Abstract
We analyzed temporal patterns of alcohol misuse, smoking, and depression among veterans in care to determine whether these conditions vary concordantly or sequentially. Using the Veterans Aging Cohort Study, harmful alcohol use (AUDIT-C ≥ 4), current smoking, and depression (PHQ-9 ≥ 8), were measured. In regression analyses, predictors included each outcome condition at baseline, the other two conditions in the same survey, the other two conditions in the immediately preceding survey, number of years since enrollment, and HIV status. We found that current smoking and depression were more common among HIV infected individuals. Harmful alcohol use was more common among uninfected individuals. Temporal analyses suggested a concurrent pattern: each condition was associated with the other two conditions (p < 0.03, OR 1.12–1.66) as well as with the prior presence of the same condition (p < 0.0001; OR 6.38–22.02). Smoking was associated with prior depression after controlling for current depression (OR 1.16; p = 0.003). In conclusion, alcohol misuse, smoking, and depression were temporally concordant and persistent, raising the question of whether they constitute a common syndrome in HIV infected patients and others with chronic diseases.
Keywords: Alcohol use, Aging, Temporal analysis, Depression, HIV, Smoking, Veterans
Introduction
It remains unclear when screening, surveillance, and treatment strategies should be tailored to the distinct risk profiles of individuals with HIV [1]. For example, the Unites States Preventive Services Task Force (USPSTF) recommends that adults in primary care are screened for smoking (Grade A, meaning the USPSTF recommends the service and there is high certainty of substantial benefit), alcohol misuse (Grade B, meaning the USPSTF recommends the service and there is high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial), and depression (Grade B or C, depending on setting; Grade C meaning the USPSTF recommends selectively offering or providing this service to individual patients based on professional judgment and patient preferences and there is at least moderate certainty of a small net benefit) [1, 2]. However, the USPSTF recommendations do not reflect a high level of personalization, with age and sex typical criteria determining when screening is appropriate, rather than particular risk profiles such alcohol misuse, smoking, and depression; or particular comorbidities such as HIV. While this simplicity has helped to keep clinical guidelines feasible and implementable at the point of care, newer care models (e.g., patient-centered medical homes) and information system models (e.g., patient-level information feeding into decision supports) may permit future guidelines to increase prioritization and personalization.
Alcohol misuse, smoking, and depression are substantial sources of preventable morbidity and mortality in HIV and non-HIV populations, and are commensurately represented in clinical guidelines. It has been estimated that these three account for 21.6 % of the population mortality burden and 23.1 % of the population morbidity burden in the United States [3–5]. Furthermore, they frequently co-occur, concentrating their morbidity and mortality burden in a subgroup. Data among 50,028 persons in active military service suggest that new mental disorders including depression were heralded by concordant new smoking and/ or hazardous drinking [6]. Among 2436 Filipinos, depressive symptoms were predicted by frequency of smoking and frequency of drinking [7]. The large potential health benefit from tailored care strategies in the subgroup with co-occurring alcohol misuse, smoking, and depression could warrant additional systems and provider burdens they may impose. Indeed, it has been suggested that refining screening, surveillance, and treatment strategies for this subgroup such as making them more patient-centered and prioritized, has great potential to improve population health [8]. But no group has considered whether discontinuation of one behavior is associated with the discontinuation of these other behaviors, a question of central importance in the efficient and effective design of interventions.
In order to inform the future development of patient-centered screening care strategies for individuals with HIV, we analyzed temporal patterns of alcohol misuse, smoking, and depression in a large longitudinal cohort of HIV-infected and matched uninfected patients in care. Our underlying question was whether screening and treatment strategies for one of these conditions should be impacted by HIV or by the current or prior presence of any of the other disorders. Correspondingly, we sought to determine whether there were concurrent or temporal relationships between these conditions, and if so, if their effect size was sufficiently large to warrant distinct specifications of screening and treatment strategies.
Methods
Sample
We used data from the Veterans Aging Cohort Study (VACS) [9–11] a longitudinal observational study focused on comorbidities and behaviors affecting medical outcomes in the broader context of aging and HIV [9, 11]. The long term objective is to use these data to design and implement effective interventions to improve outcomes among patients with HIV infections [11]. HIV infected patients were enrolled from VA infectious disease clinics and age-, race-, gender-, site-matched uninfected comparators were enrolled from general medical clinics located at the same VA centers [11].
At study enrollment (beginning in 2002), patients completed baseline surveys. Thereafter, they completed follow-up studies on an approximately annual basis. The first annual follow-up survey occurred from September 2003 to September 2004. For the analyses in the current project, our inclusion criteria only considered those individuals who reported at baseline survey that they had ever consumed alcohol and ever smoked. Our rationale was to ensure that included patients were eligible for the outcome of greatest interest (cessation of alcohol misuse, smoking, or depression symptoms).
Measures
We measured alcohol consumption using the Alcohol Use Disorders Identification Test Consumption (AUDIT-C) score, the sum of scores for the 3 questions ranging from 0 to 12 [12]. We dichotomized AUDIT-C scores based on Bradley et al. [12], who suggested that AUDIT-score C 4 alcohol misuse.
We measured smoking using the VACS variable “Smoking status” considering the categorical responses (0 = never; 1 = current smoker; 2 = past smoker).
We measured depression using the Patient Health Questionnaire (PHQ-9) score, with scores ranging from 0 to 27 [13]. Manea et al. found that the PHQ-9 score has acceptable diagnostic properties for detecting major depressive disorder for cut-off scores between 8 and 11 [14]. Accordingly, we considered PHQ-9 score C 8 as indicator of depressive disorder.
Other characteristics considered in analyses include age, gender, race, hepatitis C (HCV) status, and HIV status.
Descriptive Analyses
Baseline characteristics were compared between HIV infected participants and uninfected participants. T test was used for continuous variables and Chi square test for categorical variables.
Mean-curves of AUDIT-C score and PHQ-9 score and proportion-curve of smoking were evaluated to determine the overall change of these variables over the seven surveys. The subjects were divided into seven subgroups according to the numbers of surveys they took. Subgroup 0 consisted of subjects who only took the baseline survey; Subgroup k consisted of subjects by whom the last survey taken was follow-up survey k, k = 1, …, 6. Subgroup curves were also displayed to assess whether the missing data problem of not completing all seven surveys due to death or late entering was “ignorable”.
Quantitative Analyses
Multiple logistic regression analyses were performed in which each one of the three variables (indicators of AUDIT-C score C 4, current smoker and PHQ-9 score C 8) was the outcome variable, and predictors included the outcome variable at baseline, the other two characteristics in the same survey, the other two characteristics in the immediately preceding survey, the number of years from enrollment to the current survey, and HIV status. Repeated measures across six follow-up surveys were addressed using generalized-estimation-equation methods (GEE) for binary outcomes [15].
Missing data were addressed using multiple-imputations methods [13]. First, using Markov-Chain Monte Carlo models, ten imputations were obtained including HIV status and the three variables of greatest interest (AUDIT-C score, smoking status, and PHQ-9 score) from all available surveys. Second, a generalized estimating equation (GEE) regression analysis was performed maintaining the “missing” status of the outcome while incorporating the imputed values for the predictors. Third, the results from the ten imputations were combined and the final p values were reported. GEE method was implemented by PROC GENMOD, and multiple-imputation method was implemented by PROCs MI and MIANALYZE in SAS 9.3. When we compared results with versus without imputation, they were qualitatively similar.
We decided not to control for random effects of sites for several reasons [1]. Because the number of sites was vastly exceeded by the number of subjects (8 sites and 5609 subjects), it seemed preferable to consider fixed site effect than random site effect [2]; Because we were interested in the marginal associations among those three characteristics across the cohort, adding any main effect would have minor impact on such associations; and [3] when we compared values of baseline variables across sites, we found that they were similar.
Results
Sample
Among the 7327 VACS participants, 3632 were HIV infected and 3695 were uninfected. Among the 5609 VACS patients meeting study inclusion criteria, 2892 were HIV infected and 2717 were uninfected. A total of 1718 patients were excluded because 1146 participants had never smoked, 571 participants had ever smoked but never drank, and one participant had no baseline data. Participants were, on average, age 50.6 (range 23–91), primarily male (96.0 %) and African-American (65.7 %). Table 1 summarizes the baseline characteristics by HIV status. Although HIV infected individuals were statistically significantly younger, more likely to be male and African-American, the differences were not clinically significant. But HIV infected individuals were both statistically and clinically significantly more likely to be HCV positive and to die during the study.
Table 1.
Baseline characteristics of the VACS participants who ever drank and smoked
| HIV+ | HIV− | p value | |||
|---|---|---|---|---|---|
|
|
|
||||
| N | Mean or % | N | Mean or % | ||
| Age, mean (SD) | 2892 | 49.8 (8.2) | 2717 | 51.3 (9.1) | <0.0001 |
| Male, % | 2813 | 97.3 % | 2570 | 94.6 % | <0.0001 |
| Race White, % | 549 | 19.0 % | 624 | 23.0 % | 0.001 |
| Race Black, % | 1953 | 67.5 % | 1733 | 63.8 % | - |
| Race other, % | 390 | 13.5 % | 360 | 13.2 % | - |
| HCV+, % | 1609 | 55.6 % | 919 | 33.8 % | <0.0001 |
| Died, % | 857 | 29.6 % | 478 | 16.6 % | <0.0001 |
| AUDIT-C, mean (SD) | 2481 | 4.0 (3.3) | 2251 | 4.5 (3.5) | <0.0001 |
| Current smoke, % | 1852 | 64.5 % | 1484 | 58.1 % | <0.0001 |
| PHQ-9, mean (SD) | 2866 | 6.0 (6.3) | 2693 | 5.9 (6.6) | 0.53 |
| AUDIT-C ≥ 4 | 782 | 28.1 % | 783 | 31.6 % | 0.005 |
| PHQ-9 ≥ 8 | 908 | 31.7 % | 798 | 29.6 % | 0.10 |
| Smoker and AUDIT-C ≥ 4, % | 610 | 21.9 % | 537 | 21.7 % | 0.84 |
| Smoker and PHQ-9 ≥ 8, % | 652 | 22.9 % | 506 | 20.0 % | 0.01 |
| AUDIT-C ≥ 4 and PHQ-9 C 8, % | 293 | 10.6 % | 241 | 9.8 % | 0.35 |
| At least two conditions, % | 1058 | 38.3 % | 905 | 36.9 % | 0.29 |
| All three conditions, % | 238 | 8.6 % | 179 | 7.3 % | 0.08 |
Follow-up
All 5609 patients completed the first survey. Thereafter, numbers completing subsequent numbers of surveys declines, as would be expected in a “living” cohort (people are continually enrolled and have not had an opportunity to complete all survey waves), with 3183 completing the 3rd survey and 1066 completing the 6th survey.
Temporal Patterns of Alcohol Misuse, Smoking, and Depression
Table 2 shows the relationship between current drinking status and the current and prior status of smoking and depression, controlling for HIV status and number of years from enrollment. We find that current drinking was associated with prior drinking (p < 0.0001, OR 9.72) and was associated with current smoking (p < 0.0001, OR 1.66) and current depression (p < 0.03, OR 1.12). It was not associated with prior smoking or prior depression.
Table 2.
Logistic regression analysis of current harmful alcohol use status and the current and prior status of smoking and depression (c-stat = 0.79)
| Predictor | Odds ratioa | 95 % CI of OR | p value |
|---|---|---|---|
| Number of years from enrollment | 0.95 | (0.93, 0.96) | <0.0001 |
| HIV+ status | 0.88 | (0.78, 0.99) | 0.04 |
| Harmful alcohol use at baseline | 9.72 | (8.51, 11.1) | <0.0001 |
| Current smoking status | 1.66 | (1.46, 1.88) | <0.0001 |
| Current smoking status at preceding surveyb | 1.12 | (0.99, 1.26) | 0.07 |
| Positive depression status | 1.12 | (1.01, 1.24) | 0.03 |
| Positive depression status at preceding surveyc | 0.99 | (0.90, 1.09) | 0.80 |
Adjusted for HIV status and number of years from enrollment
Controlling for current smoking
Controlling for current depression
Table 3 shows the relationship between current smoking and the current and prior status of alcohol and depression, controlling for HIV status and number of years from enrollment. We find that current smoking was associated with prior smoking (p < 0.0001, OR 22.02) and was associated with current drinking (p < 0.0001, OR = 1.64) and current depression (p < 0.0001, OR 1.23). It was not associated with prior alcohol misuse, but was associated with prior depression (p = 0.003, OR 1.16) even after controlling for current depression.
Table 3.
Logistic regression analysis of current smoking and the current and prior status of alcohol and depression (c-stat = 0.85)
| Predictor | Odds ratioa | 95 % CI of OR | p value |
|---|---|---|---|
| Number of years from enrollment | 0.91 | (0.89, 0.92) | <0.0001 |
| HIV+ status | 1.08 | (0.96, 1.22) | 0.22 |
| Current smoking status at baseline | 22.02 | (19.31, 25.10) | <0.0001 |
| Harmful alcohol use | 1.64 | (1.45, 1.85) | <0.0001 |
| Harmful alcohol use at preceding surveyb | 1.10 | (0.97, 1.23) | 0.13 |
| Positive depression status | 1.23 | (1.11, 1.35) | <0.0001 |
| Positive depression status at preceding surveyc | 1.16 | (1.05, 1.28) | 0.003 |
Adjusted for HIV status and number of years from enrollment
Controlling for current alcohol use
Controlling for current depression
Table 4 shows the relationship between current depression status and the current and prior status of smoking and drinking, controlling for HIV status and number of years from enrollment. We find that current depression was associated with prior depression (p < 0.0001, OR 6.38) and was associated with current smoking (p < 0.0001, OR 1.31) and current drinking (p = 0.01, OR 1.16). It was not associated with prior smoking or prior drinking.
Table 4.
Logistic regression analysis of current depression status and the current and prior status of smoking and drinking (c-stat = 0.74)
| Predictor | Odds ratioa | 95 % CI of OR | p value |
|---|---|---|---|
| Number of years from enrollment | 0.99 | (0.97, 1.00) | 0.05 |
| HIV+ status | 0.76 | (0.69, 0.84) | <0.0001 |
| Positive depression status at baseline | 6.38 | (5.72, 7.11) | <0.0001 |
| Harmful alcohol use | 1.16 | (1.04, 1.29) | 0.01 |
| Harmful alcohol use at preceding surveyb | 1.01 | (0.91, 1.12) | 0.84 |
| Current smoking status | 1.31 | (1.17, 1.46) | <0.0001 |
| Current smoking status at preceding surveyc | 1.03 | (0.92, 1.14) | 0.64 |
Adjusted for HIV status and number of years from enrollment
Controlling for current alcohol use
Controlling for current smoking
Discussion
Our study is the largest published study of finely-grained temporal associations between alcohol misuse, smoking, and depression. Our results show three important findings. First, current manifestation of any of the three conditions was predicted in a markedly strong fashion by prior presence of that factor. Although it is common clinical wisdom that “the best predictor of future behavior is past behavior,” the magnitudes of effect are striking, particularly for smoking (OR 22.02), and are sufficiently high to raise the question of whether there should be more intense screening and surveillance strategies for individuals with predispositions for alcohol misuse, smoking, or depression. Second, it also underscores the relative intransigence of smoking behavior in adult life and the value of finding any means possible for facilitating quitting. Third, our results show that prior depression is predictive of current smoking, even when controlling for current depression. This observation raises the question of whether depression is a “leading indicator” of smoking, and of whether former smokers who screen positive for depression should be screened more frequently for smoking than would occur otherwise.
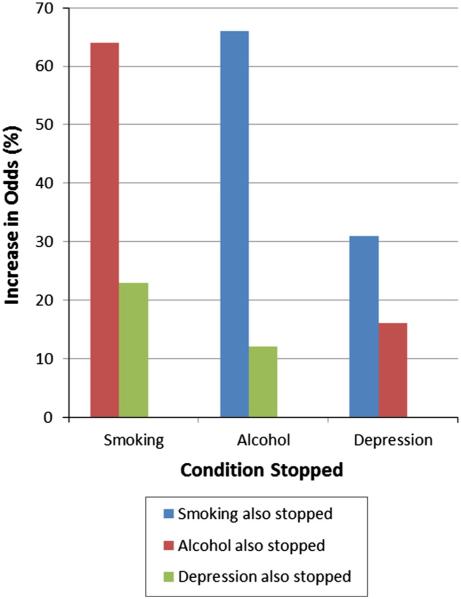
Discontinuation of any of the three conditions was associated with discontinuation of any of the other two conditions (Fig. 1), if a patient stopped smoking, the odds that they also stopped misuse of alcohol increased by 66 %, even controlling for their predisposition to be a smoker and to misuse. If a patient stopped smoking, the odds that their depression would resolve increased by 31 %, even controlling for a patient’s predisposition to be a smoker and be depressed. If a patient stopped misusing alcohol, the odds that they stopped smoking increased 64 % and the odds of their depression resolving increased 16 %, even controlling for a patients predisposition to misuse alcohol and to be a smoker and/or depressed. If a patient’s depression was controlled, the odds of their stopping smoking increased 23 % and the odds of their misuse of alcohol increased by 12 %, even controlling for a patient’s predisposition to be depressed and to be a smoker and/or alcohol misuser.
Fig. 1.
Odds that one condition stops conditional upon another condition also stopping. Likelihoods of stopping alcohol and smoking were most correlated, but stopping each condition was more likely if one or more other conditions stopped also
Our findings raise the question of whether there is a distinct syndrome of alcohol misuse, smoking, and depression that is highly prevalent in HIV patients and in many other patients with chronic diseases such as diabetes [16]. In the particular case of HIV, alcohol misuse plays an important role in infection and disease progression [17, 18] and therefore such a syndrome might be particularly germane to tailoring screening, surveillance, and treatment strategies. Our results add to a growing literature of interrelationships between drinking, smoking, and depression [19]; with our study designed to minimize likely biases [20] that prior drinkers may be more likely to be depressed than current drinkers (“unhealthy quitter” effect) and very low-quantity drinkers may be less likely to be depressed than nondrinkers (potential selection bias from individuals who successfully limit alcohol exposure).
The clinical implications of our results raise questions regarding optimal screening, surveillance, and treatment strategies for individuals with HIV, particularly those with alcohol misuse or other co-syndromic conditions. For example, should a positive screen for smoking or alcohol misuse elevate the pre-screen probability of depression sufficiently so that it would merit a “B” rating by the USPSTF rather than a “C” rating, regardless of whether screening support services are available on site? Should depression lead to a higher subsequent frequency of screening for smoking than would otherwise occur? More generally, should any patient who screens positive for one condition be screened for the other two automatically, and/ or should have a subsequent period of enhanced surveillance for all three conditions? If our results are generalizable, these questions can be addressed by applying the effect sizes to decision analyses that weigh the comparative benefits and harms of alternative screening strategies, ideally considering patient-centered preferences.
Additional clinical implications concern the treatment of alcohol misuse, smoking, and depression. If they simultaneously occur in the same patient, is treatment of one condition more likely to be effective when combined with treatment of the others conditions? Counseling strategies such as cognitive behavioral therapy have demonstrated efficacy for both mood and substance use disorders. In addition, medications for one disorder (e.g., depression), may have efficacy in the treatment of a co-syndromic condition (e.g., nicotine dependence). Specific examples include the use of bupropion which has Food and Drug Administration approval as a treatment for both depression and smoking cessation. Similarly, naltrexone, approved for the treatment of alcohol dependence, has been shown to decrease smoking behavior [21]. Finally, varenicline, a smoking cessation medication has been shown to decrease alcohol consumption [22], and smoking cessation itself is associated with reductions in subsequent alcohol use disorders and mood/anxiety disorder [23].
Our study highlights the importance of trials that can identify best the integrated treatment strategies for the many HIV-infected persons with a syndromic predisposition for alcohol misuse, smoking, and depression. Our results have notable limitations. Not every patient completed every survey. There were variable intervals between surveys. Our results may be impacted by survivor bias and potential attrition bias, particularly for the HIV-infected persons with depression, although the bulk of our qualitative analyses suggest that this was not likely to be a major consideration. It is possible that our results involving veterans are not generalizable to the larger population; however, co-occurrence of these conditions is not unique to veterans [24, 25]. We suspect that the patterns of association we report are generalizable. Our results for alcohol misuse, and to a lesser extent, smoking, were likely affected by desirability bias, although this would likely bias results towards the null affect. Finally, it is important to note that this is an observational study, and association does not imply causation.
In conclusion, alcohol misuse, smoking and depression were common among those with and without HIV infection, temporally concordant, persistent, and depression anteceded smoking. Those with HIV were particularly likely to have more than one of these conditions present. These results raise the question of whether any patient who screens positive for one condition should (1) be screened for the other two (2) should have a period of increased surveillance for all conditions, and (3) should have integrated treatment strategies when co-occurrence is observed. More generally, they raise the question of whether there is a distinct syndrome of alcohol misuse, depression, and smoking in HIV infected patients, and whether the screening and treatment of these conditions should be integrated.
Acknowledgment
Research reported in this publication was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under Award Number U24AA022007. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest The authors have no conflicts of interest to declare.
References
- 1.U.S. Preventive Services Task Force Recommendations for primary care practice 2014. http://www.uspreventiveservicestaskforce.org/Page/Name/recommendations.
- 2.U.S. Preventive Services Task Force Grade definitions 2014. http://www.uspreventiveservicestaskforce.org/Page/Name/grade-definitions. Accessed 24 Mar 2015.
- 3.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289(23):3095–105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 4.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291(10):1238–45. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 5.US Burden of Disease Collaborators The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310(6):591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith TC, LeardMann CA, Smith B, Jacobson IG, Miller SC, Wells TS, et al. Longitudinal assessment of mental disorders, smoking, and hazardous drinking among a population-based cohort of US service members. J Addict Med. 2014;8(4):271–81. doi: 10.1097/ADM.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 7.Lee RB, Sta Maria M, Estanislao S, Rodriguez C. Factors associated with depressive symptoms among Filipino university students. PLoS ONE. 2013;8(11):e79825. doi: 10.1371/journal.pone.0079825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taksler GB, Keshner M, Fagerlin A, Hajizadeh N, Braithwaite RS. Personalized estimates of benefit from preventive care guidelines: a proof of concept. Ann Intern Med. 2013;159(3):161–8. doi: 10.7326/0003-4819-159-3-201308060-00005. [DOI] [PubMed] [Google Scholar]
- 9.Justice AC, Landefeld CS, Asch SM, Gifford AL, Whalen CC, Covinsky KE. Justification for a new cohort study of people aging with and without HIV infection. J Clin Epidemiol. 2001;54(Suppl 1):S3–8. doi: 10.1016/s0895-4356(01)00440-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conigliaro J, Madenwald T, Bryant K, Braithwaite S, Gordon A, Fultz SL, et al. The Veterans Aging Cohort Study: observational studies of alcohol use, abuse, and outcomes among human immunodeficiency virus-infected veterans. Alcohol Clin Exp Res. 2004;28(2):313–21. doi: 10.1097/01.alc.0000113414.73220.21. [DOI] [PubMed] [Google Scholar]
- 11.Justice AC, Dombrowski E, Conigliaro J, Fultz SL, Gibson D, Madenwald T, et al. Veterans Aging Cohort Study (VACS): overview and description. Med Care. 2006;44(8 Suppl 2):S13–24. doi: 10.1097/01.mlr.0000223741.02074.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bradley KA, DeBenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol Clin Exp Res. 2007;31(7):1208–17. doi: 10.1111/j.1530-0277.2007.00403.x. [DOI] [PubMed] [Google Scholar]
- 13.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manea L, Gilbody S, McMillan D. Optimal cut-off score for diagnosing depression with the Patient Health Questionnaire (PHQ-9): a meta-analysis. CMAJ. 2012;184(3):E191–6. doi: 10.1503/cmaj.110829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 16.Chowdhury PP, Balluz LS, Zhao G, Town M. Health behaviors and obesity among Hispanics with depression, United States 2006. Ethn Dis. 2014;24(1):92–6. [PMC free article] [PubMed] [Google Scholar]
- 17.Braithwaite RS, McGinnis KA, Conigliaro J, Maisto SA, Crystal S, Day N, et al. A temporal and dose-response association between alcohol consumption and medication adherence among veterans in care. Alcohol Clin Exp Res. 2005;29(7):1190–7. doi: 10.1097/01.alc.0000171937.87731.28. [DOI] [PubMed] [Google Scholar]
- 18.Braithwaite RS, Conigliaro J, McGinnis KA, Maisto SA, Bryant K, Justice AC. Adjusting alcohol quantity for mean consumption and intoxication threshold improves prediction of nonadherence in HIV patients and HIV-negative controls. Alcohol Clin Exp Res. 2008;32(9):1645–51. doi: 10.1111/j.1530-0277.2008.00732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sullivan LE, Goulet JL, Justice AC, Fiellin DA. Alcohol consumption and depressive symptoms over time: a longitudinal study of patients with and without HIV infection. Drug Alcohol Depend. 2011;117(2–3):158–63. doi: 10.1016/j.drugalcdep.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noh JW, Juon HS, Lee S, Kwon YD. Atypical epidemiologic finding in association between depression and alcohol use or smoking in Korean male: Korean Longitudinal Study of Aging. Psychiatry Investig. 2014;11(3):272–80. doi: 10.4306/pi.2014.11.3.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fridberg DJ, Cao D, Grant JE, King AC. Naltrexone improves quit rates, attenuates smoking urge, and reduces alcohol use in heavy drinking smokers attempting to quit smoking. Alcohol Clin Exp Res. 2014;38(10):2622–9. doi: 10.1111/acer.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erwin BL, Slaton RM. Varenicline in the treatment of alcohol use disorders. Ann Pharmacother. 2014;48(11):1445–55. doi: 10.1177/1060028014545806. [DOI] [PubMed] [Google Scholar]
- 23.Cavazos-Rehg PA, Breslau N, Hatsukami D, Krauss MJ, Spitznagel EL, Grucza RA, et al. Smoking cessation is associated with lower rates of mood/anxiety and alcohol use disorders. Psychol Med. 2014;44(12):2523–35. doi: 10.1017/S0033291713003206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264(19):2511–8. [PubMed] [Google Scholar]
- 25.Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51(1):8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]