Abstract
To assess chemical toxicity, current high throughput screening (HTS) assays rely primarily on in vitro measurements using cultured cells. Responses frequently differ from in vivo results due to the lack of physical and humoral interactions provided by the extracellular matrix, cell-cell interactions, and other molecular components of the native organ. To more accurately reproduce organ complexity in HTS, we developed an organotypic assay using the cryopreserved precision cut lung slice (PCLS) from rats and mice. Compared to the never-frozen PCLS, their frozen-thawed counterpart slices showed viability or metabolic activity that is decreased to an extent comparable to that observed in other cryopreserved cells and tissues, but shows no differences in further changes in cell viability, mitochondrial integrity, and glutathione activity in response to the model toxin zinc chloride (ZnCl2). Notably, these measurements were successfully miniaturized so as to establish HTS capacity in a 96-well plate format. Finally, PCLS responses correlated with common markers of lung injury measured in lavage fluid from rats intratracheally instilled with ZnCl2. In summary, we establish that the cryopreserved PCLS is a feasible approach for HTS investigations in predictive toxicology.
Key words: precision cut lung slices, toxicology, zinc chloride, cryopreservation, high throughput screening, organotypic
As a result of industrial breakthroughs and growing technology, we are surrounded by many novel chemicals and materials. To screen for their toxic potential, current measurements are typically performed using the cultured cell or the living animal. The cultured cell allows direct and unambiguous measurements of biological endpoints and is well suited for high throughput screening (HTS) assays. Indeed, multiplexing or utilizing different detection reagents in tandem has enhanced the simultaneous detection of multiple markers of biological processes. However, the lack of physical and humoral interactions provided by the extracellular matrix, cell-cell interactions, and other molecular components of the intact organ may underestimate or overestimate biological responses (Godoy et al., 2013). This underscores the need for alternative testing (McKim, 2010). To this end, “organ-on-a-chip” approaches are emerging (Ahmad et al., 2014; Bhise et al., 2014; Huh et al., 2010) but are still limited to a small number of interacting cells. The living animal more accurately reproduces organ complexity but inference of toxicological responses is complex and sometimes ambiguous. Moreover, animal testing is time-consuming, expensive, and is limited by ethical concerns (de Kanter et al., 2002). For these reasons, animal studies are increasingly curtailed in toxicological research (Morin et al., 2013).
To fill the gap between cell-based HTS assays and the intact animal, we describe here an assay using the cryopreserved precision cut lung slice (PCLS) with HTS potential. PCLS are viable, functional, and retain nearly all lung cell types (Rosner et al., 2014; Sanderson, 2011). The PCLS is well suited to assess toxicity of chemical allergens (Henjakovic et al., 2008). In addition, the PCLS has been used to examine airway responsiveness to biological toxins such as fungal metabolites (Balenga et al., 2015) as well as to evaluate the potential hazards of nanomaterials such as TiO2, ZnO, CeO2, SiO2, and nanosilver (Sauer et al., 2014) and the anticancer drug 2-[4-amino-3-methylphenyl]-5-fluorobenzothiazole lysylamide dihydrochloride (Behrsing et al., 2013). Despite its advantages, adoption of the PCLS in toxicology has been constrained by the limited time window within which the PCLS stays viable, which is typically 2–5 days from the time of harvest.
To overcome time-limited PCLS viability, we and others have developed the approach of cryopreservation (Bai et al., 2015; Rosner et al., 2014). This approach offers practical advantages in planning and execution of experiments as well as enables expanded use for HTS applications. Here, using cryopreservation, we established mouse and rat PCLS as suitable models for HTS screening in predictive toxicology. As our model toxin, we chose a well-characterized chemical with known mechanisms of lung toxicity, ZnCl2. For comparison, we also used 2 distinct cell lines commonly employed in toxicity screening applications. Primary human small airway epithelial (SAEC) and the human monocytic cell line (THP-1) were chosen due to their relevance in pulmonary exposures to toxins. Finally, we correlated the rat PCLS data with common markers of lung injury measured in lavage fluid from ZnCl2-exposed rats.
MATERIALS AND METHODS
Preparation of mouse and rat PCLS
PCLS were prepared from male 8-week old C57BL/6 mice and 9-week old Wistar Han rats. Animals were humanely killed with 4% isoflurane followed by exsanguination. A skin incision was performed on the ventral side of the neck, and the trachea was exposed. A small incision was made on the trachea to allow insertion of an 18G catheter (mice) or a 16G catheter (rat). Once the chest and abdominal cavities were opened, the collapsed lung lobes were infused with 1.5 ml (mouse) or 11 ml (rat) of warmed (37°C) 1.5% agarose in Hanks’ balanced salt solution (HBSS) (Life Technologies, Carlsbad, California) through the inserted catheter. The agarose infusion was followed by 0.5 ml (mouse) and 2 ml (rat) of air to clear the airways of agarose. The whole mouse/rat was placed at 4°C for 10–15 min to allow the agarose to solidify. The lungs were then carefully dissected out of the chest cavity and kept in cold HBSS prior to slicing.
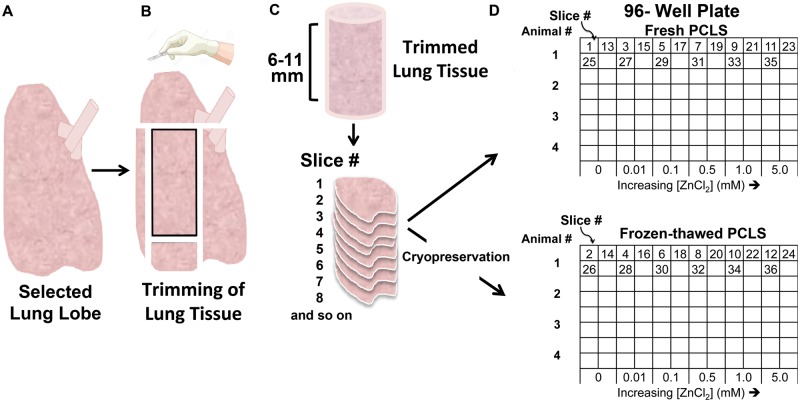
The protocol developed for PCLS preparation is illustrated in Figure 1. For this study, we used a designated lobe for each assay (rat: left lobe for all assays; mouse: left lobe for MTS, and right lobes for GSH and TMRE) to minimize the potential contribution of lobar differences in ZnCl2 response in each assay. The selected lobes were separated and trimmed such that tissue slabs (6–8 mm for mice, 8–11 mm for rats) from each lobe were created. These slabs were then sectioned into 24 (mouse) or 42 (rat) slices of 250 µm thickness using a VF-300 tissue slicer (Precisionary Instruments, Greenville, North Carolina). The lung slices were sorted serially during the slicing process to ensure that every exposure group consisted of adjacent PCLS from throughout (distal, middle, and proximal regions) the slab (Figure 1). Additionally, each pair of adjacent PCLS was sorted such that 1 slice of the pair was assigned to the “fresh” and the other to the “frozen-thawed” groups (Figure 1). After slicing, the sorted PCLS were incubated (3 PCLS per well in a 24-well plate) overnight in Dulbecco’s modified Eagle/Ham’s F-12 Medium (Corning, Corning, New York) supplemented with 0.05 mg/ml Kanamycin (Sigma Aldrich, St. Louis, MO), 1% Gibco Antibiotic-Antimycotic, containing 10 000 units/ml of penicillin, 10 mg/ml of streptomycin, and 25 µg/ml of Amphotericin B (Life Technologies) at 37°C. The PCLS designated for cryopreservation were placed in separate cryovials containing 10% dimethyl sulfoxide (DMSO) (Sigma Aldrich, St. Louis, Missouri) diluted in media and stored at −80°C (Mr. Frosty Freezing Container, Thermo Scientific, Tewksbury, Massachusetts). DMSO was employed to inhibit crystal formation during the freezing process, which can reduce viability.
FIG. 1.
Standardized protocol for precision cut lung slice (PCLS) preparation. A, The agarose-infused left lung lobe was removed. B, The left lung lobe was then trimmed. C, A slab of lung tissue was sliced using a vibratome. Consecutive lung slices were generated from the trimmed slab. D, Sequential slices were assigned alternately to each experimental condition as shown. Complementary PCLS were cryopreserved in cryovials prior to experimentation.
Determination of metabolic activity and competency of frozen-thawed PCLS
Frozen PCLS were thawed and used within 2–3 days after freezing. On the day of each experiment, the frozen PCLS were quickly thawed in a 37°C water bath, and then placed in a 24-well plate containing the same DMEM/F12 media as described above. The thawed PCLS were rinsed twice and were allowed to recover in the incubator for 0, 2, and 6 h in working media consisting of 0.5% amphotericin B and 1% Gibco Antibiotic-Antimycotic and kanamycin. After each recovery period, the PCLS were tested for metabolic activity using CellTiter 96 AQueous One Solution Reagent (MTS). MTS contains a tetrazolium compound [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] and an electron coupling reagent, phenazine ethosulfate, which is reduced in the cytoplasm and on the plasma membrane providing an overall assessment of metabolic health or activity (Berridge et al., 2005). We also measured mitochondrial membrane potential using tetramethylrhodamine ethyl esther perchlorate (TMRE) assay (Life Technologies). TMRE is a cell-permeable, positively charged dye that accumulates in active and polarized mitochondria due to the negative charge maintained within functional mitochondrial membranes. Depolarized or damaged mitochondria retain less dye and generate less fluorescence signal.
To further assess whether frozen-thawed PCLS retained their metabolic competency, we tested for the levels of cytochrome p450 2B1 protein with an enzyme linked immunosorbent assay (ELISA) (US Biological, Salem, Massachusetts) and glutathione S-transferase (GST) activity using the GST activity assay (Sigma Aldrich) of supernatants obtained from multiple freeze-thaws of PCLS in PBS containing 2 mM EDTA.
Cell culture and exposure to ZnCl2
SAEC were purchased from Lonza (Walkersville, Maryland) and cultured in complete small airway epithelial growth media (SAGM) (Lonza, Walkersville, Maryland) and incubated at 37°C and 5% CO2 atmosphere. SAEC were subcultured in 96-well plates at a cell density of 600 cells/well in SAGM for a period of 3-4 days or until 80%–90% confluence. Then, SAEC were serum starved for 24 h before treatment with 0.01, 0.1, 0.5, 1, 5 mM ZnCl2 diluted in SAGM media for 4 h. Zinc chloride (ZnCl2) was obtained from Mallinckrodt Chemicals, (St.Louis, Missouri). Human monocytic cell line (THP-1) was purchased from American Type Culture Collection (ATCC, Manassas, Virginia) and cultured in RPMI-1640 (MediaTech, Herndon, Virginia) supplemented with 10% heat inactivated fetal bovine serum (ATCC) and incubated at 37°C and 5% CO2. THP-1 cells were subcultured in 96-well plates at a cell density 1 × 104 cells/well in complete RPMI media supplemented with PMA (200 nM) and allowed to mature for 4–5 days. After maturation, THP-1 cells were washed once with PBS and were allowed to recover for 4 days before exposure to ZnCl2 in RPMI media (100 µl) for 4 h.
PCLS culture and exposure to ZnCl2
ZnCl2 solutions were prepared in culture DMEM/F12 1:1 media at 0, 0.01, 0.1, 0.5, 1, and 5 mM concentrations. One PCLS was placed in each well of a 96-well plate and submerged in 200 µl of ZnCl2 media solution. Media was used as negative controls. As positive controls, 0.01% Triton X-100 was used for the MTS assay and carbonyl cyanide p-trifluoro-methoxyphenyl hydrazine (FCCP) (1 µM) for the TMRE assay. Each exposure group consisted of a triplicate PCLS for rats (3 PCLS per rat, 6 rats) and for mice (3 slices per mouse, 6 mice).
Assessment of cell viability
Cell and PCLS viability was measured using the Cell Titer 96 AQueous One Solution Reagent (MTS) (Promega, Madison, WI). After a 4-h incubation period with ZnCl2, PCLS were washed twice in a 6-well plate with 2 ml of phosphate-buffered saline containing Ca++ and Mg++ (Mediatech, Corning, New York). Washed PCLS were placed in a new 96-well plate containing 200 µl/well of media with MTS reagent. Plates were incubated at 37°C for 1 h. Absorbance (490 nm) was measured on the supernatants using the SpectraMax M5 microplate reader (Molecular Devices, Sunnyvale, California).
Mitochondrial membrane potential (ΔΨm)
Mitochondrial membrane potential of cells and PCLS was measured using the TMRE assay (Life Technologies). After incubation with ZnCl2, cells and PCLS were washed twice in PBS and incubated in media with TMRE (200 µM for slices and 200 nM for cells) at 37°C for 1 h. Cells and PCLS were washed twice with PBS before obtaining fluorescence measurements in a microplate reader (SpectraMax M5, Molecular Devices) (peak excitation = 549 nm, peak emission = 575 nm). The auto fluorescence of unstained cells and PCLS was measured then subtracted from the raw data.
Measurement of oxidative stress
The induction of oxidative stress was measured by evaluating changes in the cellular content of glutathione (GSH), an important cellular antioxidant. To measure intracellular GSH, we used the GSH-Glo Glutathione Assay (Promega, Madison, Wisconsin), a chemiluminescence-based assay. The reactive oxygen species induced by ZnCl2 treatment decrease glutathione levels by oxidation or by reaction of constituent thiol groups within glutathione. ZnCl2-exposed cells were washed with PBS, then GSH-Glo Reagent 1X (100 µl/well) was added and incubated at room temperature for 30 min. Then, luciferin detection reagent (100 µl/well) was added, followed by 15 min incubation. The protocol for PCLS included 3 cycles of freeze-thaws in 200 µl PBS containing 2 mM EDTA. The PCLS were frozen at −80°C, thawed at room temperature then centrifuged at 16 000 × g at 4°C for 20 min. The supernatants (50 µl) were collected and added to individual wells containing 50 µl of GSH-Glo Reagent twice, then incubated for 30 min at room temperature. Next, 100 µl luciferin detection reagent was added followed by a 15-min incubation. Luminescence was measured immediately using SpectraMax M5 microplate reader.
Pulmonary responses to intratracheally instilled ZnCl2 in vivo
The protocols used in this experiment were approved by the Harvard Medical Area Animal Care and Use Committee. Nine-week-old male CD rats were purchased from Harlan Laboratories (South Easton, Massachusetts). Rats were housed in pairs in polypropylene cages and allowed to acclimatize for 1 week before the studies were initiated. Rats were maintained on a 12-h light/dark cycle. Food and water were provided ad libitum. A group of rats (mean wt. 265 ± 21 g) was intratracheally instilled with ZnCl2 solution at a 0, 0.08, 0.4, or 2.0 mg/kg ZnCl2 dose. The doses were chosen to encompass the range from no-response to maximum responses. For in vivo experiments, a maximum dose of 2 mg/kg was used in careful consideration of its impact on rat respiratory function. The ZnCl2 solution was delivered to the lungs through the trachea in a volume of 1.5 ml/kg. Twenty-four hours later, rats were euthanized via exsanguination with a cut in the abdominal aorta while under anesthesia. The trachea was exposed and cannulated. The lungs were then lavaged 12 times, with 3 ml of 0.9% sterile PBS, without calcium and magnesium ions. The cells of all washes were separated from the supernatant by centrifugation (350 × g at 4°C for 10 min). Total cell count and hemoglobin measurements were made from the cell pellets. After staining the cells, a differential cell count was performed. The supernatant of the two first washes was clarified via centrifugation (14 500 × g at 4°C for 30 min), and used for standard spectrophotometric assays for lactate dehydrogenase (LDH), myeloperoxidase (MPO), and albumin. Total GSH was also measured in clarified lavage supernatants obtained from a separate cohort of rats intratracheally instilled with ZnCl2 at 0, 0.05, and 0.5 mg/kg dose (n = 3 rats/dose).
RESULTS
Metabolic Activity and Competency of Frozen-Thawed PCLS
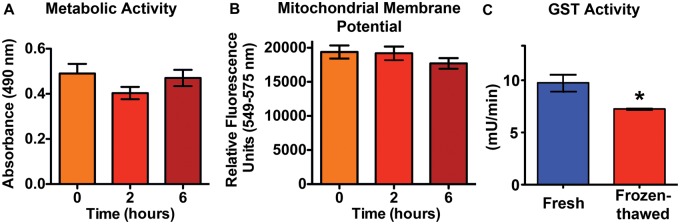
As shown in Figure 2, there were no significant differences in either metabolic activity (2A) or mitochondrial membrane potential (2B) in mouse PCLS at 0, 2, or 6 h after thawing. Based on these data, we adopted a 2-h postthawing recovery period in our mouse and rat PCLS experiments. This recovery period was also found optimal for cryopreserved PCLS in our previous study (Rosner et al., 2014). The metabolic competency of rat frozen-thawed PCLS were evaluated by measuring cytochrome p450 2B1 protein levels after the standard 2-h recovery period. The protein levels of cytochrome p450 2B1, a Phase 1 enzyme in xenobiotic detoxification pathways, were significantly different between rat fresh (5.2 ng/ml) and frozen-thawed PCLS (8.6 ng/ml) (Supplementary Figure 1). A phase 2 enzyme, glutathione-S-transferase, was lower in frozen-thawed versus fresh rat PCLS (Figure 2C).
FIG. 2.
Viability and metabolic competency are maintained in cryopreserved PCLS. A, Metabolic activity of frozen-thawed mouse PCLS measured by the MTS assay at 0, 2, and 6 h postthawing were not significantly different. B, There was also no significant change in mitochondrial membrane potential. Data are mean ± SE. Each exposure time consisted of a total of 12 PCLS (3 slices per mouse). C, GST activity was higher in fresh PCLS in comparison to frozen-thawed rat PCLS. Data are mean ± SE. n = 6 PCLS (2 slices per rat) (*P < .05, Student t test).
PCLS Response to ZnCl2: Comparison of Fresh and Frozen-Thawed PCLS
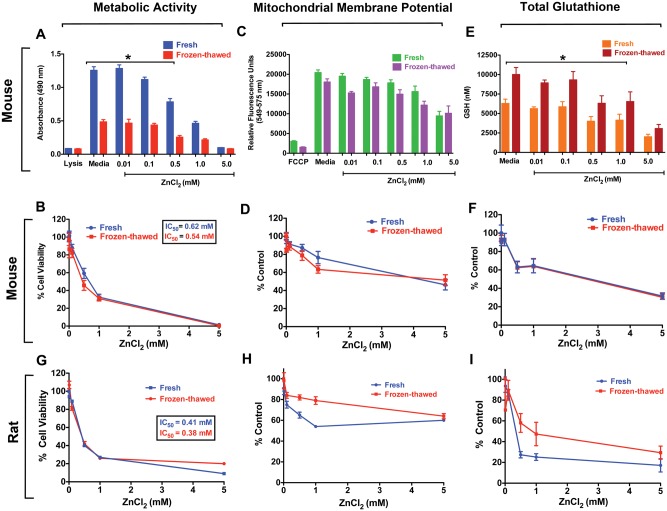
The cryopreserved mouse PCLS showed lower initial metabolic activity compared to their fresh counterpart slices (Figure 3A). However, when expressed as percentage of media control, the slopes were nearly identical, indicating that their responses to ZnCl2 were similar (Figure 3B). The IC50 were 0.54 and 0.62 mM for frozen-thawed PCLS and fresh PCLS, respectively. The mitochondrial membrane potential values were lower in frozen-thawed than fresh PCLS (Figure 3C), but also declined similarly in response to increasing concentrations of ZnCl2 when data were normalized (Figure 3D). Interestingly, we observed higher glutathione levels in frozen-thawed PCLS than in fresh PCLS (Figure 3E). Although there were significant differences in basal glutathione levels, the normalized data showed identical declines in response to ZnCl2 (Figure 3F).
FIG. 3.
Cryopreservation of PCLS does not alter responses to ZnCl2. A, As measured by the MTS, there was a difference in the raw absorbance values between fresh and frozen-thawed PCLS in response to ZnCl2 (0.01–5 mM). B, Normalized data showed identical dose-response between fresh and frozen-thawed mouse PCLS. C, Mitochondrial membrane potential assessed by TMRE assay revealed no significant differences between fresh and frozen-thawed mouse PCLS. D, Normalized data showed similar dose-response between fresh and frozen-thawed mouse PCLS in mitochondrial function. E, Fresh PCLS had lower glutathione levels than frozen-thawed PCLS. F, Normalized data showed identical dose-response between fresh and frozen-thawed mouse PCLS in glutathione levels. G, Metabolic activity of rat fresh and frozen-thawed PCLS both revealed similar reductions with increasing ZnCl2 concentrations. H, The mitochondrial membrane potential was reduced in both fresh and frozen-thawed rat PCLS. I, Significant reduction in total glutathione levels in both fresh and frozen-thawed rat PCLS was observed in response to ZnCl2. Positive controls for the MTS assay were 0.01% Triton X-100 and carbonyl cyanide-4-(trifluoromethoxy) phenyl hydrazone (FCCP) (1 µM), a potent ATP synthesis disruptor, for the TMRE assay. Data are mean ± SE. Each exposure group consisted of a total of 18 PCLS (3 PCLS per rat, 6 rats) and 18 PCLS (3 slices per mouse, 6 mice). (*P < .05, ANOVA followed by Bonferroni’s post hoc test).
The frozen-thawed rat PCLS also showed lower metabolic activity and mitochondrial membrane potential than fresh PCLS (Supplementary Figures 2A and 2B). However, normalized MTS data showed similar dose-response relationships (Figure 3G). We found that rat PCLS also showed ZnCl2 dose-dependent decrease in mitochondrial membrane potential (Figure 3H). Interestingly, normalized data from fresh PCLS had a different slope within the lower dose range (0.01–1 mM) compared to the frozen-thawed rat PCLS (Figure 3H). The total glutathione levels were significantly lower in frozen-thawed rat PCLS (Supplementary Figure 2C). Consequently, the normalized data showed less reduction in response to ZnCl2 than the fresh PCLS (Figure 3I).
Responses to ZnCl2: Frozen-Thawed PCLS Versus Cells
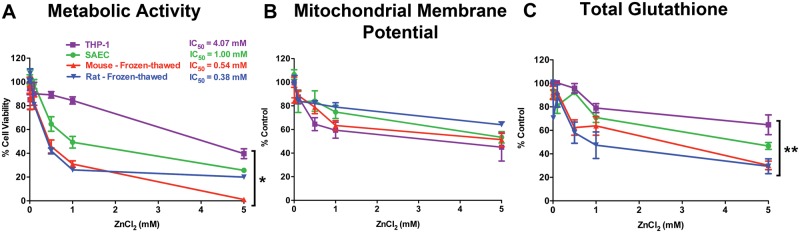
We evaluated the cytotoxic effects of the same concentrations of ZnCl2 on SAEC and THP-1 cells. Figure 4A shows the normalized MTS data for THP-1, SAEC, frozen-thawed mouse and rat PCLS. Exposure to ZnCl2 caused significant dose-dependent cytotoxicity in frozen-thawed PCLS and cells. However, SAEC showed a higher tolerance to ZnCl2 with an IC50 value of 1.00 mM compared to mouse (IC50 = 0.54 mM) and rat (IC50 = 0.38 mM) PCLS. THP-1 cells were also significantly more tolerant to increasing concentrations of ZnCl2 (IC50 = 4.01 mM). Interestingly, the effects of ZnCl2 on mitochondrial integrity were not different between the cells and frozen-thawed PCLS (Figure 4B). At 5 mM concentration, a decrease of nearly 50% in both cell types and frozen-thawed PCLS was observed (Figure 4B). Similarly, we found a dose-dependent reduction in glutathione levels in cells and in frozen-thawed PCLS (Figure 4C). There was a significant difference in glutathione reduction between frozen-thawed PCLS and both cell types at each exposure dose. THP-1 cells showed greater resistance to oxidative stress, as decreases in GSH levels were observed at 1 mM and higher concentrations (Figure 4C).
FIG. 4.
Frozen-thawed PCLS are more sensitive than cells in response to ZnCl2. A, Mouse and rat frozen-thawed PCLS showed higher sensitivity to increasing doses of ZnCl2 compared to THP-1 and SAEC cells. B, As measured by the TMRE assay, the mitochondrial membrane potential of both mouse and rat frozen-thawed PCLS was reduced similarly as the cell lines in response to increasing concentrations of ZnCl2. C, Both mouse and rat frozen-thawed PCLS exhibited a higher sensitivity to increasing doses of ZnCl2 as indicated by the steeper decline in cellular glutathione levels. Data are mean ± SE, where each exposure group consisted of a total of 18 PCLS (3 PCLS per rat, 6 rats) and 18 PCLS (3 slices per mouse, 6 mice). (*P = .0001, **P < .05, ANOVA followed by Bonferroni’s post hoc test).
In Vivo Pulmonary Responses to ZnCl2
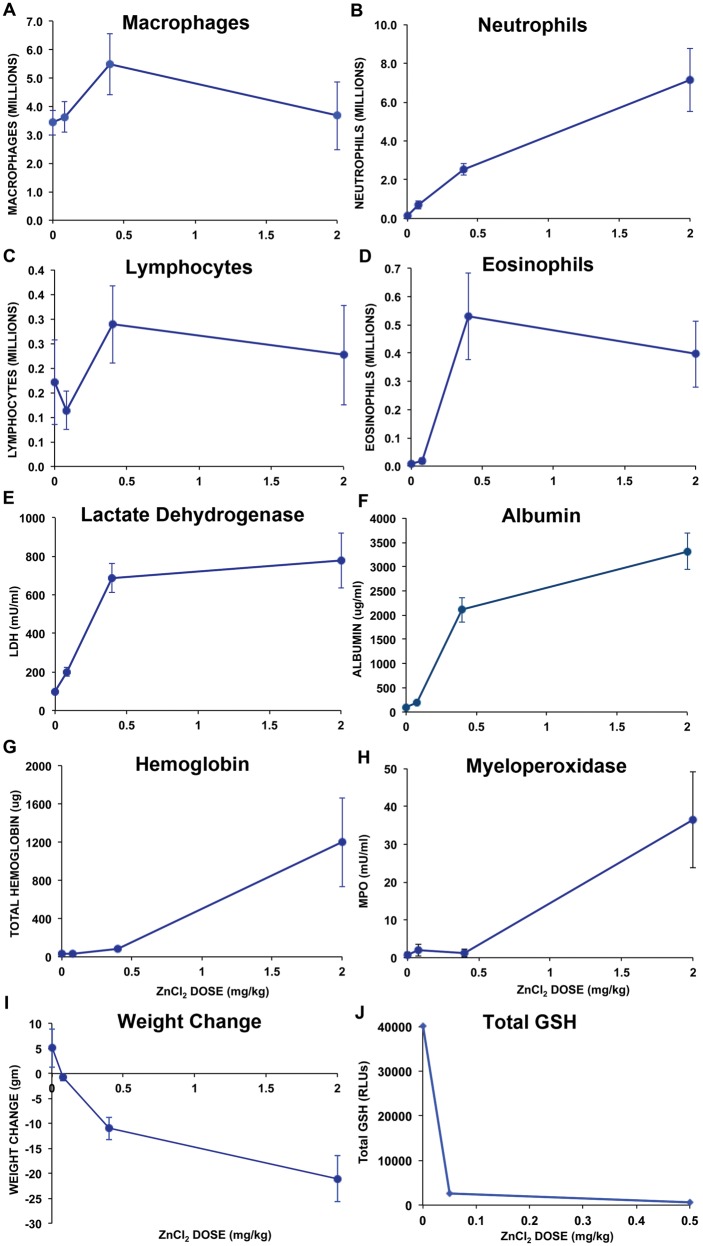
We evaluated acute responses to instilled ZnCl2 by analyzing common cellular and biochemical parameters in injury and inflammation in bronchoalveolar lavage at 24 h postinstillation. Figure 5 shows bronchoalveolar lavage (BAL) parameters after instillation of increasing doses of ZnCl2 solution. An increase in macrophages (Figure 5A), neutrophils (Figure 5B), and lymphocytes (Figure 5C) were observed in response to increasing doses of ZnCl2. Dose-dependent increases in LDH (Figure 5E), albumin (Figure 5F), hemoglobin (Figure 5G) and MPO (Figure 5H) are shown as function of ZnCl2 doses. All of these BAL measurements significantly increased with ZnCl2 dose (MANOVA, P < .05). There were also significant dose-dependent decreases in body weights in 24 h, which might be related to decreased water and food intake resulting from the effects of ZnCl2 (Figure 5I). Total glutathione levels in lung lavage fluids were reduced in response to low doses of ZnCl2 (0.05 and 0.5 mg/kg) indicating oxidative stress (Figure 5J).
FIG. 5.
Responses to ZnCl2 measured in living rats. A, Lavaged macrophages, B, neutrophils, C, lymphocytes, D, eosinophils, E, lactate dehydrogenase, F, albumin, G, hemoglobin, and H, myeloperoxidase were significantly increased with increasing dose of ZnCl2 (0–2 mg/kg). I, Rat body weight decreased with increasing ZnCl2 doses. J, Total glutathione levels in BAL significantly decreased in response to ZnCl2 (0.05 and 0.5 mg/kg). Data are mean ± SE (5 rats/group; GSH analysis, 3 rats/group).
DISCUSSION
While the PCLS has been previously identified as a useful model for toxicological assessment, its short-term viability in culture, typically 2–5 days from harvest, has imposed scheduling constraints as well as restricted the number of slices that can be used per animal. Recent advances in cryopreservation (Bai et al., 2015; Rosner et al., 2014) have enabled expanded PCLS use in measurements of airway reactivity. Here, we validate the cryopreserved PCLS as a feasible model for HTS applications in toxicology.
While optimizing our methodology, we identified some technical parameters that should be controlled to improve the reproducibility of PCLS results. Using freshly opened bottles of media significantly minimizes the reduction in viability as well as the variability in different days of experimentation. Preincubating the media at 37°C with 5% CO2 for at least 20 min allows the media to reach a neutral pH, a parameter which can influence viability. In addition, preparing cryopreservation media on the day of freezing with both newly opened DMSO and media also decreases variability across experiments. We also recommend that the chest cavity of the animals be kept intact until the agarose has solidified. This prevents the potential hyperinflation of the lungs with agarose. It also reduces the potential contamination of the lungs. To reduce the variability resulting from differences in PCLS size/mass as well as potential regional lung heterogeneity in toxicological responses, we recommend the strategy similar to that illustrated in Figure 1. This approach ensures that each experimental condition contains a representative slice from each lung region.
For in vitro testing of chemicals, the MTS assay is the gold standard for assessing metabolic activity. Its accuracy in determining the health of individual cells and tissues in response to chemical exposures has been well documented (Malich et al., 1997). In our experiments, we found that while freezing and thawing reduce the initial metabolic activity of mouse and rat PCLS, the dose-response of toxicity to ZnCl2 are not different when expressed as a percent of the negative control values. Contributing factors to differences in basal metabolic activity could be plasma membrane injury from the initial lung slicing (de Kanter et al., 20012) and from subsequent cryopreservation (Baust et al., 2009). The specific cell types that exhibit reduced metabolic activity associated with cryopreservation remains unclear. The reductions may differ among cell types or might be a common feature of all major lung cell types.
In addition to metabolic activity, we showed that the basal protein levels of Phase 1 metabolic enzyme, cytochrome p450 2B1 in frozen-thawed PCLS were maintained. This is an important finding as cytochrome 2B monooxygenases are crucial in the biotransformation of xenobiotics within the lung (Castell et al., 2005). We also measured glutathione-S-transferase activity in frozen-thawed PCLS. Glutathione S-Transferase is a Phase 2 metabolic enzyme responsible for the conjugation of reduced glutathione with various substrates from environmental toxicants and pharmaceuticals (Zhang et al., 2006).
While zinc is an essential trace element, it is both proapoptotic and antiapoptotic at high concentrations (Plum et al., 2010). Interestingly, while we observed reductions in metabolic activity in both fresh and frozen-thawed PCLS using the MTS assay, we saw a decline in caspase-3 activity with increasing ZnCl2 concentrations (data not shown). This finding is in agreement with a study that showed zinc (Zn2+) as a potent inhibitor of caspase-3 (Perry et al., 1997). Other studies have shown that treatment with Zn2+ at 1 mM can offset important mediators of apoptosis such as Bcl-2-like and Bax-like protein (Zalewski et al., 1991) causing disruption of the mitochondrial membrane potential and thereby releasing cytochrome c into the cytoplasm. In line with these observations, we observed a decline in mitochondrial membrane potential with increasing concentrations of ZnCl2. These findings correlated with our MTS results. However, the TMRE assay was less sensitive to ZnCl2 effects in comparison to the MTS assay. The difference may be due to the confounding effects of ZnCl2 on cellular metabolism (Kumar et al., 2010) or cellular necrosis. Additional endpoints such as LDH release might have provided further insight into the mode of cell death; however, we were unable to determine LDH levels due to Zn2+ interference with the testing reagents (data not shown).
We observed that primary SAEC and THP-1 cell line were more tolerant to the toxic effects of ZnCl2 than PCLS. A possible explanation is differences in metabolic competency (Garcia-Canton et al., 2013). Metabolic competency is essential for bioactivation, a process by which certain protoxicant chemicals are metabolized into reactive metabolites by Phase 1 enzymes and detoxified by Phase 2 enzymes. Although not known as a Phase 1 enzyme inducer or protoxicant, ZnCl2 has been shown to induce Phase 2 enzymes in metabolically competent cell types (Prestera et al., 1993). Both mouse and rat frozen-thawed PCLS maintain Phase 1 and 2 enzymes further supporting their utility in toxicological assessments of toxicants.
Employing different and complementary assays to understand the biological activity of toxicants is essential in chemical testing. We utilized the GSH-Glo assay to determine whether reactive oxygen species contributed to the observed toxicity. The raw data of total intracellular glutathione for mouse frozen-thawed PCLS were notably higher than fresh PCLS. It is possible that the higher glutathione levels observed in the frozen-thawed PCLS may be compensatory mechanisms elicited by the cryopreservation process (Mazur, 2004). Alternatively, this may be due to the freezing process, which may damage cell membranes, thereby releasing GSH into the surrounding tissues. Interestingly, the rat frozen-thawed PCLS displayed lower concentrations of total glutathione in comparison to fresh rat PCLS. However, fresh and frozen-thawed PCLS from mice and rats showed similar trends in the reduction of glutathione in response to increasing ZnCl2, when data were expressed as percentage of negative control. These results agree with previous reports on the effects of high concentrations of ZnCl2 on oxidative stress and production of reactive oxygen species (Maret, 2003). Both fresh and frozen-thawed PCLS from both murine species displayed greater sensitivity to ZnCl2-mediated oxidative stress in comparison to SAEC and THP-1 cell lines.
Finally, to explore whether the effects of ZnCl2 in cryopreserved rat PCLS correlate with pulmonary effects in intact animals, we performed in vivo experiments on adult rats using intratracheal instillation of ZnCl2 followed by bronchoalveolar lavage and analysis. We found that our in vivo data showed the same trend observed in the ex vivo PCLS experiments—ZnCl2 cause dose-dependent increases in lung injury and inflammation as well as reduction in total glutathione levels in BAL indicating oxidative stress. We also found elevated BAL levels of hemoglobin as well as MPO and albumin, both of which have been linked to oxidative stress and inflammation (Buss et al., 2000; Ziouzenkova et al., 1999). The observed lung injury and inflammation with accompanying clinical manifestations led to weight loss further demonstrating the toxicity of ZnCl2.
In conclusion, the data reported here establish the frozen-thawed rodent PCLS as a useful preparation for HTS investigations in predictive toxicology. Establishment of strict protocols and optimization was needed for consistent and reproducible results. In HTS of emerging toxicants, our approach is well suited to fill the gap between cell-based assays and complex intact animal model systems.
FUNDING
National Institutes of Health Grants (ES00002 and HL007118).
Supplementary Material
ACKNOWLEDGMENTS
F.D., S.R. and P.M.Q. received support from the Science Without Borders Program of Brazil administered by CAPES, the Brazilian Coordinating Office for the Advancement of Higher Education. CW received support from the Harvard Yerby Fellowship. We thank Nagarjun Konduru and Hugo Hirano for technical help and Melissa Curran for editorial assistance.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
REFERENCES
- Ahmad A. A., Wang Y., Gracz A. D., Sims C. E., Magness S. T., Allbritton N. L. (2014). Optimization of 3-D organotypic primary colonic cultures for organ-on-chip applications. J. Biol. Eng. 8, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y., Krishnamoorthy N., Patel K. R., Rosas I., Sanderson M. J., Ai X. (2015). Cryopreserved human precision-cut lung slices as a bioassay for live tissue banking: A viability study of bronchodilation with bitter-taste receptor agonists. Am. J. Respir. Cell Mol. Biol. doi: 10.1165/rcmb.2015-0290MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balenga N. A., Klichinsky M., Xie Z., Chan E. C., Zhao M., Jude J., Laviolette M., Panettieri R. A., Jr, Druey K. M. (2015). A fungal protease allergen provokes airway hyper-responsiveness in asthma. Nat. Commun. 6:6763 doi: 10.1038/ncomms7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baust J. G., Gao D., Baust J. M. (2009). Cryopreservation: An emerging paradigm change. Organogenesis 5, 90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrsing H. P., Furniss M. J., Davis M., Tomaszewski J. E., Parchment R. E. (2013). In vitro exposure of precision-cut lung slices to 2-(4-amino-3-methylphenyl)-5-fluorobenzothiazole lysylamide dihydrochloride (NSC 710305, Phortress) increases inflammatory cytokine content and tissue damage. Toxicol. Sci. 131, 470–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. V., Herst P. M., Tan A. S. (2005). Tetrazolium dyes as tools in cell biology: new insights into their cellular reduction. Biotechnol. Annu. Rev. 11, 127–152. [DOI] [PubMed] [Google Scholar]
- Bhise N. S., Ribas J., Manoharan V., Zhang Y. S., Polini A., Massa S., Dokmeci M. R., Khademhosseini A. (2014). Organ-on-a-chip platforms for studying drug delivery systems. J. Control. Release 190, 82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss I. H., Darlow B. A., Winterbourn C. C. (2000). Elevated protein carbonyls and lipid peroxidation products correlating with myeloperoxidase in tracheal aspirates from premature infants. Pediatr. Res. 47, 640–645. [DOI] [PubMed] [Google Scholar]
- Castell J. V., Donato M. T., Gomez-Lechon M. J. (2005). Metabolism and bioactivation of toxicants in the lung. The in vitro cellular approach. Exp. Toxicol. Pathol. 57(Suppl 1), 189–204. [DOI] [PubMed] [Google Scholar]
- de Kanter R., Monshouwer M., Meijer D. K., Groothuis G. M. (2002). Precision-cut organ slices as a tool to study toxicity and metabolism of xenobiotics with special reference to non-hepatic tissues. Curr. Drug Metab. 3, 39–59. [DOI] [PubMed] [Google Scholar]
- Garcia-Canton C., Minet E., Anadon A., Meredith C. (2013). Metabolic characterization of cell systems used in in vitro toxicology testing: Lung cell system BEAS-2B as a working example. Toxicol. In Vitro 27, 1719–1727. [DOI] [PubMed] [Google Scholar]
- Godoy P., Hewitt N. J., Albrecht U., Andersen M. E., Ansari N., Bhattacharya S., Bode J. G., Bolleyn J., Borner C., Böttger J., et al. (2013). Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch. Toxicol. 87, 1315–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henjakovic M., Martin C., Hoymann H. G., Sewald K., Ressmeyer A. R., Dassow C., Pohlmann G., Krug N., Uhlig S., Braun A. (2008). Ex vivo lung function measurements in precision-cut lung slices (PCLS) from chemical allergen–sensitized mice represent a suitable alternative to in vivo studies. Toxicol. Sci. 106, 444–453. [DOI] [PubMed] [Google Scholar]
- Huh D., Matthews B. D., Mammoto A., Montoya-Zavala M., Hsin H. Y., Ingber D. E. (2010). Reconstituting organ-level lung functions on a chip. Science 328, 1662–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Abbas A., Fausto N., Aster J. (2010). Robbins and Cotran Pathologic Basis of Disease, 8th ed Saunders/Elsevier, Philadelphia, PA. [Google Scholar]
- Malich G., Markovic B., Winder C. (1997). The sensitivity and specificity of the MTS tetrazolium assay for detecting the in vitro cytotoxicity of 20 chemicals using human cell lines. Toxicology 124, 179–192. [DOI] [PubMed] [Google Scholar]
- Maret W. (2003). Cellular zinc and redox states converge in the metallothionein/thionein pair. J. Nutr. 133(Suppl. 1), 1460S–1462S. [DOI] [PubMed] [Google Scholar]
- Mazur P. (2004). In Life in the Frozen State. In Fuller B.J., Lane N., Benson E.E., eds. Principles of Cryobiology. CRC Press, Boca Raton, FL, USA Academic Press; Boca Raton: pp. 3–65. [Google Scholar]
- McKim J. M. (2010). Building a tiered approach to in vitro predictive toxicity screening: a focus on assays with in vivo relevance. Comb. Chem. High Throughput Screen 13, 188–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin J. P., Baste J. M., Gay A., Crochemore C., Corbière C., Monteil C. (2013). Precision cut lung slices as an efficient tool for in vitro lung physio-pharmacotoxicology studies. Xenobiotica 43, 63–72. [DOI] [PubMed] [Google Scholar]
- Perry D. K., Smyth M. J., Stennicke H. R., Salvesen G. S., Duriez P., Poirier G. G., Hannun Y. A. (1997). Zinc is a potent inhibitor of the apoptotic protease, caspase-3. A novel target for zinc in the inhibition of apoptosis. J. Biol. Chem. 272, 18530–18533. [DOI] [PubMed] [Google Scholar]
- Plum L. M., Rink L., Haase H. (2010). The essential toxin: impact of zinc on human health. Int. J. Environ. Res. Public Health 7, 1342–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestera T., Holtzclaw W. D., Zhang Y., Talalay P. (1993). Chemical and molecular regulation of enzymes that detoxify carcinogens. Proc. Natl. Acad. Sci. U.S.A. 90, 2965–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner S. R., Ram-Mohan S., Paez-Cortez J. R., Lavoie T. L., Dowell M. L., Yuan L., Ai X., Fine A., Aird W. C., Solway J., et al. (2014). Airway contractility in the precision-cut lung slice after cryopreservation. Am. J. Respir. Cell Mol. Biol. 50, 876–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson M. J. (2011). Exploring lung physiology in health and disease with lung slices. Pulm. Pharmacol. Ther. 24, 452–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer U. G., Vogel S., Aumann A., Hess A., Kolle S. N., Ma-Hock L., Wohlleben W., Dammann M., Strauss V., Treumann S., et al. (2014). Applicability of rat precision-cut lung slices in evaluating nanomaterial cytotoxicity, apoptosis, oxidative stress, and inflammation. Toxicol. Appl. Pharmacol. 276, 1–20. [DOI] [PubMed] [Google Scholar]
- Zalewski P. D., Forbes I. J., Giannakis C. (1991). Physiological role for zinc in prevention of apoptosis (gene-directed death). Biochem. Int. 24, 1093–1101. [PubMed] [Google Scholar]
- Zhang J. Y., Wang Y., Prakash C. (2006). Xenobiotic-metabolizing enzymes in human lung. Curr. Drug Metab. 7, 939–948. [DOI] [PubMed] [Google Scholar]
- Ziouzenkova O., Asatryan L., Sevanian A. (1999). Oxidative stress resulting from hemolysis and formation of catalytically active hemoglobin: protective strategies. Int. J. Clin. Pharmacol. Ther. 37, 125–132. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.