Abstract
Nociceptin/Orphanin FQ (N/OFQ) appears to contribute to the development of morphine tolerance, as blockade of its actions will block or reverse the process. To better understand the contribution of N/OFQ to the development of morphine tolerance, this study examined the effect of chronic morphine treatment on levels of N/OFQ and levels and activity of the N/OFQ peptide (NOP) receptor in spinal cord (SC) from male and female rats. Both male and female Wistar rats showed less responsiveness to morphine after subcutaneous injection of escalating doses of morphine (10, 20, 40, 60 and 80 mg/kg, respectively) twice daily for five consecutive days. Male rats were more tolerant to the antinociceptive actions of morphine than females. The N/OFQ content of SC extracts was higher in females than in males, regardless of treatment; following chronic morphine treatment the difference in N/OFQ levels between males and females was more pronounced. N/OFQ content in cerebrospinal fluid (CSF) was reduced 40% in male and 16% in female rats with chronic morphine exposure, but increased in periaqueductal grey of both sexes. Chronic morphine treatment increased NOP receptor levels 173% in males and 137% in females, while decreasing affinity in both. Chronic morphine increased the efficacy of N/OFQ-stimulated [35S]GTPγS binding to SC membranes from male rats, consistent with increased receptor levels. Taken together, these findings demonstrate sex differences in N/OFQ–NOP receptor expression and NOP receptor activity following chronic morphine treatment. They also suggest interplay between endogenous N/OFQ and chronic morphine treatment that results in nociceptive modulation.
Keywords: N/OFQ, Morphine, Sex difference, Tolerance, Spinal cord, NOP receptor
1. Introduction
Numerous studies support the existence of sex differences in the response to opioid drugs in both humans and laboratory animals. In general, females have been observed to be more sensitive to experimental pain and less sensitive to morphine analgesia (Kepler et al., 1991; Craft et al., 1999; Sarton et al., 2000; Cook and Nickerson, 2005; Ji et al., 2006; Wang et al., 2006; Dahan et al., 2008). Female and male gonadal steroids are key factors influencing sexual dimorphism in pain and analgesia (for reviews, Aloisi and Bonifazi, 2006; Dahan et al., 2008). Sex differences in the development of tolerance to morphine in rodents have been reported, though with some controversy. Some studies revealed greater morphine tolerance in male rats (Badillo-Martinez et al., 1984; Craft et al., 1999; South et al., 2001), which was challenged by other studies (Barrett et al., 2001; Holtman et al., 2004). The discrepancy between studies may be due to several factors such as means of drug administration, type of nociceptive assay utilized, hormonal status, species, and experimental design.
The NOP receptor was first cloned and identified as LC132 (Bunzow et al., 1994), opioid receptor-like 1 receptor (ORL-1;Mollereau et al., 1994), XOR1 (Chen et al., 1994), ROR-C (Fukuda et al., 1994) and KOR-3 (Pan et al., 1995). Its endogenous ligand, N/OFQ was identified within the next year by two groups. One group named it Nociceptin (Meunier et al., 1995) and the other called it Orphanin FQ (Reinscheid et al., 1995). N/OFQ exhibits a variety of complex actions; in particular, it is an important endogenous modulator of nociceptive processing (Lambert, 2008). Contrary to its anti-opioid activity at the supraspinal level (Mogil et al., 1996; Tian et al., 1997; Murphy et al., 1999; Ciccocioppo et al., 2000), N/OFQ possesses analgesic properties when administered spinally (Tian et al., 1997; King et al., 1997; Yamamoto et al., 1997). Several lines of evidence strongly suggest a role for N/OFQ in the development and maintenance of morphine tolerance at the supraspinal level. For example, intracerebroventricular (i.c.v.) injections of anti-sera directed against N/OFQ reduced analgesic tolerance associated with chronic morphine treatment (Tian and Han, 2000). Morphine tolerance was blocked in N/OFQ knockout mice or by systemic administration of the NOP receptor antagonist J-113397 (Ueda et al., 2000; Chung et al., 2006), and intravenous administration of NOP receptor antagonist SB-612111 reversed morphine tolerance (Zaratin et al., 2004). There is a dynamic regulation of the gene itself during exposure to morphine. Pro-N/OFQ mRNA was increased in rat brain regions following both acute and chronic morphine administration (Romualdi et al., 2002). Chronic morphine treatment increased levels of N/OFQ in rat brain perfusate and in the periaqueductal gray (PAG), a region of rat brain associated with activation of the descending analgesic pathway (Yuan et al., 1999). However, it is still not clear how the production and release of endogenous N/OFQ in brain is involved in morphine tolerance, or how the development of morphine tolerance alters the pharmacological characteristics of the NOP receptor and N/OFQ.
Recent studies noted that intrathecal N/OFQ produces antinociception to acute heat, NMDA administration or mustard oil stimuli in male rats, diestrous or OVX female rats but not in proestrous or estradiol-treated OVX female rats (Claiborne et al., 2006,2009). This supports the hypothesis that the endogenous N/OFQ–NOP system is involved in the sexual dimorphism of pain. However, it is unclear whether N/OFQ is involved in the more clinically significant problems of sex-related differences in response to chronic morphine treatment. The present study approached this question from the perspective of the spinal cord to investigate the effect of chronic morphine treatment on the N/OFQ–NOP receptor system. We provide new evidence of plastic changes of N/OFQ and NOP receptor in morphine tolerance, and confirmed that the N/OFQ system as a valuable candidate for development of new strategies for better use of existing pain medications.
2. Methods
2.1. Materials
The following drugs and materials were purchased from or provided by the sources indicated: [3H]N/OFQ and morphine HCl (Chemistry and Physiological Systems Research Branch of the National Institute on Drug Abuse, Bethesda, MD); N/OFQ RIA kit (Phoenix Pharmaceuticals, Belmont, CA).
2.2. Animals
Adult male and female Wistar rats weighing 200–250 g were provided by Charles River Labs (Wilmington, MA). Animals were housed in the animal facility under a 12-h light: 12-h dark cycle (lights on at 0600 h) with free access to food and water. After arrival, rats were acclimated to the animal facility for at least 1 week before experiments were initiated. Experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Oklahoma Health Sciences Center. All experiments conformed to the guidelines of the International Association for the Study of Pain. Every effort was made to minimize animal discomfort and reduce the number of animals used.
2.3. Behavioral testing
Experiments were performed in a climate-controlled room. A tail flick unit including a tail temperature sensor (IITC Life Science Inc., Woodland Hills, CA) was used to assess the nociceptive sensitivity by radiant heat tail-flick latency (TFL) assay, with the lamp set at 25% active intensity. Rats were kept in a plastic restrainer with hind limbs and tail extended. The lamp in the tail flick unit was turned off as soon as the rat flicked its tail and the time lapse between the onset of irradiation and the flick of the tail was noted. Values from 3 measurements with 5 min intervals were averaged as the basal TFL. Tail temperature was monitored by Tail Temperature probe for every test, and a cut-off limit of 12 s was set to prevent any tissue damage.
2.4. Chronic morphine treatment
Female (n = 15) and male (n = 14) Wistar rats (200–250 g) received subcutaneous (s.c.) injections of escalating doses of morphine (10, 20, 40, 60 and 80 mg/kg, respectively) twice a day (08:30 h and 17:30 h) for 5 days similar to the procedure described by Yuan et al. (1999). Saline-treated control animals (female: n = 15; male: n = 14) received an equivalent volume of saline. The development of morphine tolerance was tested between 09:00 h and 12:00 h on the 6th day. Basal TFL in all rats was measured after s.c. injection of saline. Animals then received s.c. injection of 10 mg/kg morphine and TFL was measured again after 30 min. At the end of the experiment, animals were euthanized with an overdose of inhaled isoflurane. CSF and spinal cord tissues were immediately taken and kept in −80 °C for analysis.
2.5. Radioimmunoassay of N/OFQ-immunoreactivity (N/OFQ-IR)
The procedure for peptide extraction has been described previously (Walker et al., 2002) and modified. After TFL was determined in the 6th day, rats were anesthetized as described above. CSF from each rat was withdrawn by inserting a 26-gauge needle into the cisterna magna and immediately stored at −80 °C. Acetic acid (0.5 M, 200 μl) was preheated to ~95 °C, added to each sample of spinal cord tissue and boiled for 10 min before cooling on ice for 2 min. Samples were homogenized and reheated at 95 °C for 5 min, cooled on ice, then centrifuged at 15,000 g for 15 min at 4 °C. Supernatant was dried in a vacuum centrifuge, and stored at −80 °C until assay. N/OFQ content in CSF and tissue was determined by RIA kit, according to the protocol suggested by manufacturer, and is presented as N/OFQ-IR. All samples and standards were assayed in duplicate. The sensitivity of the assay was <10 pg/ml; non-specific binding was 2.9%. There was no cross-reactivity with Dynorphin A (1–17), enkephalin or β-endorphin. Concentration of soluble protein present in the spinal cord extract was determined by the Bradford method (Bradford, 1976) using a Pierce protein assay kit. Total amount of N/OFQ was calculated and expressed as pg/μg protein. RIA curves and data have been analyzed using GraphPad Prism 5.0 software.
2.6. [3H]N/OFQ receptor binding assay
Spinal cord dorsal horn tissue was thawed and homogenized in 50 mM Tris–HCl (pH 7.4). The homogenates were centrifuged at 35,000× g for 25 min at 4 °C and the membrane pellets were suspended in assay buffer (50 mM Tris–HCl pH 7.4, 0.5% bovine serum albumin, 0.1% bacitracin). Membrane proteins (40–50 μg) were incubated with various concentrations of [3H]N/OFQ from 0.9 nM to 6 nM in 250 μl assay buffer at 30 °C for 1 h. The reaction was terminated by filtration and washing through GF/C filters presoaked with 0.1% polyethyleneimine. [3H]N/OFQ binding was determined by scintillation spectroscopy. Non-specific binding (<20% of the total binding) was determined in the presence of 50 μM cold N/OFQ and was subtracted from the total binding.
2.7. [35S]GTPγS binding assay
NOP receptor activity was determined by [35S]GTPγS binding assay. Spinal cord dorsal horn tissue was dissected on ice from 6 rats in each group. Membrane protein was prepared (Odagaki and Toyoshima, 2006) and [35S]GTPγS binding assay was conducted as described (Baker et al., 2000). Aliquots (25 μl) of the diluted rat spinal cord membranes equivalent to 3 μg protein were incubated at 30 °C for 60 min in 100 μl of 50 mM Tris–HCl buffer containing 0.2 nM [35S]GTPγS, 0.5% BSA, 10 μM GDP, 0.1 mM EDTA, 0.2 mM DTT, 5 mM MgCl2, and 100 mM NaCl in the presence of 10−9–10−5 M N/OFQ. The reaction was terminated by rapid filtration through glass fiber filters using a Brandel cell harvester with three washing with 5 ml of ice-cold washing buffer (5 mM KPO4, pH 7.4). Radioactivity was determined by liquid scintillation spectroscopy. Non-specific binding was measured in the presence of 100 μM unlabeled GTPγS, which was subtracted from the total binding to define the specific [35S]GTPγS binding.
2.8. Data analysis
Data are expressed as mean ± S.E.M. Statistical comparisons of behavioral and neurochemical data were performed with two-way analysis of variance (ANOVA) followed by Bonferroni’s posttest. Where necessary, Bonferroni correction was employed to adjust for multiple comparisons. All analyses were conducted with GraphPad Prism 5.0 software. A p < 0.05 was considered statistically significant.
3. Results
3.1. Sex difference in the development of chronic morphine tolerance in rats
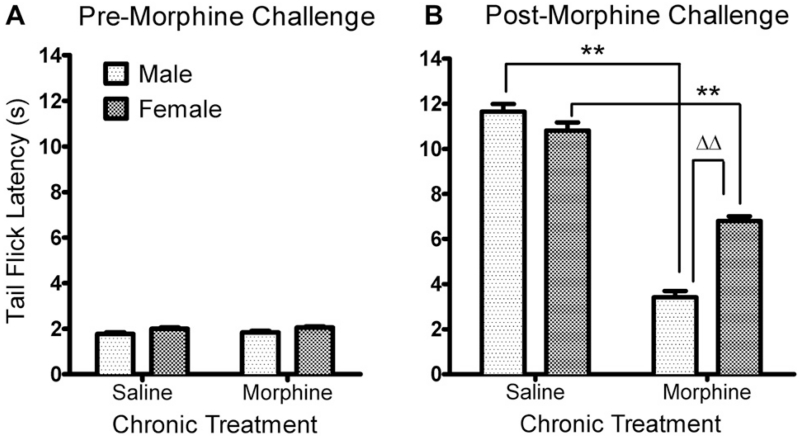
After five consecutive days of saline or morphine (10, 20, 40, 60, 80 mg/kg, s.c., twice daily) treatment, rats of both sexes and in both treatment groups showed similar baseline TFL (Saline: Female vs. male, 2.00 ± 0.06 s vs. 1.77 ± 0.06 s; chronic morphine: Female vs. male, 2.05 ± 0.05 s vs. 1.83 ± 0.07 s), indicating that neither gender nor chronic morphine altered the basal pain threshold in this study (Fig. 1A). Tolerance to the antinociceptive actions of chronic morphine was verified by administration of a challenge dose of morphine (10 mg/kg, s.c.) administered on day 6. Two-way ANOVA revealed a significant interaction between sex and chronic morphine treatment [F(3, 108) = 165.59, p < 0.01]. There were significant effects of sex [F(3, 108) = 599.48, p < 0.01] and chronic morphine [F(1, 108) = 393.73, p < 0.01] on the TFL (Fig. 1B). The challenge dose of morphine-induced significant antinociceptive effects in saline- and morphine-treated rats of both sexes (p < 0.01 in all comparisons) but the antinociceptive effect was greater in saline-treated rats, as indicated by longer TFL (saline vs. chronic morphine: 11.05 ± 0.31 s vs. 7.04 ± 0.32 s in females; 11.71 ± 0.28 s vs. 3.42 ± 0.29 s in males, **p < 0.01 by Bonferroni post-test). This confirms the development of morphine tolerance in male and female rats. Moreover, male rats were significantly less responsive to the morphine challenge after chronic morphine treatment than female rats (Bonferroni post-test, ΔΔp < 0.001), indicating a sex difference in the development of chronic morphine tolerance in rats.
Fig. 1.
Differential development of antinociceptive tolerance to chronic morphine in male and female rats. Escalating doses of morphine were given for five consecutive days to groups of male (n = 14) and female (n = 15) rats. Control rats received an equivalent volume of saline (male, n = 14; female, n = 15). Tolerance was verified by measuring TFL before (A) and after (B) a challenge dose of morphine administered on the 6th day. Both male and female rats in the chronic morphine-treated groups showed less responsiveness to 10 mg/kg morphine than rats that received saline, but the degree of antinociceptive tolerance was greater in male rats. Asterisks (**) indicate decreased TFL compared to controls of the same sex (p < 0.01) and triangles (ΔΔ) indicate significantly less TFL compared to female rats (p < 0.01). Data were analyzed by two-way ANOVA with Bonferroni post-tests.
3.2. N/OFQ content in spinal dorsal horn, CSF and PAG after chronic morphine treatment
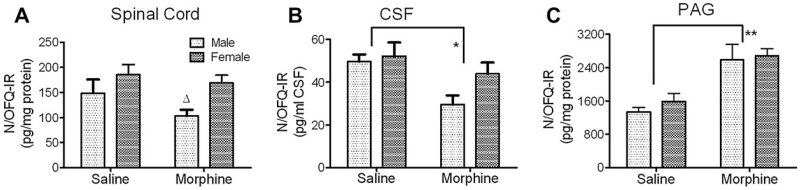
N/OFQ content in dorsal horn tissue, CSF and PAG was measured by RIA. Two-way ANOVA revealed significant effect of sex [F(1, 29) = 6.80, *p < 0.05], but no interaction between sex and chronic morphine [F(1, 29) = 0.58, p = 0.45] and no effect of chronic morphine on the N/OFQ content in spinal cord [F(1, 29) = 2.54, p = 0.12] was seen (Fig. 2A; Female, saline vs. morphine: 185 ± 21 vs. 158 ± 13 pg/mg; Male, saline vs. morphine: 149 ± 27 vs. 103 ± 13 pg/mg, n = 8/each group). Therefore, while N/OFQ levels were higher in females, regardless of treatment, this difference was more pronounced in rats that received chronic morphine treatment (Δp < 0.05, Fig. 2A).
Fig. 2.
Levels of N/OFQ-IR decreased in male rat spinal cord (A) and in male and female CSF (B), but increased in male and female PAG (C) after chronic morphine treatment. A. There was a significant effect of sex on N/OFQ content of dorsal spinal cord extracts, with higher levels of N/OFQ in females than males. This difference was even more pronounced following chronic morphine treatment with N/OFQ content of spinal cord extracts in males dropping even lower (n = 8–9/group; Δp < 0.05). B. N/OFQ content in CSF was reduced significantly in male and female rats following chronic morphine exposure compared to saline-treated controls (*p < 0.05; n = 5–6). No significant difference was detected between male and female CSF (n = 6/group). C. N/OFQ levels increased in PAG of male and female rats receiving chronic morphine treatment compared to saline-treated rats (**p < 0.001; n = 4–6). Data were analyzed by two-way ANOVA with Bonferroni post-tests.
N/OFQ content in CSF also was examined. Similar to results in SC dorsal horn tissue, there was no significant interaction between sex and treatment by two-way ANOVA in N/OFQ levels in CSF [F(1, 19) = 1.38, p = 0.25]. In contrast to SC dorsal horn results, there was a significant effect of chronic morphine treatment [F(1, 19) = 7.90, p < 0.05] on the N/OFQ content in CSF (Fig. 2B), but no effect of sex [F(1, 19) = 2.83, p = 0.11]. Therefore, chronic morphine reduced N/OFQ levels in the CSF of both male and female rats, but the decrease in males was 40% (Saline, 49.6 ± 3.4 pg/ml; Morphine, 29.5 ± 4.3 pg/ml) compared to only a 16% decrease in females (Saline, 52.2 ± 6.4 pg/ml; Morphine, 43.9 ± 5.3 pg/ml).
As a positive control for the ability of chronic morphine to alter N/OFQ levels (Yuan et al., 1999), levels of N/OFQ in PAG also were measured in female and male rats treated chronically with saline or morphine (Fig. 2C). Yuan et al. (1999) previously reported that chronic morphine increased N/OFQ levels in PAG of female Wistar rats; our results confirmed this finding and extended it to male rats, as well. Two-way ANOVA analysis revealed a significant effect of chronic morphine treatment [F (1, 16) = 25, **p < 0.0001] on N/OFQ levels in males and females. Chronic morphine treatment increased N/OFQ levels in both males (Saline, 1332 ± 111 pg/mg; Morphine, 2582 ± 370 pg/mg) and females (Saline, 1590 ± 190 pg/mg; Morphine, 2680 ± 170 pg/mg). Therefore, in the PAG both male and female rats express similar N/OFQ levels in a basal state and respond to chronic morphine treatment by increasing N/OFQ levels to the same extent.
3.3. [3H]N/OFQ binding in spinal dorsal horn
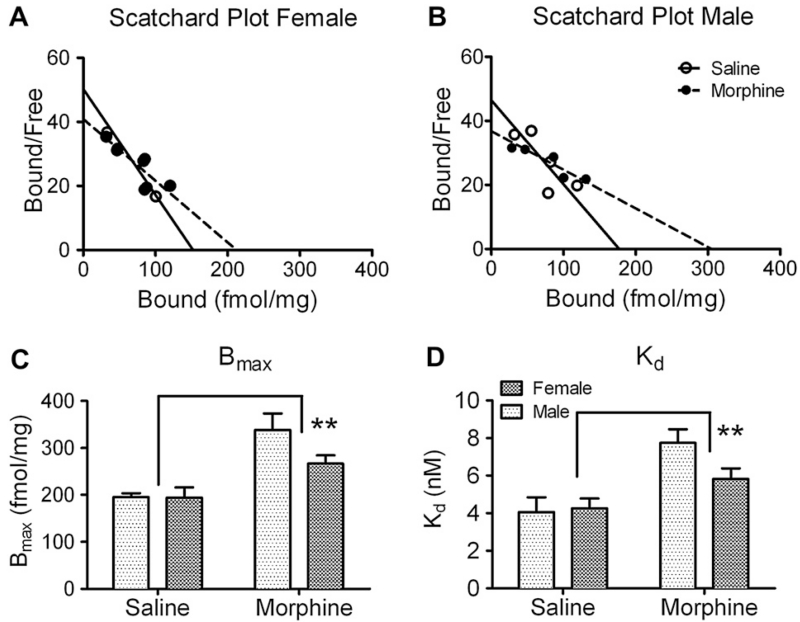
Membrane protein extracted from SC dorsal horn (3 μg) was subjected to saturation binding assay with [3H]N/OFQ. Membrane binding of [3H]N/OFQ, the specific ligand of the NOP receptor, was saturable and specific. The dissociation constant (Kd) of N/OFQ for the receptor and the expression level of the receptor (Bmax) were calculated for each experiment by Scatchard analysis of the saturation binding curve. Representative Scatchard plots from male and female spinal cord binding are shown (Fig. 3A, B). Our results are comparable to those previously reported from rat brain homogenates (Dooley and Houghten, 1996). NOP receptor Bmax and Kd were similar in saline-treated female and male rats. Two-way ANOVA revealed a significant effect of chronic morphine on Kd and Bmax (Kd: F(1, 20) = 15.74, p < 0.01; Bmax: F(1, 20) = 22.12, p < 0.01. Fig. 3C, D) with no significant effect of sex and no significant interaction between sex and chronic morphine. NOP receptor affinity decreased in both male and female rat SC dorsal horn membranes compared to same sex controls (Female, morphine vs. saline: 5.8 ± 0.6 vs. 4.3 ± 0.5 nM; Male, morphine vs. saline: 7.7 ± 0.7 vs. 4.1 ± 0.8 nM; n = 6 per group). NOP receptor levels increased 173% after chronic morphine treatment in males (morphine vs. saline: 338 ± 35 vs. 195 ± 8 fmol/mg, n = 6 per group) and 137% in females (morphine vs. saline: 267 ± 18 vs. 194 ± 22 fmol/mg, n = 6 per group).
Fig. 3.
Receptor binding characteristics of [3H]N/OFQ to spinal cord membranes of male and female rats. Membrane preparations (n = 6 in each group) were incubated with 0.9–6 nM [3H]N/OFQ; specific binding was determined and results subjected to Scatchard analysis as described in Methods. A, B. Scatchard transformations of representative binding data. C. NOP receptor levels increased in SC membranes from morphine-treated rats (**p < 0.001) compared to saline-treated rats; no significant effect of sex was noted. D. NOP receptor affinity in spinal cord membranes decreased after chronic morphine treatment (**p < 0.001), with no significant effect of sex. Data were analyzed by two-way ANOVA.
3.4. N/OFQ-stimulated [35S]GTPγS binding in spinal cord
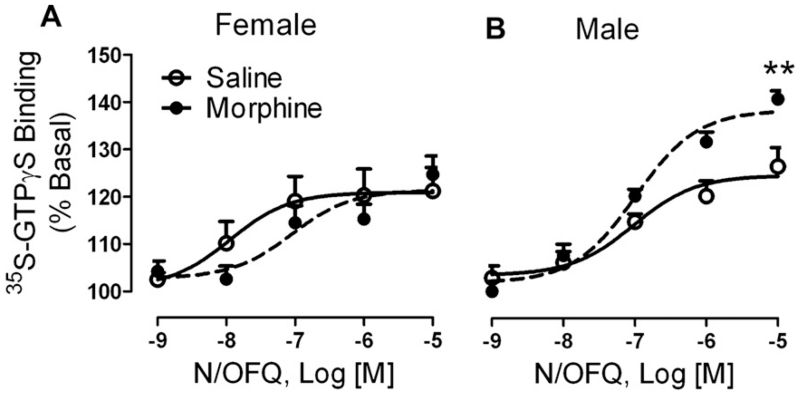
We further assessed NOP receptor function by the ability of N/OFQ to stimulate [35S]GTPγS binding in membranes prepared from lumbar spinal cord tissue from saline- and morphine-treated rats after pain assessment on day 6. Two-way ANOVA indicated that there was a significant interaction between sex and chronic morphine treatment on the Emax of the NOP receptor [F(1, 30) = 7.41, p < 0.05]. As illustrated in Fig. 4, N/OFQ-stimulated [35S] GTPγS binding in naïve male and female rats to the same maximal extent (male: 125 ± 2%, n = 9; female: 121 ± 3%, n = 7). However, chronic morphine treatment significantly increased N/OFQ efficacy in membranes from male rats (139 ± 2%, **p < 0.001 by Bonferroni post-test) compared to saline-treated controls, but had no effect on the maximum effect Emax in females (121 ± 3%). Though N/OFQ appeared more potent in spinal cord membranes from naïve female rats (Log EC50: −7.9 ± 0.6) than in membranes from males (Log EC50: −7.0 ± 0.3), no significant interaction or differences were noted between males and females or as a result of chronic morphine treatment (females: Log EC50: −7.0 ± 0.4; males: Log EC50: −7.0 ± 0.1).
Fig. 4.
NOP receptor efficacy increased in male rat spinal cord membranes following chronic morphine treatment. Dorsal SC membranes (3–10 μg) from male and female rats (n = 7–9 per group) treated chronically with saline or morphine were assayed for the ability of N/OFQ to stimulate [35S]GTPγS binding. N/OFQ was significantly more efficacious in membranes from male rats treated with morphine than in saline-treated controls (**p < 0.001) while this treatment failed to alter N/OFQ efficacy in females. N/OFQ potency did not significantly differ by sex or treatment. Data were analyzed by two-way ANOVA with Bonferroni post-test.
4. Discussion
The morphine administration strategy in the current study successfully induced tolerance in both male and female rats. While chronic morphine treatment didn’t induce TFL change in response to a saline challenge, consistent with previous reports (Bartok and Craft, 1997; Holtman et al., 2003), both male and female rats were less responsive to 10 mg/kg morphine challenge. We also found that the extent of tolerance development was significantly greater in male rats than females. Our result is in accord with previous studies that observed similar sex-related differences in development of morphine tolerance (Badillo-Martinez et al., 1984; Craft et al., 1999; South et al., 2001; Loyd et al., 2008). However, this difference was not noted in other reports (Kest et al., 2000; Barrett et al., 2001; Holtman et al., 2004). Our study utilized a relatively high dose (10–80 mg/kg, twice daily for 5 days) of morphine that likely induced greater tolerance than other paradigms, and challenged with only a single, moderate morphine dose. Craft et al. showed that after chronic morphine treatment over 2 weeks (10–20 mg/kg, twice daily) the ED50 for morphine antinociception increased approximately 6.9-fold in males versus 3.7-fold in females as measured by hot-plate and tail withdrawal tests (Craft et al., 1999). A shorter term and lower dose of morphine (7 days, 5–10 mg/kg, twice daily) resulted in a similar, but statically non-significant change, where ED50 increased 3.3- versus 2.4-fold in male versus female Sprague–Dawley rats, respectively, using a tail flick assay (Kasson and George, 1984). The other study suggested that when the functional chronic morphine dose (chronic morphine dose/acute morphine potency) was adjusted, the development of morphine tolerance was comparable in male and female Lewis and F344 rats (Barrett et al., 2001). In contrast to rats, female mice undergo greater reductions in morphine analgesia relative to males following chronic morphine (Kest et al., 2000). Therefore, it seems that morphine dose, species and method of pain measurement account for the discrepancy.
Several lines of evidence suggest endogenous N/OFQ plays an important role in the development of morphine tolerance (Tian and Han, 2000; Yuan et al., 1999; Ueda et al., 2000, 2001; Zaratin et al., 2004; Chung et al., 2006) therefore we analyzed levels of N/OFQ by RIA in spinal cord, CSF and PAG samples. Spinal N/OFQ levels were higher in opioid naïve and chronic morphine-treated female rats than male rats (Fig. 2A). However, following chronic morphine treatment the difference in N/OFQ levels between the sexes became more pronounced. Chronic morphine treatment had a general effect to lower levels of N/OFQ in CSF of male and female rats (Fig. 2B), but increased N/OFQ levels to the same extent in males and females in the PAG (Fig. 2C). Yuan et al. (1999) reported that N/OFQ levels were elevated in PAG, amygdala and brain perfusate of female rats using a chronic morphine paradigm that involved 3 injections of morphine per day that increased from 10 mg/kg/injection on day 1–60 mg/kg/injection on day 5. Therefore, those rats received 29% more morphine than ours did. In that study, the level of N/OFQ in the spinal cord was not determined. Further, cerebroventricular perfusate was collected over a 30 min period of perfusion with artificial CSF on day 5 of chronic morphine treatment from a cannula in the lateral cerebral ventricle, 20 min after the last large dose of morphine (60 mg/kg). It is not clear whether the increase in N/OFQ from brain perfusate resulted from the chronic morphine treatment, per se, or the acute 60 mg/kg dose of morphine administered 20 min before collection of the perfusate. The timing of our study was to determine the effect of chronic morphine, thus we collected CSF on day 6, 18 h after the last chronic dose of morphine and 30 min after the small (10 mg/kg) challenge dose of morphine. The difference in CSF findings between our study and that of Yuan et al. (1999) likely is due to differences in loci, method and time of sample collection. The similarity between our PAG results and that of Yuan et al. (1999) suggests that chronic morphine treatment increases N/OFQ levels in the PAG in males as well as females.
The nature and level of the neuropeptides present in the brain, spinal cord and CSF reflect the metabolic events of the underlying CNS pathophysiology. In the present case, the reduced N/OFQ level in CSF and spinal cord may indicate modulation of N/OFQ biosynthesis, release, and/or degradation by chronic morphine. In vivo and in vitro findings suggest that morphine increases N/OFQ expression at the mRNA level. For instance, intrathecal morphine administration increased the levels of proN/OFQ and NOP receptor mRNAs in spinal dorsal horn in a sciatic nerve ligation-induced neuropathic pain model (Mika et al., 2004). Morphine dose-dependently enhanced proN/OFQ mRNA level in primary cultures of rat brain astrocytes, but not in neurons or microglia (Takayama and Ueda, 2005). In contrast, chronic morphine treatment substantially increased endopeptidase activity in cultured cells, and enhanced N/OFQ cleavage into N-terminal fragments (Vlaskovska et al., 1999;Terenius et al., 2000). It is possible that the chronic morphine-induced N/OFQ degradation over-shadowed increased N/OFQ expression, thereby reducing N/OFQ levels in spinal cord and in CSF, with perhaps degradation playing a greater role in male rats than females. Indeed, a recent clinical study found higher levels of N/OFQ in the CSF of chronic pain patients compared to controls. In that study, N/OFQ levels were reduced over 75% in chronic pain patients (mainly males) who received prolonged intrathecal morphine compared to male and female patients receiving no morphine (Raffaeli et al., 2006).
Several studies support the idea that plastic change of NOP receptor is involved in the development of morphine tolerance. NOP receptor knock-out mice were resistant to morphine tolerance and dependence; single subcutaneous or intrathecal, but not intracerebroventricular, injection of the NOP receptor antagonist, J-113397, markedly attenuated morphine tolerance in mice (Ueda et al., 2000; Chung et al., 2006). A parallel enhancement of NOP receptor gene expression was seen in the spinal cord (Gouarderes et al., 1999; Ueda et al., 2000), but not in various brain regions including locus coeruleus, ventral tegmental area, amygdala, and PAG (Ueda et al., 2000). NOP receptor up-regulation also was noted in many supraspinal brain regions associated with the descending analgesic pathway in N/OFQ knock-out mice (Clarke et al., 2003). However, the in vivo pharmacological characteristics of the NOP receptor following chronic morphine treatment have not been reported. Our data revealed that [3H]N/OFQ binding to spinal cord dorsal horn membranes increased in male and female rats following chronic morphine treatment (Fig. 3). Perhaps the up-regulation of NOP receptor expression in the spinal cord of the chronic morphine-treated rats (Fig. 3C) was, in part, in response to reduced levels of N/OFQ in the CSF (Fig. 2B). In fact, there was a trend toward a greater increase in NOP receptor binding in males than females and towards a greater decrease in N/OFQ peptide in CSF, though they never reached significance. It is interesting that the increased binding was accompanied by decreased receptor affinity (Fig. 3D) as it was previously reported that the N/OFQ dose–response curve for antinociception was shifted to the right in morphine tolerant rats (Jhamandas et al., 1998). Repeated intrathecal injection of N/OFQ or morphine produced a significant decline in the antinociceptive effect of N/OFQ (Jhamandas et al., 1998), indicating NOP receptor desensitization followed chronic stimulation of the μ receptor. Our previous study also found that a 24 h stimulation of either μ or NOP receptors in two different human neuroblastoma cell lines desensitized the responses to both receptors (Thakker and Standifer, 2002), consistent with loss of binding affinity in membranes from chronic morphine-treated spinal cord. Therefore, increased NOP receptor expression observed in this study may serve as a compensatory mechanism for reduced NOP receptor efficacy and increased synthesis and metabolism of N/OFQ in the spinal cord.
In line with that trend towards increased binding of [3H]N/OFQ to spinal cord membranes of male rats compared to females, the efficacy of N/OFQ-stimulated [35S]GTPγS binding was significantly increased in males and unchanged in females (Fig. 4). This significant interaction between sex and chronic morphine treatment at the functional NOP receptor level supports the idea that chronic morphine treatment differentially affects the N/OFQ/NOP receptor system in males and female rats. The increase in N/OFQ efficacy following morphine treatment is consistent with our previous report and that of others that morphine agonists increase expression of NOP receptor mRNA and protein (Gouarderes et al., 1999;Ueda et al., 2000; Mandyam et al., 2002), and demonstrates that the additional receptors are functional in males. Since the point of these studies was to determine whether differences in spinal N/OFQ expression and response following chronic morphine treatment existed between male and female rats, no attempt to correlate any of the results with a particular point in the estrous cycle was made. The fact that such differences were noted at all suggests that the findings will be much more pronounced in subsequent studies as they are correlated with different times in the estrous cycle.
In summary, our study demonstrated sex differences and plastic changes in N/OFQ–NOP receptor expression and activity following the development of morphine tolerance. We found that the pharmacological characteristics of the NOP receptor are differentially affected by chronic morphine treatment, especially in male rats. This implies a role for endogenous N/OFQ in the actions of chronic morphine and in nociceptive modulation.
Acknowledgments
This study was supported by grants from NIH (R01-DA017380) and the Presbyterian Health Foundation to KMS. The sponsors had no involvement in study design, analysis, collection and interpretation of the data, writing the report or in the decision to submit this paper for publication.
Footnotes
The authors have no conflict of interest.
References
- Aloisi AM, Bonifazi M. Sex hormones, central nervous system and pain. Horm. Behav. 2006;50:1–7. doi: 10.1016/j.yhbeh.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Badillo-Martinez D, Kirchgessner AL, Butler PD, Bodnar RJ. Monosodium glutamate and analgesia induced by morphine. Test-specific effects. Neuropharmacology. 1984;23:1141–1149. doi: 10.1016/0028-3908(84)90231-4. [DOI] [PubMed] [Google Scholar]
- Baker SP, Scammells PJ, Belardinelli L. Differential A(1)-adenosine receptor reserve for inhibition of cyclic AMP accumulation and G-protein activation in DDT(1) MF-2 cells. Br. J. Pharmacol. 2000;130:1156–1164. doi: 10.1038/sj.bjp.0703405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett AC, Cook CD, Terner JM, Craft RM, Picker MJ. Importance of sex and relative efficacy at the mu opioid receptor in the development of tolerance and cross-tolerance to the antinociceptive effects of opioids. Psychopharmacology (Berl.) 2001;158:154–164. doi: 10.1007/s002130100821. [DOI] [PubMed] [Google Scholar]
- Bartok RE, Craft RM. Sex differences in opioid antinociception. J. Pharmacol. Exp. Ther. 1997;282:769–778. [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bunzow JR, Saez C, Mortrud M, Bouvier C, Williams JT, Low M, Grandy DK. Molecular cloning and tissue distribution of a putative member of the rat opioid receptor gene family that is not a mu, delta or kappa opioid receptor type. FEBS Lett. 1994;347:284–288. doi: 10.1016/0014-5793(94)00561-3. [DOI] [PubMed] [Google Scholar]
- Chen Y, Fan Y, Liu J, Mestek A, Tian M, Kozak CA, Yu L. Molecular cloning, tissue distribution and chromosomal localization of a novel member of the opioid receptor gene family. FEBS Lett. 1994;347:279–283. doi: 10.1016/0014-5793(94)00560-5. [DOI] [PubMed] [Google Scholar]
- Chung S, Pohl S, Zeng J, Civelli O, Reinscheid RK. Endogenous orphanin FQ/Nociceptin is involved in the development of morphine tolerance. J. Pharmacol. Exp. Ther. 2006;318:262–267. doi: 10.1124/jpet.106.103960. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Angeletti S, Sanna PP, Weiss F, Massi M. Effect of nociceptin/orphanin FQ on the rewarding properties of morphine. Eur. J. Pharmacol. 2000;404:153–159. doi: 10.1016/s0014-2999(00)00590-2. [DOI] [PubMed] [Google Scholar]
- Claiborne J, Nag S, Mokha SS. Activation of opioid receptor like-1 receptor in the spinal cord produces sex-specific antinociception in the rat: estrogen attenuates antinociception in the female, whereas testosterone is required for the expression of antinociception in the male. J. Neurosci. 2006;26:13048–13053. doi: 10.1523/JNEUROSCI.4783-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claiborne JA, Nag S, Mokha SS. Estrogen-dependent, sex-specific modulation of mustard oil-induced secondary thermal hyperalgesia by orphanin FQ in the rat. Neurosci. Lett. 2009;456:59–63. doi: 10.1016/j.neulet.2009.03.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S, Chen Z, Hsu M-S, Hill RG, Pintar JE, Kitchen I. Nociceptin/orphanin FQ knockout mice display up-regulation of the opioid receptor-like 1 receptor and alterations in opioid receptor expression in the brain. Neuroscience. 2003;117:157–168. doi: 10.1016/s0306-4522(02)00750-9. [DOI] [PubMed] [Google Scholar]
- Cook CD, Nickerson MD. Nociceptive sensitivity and opioid antinociception and antihyperalgesia in Freund’s adjuvant-induced arthritic male and female rats. J. Pharmacol. Exp. Ther. 2005;313:449–459. doi: 10.1124/jpet.104.077792. [DOI] [PubMed] [Google Scholar]
- Craft RM, Stratmann JA, Bartok RE, Walpole TI, King SJ. Sex differences in development of morphine tolerance and dependence in the rat. Psychopharmacology. 1999;143:1–7. doi: 10.1007/s002130050911. [DOI] [PubMed] [Google Scholar]
- Dahan A, Kest B, Waxman AR, Sarton E. Sex-specific responses to opiates: animal and human studies. Anesth. Analg. 2008;107:83–95. doi: 10.1213/ane.0b013e31816a66a4. [DOI] [PubMed] [Google Scholar]
- Dooley CT, Houghten RA. Orphanin FQ: receptor binding and analog structure activity relationships in rat brain. Life Sci. 1996;59:PL23–29. doi: 10.1016/0024-3205(96)00261-5. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Kato S, Mori K, Nishi M, Takeshima H, Iwabe N, Miyata T, Houtani T, Sugimoto T. cDNA cloning and regional distribution of a novel member of the opioid receptor family. FEBS Lett. 1994;343:42–46. doi: 10.1016/0014-5793(94)80603-9. [DOI] [PubMed] [Google Scholar]
- Gouarderes C, Tafani JAM, Meunier JC, Jhamandas K, Zajac JM. Nociceptin receptors in the rat spinal cord during morphine tolerance. Brain Res. 1999;838:85–94. doi: 10.1016/s0006-8993(99)01713-8. [DOI] [PubMed] [Google Scholar]
- Holtman JR, Jr., Jing X, Wala EP. Sex-related differences in the enhancement of morphine antinociception by NMDA receptor antagonists in rats. Pharmacol. Biochem. Behav. 2003;76:285–293. doi: 10.1016/j.pbb.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Holtman JR, Jr., Sloan JW, Wala EP. Morphine tolerance in male and female rats. Pharmacol. Biochem. Behav. 2004;77:517–523. doi: 10.1016/j.pbb.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Jhamandas KH, Sutak M, Henderson G. Antinociceptive and morphine modulatory actions of spinal orphanin FQ. Can. J. Physiol. Pharmacol. 1998;76:314–324. [PubMed] [Google Scholar]
- Ji Y, Murphy AZ, Traub RJ. Sex differences in morphine-induced analgesia of visceral pain are supraspinally and peripherally mediated. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291:R307–R314. doi: 10.1152/ajpregu.00824.2005. [DOI] [PubMed] [Google Scholar]
- Kasson B, George R. Endocrine influences on the actions of morphine: IV. Effects of sex and strain. Life Sci. 1984;34:1627–1634. doi: 10.1016/0024-3205(84)90633-7. [DOI] [PubMed] [Google Scholar]
- Kepler KL, Standifer KM, Paul D, Kest B, Pasternak GW, Bodnar RJ. Gender effects and central opioid analgesia. Pain. 1991;45:87–94. doi: 10.1016/0304-3959(91)90168-W. [DOI] [PubMed] [Google Scholar]
- Kest B, Palmese C, Hopkins E. A comparison of morphine analgesic tolerance in male and female mice. Brain Res. 2000;879:17–22. doi: 10.1016/s0006-8993(00)02685-8. [DOI] [PubMed] [Google Scholar]
- King MA, Rossi GC, Chang AH, Williams L, Pasternak GW. Spinal analgesic activity of orphanin FQ/nociceptin and its fragments. Neurosci. Lett. 1997;223:113–116. doi: 10.1016/s0304-3940(97)13414-0. [DOI] [PubMed] [Google Scholar]
- Lambert DG. The nociceptin/orphanin FQ receptor: a target with broad therapeutic potential. Nat. Rev. Drug Discov. 2008;7:694–710. doi: 10.1038/nrd2572. [DOI] [PubMed] [Google Scholar]
- Loyd DR, Morgan MM, Murphy AZ. Sexually dimorphic activation of the periaqueductal gray-rostral ventromedial medullary circuit during the development of tolerance to morphine in the rat. Eur. J. Neurosci. 2008;27:1517–1524. doi: 10.1111/j.1460-9568.2008.06100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandyam CD, Thakker DR, Christensen JL, Standifer KM. Orphanin FQ/nociception mediated desensitization of opioid receptor-like 1 receptor and μ opioid receptors involves protein kinase C: a molecular mechanism for heterologous cross-talk. J. Pharmacol. Exp. Ther. 2002;302:502–509. doi: 10.1124/jpet.102.033159. [DOI] [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B, Mazarguil H, Vassart G, Parmentier M, Costentin J. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- Mika J, Schäfer MK, Obara I, Weihe E, Przewlocka B. Morphine and endomorphin-1 differently influence pronociceptin/orphanin FQ system in neuropathic rats. Pharmacol. Biochem. Behav. 2004;78:171–178. doi: 10.1016/j.pbb.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Grisel JE, Reinscheid RK, Civelli O, Belknap JK, Grandy DK. Orphanin FQ is a functional anti-opioid peptide. Neuroscience. 1996;75:333–337. doi: 10.1016/0306-4522(96)00338-7. [DOI] [PubMed] [Google Scholar]
- Mollereau C, Parmentier M, Mailleux P, Butour JL, Moisand C, Chalon P, Caput D, Vassart G, Meunier JC. ORL1, a novel member of the opioid receptor family. Cloning, functional expression and localization. FEBS. Lett. 1994;341:33–38. doi: 10.1016/0014-5793(94)80235-1. [DOI] [PubMed] [Google Scholar]
- Murphy NP, Lee Y, Maidment NT. Orphanin FQ/nociceptin blocks acquisition of morphine place preference. Brain Res. 1999;832:168–170. doi: 10.1016/s0006-8993(99)01425-0. [DOI] [PubMed] [Google Scholar]
- Odagaki Y, Toyoshima R. Dopamine D2 receptor-mediated G protein activation assessed by agonist-stimulated [35S]guanosine 5’-O-(gamma-thiotriphosphate) binding in rat striatal membranes. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2006;30:1304–1312. doi: 10.1016/j.pnpbp.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Pan YX, Cheng J, Xu J, Rossi G, Jacobson E, Ryan-Moro J, Brooks AI, Dean GE, Standifer KM, Pasternak GW. Cloning and functional characterization through antisense mapping of a kappa 3-related opioid receptor. Mol. Pharmacol. 1995;47:1180–1188. [PubMed] [Google Scholar]
- Raffaeli W, Dekel BGS, Landuzzi D, Caminit A, Righetti D, Balestri M, Montanari F, Romualdi P, Candeletti S. Nociceptin levels in the cerebrospinal fluid of chronic pain patients with or without intrathecal administration of morphine. J. Pain. Symptom. Manage. 2006;32:372–377. doi: 10.1016/j.jpainsymman.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Jr., Civelli O. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- Romualdi P, Landuzzi D, D’Addario C, Candeletti S. Modulation of proorphaninFQ/N gene expression by morphine in the rat mesocorticolimbic system. Neuroreport. 2002;13:645–648. doi: 10.1097/00001756-200204160-00022. [DOI] [PubMed] [Google Scholar]
- Sarton E, Olofsen E, Romberg R, den Hartigh J, Kest B, Nieuwenhuijs D, Burm A, Teppema L, Dahan A. Sex differences in morphine analgesia: an experimental study in healthy volunteers. Anesthesiology. 2000;93:1245–1254. doi: 10.1097/00000542-200011000-00018. [DOI] [PubMed] [Google Scholar]
- South SM, Wright AW, Lau M, Mather LE, Smith MT. Sex-related differences in antinociception and tolerance development following chronic intravenous infusion of morphine in the rat: modulatory role of testosterone via morphine clearance. J. Pharmacol. Exp. Ther. 2001;297:446–457. [PubMed] [Google Scholar]
- Takayama N, Ueda H. Morphine-induced overexpression of prepronociceptin/orphanin FQ in cultured astrocytes. Peptides. 2005;26:2513–2517. doi: 10.1016/j.peptides.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Terenius L, Sandin J, Sakurada T. Nociceptin/orphanin FQ metabolism and bioactive metabolites. Peptides. 2000;21:919–922. doi: 10.1016/s0196-9781(00)00228-x. [DOI] [PubMed] [Google Scholar]
- Thakker DR, Standifer KM. Induction of G protein-coupled receptor kinases 2 and 3 contributes to the cross-talk between opioid receptor-like 1 and μ opioid receptors following prolong agonist exposure. Neuropharmacology. 2002;43:979–990. doi: 10.1016/s0028-3908(02)00145-4. [DOI] [PubMed] [Google Scholar]
- Tian JH, Han JS. Functional studies using antibodies against orphanin FQ/nociceptin. Peptides. 2000;21:1047–1050. doi: 10.1016/s0196-9781(00)00242-4. [DOI] [PubMed] [Google Scholar]
- Tian JH, Xu W, Fang Y, Mogil JS, Grisel JE, Grandy DK, Han JS. Bidirectional modulatory effect of orphanin FQ on morphine-induced analgesia: antagonism in brain and potentiation in spinal cord of the rat. Br. J. Pharmacol. 1997;120:676–680. doi: 10.1038/sj.bjp.0700942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H, Inoue M, Takeshima H, Iwasawa Y. Enhanced spinal Nociceptin receptor expression develops morphine tolerance and dependence. J. Neurosci. 2000;20:7640–7647. doi: 10.1523/JNEUROSCI.20-20-07640.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H, Inoue M, Matsumoto T. Protein kinase C-mediated inhibition of mu-opioid receptor internalization and its involvement in the development of acute tolerance to peripheral mu-agonist analgesia. J. Neurosci. 2001;12:2967–2973. doi: 10.1523/JNEUROSCI.21-09-02967.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaskovska M, Kasakov L, Suder P, Silberring J, Terenius L. Biotransformation of nociceptin/orphanin FQ by enzyme activity from morphine-naive and morphine-treated cell cultures. Brain Res. 1999;818:212–220. doi: 10.1016/s0006-8993(98)01266-9. [DOI] [PubMed] [Google Scholar]
- Walker JR, Terenius L, Koob GF. Conditioned opioid withdrawal decreases nociceptin/orphanin FQ levels in the frontal cortex and olfactory tubercle. Neuropsychopharmacology. 2002;27:203–211. doi: 10.1016/S0893-133X(02)00292-0. [DOI] [PubMed] [Google Scholar]
- Wang X, Traub RJ, Murphy AZ. Persistent pain model reveals sex difference in morphine potency. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291:R300–R306. doi: 10.1152/ajpregu.00022.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Nozaki-Taguchi N, Kimura S. Analgesic effect of intrathecally administered Nociceptin, an opioid receptor-like1 receptor agonist, in the rat formalin test. Neuroscience. 1997;81:249–254. doi: 10.1016/s0306-4522(97)00166-8. [DOI] [PubMed] [Google Scholar]
- Yuan L, Han Z, Chang JK, Han JS. Accelerated release and production of orphanin FQ in brain of chronic morphine tolerant rats. Brain Res. 1999;826:330–334. doi: 10.1016/s0006-8993(99)01337-2. [DOI] [PubMed] [Google Scholar]
- Zaratin PF, Petrone G, Sbacchi M, Garnier M, Fossati C, Petrillo P, Ronzoni S, Giardina GA, Scheideler MA. Modification of nociception and morphine tolerance by the selective opiate receptor-like orphan receptor antagonist (–)-cis-1-methyl-7-[[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl]-6,7,8,9-tetrahydro-5H-benzocyclohepten-5-ol (SB-612111) J. Pharmacol. Exp. Ther. 2004;308:454–461. doi: 10.1124/jpet.103.055848. [DOI] [PubMed] [Google Scholar]