Abstract
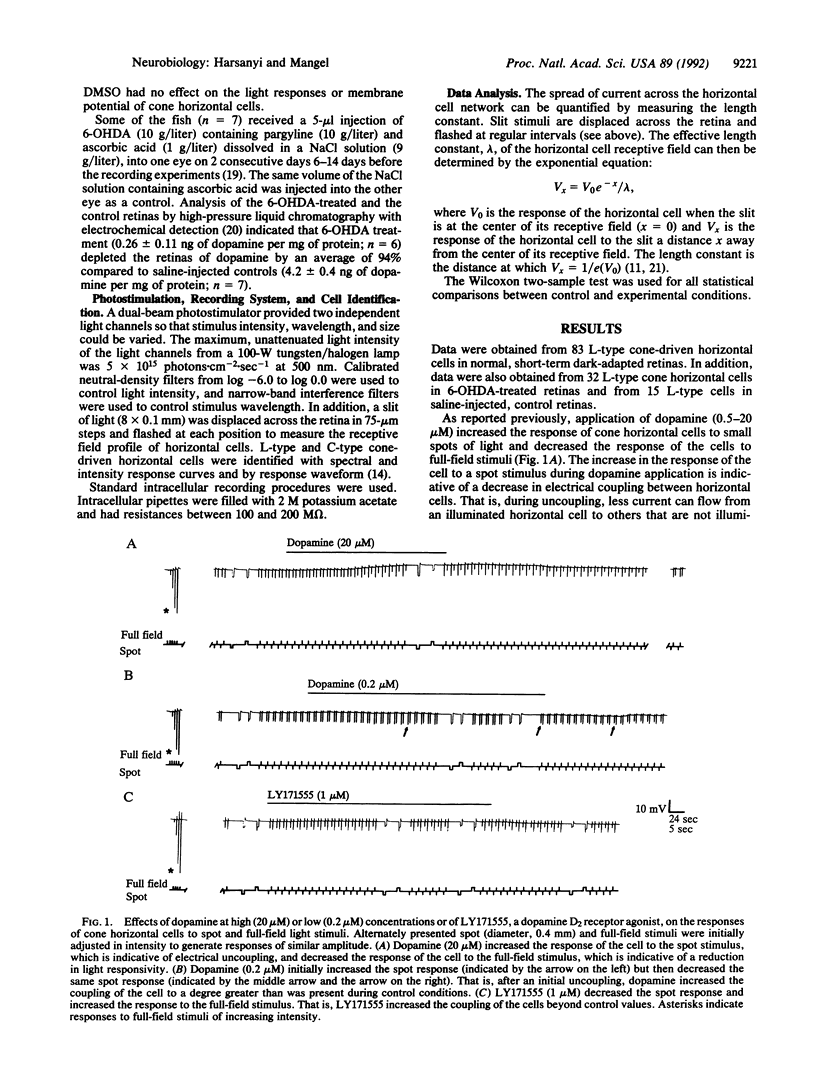
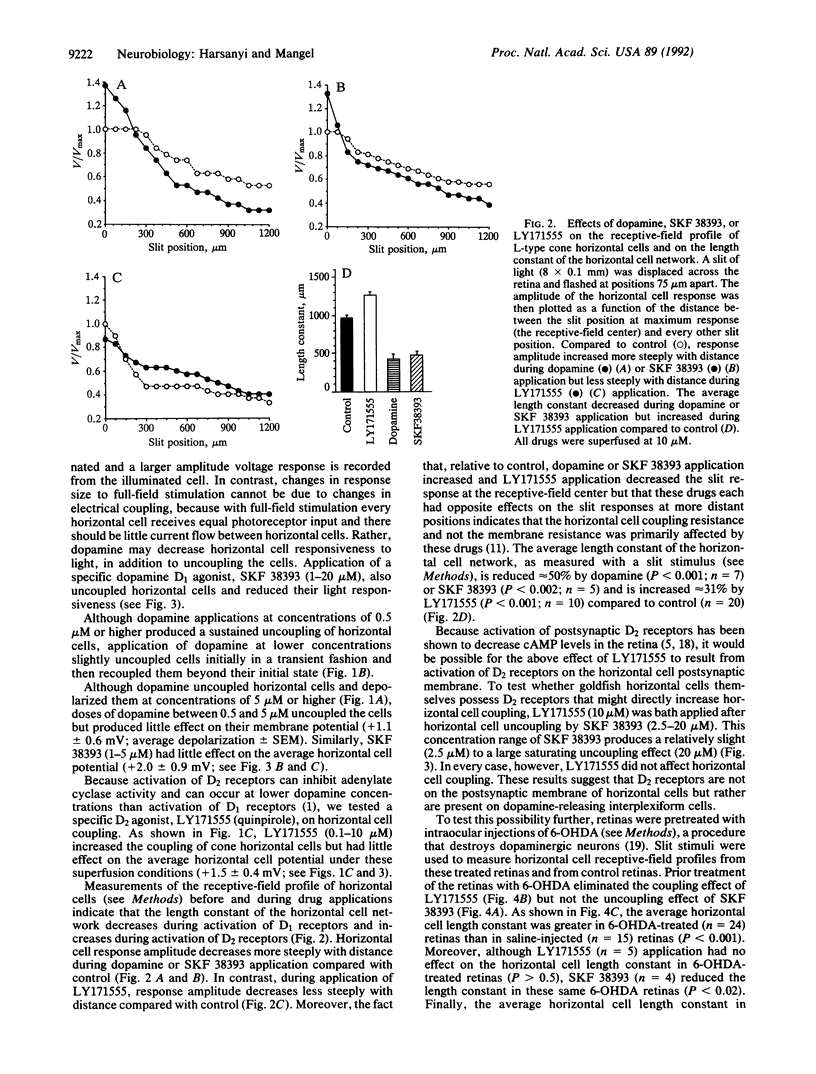
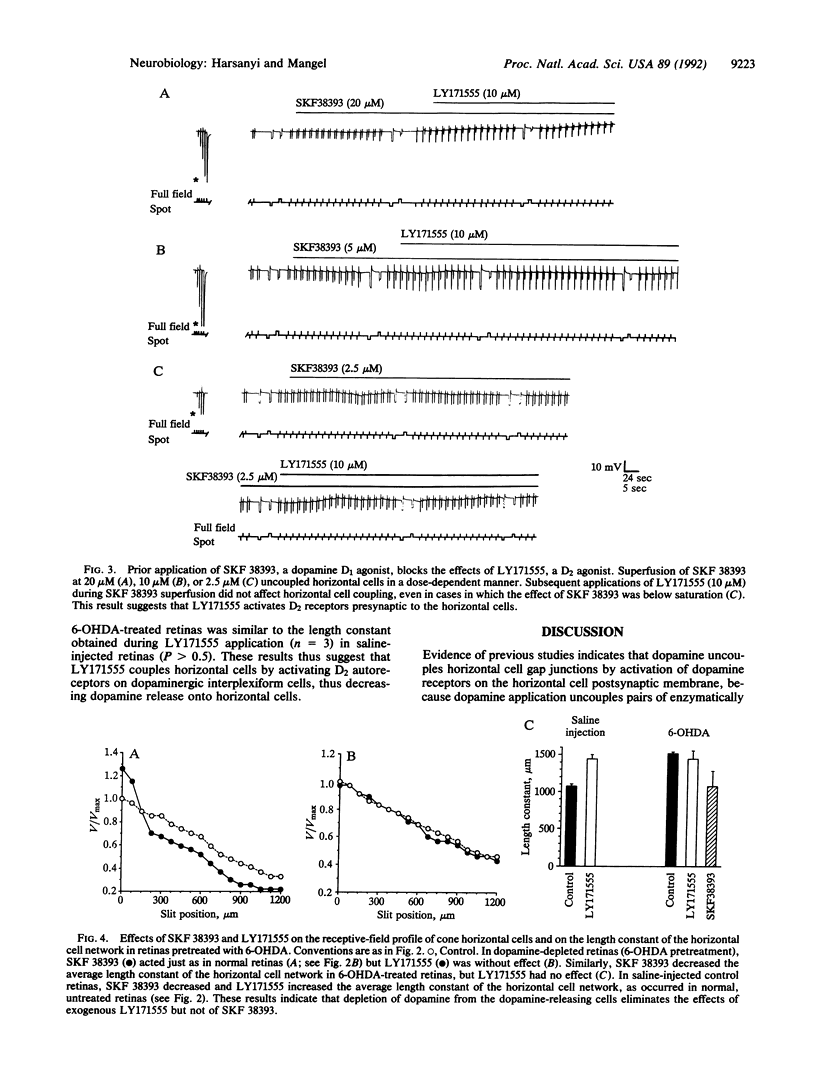
In the fish retina, interplexiform cells release dopamine onto cone-driven horizontal cells. Dopamine decreases the electrical coupling between horizontal cells by activating adenylate cyclase through dopamine D1 receptors. Using intracellular recording, we have studied the effect of dopamine D2 receptor activation on horizontal cell electrical coupling in the intact goldfish retina. Superfusion of the D2 agonist LY171555 (quinpirole; 0.2-10 microM) increased horizontal cell coupling, as indicated by a decrease in responses to centered spots or slits of light. The length constant of the horizontal cell network increased an average of 31%. Although dopamine (0.5-20 microM) uncoupled horizontal cells, lower concentrations (e.g., 0.2 microM) initially uncoupled and then subsequently increased coupling beyond initial control levels. The coupling effect of LY171555 (10 microM) was blocked completely by prior application of the D1 agonist SKF 38393 at saturating (20 microM) or nonsaturating (2.5-5.0 microM) doses. Prior treatment of the retinas with 6-hydroxydopamine, which destroyed dopaminergic neurons, eliminated the coupling effect of LY171555 but not the uncoupling effect of SKF 38393. These results suggest that goldfish horizontal cells contain D1, but not D2, receptors and that dopamine activation of D2 autoreceptors on interplexiform cells inhibits dopamine release onto horizontal cells so that the electrical coupling between horizontal cells increases.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brann M. R., Young W. S., 3rd Dopamine receptors are located on rods in bovine retina. Neurosci Lett. 1986 Sep 12;69(3):221–226. doi: 10.1016/0304-3940(86)90483-0. [DOI] [PubMed] [Google Scholar]
- Cohen J., Iuvone P. M., Neff N. H. Neuroleptic drugs activate tyrosine hydroxylase of retinal amacrine cells. J Pharmacol Exp Ther. 1981 Aug;218(2):390–394. [PubMed] [Google Scholar]
- Dearry A., Burnside B. Dopaminergic regulation of cone retinomotor movement in isolated teleost retinas: I. Induction of cone contraction is mediated by D2 receptors. J Neurochem. 1986 Apr;46(4):1006–1021. doi: 10.1111/j.1471-4159.1986.tb00612.x. [DOI] [PubMed] [Google Scholar]
- Dearry A., Falardeau P., Shores C., Caron M. G. D2 dopamine receptors in the human retina: cloning of cDNA and localization of mRNA. Cell Mol Neurobiol. 1991 Oct;11(5):437–453. doi: 10.1007/BF00734808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C. J., McReynolds J. S. The relationship between light, dopamine release and horizontal cell coupling in the mudpuppy retina. J Physiol. 1991;440:291–309. doi: 10.1113/jphysiol.1991.sp018709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J. E., Ehinger B. The interplexiform cell system. I. Synapses of the dopaminergic neurons of the goldfish retina. Proc R Soc Lond B Biol Sci. 1978 Apr 13;201(1142):7–26. doi: 10.1098/rspb.1978.0030. [DOI] [PubMed] [Google Scholar]
- Dubocovich M. L., Weiner N. Pharmacological differences between the D-2 autoreceptor and the D-1 dopamine receptor in rabbit retina. J Pharmacol Exp Ther. 1985 Jun;233(3):747–754. [PubMed] [Google Scholar]
- Iuvone P. M., Boatright J. H., Bloom M. M. Dopamine mediates the light-evoked suppression of serotonin N-acetyltransferase activity in retina. Brain Res. 1987 Aug 25;418(2):314–324. doi: 10.1016/0006-8993(87)90098-9. [DOI] [PubMed] [Google Scholar]
- Iuvone P. M. Evidence for a D2 dopamine receptor in frog retina that decreases cyclic AMP accumulation and serotonin N-acetyltransferase activity. Life Sci. 1986 Jan 27;38(4):331–342. doi: 10.1016/0024-3205(86)90080-9. [DOI] [PubMed] [Google Scholar]
- Kaneko A. Electrical connexions between horizontal cells in the dogfish retina. J Physiol. 1971 Feb;213(1):95–105. doi: 10.1113/jphysiol.1971.sp009370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebabian J. W., Calne D. B. Multiple receptors for dopamine. Nature. 1979 Jan 11;277(5692):93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- Lamb T. D. Spatial properties of horizontal cell responses in the turtle retina. J Physiol. 1976 Dec;263(2):239–255. doi: 10.1113/jphysiol.1976.sp011630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasater E. M., Dowling J. E. Dopamine decreases conductance of the electrical junctions between cultured retinal horizontal cells. Proc Natl Acad Sci U S A. 1985 May;82(9):3025–3029. doi: 10.1073/pnas.82.9.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangel S. C., Dowling J. E. Responsiveness and receptive field size of carp horizontal cells are reduced by prolonged darkness and dopamine. Science. 1985 Sep 13;229(4718):1107–1109. doi: 10.1126/science.4035351. [DOI] [PubMed] [Google Scholar]
- Mangel S. C., Dowling J. E. The interplexiform-horizontal cell system of the fish retina: effects of dopamine, light stimulation and time in the dark. Proc R Soc Lond B Biol Sci. 1987 Jun 22;231(1262):91–121. doi: 10.1098/rspb.1987.0037. [DOI] [PubMed] [Google Scholar]
- O'Connor P. M., Zucker C. L., Dowling J. E. Regulation of dopamine release from interplexiform cell processes in the outer plexiform layer of the carp retina. J Neurochem. 1987 Sep;49(3):916–920. doi: 10.1111/j.1471-4159.1987.tb00980.x. [DOI] [PubMed] [Google Scholar]
- Piccolino M., Demontis G., Witkovsky P., Strettoi E., Cappagli G. C., Porceddu M. L., De Montis M. G., Pepitoni S., Biggio G., Meller E. Involvement of D1 and D2 Dopamine Receptors in the Control of Horizontal Cell Electrical Coupling in the Turtle Retina. Eur J Neurosci. 1989 May;1(3):247–257. doi: 10.1111/j.1460-9568.1989.tb00792.x. [DOI] [PubMed] [Google Scholar]
- Piccolino M., Neyton J., Gerschenfeld H. M. Decrease of gap junction permeability induced by dopamine and cyclic adenosine 3':5'-monophosphate in horizontal cells of turtle retina. J Neurosci. 1984 Oct;4(10):2477–2488. doi: 10.1523/JNEUROSCI.04-10-02477.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolino M., Witkovsky P., Trimarchi C. Dopaminergic mechanisms underlying the reduction of electrical coupling between horizontal cells of the turtle retina induced by d-amphetamine, bicuculline, and veratridine. J Neurosci. 1987 Aug;7(8):2273–2284. [PMC free article] [PubMed] [Google Scholar]
- Starke K., Göthert M., Kilbinger H. Modulation of neurotransmitter release by presynaptic autoreceptors. Physiol Rev. 1989 Jul;69(3):864–989. doi: 10.1152/physrev.1989.69.3.864. [DOI] [PubMed] [Google Scholar]
- Teranishi T., Negishi K., Kato S. Dopamine modulates S-potential amplitude and dye-coupling between external horizontal cells in carp retina. Nature. 1983 Jan 20;301(5897):243–246. doi: 10.1038/301243a0. [DOI] [PubMed] [Google Scholar]
- Van Buskirk R., Dowling J. E. Isolated horizontal cells from carp retina demonstrate dopamine-dependent accumulation of cyclic AMP. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7825–7829. doi: 10.1073/pnas.78.12.7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watling K. J., Dowling J. E. Dopaminergic mechanisms in the teleost retina. I. Dopamine-sensitive adenylate cyclase in homogenates of carp retina; effects of agonists, antagonists, and ergots. J Neurochem. 1981 Feb;36(2):559–568. doi: 10.1111/j.1471-4159.1981.tb01628.x. [DOI] [PubMed] [Google Scholar]
- Weiler R., Kohler K., Kirsch M., Wagner H. J. Glutamate and dopamine modulate synaptic plasticity in horizontal cell dendrites of fish retina. Neurosci Lett. 1988 May 3;87(3):205–209. doi: 10.1016/0304-3940(88)90449-1. [DOI] [PubMed] [Google Scholar]
- Yazulla S. Evoked efflux of [3H]GABA from goldfish retina in the dark. Brain Res. 1985 Jan 28;325(1-2):171–180. doi: 10.1016/0006-8993(85)90313-0. [DOI] [PubMed] [Google Scholar]
- Yazulla S., Zucker C. L. Synaptic organization of dopaminergic interplexiform cells in the goldfish retina. Vis Neurosci. 1988;1(1):13–29. doi: 10.1017/s0952523800000997. [DOI] [PubMed] [Google Scholar]



