avidin biotin complex solution (Vector Laboratories, Inc., Burlingame, CA). ImmPACT NovaRED peroxidase substrate solution (Vector Laboratories, Inc., Burlingame, CA) then was applied to all samples for an equal amount of time until color optimally developed, and the slides were counterstained with a 1:10 dilution of hematoxylin. The negative control was incubated with a negative control rabbit immunoglobulin fraction (Dako, Carpinteria, CA) at the same concentration of the primary antibodies but was otherwise subjected to the same methods.
Staining for BAX and BCL2 was chosen because BAX is a pro-apoptotic protein that induces follicular atresia, and BCL2 is an antiapoptotic protein that protects the follicle from atresia (Flaws et al., 2001; Greenfeld et al., 2007; Hsu and Hsueh, 2000; Ratts et al., 1995). Further, BCL2 has been shown to cross talk with autophagy, in which BCL2 may inhibit autophagy-induced follicle atresia (Maiuri et al., 2010). Both apoptosis- and autophagy-induced atresia are potential mechanisms by which the primordial follicle reserve can be depleted (Rodrigues et al., 2009; Tingen et al., 2009).
Analysis of the Levels of Protein Staining
Following immunohistochemistry, the levels of BAX and BCL2 staining were quantified in the whole ovary and primordial follicles using previously published methods (Barnett et al., 2007; Hannon et al., 2014, 2015b; Masters et al., 2007; Paulose et al., 2011). Briefly, five representative images of each ovary were digitally captured using a Leica DFC 290 camera and analyzed using the ImageJ software (http://rsb.info.nih.gov/nih-image/) (n = 3 ovaries/group). Digital images were converted to 8-bit grayscale images and then converted to pseudocolored images. The colors from these images were based on relative stain intensity, as defined digitally. Areas with no positive staining appeared black and dark blue, while areas with positive staining appeared as bright, neon colors. Percentages of pixels corresponding to positive staining of BAX and BCL2 were calculated by dividing the number of pixels corresponding to positive staining by the total number of pixels in the whole ovary and primordial follicles and multiplying that value by 100. Further, the BAX/BCL2 ratios in the whole ovary and primordial follicles were calculated by dividing the number of pixels corresponding to positive staining of BAX by the number of pixels corresponding to positive staining of BCL2.
Statistical Analysis
Data analysis was conducted using SPSS statistical software (SPSS, Inc., Chicago, IL). Data were expressed as means and standard error of the means (SEM). Multiple comparisons between normally distributed experimental groups were made using one-way analysis of variance followed by Tukey post hoc comparison. Multiple comparisons between nonnormally distributed experimental groups were made using Kruskal-Wallis tests when appropriate. Statistical significance was assigned at P ≤ .05.
RESULTS
Effect of Acute DEHP Exposure on Breeding Outcomes at 6 Months Postdosing
Previously, we have shown that oral exposure to DEHP for 10 days disrupts folliculogenesis by accelerating primordial follicle recruitment in adult mice (Hannon et al., 2014). The depletion of quiescent primordial follicles by accelerated recruitment to the primary stage of development can have lasting effects on fertility. Thus, this study evaluated if 10-day exposure to DEHP, an exposure window known to cause defects in folliculogenesis, disrupts breeding outcomes later in life. Specifically, the mice were subjected to breeding trials at 6 months postdosing, and their fertility was assessed. Oral exposure to DEHP for 10 days did not significantly alter the time to pregnancy, the number of mice that lost a pregnancy, the number of mice that never became pregnant, the average number of pups per litter, the percentages of live and dead pups at birth, or the offspring’s male to female sex ratio when compared to the vehicle control group at the 6 months postdosing time-point (Table 1, n = 3–5/group). However, the 500 mg/kg/day dose trended toward causing a decrease in the percentage of live pups (78.05%) and increasing the percentage of dead pups (21.95%) at birth when compared to the vehicle control group, but this was not statistically significant (Table 1, n = 3–4/group). Conversely, DEHP exposure significantly increased the average pup weight at the 20 and 200 µg/kg/day doses when compared to the vehicle control group (bold values in Table 1, n = 3–4/group, P ≤ .05).
TABLE 1.
Effect of Acute DEHP Exposure on Breeding Outcomes at 6 Months Postdosing
| Treatment | Breeding Pairs | Time to Pregnancy (days) | Number of Mice that Lost Pregnancy | Number of Mice that Never Became Pregnant | Average Number of Live Pups/Litter | Percentage of Live Pups at Birth | Percentage of Dead Pups at Birth | Male to Female Ratio | Average Pup Weight (g) |
|---|---|---|---|---|---|---|---|---|---|
| Vehicle | 6 | 2.80 ± 0.58 | 1 | 0 | 10.25 ± 0.75 | 100 | 0 | 0.8 ± 0.12 | 1.63 ± 0.02 |
| 20 µg/kg/day | 6 | 2.00 ± 0.58 | 0 | 1 | 7.25 ± 2.39 | 100 | 0 | 0.97 ± 0.23 | 1.87 ± 0.03* |
| 200 µg/kg/day | 6 | 2.83 ± 0.48 | 1 | 0 | 8.50 ± 3.23 | 97.14 | 2.86 | 1.17 ± 0.10 | 1.83 ± 0.08* |
| 20 mg/kg/day | 6 | 2.83 ± 0.65 | 1 | 0 | 10.20 ± 0.80 | 100 | 0 | 1.29 ± 0.23 | 1.69 ± 0.08 |
| 200 mg/kg/day | 6 | 3.00 ± 1.29 | 1 | 0 | 9.00 ± 1.10 | 97.83 | 2.17 | 1.03 ± 0.13 | 1.75 ± 0.04 |
| 500 mg/kg/day | 4 | 3.00 ± 1.15 | 0 | 0 | 8.00 ± 3.58 | 78.05 | 21.95 | 1.33 ± 0.33 | 1.53 ± 0.06 |
Effect of Acute DEHP Exposure on Estrous Cyclicity at 6 and 9 Months Postdosing
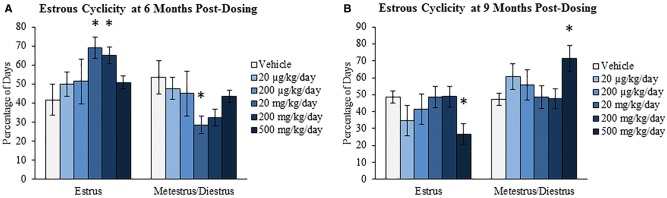
Previously, we have shown that oral exposure to DEHP for 10 days alters estrous cyclicity by increasing the percentage of days the mice were in estrus (Hannon et al., 2014). Altered estrous cyclicity can disrupt fertility and can be an indicator of altered steroidogenesis and/or a disruption in the HPO axis. Further, estrous cycles become irregular during reproductive aging. Thus, this study evaluated if the effects on estrous cyclicity following 10 days of exposure were evident later in life. Specifically, estrous cyclicity was assessed at 6 months postdosing, prior to the breeding trial, and at 9 months postdosing, prior to tissue collection. At the 6-month postdosing time-point, DEHP exposure significantly increased the percentage of days the mice were in estrus at the 20 and 200 mg/kg/day doses and significantly decreased the percentage of days the mice were in metestrus/diestrus at the 20 mg/kg/day dose when compared to the vehicle control group (Fig. 1A, n = 4–6/group, P ≤ .05). At the 9-month postdosing time-point, DEHP exposure significantly decreased the percentage of days the mice were in estrus and significantly increased the percentage of days the mice were in metestrus/diestrus at the 500 mg/kg/day dose when compared to the vehicle control group (Fig. 1B, n = 4–6/group, P ≤ 0.05).
FIG. 1.
Effect of acute di(2-ethylhexyl) phthalate (DEHP) exposure on estrous cyclicity at 6 and 9 months postdosing. Adult CD-1 mice were orally dosed with vehicle (tocopherol-stripped corn oil) or DEHP (20 µg/kg/day–500 mg/kg/day) daily for 10 days. Vaginal swabs were taken daily for 14 consecutive days prior to the breeding trial (6 months postdosing; panel A) and prior to tissue collection (9 months postdosing; panel B). Percentage of days in estrus and metestrus/diestrus were calculated and compared in each treatment group at each time-point. Graphs represent means ± SEM (n = 4–6 mice/treatment group). Asterisks (*) represent significant difference from vehicle control (P ≤ 0.05).
Effect of Acute DEHP Exposure on Serum Sex Steroid, Gonadotropin, and Peptide Hormone Levels at 9 Months Postdosing
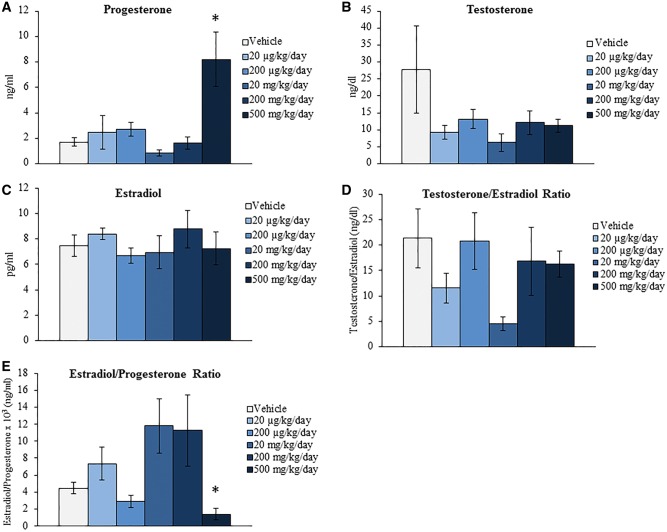
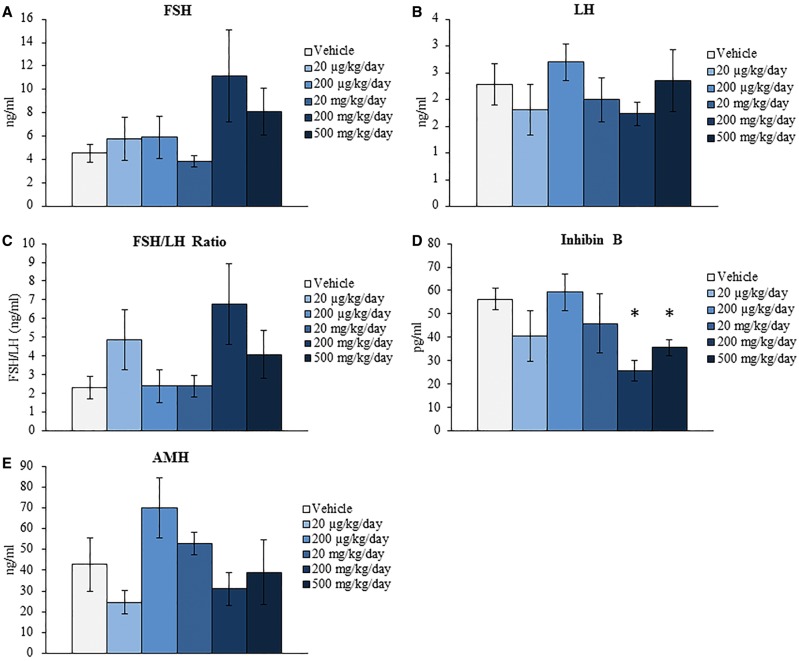
Previous studies have determined that DEHP exposure inhibits steroidogenesis in vivo (Davis et al., 1994; Hirosawa et al., 2006; Ma et al., 2011) and in cultured ovarian cell types and follicles in vitro (Gupta et al., 2010; Hannon et al., 2015b; Laskey et al., 1993; Svechnikova et al., 2007). A decrease in necessary sex steroid, gonadotropin, and peptide hormones can have adverse effects on reproductive and hormone regulated nonreproductive health. Further, the levels and ratios of these hormones begin to fluctuate in response to reproductive aging. Specifically, ovarian-derived hormones decrease and gonadotropins increase during reproductive senescence (Kevenaar et al., 2006; Nelson et al., 1987; Santoro et al., 2003). Thus, this study investigated if acute exposure to DEHP for 10 days alters the levels of hormones associated with reproductive health and aging at the 9-month postdosing time-point. Beginning with the sex steroid hormones, DEHP exposure significantly increased the serum levels of progesterone and decreased the estradiol/progesterone ratio at the 500 mg/kg/day dose when compared to the vehicle control group (Fig. 2A and E, n = 4–6/group, P ≤ 0.05). Conversely, DEHP exposure did not statistically alter the serum levels of testosterone and estradiol or the testosterone/estradiol ratio when compared to the vehicle control group (Figs. 2B–D, n = 4–6/group). When observing the gonadotropin hormone results, DEHP exposure did not statistically alter the serum levels of FSH and LH or the FSH/LH ratio when compared to the vehicle control group (Figs. 3A–C, n = 4–6/group). However, DEHP exposure significantly decreased the serum levels of the peptide hormone inhibin B at the 200 and 500 mg/kg/day doses when compared to the vehicle control group (Fig. 3D, n = 3–5/group, P ≤ 0.05) but did not statistically alter the levels of AMH at any selected dose when compared to the vehicle control group (Fig. 3E, n = 3–5/group).
FIG. 2.
Effect of acute di(2-ethylhexyl) phthalate (DEHP) exposure on serum sex steroid hormone levels at 9 months postdosing. Adult CD-1 mice were orally dosed with vehicle (tocopherol-stripped corn oil) or DEHP (20 µg/kg/day–500 mg/kg/day) daily for 10 days. Following 9 months postdosing, serum was collected, sent to the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core, and subjected to enzyme-linked immunosorbent assays (ELISAs) for the measurements of progesterone (panel A), testosterone (panel B), and estradiol (panel C). Further, the testosterone/estradiol (panel D) and estradiol/progesterone (panel E) ratios were calculated. Graphs represent means ± SEM (n = 4–6 mice/treatment group). Asterisks (*) represent significant difference from vehicle control (P ≤ 0.05).
FIG. 3.
Effect of acute di(2-ethylhexyl) phthalate (DEHP) exposure on serum gonadotropin and peptide hormone levels at 9 months postdosing. Adult CD-1 mice were orally dosed with vehicle (tocopherol-stripped corn oil) or DEHP (20 µg/kg/day-500 mg/kg/day) daily for 10 days. Following 9 months postdosing, serum was collected, sent to the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core, and subjected to enzyme-linked immunosorbent assays (ELISAs) or radioimmunoassays (RIAs) for the measurements of follicle-stimulating hormone (FSH) (panel A), luteinizing hormone (LH) (panel B), inhibin B (panel D), and AMH (panel E). Additionally, the FSH/LH ratio was calculated (panel C). Graphs represent means ± SEM (n = 3–6 mice/treatment group). Asterisks (*) represent significant difference from vehicle control (P ≤ 0.05).
Effect of Acute DEHP Exposure on Folliculogenesis at 9 Months Postdosing
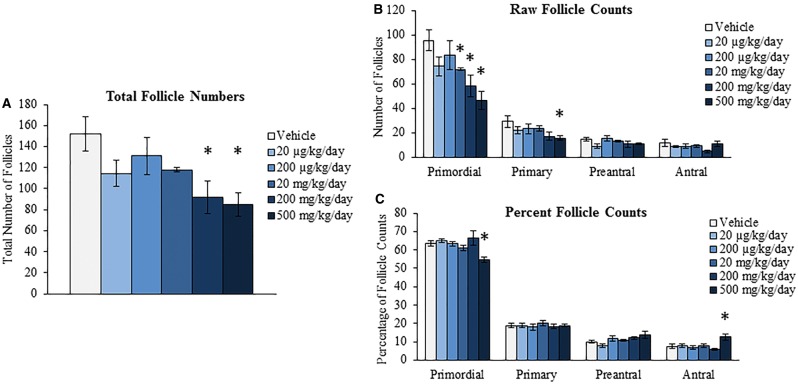
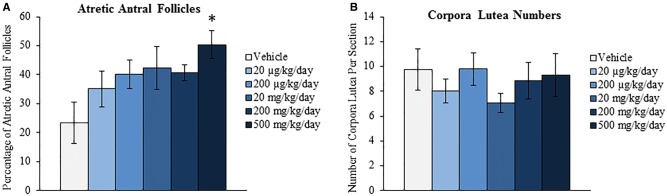
We have previously shown that oral exposure to DEHP for 10 days disrupts folliculogenesis by decreasing the number of primordial follicles and increasing the number of primary follicles (Hannon et al., 2014). Further, we showed that this acceleration of primordial follicle recruitment is a direct effect on the ovary as seen in a whole ovarian culture system (Hannon et al., 2015a). Since the primordial follicle reserve is set at birth and represents a female’s reproductive lifespan, this defect in folliculogenesis has the potential to cause infertility and premature ovarian failure. Thus, this study investigated if acute exposure to DEHP for 10 days, a time-point known to decrease primordial follicle numbers, alters folliculogenesis later in life to perhaps advance the age of reproductive senescence. DEHP exposure for 10 days significantly decreased the total number of all follicles counted at the 9-month postdosing time-point at the 200 and 500 mg/kg/day doses when compared to the vehicle control group (Fig. 4A, n = 4–6/group, P ≤ 0.05). When observing the total follicle counts per follicle type, DEHP exposure significantly decreased the number of primordial follicles counted at the 20, 200, and 500 mg/kg/day doses and decreased the number of primary follicles counted at the 500 mg/kg/day dose when compared to the vehicle control group (Fig. 4B, n = 4–6/group, P ≤ 0.05). When observing the percentages of follicles counted per follicle type, DEHP exposure significantly decreased the percentage of primordial follicles counted and increased the percentage of antral follicles counted at the 500 mg/kg/day dose when compared to the vehicle control group (Fig. 4C, n = 4–6/group, P ≤ 0.05). DEHP exposure at the 500 mg/kg/day dose also significantly increased the percentage of atretic antral follicles counted when compared to the vehicle control group (Fig. 5A, n = 4–6/group, P ≤ 0.05). On the contrary, DEHP exposure did not statistically alter the number of corpora lutea counted per section when compared to the vehicle control group (Fig. 5B, n = 4–6/group).
FIG. 4.
Effect of acute di(2-ethylhexyl) phthalate (DEHP) exposure on folliculogenesis at 9 months postdosing. Adult CD-1 mice were orally dosed with vehicle (tocopherol-stripped corn oil) or DEHP (20 µg/kg/day–500 mg/kg/day) daily for 10 days. Following 9 months postdosing, ovaries were processed for histological evaluation of follicle counts. Total follicles (panel A) and total follicles separated by stage of development (panel B) were compared in each treatment group. Further, percentages of each follicle type (panel C) were calculated and compared in each treatment group. Graphs represent means ± SEM (n = 4–6 ovaries/treatment group). Asterisks (*) represent significant difference from vehicle control (P ≤ 0.05).
FIG. 5.
Effect of acute di(2-ethylhexyl) phthalate (DEHP) exposure on atresia and corpora lutea numbers at 9 months postdosing. Adult CD-1 mice were orally dosed with vehicle (tocopherol-stripped corn oil) or DEHP (20 µg/kg/day–500 mg/kg/day) daily for 10 days. Following 9 months postdosing, ovaries were processed for histological evaluation of antral follicle atresia (panel A) and corpora lutea numbers (panel B). Graphs represent means ± SEM (n = 4–6 ovaries/treatment group). Asterisks (*) represent significant difference from vehicle control (P ≤ 0.05).
Effect of Acute DEHP Exposure on Ovarian Protein Levels of BAX and BCL2 at 9 Months Postdosing
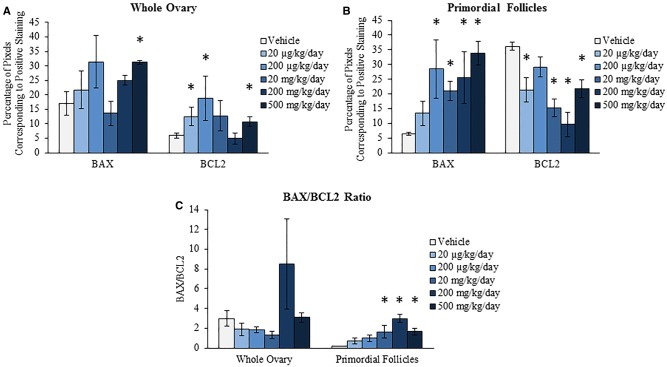
During reproductive aging, the ovary loses follicles at an accelerated rate, likely due to an increase in atresia (Faddy et al., 1992; Gougeon et al., 1994; Tatone et al., 2008). This apoptosis- and/or autophagy-induced atresia is partially mediated by the pro-apoptotic protein BAX and the antiapoptotic and antiautophagic protein BCL2 (Flaws et al., 2001; Greenfeld et al., 2007; Hsu and Hsueh, 2000; Maiuri et al., 2010; Ratts et al., 1995). This study investigated if acute exposure to DEHP alters the levels of BAX and BCL2 at the 9-month postdosing time-point, perhaps to serve as a mechanism by which 10-day DEHP exposure accelerates ovarian aging later in life. In the whole ovary, DEHP exposure significantly increased the staining of BAX at the 500 mg/kg/day dose and significantly increased the staining of BCL2 at the 20 and 200 µg/kg/day and the 500 mg/kg/day doses when compared to the vehicle control group (Fig. 6A, n = 3/group, P ≤ 0.05). In the primordial follicles, DEHP exposure significantly increased the staining of BAX at the 200 µg/kg/day and the 20, 200, and 500 mg/kg/day doses when compared to the vehicle control group (Fig. 6B, n = 3/group, P ≤ 0.05). In accordance, DEHP exposure significantly decreased the staining of BCL2 in the primordial follicles at the 20 µg/kg/day and the 20, 200, and 500 mg/kg/day doses when compared to the vehicle control group (Fig. 6B, n = 3/group, P ≤ 0.05). In contrast, DEHP exposure did not statistically alter the BAX/BCL2 ratio in the whole ovary at any selected dose when compared to the vehicle control group; however, DEHP significantly increased the BAX/BCL2 ratio in the primordial follicles at the 20, 200, and 500 mg/kg/day doses when compared to the vehicle control group (Fig. 6C, n = 3/group, P ≤ 0.05).
FIG. 6.
Effect of acute di(2-ethylhexyl) phthalate (DEHP) exposure on ovarian protein levels of BAX and BCL2 at 9 months postdosing. Adult CD-1 mice were orally dosed with vehicle (tocopherol-stripped corn oil) or DEHP (20 µg/kg/day–500 mg/kg/day) daily for 10 days. Following 9 months postdosing, ovaries were subjected to immunohistochemistry for quantification of BAX and BCL2. Percentage of pixels corresponding to positive staining in the whole ovary (panel A), percentage of pixels corresponding to positive staining in the primordial follicles (panel B), and the BAX/BCL2 ratios in the whole ovary and primordial follicles (panel C) were calculated and compared in each treatment group. Graphs represent means ± SEM (n = 3/group). Asterisks (*) represent significant difference from vehicle control (P ≤ 0.05).
DISCUSSION
This study was conducted to investigate if acute DEHP exposure for 10 days, an exposure period known to accelerate primordial follicle recruitment and disrupt estrous cyclicity (Hannon et al., 2014), has permanent effects on reproductive health later in life and/or accelerates reproductive aging. Our main findings indicate that acute exposure to DEHP at the higher doses tested affects certain reproductive parameters later in life, in that estrous cyclicity remains disrupted, ovarian sex steroid and peptide hormones are altered, and follicle numbers are decreased due to an increase in atresia.
Interestingly, acute DEHP exposure for 10 days does not significantly alter fertility outcomes at the 6-month postdosing time-point. This was surprising because previous studies have shown that DEHP accelerates recruitment of follicles and that (Hannon et al., 2014) can compromise both the development of growing follicles and the competence of the oocyte (Bristol-Gould et al., 2006; McLaughlin et al., 2014). The follicles that were prematurely developed in this study must have been cleared or are arrested in development because, aside from one mouse, all other DEHP exposed mice were able to successfully breed within a time span comparable to controls. In addition, the majority of the DEHP exposed mice were able to carry their pregnancy to term with a comparable amount of live pups at birth to the controls.
The only significant finding in the breeding trial was an increase in average pup weight at the 20 and 200 µg/kg/day doses. Although the reason for this increase in birth weight is unclear, high birth weight in newborns is associated with an increased risk of obesity, diabetes, cardiovascular disorders, and neurological disorders in the offspring (Kuchlbauer et al., 2014; Skilton et al., 2014; Wegelius et al., 2011). Future studies should address how acute DEHP exposure affects offspring birth weights later in life and whether this increased birth weight can lead to adverse health outcomes in the offspring.
Similar to the disruption in estrous cyclicity immediately following 10 days of dosing (Hannon et al., 2014), acute DEHP exposure interferes with normal cyclicity at the 6- and 9-month postdosing time-points. During reproductive aging, rodents experience a state of persistent estrus sometimes followed by complete acyclicity characterized by the presence of leukocytes (diestrus) (Gee et al., 1983; Lerner et al., 1990; Nass et al., 1984). Although mice in the 20 and 200 mg/kg/day doses appear to be in estrus longer than controls, an indicator of reproductive aging, this effect is alleviated by the 9-month postdosing time-point. At the 9-month postdosing time-point, mice in the 500 mg/kg/day appear to be in diestrus longer than controls, an indicator of complete acyclicity; however, these mice still experience some days of estrus, albeit for a shorter duration than controls. The prolonged period of time in diestrus may be indicative of a lengthened estrous cycle and/or a state of repetitive pseudopregnancy, which is a less common cycle abnormality than persistent estrus/anestrus that manifests with age (Felicio et al., 1984). Perhaps the existence of a repetitive pseudopregnant state explains why mice in the 500 mg/kg/day group have increased levels of progesterone when compared to the vehicle control group. Importantly, the estrous cyclicity data in this study suggest that acute exposure to DEHP for 10 days, an exposure window known to increase the percentage of days in estrus, can have permanent effects on cyclicity.
Acute exposure to DEHP for 10 days can also alter the levels of ovarian-derived hormones at 9-month postdosing. The most compelling hormone data in support of accelerated reproductive aging in this study are the decreased inhibin B levels at the 200 and 500 mg/kg/day doses. Inhibin B is secreted by small, growing follicles, and inhibin B levels decrease during reproductive aging (Burger 1999; MacNaughton et al., 1992). This decrease in inhibin B levels precedes the monotropic rise in FSH levels that is characteristic of later stage reproductive aging (Burger 1999; MacNaughton et al., 1992). Further, the combination of these two endocrine events is thought to reflect the declining number of primordial follicles in the ovary during aging (Santoro et al., 1999). Interestingly, the decreases in inhibin B levels observed in this study are at doses in which we observe decreased primordial follicle numbers, suggesting accelerated reproductive aging.
In contrast to our findings, previous studies have reported that reproductive aging leads to an increased estradiol/progesterone ratio (Nass et al., 1984). However, this increase in the estradiol/progesterone ratio appears to occur later in reproductive aging when the mice are in a state of persistent estrus, an observation that was not made in this study. Further, reproductive aging is characterized by an increase in FSH levels and the FSH/LH ratio, a decrease in AMH and estradiol levels (Kevenaar et al., 2006; Nelson et al., 1987; Santoro et al., 2003), and an increased testosterone/estradiol ratio (Ala-Fossi et al., 1998). These later stage reproductive aging events were not observed in this study, although there is a trend for an increase in FSH levels at the 200 and 500 mg/kg/day doses. We likely do not observe a change in AMH levels because acute DEHP exposure did not alter the number of preantral and antral follicles. Thus, it appears that the DEHP exposed mice in the 200 and 500 mg/kg/day groups with decreased inhibin B levels are exhibiting the preliminary endocrine event associated with early reproductive aging (Burger 1999; MacNaughton et al., 1992).
Acute DEHP exposure, for a period known to accelerate primordial follicle recruitment, causes permanent effects on folliculogenesis at the 9-month postdosing time-point that are consistent with reproductive aging. DEHP exposure for 10 days decreased total follicle numbers at the 200 and 500 mg/kg/day doses, and this decrease is likely attributed to the decrease in total primordial follicle numbers at the same doses. In our previous study, 10-day DEHP exposure changed the dynamics of folliculogenesis by accelerating primordial follicle recruitment rather than decreasing total follicle numbers (Hannon et al., 2014). Nonetheless, 10-day DEHP exposure did decrease primordial follicle numbers, and because these follicles are nonrenewable, it is expected that a decrease in primordial follicle numbers would be observed at 9 months postdosing if folliculogenesis did not undergo a compensatory salvation of primordial follicles. However, the rate by which these primordial follicles are lost appears to be different in the two studies. For instance, at the 200 mg/kg/day dose, DEHP exposure causes a 13.7% decrease in primordial follicle numbers compared to the controls immediately following 10 days of dosing (Hannon et al., 2014), whereas DEHP exposure causes a 38.9% decrease in primordial follicle numbers compared to the controls at the 9-month postdosing time-point. The depletion of primordial follicles leading to a decrease in total follicle numbers, in addition to the accelerated decline by which these primordial follicles are lost, is hypothesized to be the main mechanism by which women transition into menopause (Faddy et al., 1992; Gougeon et al., 1994; Tatone et al., 2008). Thus, it appears that acute DEHP exposure can accelerate reproductive aging at the level of the ovary and can have permanent effects on folliculogenesis.
The accelerated loss of follicles during reproductive aging is attributed to an increase in apoptosis- and/or autophagy-induced atresia (Faddy et al., 1992; Flaws et al., 2001; Gougeon et al., 1994; Greenfeld et al., 2007; Hsu and Hsueh, 2000; Maiuri et al., 2010; Ratts et al., 1995; Tatone et al., 2008). Acute DEHP exposure increased the levels of pro-apoptotic BAX and antiapoptotic/antiautophagic BCL2 in the whole ovary at the 9-month postdosing time-point. These changes, however, did not result in an altered BAX/BCL2 whole ovary ratio, which is the ratio used to determine susceptibility to apoptosis and atresia (Oltvai et al., 1993). Meanwhile, DEHP exposure increased the levels of BAX and decreased the levels of BCL2 in the primordial follicles, leading to an increased BAX/BCL2 ratio at the 20–500 mg/kg/day doses. Interestingly, these are the doses that deplete the primordial follicle reserve at the 9-month postdosing time-point. Although primordial follicles are already depleted at these doses at 9 months postdosing, it appears that acute DEHP exposure promotes an environment conducive for atresia or autophagy of the remaining primordial follicles. Thus, it is possible that the depleted primordial follicles were likely lost due to apoptosis-induced atresia, indicated by the BAX/BCL2 ratio, and/or autophagy-induced atresia, indicated by the decreased levels of BCL2. These findings further suggest that acute DEHP exposure can accelerate reproductive aging at the level of the ovary via a mechanism similar to that proposed for menopause (Faddy et al., 1992; Flaws et al., 2001; Gougeon et al., 1994; Greenfeld et al., 2007; Hsu and Hsueh, 2000; Maiuri et al., 2010; Ratts et al., 1995; Tatone et al., 2008).
Other effects on folliculogenesis are also observed at the 9-month postdosing time-point in response to acute DEHP exposure. The 500 mg/kg/day dose decreased total primordial and primary follicle numbers, leading to a decrease in the total follicle numbers; however, it also caused fewer follicles to be in the primordial stage and more follicles to be in the antral stage. Further, 500 mg/kg/day DEHP increased the percentage of atretic antral follicles compared to controls. This may be the reason why the BAX levels are increased in the whole ovary only at the 500 mg/kg/day dose. Thus, as is evident immediately following 10 days of dosing (Hannon et al., 2014), acute DEHP exposure at the highest selected dose shifts the dynamics of folliculogenesis, perhaps favoring accelerated maturation.
Collectively, our results indicate that 10-day exposure to DEHP in adult mice, an exposure window known to accelerate primordial follicle recruitment and increase the percentage of days in estrus (Hannon et al., 2014), has permanent effects on folliculogenesis, estrous cyclicity, and the onset of reproductive aging at 6 and 9 months postdosing. Although the DEHP exposed mice were not in complete reproductive senescence, the decrease in inhibin B levels, the alteration in estrous cyclicity, and the accelerated depletion of the primordial follicle pool is consistent with the onset of reproductive aging (Burger, 1999; Faddy et al., 1992; Gee et al., 1983; Gougeon et al., 1994; Lerner et al., 1990; MacNaughton et al., 1992; Nass et al., 1984; Tatone et al., 2008). These findings suggest that the mechanism by which acute DEHP exposure accelerates reproductive aging is that the initial follicle loss following 10 days of exposure leads to irreversible changes in the HPO axis, thus advancing the age at which follicles begin to deplete at an accelerated rate by apoptosis/autophagy. As evidence continues to emerge that DEHP exposure induces epigenetic changes, only one study has investigated DEHP-induced epigenetic modifications in the ovary (Zhang et al., 2013). Future studies should investigate if an additional mechanism of action of accelerated reproductive aging includes DEHP-induced epigenetic modifications of genes that regulate folliculogenesis. Overall, the present findings support recent epidemiological evidence showing that higher levels of DEHP metabolites are associated with an earlier age of menopause by up to 3.8 years in women (Grindler et al., 2015).
FUNDING
The National Institute of Environmental Health Sciences Grant R01ES019178 (to J.A.F.); an Interdisciplinary Environmental Toxicology Program Fellowship (to P.R.H.); the Eunice Kennedy Shriver NICHD/NIH (NCTRI) Grant P50-HD28934 (University of Virginia).
ACKNOWLEDGMENTS
The authors thank all members of Dr. Flaws’ laboratory for dosing and technical assistance. They also thank the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core for measuring serum hormone levels.
REFERENCES
- Ala-Fossi S. L., Maenpaa J., Aine R., Punnonen R. (1998). Ovarian testosterone secretion during perimenopause. Maturitas 29, 239–245. [DOI] [PubMed] [Google Scholar]
- ATSDR (2002). Toxicological Profile for Di(2-ethylhexyl)phthalate (DEHP). U.S. Department of Health and Human Services, Public Health Service, Atlanta, GA. [Google Scholar]
- Barnett K. R., Tomic D., Gupta R. K., Miller K. P., Meachum S., Paulose T., Flaws J. A. (2007). The aryl hydrocarbon receptor affects mouse ovarian follicle growth via mechanisms involving estradiol regulation and responsiveness. Biol. Reprod. 76, 1062–1070. [DOI] [PubMed] [Google Scholar]
- Bhattacharya P., Keating A. F. (2012). Impact of environmental exposures on ovarian function and role of xenobiotic metabolism during ovotoxicity. Toxicol. Appl. Pharmacol. 261, 227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristol-Gould S. K., Kreeger P. K., Selkirk C. G., Kilen S. M., Cook R. W., Kipp J. L., Shea L. D., Mayo K. E., Woodruff T. K. (2006). Postnatal regulation of germ cells by activin: The establishment of the initial follicle pool. Dev. Biol. 298, 132–148. [DOI] [PubMed] [Google Scholar]
- Burger H. G. (1999). The endocrinology of the menopause. J. Steroid Biochem. Mol. Biol. 69, 31–35. [DOI] [PubMed] [Google Scholar]
- Craig Z. R., Wang W., Flaws J. A. (2011). Endocrine-disrupting chemicals in ovarian function: Effects on steroidogenesis, metabolism and nuclear receptor signaling. Reproduction 142, 633–646. [DOI] [PubMed] [Google Scholar]
- Davis B. J., Maronpot R. R., Heindel J. J. (1994). Di-(2-ethylhexyl) phthalate suppresses estradiol and ovulation in cycling rats. Toxicol. Appl. Pharmacol. 128, 216–223. [DOI] [PubMed] [Google Scholar]
- Devine P. J., Hoyer P. B. (2005). Ovotoxic environmental chemicals: Indirect endocrine disruptors. In Endocrine Disruptors: Effects on Male and Female Reproductive Systems, 2nd ed (Naz R., Ed.), pp. 67–100. CRC Press, Boca Raton, FL. [Google Scholar]
- Doull J., Cattley R., Elcombe C., Lake B. G., Swenberg J., Wilkinson C., Williams G., van Gemert M. (1999). A cancer risk assessment of di(2-ethylhexyl)phthalate: Application of the new U.S. EPA Risk Assessment Guidelines. Regul. Toxicol. Pharmacol. 29, 327–357. [DOI] [PubMed] [Google Scholar]
- Faddy M. J., Gosden R. G., Gougeon A., Richardson S. J., Nelson J. F. (1992). Accelerated disappearance of ovarian follicles in mid-life: Implications for forecasting menopause. Hum. Reprod. 7, 1342–1346. [DOI] [PubMed] [Google Scholar]
- FDA (2001). Safety assessment of di(2-ethylhexyl)phthalate (DEHP) released from PVC medical devices. Cent. Devices Radiol. Health. http://www.fda.gov/downloads/MedicalDevices/…/UCM080457.pdf. Accessed January 8, 2013. [Google Scholar]
- Felicio L. S., Nelson J. F., Finch C. E. (1984). Longitudinal studies of estrous cyclicity in aging C57BL/6J mice: II. Cessation of cyclicity and the duration of persistent vaginal cornification. Biol. Reprod. 31, 446–453. [DOI] [PubMed] [Google Scholar]
- Flaws J. A., Doerr J. K., Sipes I. G., Hoyer P. B. (1994). Destruction of preantral follicles in adult rats by 4-vinyl-1-cyclohexene diepoxide. Reprod.Toxicol. 8, 509–514. [DOI] [PubMed] [Google Scholar]
- Flaws J. A., Hirshfield A. N., Hewitt J. A., Babus J. K., Furth P. A. (2001). Effect of bcl-2 on the primordial follicle endowment in the mouse ovary. Biol. Reprod. 64, 1153–1159. [DOI] [PubMed] [Google Scholar]
- Gee D. M., Flurkey K., Finch C. E. (1983). Aging and the regulation of luteinizing hormone in C57BL/6J mice: Impaired elevations after ovariectomy and spontaneous elevations at advanced ages. Biol. Reprod. 28, 598–607. [DOI] [PubMed] [Google Scholar]
- Gougeon A., Ecochard R., Thalabard J. C. (1994). Age-related changes of the population of human ovarian follicles: Increase in the disappearance rate of non-growing and early-growing follicles in aging women. Biol. Reprod. 50, 653–663. [DOI] [PubMed] [Google Scholar]
- Greenfeld C. R., Pepling M. E., Babus J. K., Furth P. A., Flaws J. A. (2007). BAX regulates follicular endowment in mice. Reproduction 133, 865–876. [DOI] [PubMed] [Google Scholar]
- Grindler N. M., Allsworth J. E., Macones G. A., Kannan K., Roehl K. A., Cooper A. R. (2015). Persistent organic pollutants and early menopause in U.S. Women. PLoS One 10, e0116057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. K., Singh J. M., Leslie T. C., Meachum S., Flaws J. A., Yao H. H. (2010). Di-(2-ethylhexyl) phthalate and mono-(2-ethylhexyl) phthalate inhibit growth and reduce estradiol levels of antral follicles in vitro. Toxicol. Appl. Pharmacol. 242, 224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon P., Flaws J. A. (2015). The effects of phthalates on the ovary. Front. Endocrinol. 6(8):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon P. R., Brannick K. E., Wang W., Flaws J. A. (2015a). Mono(2-ethylhexyl) phthalate accelerates early folliculogenesis and inhibits steroidogenesis in cultured mouse whole ovaries and antral follicles. Biol. Reprod. 92, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon P. R., Brannick K. E., Wang W., Gupta R. K., Flaws J. A. (2015b). Di(2-ethylhexyl) phthalate inhibits antral follicle growth, induces atresia, and inhibits steroid hormone production in cultured mouse antral follicles. Toxicol. Appl. Pharmacol. 284, 42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon P. R., Peretz J., Flaws J. A. (2014). Daily exposure to di(2-ethylhexyl) phthalate alters estrous cyclicity and accelerates primordial follicle recruitment potentially via dysregulation of the phosphatidylinositol 3-kinase signaling pathway in adult mice. Biol. Reprod. 90, 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman C. G. (1944). Some new observations on the vaginal smear of the rat. Yale J. Biol. Med. 17, 99–112. [PMC free article] [PubMed] [Google Scholar]
- Heudorf U., Mersch-Sundermann V., Angerer J. (2007). Phthalates: Toxicology and exposure. Int. J. Hyg. Environ. Health 210, 623–634. [DOI] [PubMed] [Google Scholar]
- Hirosawa N., Yano K., Suzuki Y., Sakamoto Y. (2006). Endocrine disrupting effect of di-(2-ethylhexyl)phthalate on female rats and proteome analyses of their pituitaries. Proteomics 6, 958–971. [DOI] [PubMed] [Google Scholar]
- Hogberg J., Hanberg A., Berglund M., Skerfving S., Remberger M., Calafat A. M., Filipsson A. F., Jansson B., Johansson N., Appelgren M., et al. (2008). Phthalate diesters and their metabolites in human breast milk, blood or serum, and urine as biomarkers of exposure in vulnerable populations. Environ. Health Perspect. 116, 334–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer P. B., Sipes I. G. (1996). Assessment of follicle destruction in chemical-induced ovarian toxicity. Ann. Rev. Pharmacol. Toxicol. 36, 307–331. [DOI] [PubMed] [Google Scholar]
- Hsu S. Y., Hsueh A. J. (2000). Tissue-specific Bcl-2 protein partners in apoptosis: An ovarian paradigm. Physiol. Rev. 80, 593–614. [DOI] [PubMed] [Google Scholar]
- Kato K., Silva M. J., Reidy J. A., Hurtz D., III, Malek N. A., Needham L. L., Nakazawa H., Barr D. B., Calafat A. M. (2004). Mono(2-ethyl-5-hydroxyhexyl) phthalate and mono-(2-ethyl-5-oxohexyl) phthalate as biomarkers for human exposure assessment to di-(2-ethylhexyl) phthalate. Environ. Health Perspect. 112, 327–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavlock R., Boekelheide K., Chapin R., Cunningham M., Faustman E., Foster P., Golub M., Henderson R., Hinberg I., Little R., et al. (2002). NTP Center for the Evaluation of Risks to Human Reproduction: Phthalates expert panel report on the reproductive and developmental toxicity of di(2-ethylhexyl) phthalate. Reprod. Toxicol. 16, 529–653. [DOI] [PubMed] [Google Scholar]
- Kevenaar M. E., Meerasahib M. F., Kramer P., van de Lang-Born B. M., de Jong F. H., Groome N. P., Themmen A. P., Visser J. A. (2006). Serum anti-Mullerian hormone levels reflect the size of the primordial follicle pool in mice. Endocrinology 147, 3228–3234. [DOI] [PubMed] [Google Scholar]
- Krotz S. P., Carson S. A., Tomey C., Buster J. E. (2012). Phthalates and bisphenol do not accumulate in human follicular fluid. J. Assisted Reprod. Genet. 29, 773–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchlbauer V., Vogel M., Gausche R., Kapellen T., Rothe U., Vogel C., Pfaffle R., Kiess W. (2014). High birth weights but not excessive weight gain prior to manifestation are related to earlier onset of diabetes in childhood: ‘Accelerator hypothesis’ revisited. Pediatr. Diabetes 15, 428–435. [DOI] [PubMed] [Google Scholar]
- Laskey J. W., Berman E. (1993). Steroidogenic assessment using ovary culture in cycling rats: Effects of bis(2-diethylhexyl)phthalate on ovarian steroid production. Reprod. Toxicol. 7, 25–33. [DOI] [PubMed] [Google Scholar]
- Latini G., De Felice C., Presta G., Del Vecchio A., Paris I., Ruggieri F., Mazzeo P. (2003). Exposure to di(2-ethylhexyl)phthalate in humans during pregnancy. A preliminary report. Biol. Neonate 83, 22–24, 67012. [DOI] [PubMed] [Google Scholar]
- Lerner S. P., Meredith S., Thayne W. V., Butcher R. L. (1990). Age-related alterations in follicular development and hormonal profiles in rats with 4-day estrous cycles. Biol. Reprod. 42, 633–638. [DOI] [PubMed] [Google Scholar]
- Ma M., Zhang Y., Pei X., Duan Z. (2011). [Effects of di-(2-ethylhexyl) phthalate exposure on reproductive development and PPARs in prepubertal female rats]. Wei sheng yan jiu = J. Hyg. Res. 40, 688–692, 697. [PubMed] [Google Scholar]
- MacNaughton J., Banah M., McCloud P., Hee J., Burger H. (1992). Age related changes in follicle stimulating hormone, luteinizing hormone, oestradiol and immunoreactive inhibin in women of reproductive age. Clin. Endocrinol. 36, 339–345. [DOI] [PubMed] [Google Scholar]
- Maiuri M. C., Criollo A., Kroemer G. (2010). Crosstalk between apoptosis and autophagy within the Beclin 1 interactome. EMBO J. 29, 515–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters R. A., Crean B. D., Yan W., Moss A. G., Ryan P. L., Wiley A. A., Bagnell C. A., Bartol F. F. (2007). Neonatal porcine endometrial development and epithelial proliferation affected by age and exposure to estrogen and relaxin. Domest. Anim. Endocrinol. 33, 335–346. [DOI] [PubMed] [Google Scholar]
- McLaughlin M., Kinnell H. L., Anderson R. A., Telfer E. E. (2014). Inhibition of phosphatase and tensin homologue (PTEN) in human ovary in vitro results in increased activation of primordial follicles but compromises development of growing follicles. Mol. Hum. Reprod. 20, 736–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menken J., Trussell J., Larsen U. (1986). Age and infertility. Science 233, 1389–1394. [DOI] [PubMed] [Google Scholar]
- Nass T. E., LaPolt P. S., Judd H. L., Lu J. K. (1984). Alterations in ovarian steroid and gonadotrophin secretion preceding the cessation of regular oestrous cycles in ageing female rats. J. Endocrinol. 100, 43–50. [DOI] [PubMed] [Google Scholar]
- Nelson J. F., Bergman M. D., Karelus K., Felicio L. S. (1987). Aging of the hypothalamo-pituitary-ovarian axis: Hormonal influences and cellular mechanisms. J. Steroid Biochem. 27, 699–705. [DOI] [PubMed] [Google Scholar]
- NTP. (2000). NTP-CERHR expert panel report on di(2-ethylhexyl)phthalate. NTP-CERHR-DEHP-00. U.S. Department of Health and Human Services, National Toxicology Program, Center for the Evaluation of Risks to Human Reproduction. http://cerhr.niehs.nih.gov/news/phthalates/DEHP-final.pdf. Accessed January 8, 2013. [Google Scholar]
- Oltvai Z. N., Milliman C. L., Korsmeyer S. J. (1993). Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74, 609–619. [DOI] [PubMed] [Google Scholar]
- Paulose T., Hernandez-Ochoa I., Basavarajappa M. S., Peretz J., Flaws J. A. (2011). Increased sensitivity of estrogen receptor alpha overexpressing antral follicles to methoxychlor and its metabolites. Toxicol. Sci. 120, 447–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratts V. S., Flaws J. A., Kolp R., Sorenson C. M., Tilly J. L. (1995). Ablation of bcl-2 gene expression decreases the numbers of oocytes and primordial follicles established in the post-natal female mouse gonad. Endocrinology 136, 3665–3668. [DOI] [PubMed] [Google Scholar]
- Rodrigues P., Limback D., McGinnis L. K., Plancha C. E., Albertini D. F. (2009). Multiple mechanisms of germ cell loss in the perinatal mouse ovary. Reproduction 137, 709–720. [DOI] [PubMed] [Google Scholar]
- Santoro N., Adel T., Skurnick J. H. (1999). Decreased inhibin tone and increased activin A secretion characterize reproductive aging in women. Fertil. Steril. 71, 658–662. [DOI] [PubMed] [Google Scholar]
- Santoro N., Isaac B., Neal-Perry G., Adel T., Weingart L., Nussbaum A., Thakur S., Jinnai H., Khosla N., Barad D. (2003). Impaired folliculogenesis and ovulation in older reproductive aged women. J. Clin. Endocrinol. Metab. 88, 5502–5509. [DOI] [PubMed] [Google Scholar]
- Silva M. J., Reidy J. A., Herbert A. R., Preau J. L., Jr, Needham L. L., Calafat A. M. (2004). Detection of phthalate metabolites in human amniotic fluid. Bull. Environ. Contam. Toxicol. 72, 1226–1231. [DOI] [PubMed] [Google Scholar]
- Skilton M. R., Siitonen N., Wurtz P., Viikari J. S., Juonala M., Seppala I., Laitinen T., Lehtimaki T., Taittonen L., Kahonen M., et al. (2014). High birth weight is associated with obesity and increased carotid wall thickness in young adults: The cardiovascular risk in young Finns study. Arterioscler. Thromb. Vasc. Biol. 34, 1064–1068. [DOI] [PubMed] [Google Scholar]
- Svechnikova I., Svechnikov K., Soder O. (2007). The influence of di-(2-ethylhexyl) phthalate on steroidogenesis by the ovarian granulosa cells of immature female rats. J. Endocrinol. 194, 603–609. [DOI] [PubMed] [Google Scholar]
- Tatone C., Amicarelli F., Carbone M. C., Monteleone P., Caserta D., Marci R., Artini P. G., Piomboni P., Focarelli R. (2008). Cellular and molecular aspects of ovarian follicle ageing. Hum. Reprod. Update 14, 131–142. [DOI] [PubMed] [Google Scholar]
- Tingen C. M., Bristol-Gould S. K., Kiesewetter S. E., Wellington J. T., Shea L., Woodruff T. K. (2009). Prepubertal primordial follicle loss in mice is not due to classical apoptotic pathways. Biol. Reprod. 81, 16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegelius A., Tuulio-Henriksson A., Pankakoski M., Haukka J., Lehto U., Paunio T., Lonnqvist J., Suvisaari J. (2011). An association between high birth weight and schizophrenia in a Finnish Schizophrenia family study sample. Psychiatry Res. 190, 181–186. [DOI] [PubMed] [Google Scholar]
- Wierman M. E. (2007). Sex steroid effects at target tissues: Mechanisms of action. Adv. Physiol. Educ. 31, 26–33. [DOI] [PubMed] [Google Scholar]
- Zhang X. F., Zhang L. J., Li L., Feng Y. N., Chen B., Ma J. M., Huynh E., Shi Q. H., De Felici M., Shen W. (2013). Diethylhexyl phthalate exposure impairs follicular development and affects oocyte maturation in the mouse. Environ. Mol. Mutagen. 54, 354–361. [DOI] [PubMed] [Google Scholar]