Abstract
BK virus (BKV) infection is associated with hemorrhagic cystitis (HC) in hematopoietic stem cell transplant (HSCT) recipients and nephropathy after kidney transplant. We assessed the association between BKV and kidney and bladder complications in children developing HC by retrospectively reviewing 221 consecutive pediatric allogeneic HSCT recipients at the Children’s Hospital of Philadelphia from 2005–2011. We included all patients with BKV PCR testing performed for clinical indication from day 0 until 1 year post-HSCT (N=68). We assessed the association of any BKV infection (urine and/or blood) or peak BK viremia ≥10,000 copies/ml (high viremia) with severe HC (defined as grade IV—bladder catheterization or surgical intervention), the need for dialysis, serum creatinine-estimated glomerular filtration rate (eGFR) at the time of BKV testing, day 100, and day 365, and death. Children with high viremia more likely developed severe HC compared to those with peak viremia <10,000 copies/mL (21% versus 2%; p=0.02). BKV infection of the blood or urine was not associated with the need for dialysis, change in eGFR, or mortality. BKV infection is common after pediatric allogeneic HSCT and plasma testing in those with HC may predict patients who will develop severe bladder injury.
Keywords: BK virus, hemorrhagic cystitis, transplant, pediatrics
INTRODUCTION
BK virus (BKV) is a DNA polyoma virus that infects most of the general population, with seroprevalance rates approaching 90% by 10 years of age (1). After primary infection, the virus remains dormant in the uroepithelial cells of the kidney and bladder (2). Clinical disease from BKV infection is almost exclusively seen in immunocompromised patients, particularly kidney transplant and hematopoietic stem cell transplant (HSCT) recipients (3, 4). After HSCT, BKV infection identified in the urine (viruria) and/or blood (viremia) may be associated with hemorrhagic cystitis (HC) (5, 6). BKV viremia after renal transplant contributes to kidney injury (nephropathy), typically in the absence of symptomatic HC, and may lead to allograft loss (2).
HC is a frequent complication of allogeneic HSCT, occurring in up to 25% of patients (4). HC can be associated with significant morbidity and mortality, including prolonged hospitalization, invasive surgical procedures to manage bleeding, and acute kidney injury from urinary tract obstruction (7–9). Early-onset HC is secondary to direct toxicity from the preparative conditioning regimen (3). Late-onset HC typically occurs after the first transplant week, and is associated with infections, such as BKV, or less commonly, adenovirus and CMV (9).
Kidney and bladder injury are common complications after HSCT and chronic kidney disease is often considered idiopathic in this population (10). The relationship between BKV infection and kidney disease in HSCT recipients has not been well-studied. Case reports suggest that BKV infection may also contribute to nephropathy in the native kidneys of HSCT and non-renal solid organ transplant recipients, even in the absence of HC (11–14). Only two prior studies in patients undergoing HSCT, one in adults (15) and the other in children (16), have investigated if BKV infection identified in the blood (viremia) is a risk factor for intrinsic kidney injury. Our objective was to assess the impact of BKV infection on the severity of genitourinary outcomes in children undergoing HSCT for predominately malignant indications. We hypothesized that BK viremia would be associated with more severe kidney and bladder injury in children receiving allogeneic HSCT.
MATERIALS and METHODS
We reviewed the records of all children undergoing allogeneic HSCT at the Children’s Hospital of Philadelphia (CHOP) from January 2005, when BKV polymerase chain reaction (PCR) testing became clinically available, until March 2012. We included any patient with BKV PCR testing of blood or urine performed from day 0 until 1 year post-HSCT. We excluded patients undergoing autologous HSCT and any patient without at least one blood or urine BK PCR test. The protocol was approved by our center’s Institutional Review Board.
At our institution, BKV testing is performed only for a clinical indication (no routine screening), and almost exclusively in patients with concern for HC due to gross hematuria and/or urinary symptoms. Testing is typically performed first with a urine PCR. Subsequent plasma testing is also ordered at the discretion of the attending physician, but usually for those patients with a positive urine PCR or persistent symptoms.
Separate BKV exposures were considered. First, patients with a positive BKV urine PCR (viruria, >0 copies/mL) were compared to those without viruria. Additionally, subjects with plasma PCR testing were compared using the quantitative value of their peak level of viremia during the first year after transplant. Using viral PCR thresholds reported in kidney transplant patients with BK viremia (4) and HSCT recipients with adenoviremia (17), subjects were categorized as having a peak plasma viral load of ≥10,000 copies/mL (high viremia) or <10,000 copies/mL (low viremia). As viremia is rare in patients without viruria, patients with negative urine testing who did not have plasma testing were included in the low viremia group.
The following separate outcomes were assessed: percent change in baseline serum creatinine-based estimated GFR (eGFR) at day 100, day 365, and the time of BKV infection, the need for dialysis at any point between day 0 and end of follow-up, and the severity of HC. Overall survival and transplant-related mortality were also considered as outcomes. Transplant-related mortality was defined as non-relapse death.
Each subject’s mean serum creatinine was recorded during the 14 days before the initiation of the conditioning regimen (baseline) and during the 14 day periods around day 100 and day 365. The creatinine on the first day of BKV testing was also obtained from the medical record. The eGFR was calculated with the modified Schwartz formula (18) [0.413*height(cm)/serum creatinine(mg/dL)], using the height at time of initiation of the conditioning regimen.
Urinalyses and the clinical record from the time of BKV testing were reviewed and the severity of HC was classified according to published criteria: grade 0=no hematuria; grade I=microscopic hematuria; grade II= gross hematuria; grade III=gross hematuria with clots; grade IV=need for catheterization or surgical intervention (19, 20). We defined “severe HC” as patients with grade IV disease.
Patients with positive CMV or adenovirus blood PCR testing (>0 copies/mL) within 2 weeks before or after BKV testing were classified as having a concomitant viral infection. At our institution blood CMV and adenovirus PCR testing is sent for clinical indication, weekly surveillance in all recipients of allogeneic T-cell depleted or cord blood grafts, and in all patients receiving alemtuzumab. In addition, CMV screening occurs in all recipients of matched related donor transplants if either the donor or recipient is CMV positive.
BKV testing was performed using a real-time TaqMan quantitative PCR assay with nucleic acid primer/probe pairs specific for conserved regions of the BK virus genome (Roche Molecular Systems, Inc.). In our hospital’s clinical laboratory, the assay can quantitate the viral load in urine or blood above a lower threshold of 302 copies/ml. Hospital protocol is to first test for BKV using a qualitative PCR, followed by quantitative testing for initial positive results.
The dates and viral load of all BKV quantitative measurements in blood and urine were recorded from the start of conditioning until 1 year after transplant. Baseline demographic and transplant data were collected, including age, gender, underlying condition, conditioning regimen, stem cell source, donor type, time to neutrophil and platelet engraftment, incidence of veno-occlusive disease (VOD), incidence and grading of acute and chronic graft versus host disease (GVHD), and survival up to one year or at time of last follow-up. In addition, we reviewed documentation regarding medical and surgical management of HC, and the need for dialysis. When BKV infection was treated with cidofovir, it was administered at a low dose of 0.25 mg/kg every 2 weeks (21).
As the patient population in this study was heterogeneous in terms of underlying disease, the transplant regimen was highly variable. For patients receiving myeloablative conditioning for hematologic malignancy, the conditioning regimen consisted of either busulfan/cyclophosphamide or cyclophosphamide/total body irradiation, with or without thiotepa. A subset of patients also received horse antithymocyte globulin. Recipients of reduced intensity conditioning regimens generally received fludarabine, melphalan, and alemtuzumab. Infectious prophylaxis varied by risk factors and graft type, but in general included acyclovir for HSV/VZV, intravenous immune globulin for those at risk of CMV, foscarnet in patients at high risk for CMV, fluconazole, and oral gentamicin/amoxicillin for gut decontamination. All patients received a calcineurin inhibitor for GVHD chemoprophylaxis (infusional cyclosporine followed by enteral tacrolimus). Additional GVHD prophylaxis included short-course methotrexate in bone marrow recipients, corticosteroids in cord blood recipients, mycophenolate mofetil in recipients of reduced intensity conditioning, and partial ex vivo T-cell depletion in recipients of growth factor-mobilized peripheral blood stem cell grafts.
Baseline demographic and transplant characteristics were compared using Chi-square or Fisher exact tests for categorical data, as appropriate. Continuous variables were compared using the Wilcoxon rank-sum test. Relative risks of developing the separate outcomes of severe HC or dialysis were calculated based on the presence of viruria or the degree of viremia (high viremia versus low viremia). Analysis of covariance (ANCOVA) was used to test the association between the peak quantitative urine or plasma PCR viral load and eGFR at the time of BK testing, 100 days, or 365 days, controlling for eGFR value at baseline. Quantitative viral load measurements were logarithmically transformed to account for skewed distribution. For mortality outcomes, survival analysis was performed using the Kaplan-Meier method and the log-rank test for comparisons between groups. All statistical analyses were performed using Stata 12.1 (copyright StataCorp LP) and SAS 9.2 (copyright SAS Institute, Inc.), and a two-sided pvalue of <0.05 was considered significant.
RESULTS
Study Population
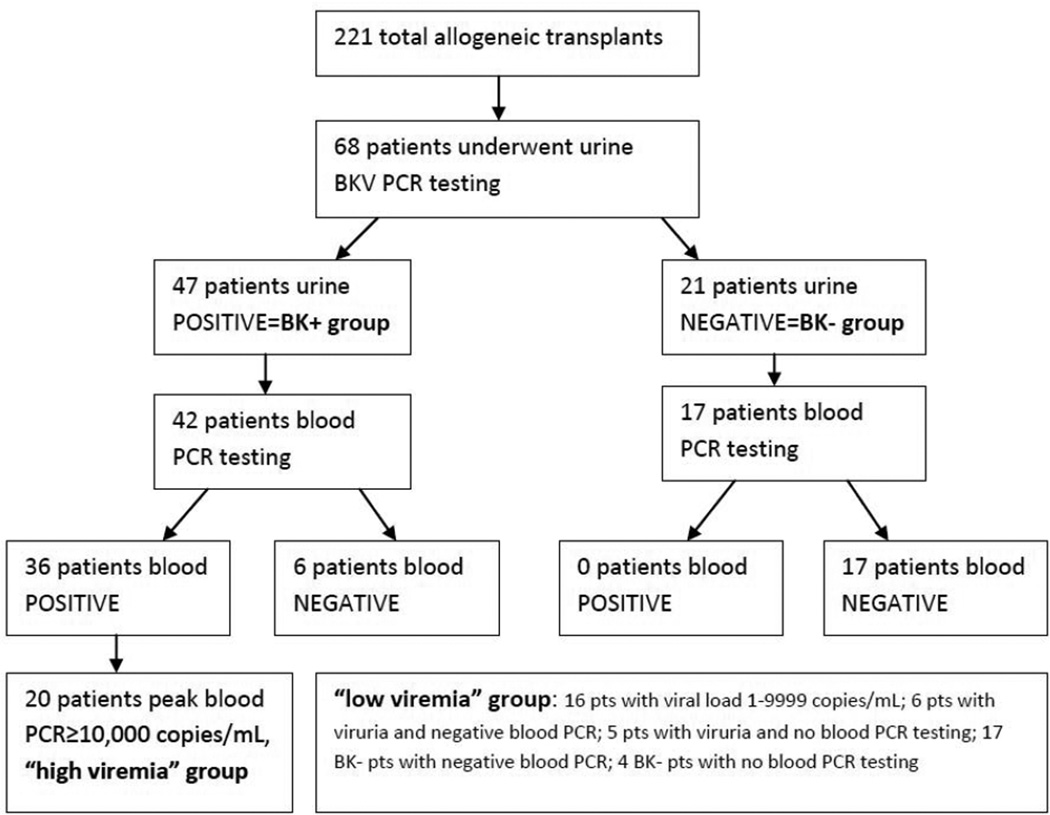
From January 2005 to March 2012, BKV testing (urine and/or plasma) was performed in 68 of 221 patients (30.8%) undergoing allogeneic HSCT at our center (Figure 1). Testing was almost exclusively performed for symptomatic HC, with only two subjects having testing sent for evaluation of persistent fever, both of whom were included in the final analyses. Of the 68 patients undergoing testing for BK viruria, 47 (69.1%) were positive in the urine. The remaining 21 subjects had negative urine testing during the study period, and comprised the BK negative group.
Figure 1.
Flowchart depicting categorization of subjects based on BKV urine and plasma testing
Of the 47 viruric patients 42 underwent plasma PCR testing for BKV and 36 were positive. Twenty of these viremic patients had a peak plasma PCR viral load ≥10,000 copies/mL (high viremia). Of the 21 patients without viruria, 17 underwent plasma PCR testing and none were positive. Therefore, a total of 59 patients underwent plasma testing. The low viremia group included subjects with peak plasma viral loads <10,000 copies/mL, those without viremia, and those without plasma testing but no viruria (Figure 1).
Most study subjects underwent myeloablative conditioning (88.2%) for a malignant indication (77.9%), and received a transplant from an unrelated donor (69.1%). There were no statistically significant differences in the clinical characteristics of age, gender, underlying disease, conditioning (myeloablative vs. reduced-intensity), donor type, and stem cell source between patients with and without viruria. Patients with high viremia were more likely to have received grafts from alternative donors compared to those with low viremia, but otherwise demographic and transplant characteristics were also similar between these viremia groups (Table 1).
Table 1.
Demographic and Clinical Characteristics by BK Viremia Status
|
High Viremia N=20 |
Low Viremia N=48 |
p-value | |
|---|---|---|---|
| Age (years) | 10.3 (5.3–17.8 ) | 11.9 (0.5–23.25) | 0.96 |
| Female gender, | 7 (35.0) | 23 (47.9) | 0.42 |
| Underlying disease | |||
| Malignant | 17 (85.0) | 36 (75.0) | 0.53 |
| Non-malignant | 3 (15.0) | 12 (25.0) | |
| Conditioning | |||
| Myeloablative | 18 (90.0) | 42 (87.5) | 1.0 |
| Reduced Intensity | 2 (10.0) | 6 (12.5) | |
| Donor type | |||
| Matched sibling | 2 (10.0) | 19 (39.6) | 0.02 |
| Alternative | 18 (90.0) | 29 (60.4) | |
| Stem Cell Source | |||
| Bone marrow | 4 (20.0) | 20 (41.7) | 0.19 |
| Peripheral blood | 13 (65.0) | 20 (41.7) | |
| Cord blood | 3 (15.0) | 8 (16.7) | |
Data shown as median (range) or n (%). P-values calculated using Fisher exact test for categorical variables and Wilcoxon rank-sum for continuous variables.
BKV Infection Characteristics
The 47 viruric patients had a median peak urinary viral load of 2.6×1010 copies/mL (range 3270–8.2×1011 copies/mL). The peak urinary viral load exceeded 1 billion copies/mL in 38 patients (80.9%). For those patients with any viremia (plasma PCR>0 copies/mL) the median peak plasma load was 12,780 copies/mL (range 202–5.5×107 copies/mL). Initial BKV testing occurred at a median of day 36 (interquartile range 8–83 days after HSCT) in the patients with viruria, and day 24 (interquartile range 8–58 days after HSCT) in the patients without viruria (p=0.15).
The first BKV urine PCR test was sent a median of 1 day (range 0–38 days) after the development of HC. A median of 1.5 urine tests (range 1–8 tests) were sent per patient, and 24 (51.1%) viruric patients had more than one urine PCR assessment. Of those with at least 2 measurements, BK viruria was monitored over a median span of 25 days (range 4–130 days). In viremic patients, the median time from development of HC to first plasma test was 2 days (range 0–76 days). Twenty-two (61.1%) viremic patients underwent repeat plasma PCR testing (median 2, range 1–9) over a median span of 23 days (range 1–137 days). Forty percent of viremic patients and 43.5% of viruric patients with multiple measurements were monitored for a span of more than 30 days. There were no differences in the time to the first urine or plasma measurement, number of samples tested, or the span over which testing was performed between patients with or without viruria. Patients with high viremia had more urine (but not plasma) samples checked (median 2 versus 1, p=0.046) over a longer time period (median 44.5 days versus 11 days, p=0.01) than those with low viremia.
Of patients with viruria, 18/47 (38.3%) received cidofovir to treat BKV infection. More patients with high viremia received cidofovir (73.3%) than those that were BK positive but with low viremia (41.2%) (p=0.067). In addition, more patients with severe HC (grade IV) received cidofovir (80%) compared to those with grades 0-III (36%) (p=0.06).
Severity of Hemorrhagic Cystitis
Among the 47 patients with viruria, 39 (83.0%) had HC grade II or worse and 5 (10.6%) had severe HC (grade IV). Two other patients had BK testing for evaluation of persistent fever and one patient was tested for indeterminate reasons. Severe HC was more likely in patients with high viremia as compared to those with low viremia (21.1% vs 2.2%, respectively; p=0.023). While the peak plasma viral load in patients with severe HC was quantitatively and significantly higher than in patients with lower grade HC (p=0.004), the peak urine viral load was not associated with the grade of HC (p=0.95) (Table 2).
Table 2.
Peak Plasma and Urine Viral Loads by Outcome
| HC | p- value |
Dialysis | p- value |
TRM | p- value |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grades 0-III N=51 |
Grade IV N=5 |
No Dialysis N=59 |
Dialysis N=7 |
Alive at 1yr N=52 |
Dead at 1yr N=16 |
|||||
| Viral Load |
Peak urine (copies/mL), median |
1.25×1010 | 1.96×1010 | 0.95 | 7.97×109 | 2.86×1010 | 0.59 | 5.43×109 | 1.31×1010 | 0.73 |
| Peak plasma (copies/mL), median |
2378 | 2,460,000 | <0.01 | 2378 | 26,110 | 0.23 | 2476 | 1826.5 | 0.79 | |
P-values calculated using Wilcoxon rank-sum tests to compare viral loads for each outcome. HC=hemorrhagic cystitis; TRM=transplant-related mortality
Kidney Injury
In all 68 patients undergoing BKV PCR testing, serum creatinine-based eGFR decreased from a median of 113.4 mL/min/1.73m2 at baseline to 102.7 mL/min/1.73m2 at day 100 (p=0.03) and to 97.0 mL/min/1.73m2 at day 365 (p<0.001). The absolute levels of eGFR and mean percent changes from baseline based on BKV status are summarized in Table 3. There were no differences in mean percent change from baseline eGFR as measured at time of BKV testing, day 100, and day 365 in those with and without viruria. Similarly, there were was no significant decrement in eGFR at the same time intervals in those with high versus low viremia. At day 100 the mean percent decrement in eGFR was significantly more in low viremia than high viremia patients, contrary to expected, but this effect was abrogated by day 365.
Table 3.
Serum creatinine-estimated glomerular filtration rate (eGFR) by BK Viruria and Viremia Status
| Viruria Status | Viremia Status | |||||||
|---|---|---|---|---|---|---|---|---|
| Viruria | No viruria | High Viremia | Low Viremia | |||||
| eGFR | % Δ | eGFR | % Δ | eGFR | % Δ | eGFR | % Δ | |
| Baseline | 119.4 | -- | 110.1 | -- | 113.0 | -- | 117.5 | -- |
| Time of BK | 108.5 | −6.8 | 105.2 | −3.4 | 110.7 | −1.8 | 109.7 | −5.2 |
| Day +100 | 103.7 | −1.3 | 96.9 | −9.4 | 126.7 | +14.6 | 104.5 | −10.9 |
| Day +365 | 92.9 | −15.7 | 97.6 | −12.1 | 102.2 | −6.3 | 97.3 | −18.0 |
eGFR estimated with the updated Schwartz formula. eGFR values presented as median ml/min/1.73m2. %Δ are the mean percent changes in in eGFR relative to baseline (i.e. a negative value indicates a decrement in eGFR).
Five patients (10.6%) with viruria and 2 patients (9.5%) without viruria required dialysis at any point post-HSCT (p=0.92). High viremia was not associated with the need for dialysis (p=0.43) There was a trend towards a higher peak plasma and urine viral load in patients requiring dialysis, but neither of these differences reached statistical significance (Table 2). The results were the same when subjects in whom dialysis was initiated prior to first BKV PCR (n=3) were excluded from the analysis (data not shown).
Additional Transplant Outcomes
Table 4 summarizes additional transplant outcomes for the study population by BK viremia status. There was a trend towards more Grade III–IV acute GVHD, chronic GVHD, and VOD in high viremia patients compared to those with low viremia, although none of these differences reached statistical significance. Similarly, there was a non-significantly higher incidence of a concomitant viral infection (CMV, adenovirus, either virus, or both viruses) in high versus low viremia patients.
Table 4.
Transplant Outcomes by BK Viremia Status
|
High Viremia N=20 |
Low Viremia N=48 |
p-value | |
|---|---|---|---|
| Engraftment—days | |||
| ANC>500 | 15 (11-19) | 17 (11-27) | 0.12 |
| Platelets>20k | 19 (16-33) | 20 (11-44) | 0.69 |
| Acute GVHD incidence | |||
| Any | 12 (66.7) | 27 (62.8) | 1.0 |
| Grade II-IV | 10 (50.0) | 19 (39.6) | 0.59 |
| Grade III-IV | 7 (35.0) | 10 (20.8) | 0.18 |
| Chronic GVHD incidence | |||
| Any | 7 (41.2) | 9 (27.3) | 0.35 |
| Extensive | 4 (20.0) | 6 (12.5) | 0.47 |
| VOD | 4 (20.0) | 3 (6.3) | 0.18 |
| Virus | |||
| CMV | 8 (40.0) | 10 (20.8) | 0.13 |
| Adenovirus | 5 (25.0) | 6 (12.5) | 0.28 |
| Either | 11 (55.0) | 14 (29.2) | 0.06 |
| Both | 2 (10.0) | 2 (4.2) | 0.58 |
| TRM at 1 year | 25.00% | 22.90% | 1.0 |
| OS at 1 year | 65.00% | 75.00% | 0.55 |
Data shown are median (interquartile range) or n (%). P-values calculated using Fisher exact test for categorical variables, Wilcoxon rank-sum for continuous variables, and log-rank test for survival. ANC=absolute neutrophil count; CMV=cytomegalovirus; GVHD=graft-versus-host disease; OS=overall survival; TRM=transplant-related mortality; VOD=veno-occlusive disease.
Overall survival (OS) at 1 year did not differ significantly between those with or without viruria (71.1% and 65.0%, respectively) or between the high and low viremia groups (65.0% and 75.0%, respectively). Neither urinary or plasma peak viral load predicted the 1 year OS, and the median urine and plasma peak viral loads was similar between subjects that survived to 1 year post-HSCT and those that did not (Table 2). Similarly, there were no statistically significant differences between transplant-related mortality based on either viruria or high viremia status (Table 4).
DISCUSSION
BKV infection is known to be associated with HC in adults and children undergoing HSCT (5, 6) and may also be associated with direct kidney injury (11–16), as seen in kidney transplant recipients (2). We found that viremia, but not viruria, was associated with more severe bladder injury (higher grade HC) in a retrospective cohort of children after allogeneic HSCT. In contrast, neither viremia nor viruria was associated with lower serum creatinine-estimated GFR, the need for dialysis, or mortality. The strengths of our analysis include follow-up to 1 year, assessment of kidney, bladder, and overall outcomes, and the inclusion of a large pediatric allogeneic HSCT population. Our study was limited by the retrospective reporting of clinical data and non-systematic measurement of BK virus infection in all subjects.
The timing and prevalence of BKV-related HC in our patients is consistent with prior reports (9, 16). The magnitude of the PCR-measured viral loads in the urine and plasma was also similar to a study in children after HSCT (16). We found no association between acute GVHD or the timing of engraftment and BKV infection, although there was a trend towards more severe acute GVHD in patients with high viremia. Others have speculated that BKV infection in HSCT recipients is related not only to the degree of immunosuppression but also direct tissue injury (22). As GVHD is associated with both augmented immunosuppression and tissue damage (23) one might expect a higher rate of GVHD in those with BKV infection. The high rate of GVHD in both viruric and nonviruric patients may explain our inability to detect an effect, possibly because most transplants were high-risk (alternative donors) compared to the general allogeneic transplant population.
The threshold for defining “high viremia” as a PCR viral load ≥10,000 copies/mL was originally described in kidney transplant recipients with biopsy-proven nephropathy (2). In children receiving HSCT, high viremia has been associated with the need for invasive procedures for HC, increased post-HSCT creatinine, dialysis, and end-stage renal disease (16). We also observed an association between high viremia and the severity of HC. Prior studies have documented BK virus (particularly viremia) as a risk factor for developing HC (7–9, 24, 25). However, to our knowledge only one prior study (16) has identified BK viral load as predictive of the severity of bladder injury after HC. The present study included children undergoing transplant primarily for a malignant indication, potentially broadening the applicability of the findings to a wider group of patients.
We were unable to detect an association between peak plasma PCR ≥10,000 copies/mL and creatinine-estimated kidney function or the need for dialysis. Subjects requiring dialysis did trend towards having a higher median peak plasma viral load, suggesting our study may have been underpowered to detect this difference. Peak urine viral loads were not associated with any outcome measure, a finding consistent with prior reports from both the adult and pediatric HSCT populations (16, 25).
Only two prior studies have reported measures of kidney function in patients with BKV infection after HSCT (15, 16). Both studies found an association between BK viremia and impaired kidney function, measured either by absolute serum creatinine level (15) or the maximum fold increase in serum creatinine relative to pre-transplant baseline (16), without calculating subjects’ eGFR. In our population, neither viruria nor the degree of viremia was associated with a need for post-transplant dialysis or a decrement in baseline eGFR, either at the time of BKV infection, day 100, or day 365.
Several factors may account for these discrepant findings. First, the prevalence of severe HC we observed is lower than that reported by others. As severe HC is often associated with obstructive and nephrotoxic (e.g. use of antivirals) kidney injury, differences in the rate and severity of HC across studies may explain the variable reporting of renal outcomes such as eGFR and the need for dialysis. Second, because kidney injury is multifactorial after HSCT, residual confounding of unmeasured exposures (including antibiotics, calcineurin inhibitors, and other infections) may also vary between different study populations. Third, by estimating kidney function at defined time periods, it is possible we missed the window of kidney injury, whereas other studies have reported the peak creatinine at any point after BK virus detection compared to the pre-transplant baseline. Fourth, the differences in the study population may influence kidney outcomes and the risk for severe BK virus infection; specifically, our cohort contained fewer patients transplanted for non-malignant indications and more peripheral blood stem cell and umbilical cord grafts. Finally, the paucity of patients requiring dialysis and differences in outcome definitions (overall, transplant-related, and BK-attributable mortality) limits our ability to make comparisons to previous studies.
The change in renal function over time observed in our study may be illustrative. Although not significant, it appeared there was only a modest decrease in eGFR at day 100, but a more pronounced decrease between day 100 and day 365. This may suggest it takes several months for patients with BKV infection to demonstrate changes in their kidney function. We were unable to directly evaluate this possibility as viral load measurements in viremic patients were made in a non-systematic fashion, and only a portion of viremic patients had longitudinal surveillance of plasma viral loads.
While serum creatinine is an established method of estimating kidney function, its concentration is highly dependent on muscle mass, potentially limiting its utility in children who have a high risk of deconditioning (after HSCT). Consistent with prior reports, it is possible serum creatinine overestimates GFR in this population (26). While this is the first study to calculate eGFR in children with BK virus infection after HCT, where height is used as a proxy for muscle mass, this adjustment is not exact. Additionally, we used a single measurement of height (taken pre-transplant) to estimate GFR throughout the transplant period, adding to the inaccuracy of GFR estimation in growing children. However, the relative paucity of vertical growth experienced during the immediate post-HSCT period likely offsets this factor. Future studies using muscle mass-independent methods to estimate kidney function may better define the degree of renal impairment after HSCT, both in those with and without BKV infection. One such method, serum cystatin C, is gaining more widespread use (26, 27).
Importantly, our cohort consisted of only patients having PCR testing performed for a clinical indication, raising the possibility these were the sickest patients. Indeed, the observed incidence of acute GVHD exceeded that typically seen in our general allogeneic HSCT population. Only larger studies using prospective surveillance for BKV infection in all HSCT recipients will be able to address this limitation. In addition, as prior studies have documented peak BK viral loads in urine and plasma that preceded the development of HC (24), it is possible that we missed a period of more severe BKV infection and therefore misclassified patients by degree of viruria or viremia.
In conclusion, we observed a high prevalence of BKV-associated HC in children undergoing allogeneic HSCT. The severity of HC correlated with the degree of BKV viremia, but not viruria. High viremia was more common in children receiving a transplant from an alternative donor. Consistent with kidney transplant guidelines, BK viremia may be more specific for clinical disease in HSCT recipients, as compared to BK viruria (28). In patients with HC or unexplained kidney injury after HSCT, we suggest initial testing of BKV with plasma and urine PCR assays to establish the diagnosis of BKV infection. Subsequently, if further monitoring is clinically indicated, testing should continue only with plasma. The development of targeted prophylaxis and/or treatment strategies may support the benefit of this monitoring approach in an effort to decrease the burden of severe HC. Although BKV infection was not associated with renal outcomes in the present study, future research is needed to address the impact of using more accurate measures of kidney function in this population at high risk of low muscle mass.
Acknowledgments
Financial Disclosure Statement: Dr. Laskin is supported by a Career Development Award in Comparative Effectiveness Research (KM1CA156715-01) and an American Society for Blood and Marrow Transplantation/Genentech New Investigator Award. Dr. Furth is supported by K24DK078737 and U01DK066174. None of these funding sources had any input in the study design, analysis, manuscript preparation, or decision to submit for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Knowles WA, Pipkin P, Andrews N, et al. Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J Med Virol. 2003;71:115–123. doi: 10.1002/jmv.10450. [DOI] [PubMed] [Google Scholar]
- 2.Ramos E, Drachenberg CB, Wali R, Hirsch HH. The decade of polyomavirus BK-associated nephropathy: state of affairs. Transplantation. 2009;87:621–630. doi: 10.1097/TP.0b013e318197c17d. [DOI] [PubMed] [Google Scholar]
- 3.Dropulic LK, Jones RJ. Polyomavirus BK infection in blood and marrow transplant recipients. Bone Marrow Transplant. 2008;41:11–18. doi: 10.1038/sj.bmt.1705886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirsch HH, Knowles W, Dickenmann M, et al. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med. 2002;347:488–496. doi: 10.1056/NEJMoa020439. [DOI] [PubMed] [Google Scholar]
- 5.Arthur RR, Shah KV, Baust SJ, Santos GW, Saral R. Association of BK viruria with hemorrhagic cystitis in recipients of bone marrow transplants. N Engl J Med. 1986;315:230–234. doi: 10.1056/NEJM198607243150405. [DOI] [PubMed] [Google Scholar]
- 6.Apperley JF, Rice SJ, Bishop JA, et al. Late-onset hemorrhagic cystitis associated with urinary excretion of polyomaviruses after bone marrow transplantation. Transplantation. 1987;43:108–112. doi: 10.1097/00007890-198701000-00024. [DOI] [PubMed] [Google Scholar]
- 7.Gaziev J, Paba P, Miano R, et al. Late-onset hemorrhagic cystitis in children after hematopoietic stem cell transplantation for thalassemia and sickle cell anemia: a prospective evaluation of polyoma (BK) virus infection and treatment with cidofovir. Biol Blood Marrow Transplant. 2010;16:662–671. doi: 10.1016/j.bbmt.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Gorczynska E, Turkiewicz D, Rybka K, et al. Incidence, clinical outcome, and management of virus-induced hemorrhagic cystitis in children and adolescents after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2005;11:797–804. doi: 10.1016/j.bbmt.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Cesaro S, Facchin C, Tridello G, et al. A prospective study of BK-virusassociated haemorrhagic cystitis in paediatric patients undergoing allogeneic haematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;41:363–370. doi: 10.1038/sj.bmt.1705909. [DOI] [PubMed] [Google Scholar]
- 10.Hingorani S. Chronic kidney disease in long-term survivors of hematopoietic cell transplantation: epidemiology, pathogenesis, and treatment. J Am Soc Nephrol. 2006;17:1995–2005. doi: 10.1681/ASN.2006020118. [DOI] [PubMed] [Google Scholar]
- 11.Raval M, Gulbis A, Bollard C, et al. Evaluation and management of BK virusassociated nephropathy following allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17:1589–1593. doi: 10.1016/j.bbmt.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verghese PS, Finn LS, Englund JA, Sanders JE, Hingorani SR. BK nephropathy in pediatric hematopoietic stem cell transplant recipients. Pediatr Transplant. 2009;13:913–918. doi: 10.1111/j.1399-3046.2008.01069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lekakis LJ, Macrinici V, Baraboutis IG, Mitchell B, Howard DS. BK virus nephropathy after allogeneic stem cell transplantation: a case report and literature review. Am J Hematol. 2009;84:243–246. doi: 10.1002/ajh.21358. [DOI] [PubMed] [Google Scholar]
- 14.Limaye AP, Smith KD, Cook L, et al. Polyomavirus nephropathy in native kidneys of non-renal transplant recipients. Am J Transplant. 2005;5:614–620. doi: 10.1046/j.1600-6143.2003.00209.x. [DOI] [PubMed] [Google Scholar]
- 15.O'Donnell PH, Swanson K, Josephson MA, et al. BK virus infection is associated with hematuria and renal impairment in recipients of allogeneic hematopoetic stem cell transplants. Biol Blood Marrow Transplant. 2009;15:1038–1048. doi: 10.1016/j.bbmt.2009.04.016. e1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haines HL, Laskin BL, Goebel J, et al. Blood, and not urine, BK viral load predicts renal outcome in children with hemorrhagic cystitis following hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:1512–1519. doi: 10.1016/j.bbmt.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Lee YJ, Chung D, Xiao K, et al. Adenovirus viremia and disease: comparison of T cell-depleted and conventional hematopoietic stem cell transplantation recipients from a single institution. Biol Blood Marrow Transplant. 2013;19:387–392. doi: 10.1016/j.bbmt.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz GJ, Munoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bedi A, Miller CB, Hanson JL, et al. Association of BK virus with failure of prophylaxis against hemorrhagic cystitis following bone marrow transplantation. J Clin Oncol. 1995;13:1103–1109. doi: 10.1200/JCO.1995.13.5.1103. [DOI] [PubMed] [Google Scholar]
- 20.Droller MJ, Saral R, Santos G. Prevention of cyclophosphamide-induced hemorrhagic cystitis. Urology. 1982;20:256–258. doi: 10.1016/0090-4295(82)90633-1. [DOI] [PubMed] [Google Scholar]
- 21.Lamoth F, Pascual M, Erard V, Venetz JP, Nseir G, Meylan P. Low-dose cidofovir for the treatment of polyomavirus-associated nephropathy: two case reports and review of the literature. Antivir Ther. 2008;13:1001–1009. [PubMed] [Google Scholar]
- 22.Mori Y, Miyamoto T, Kato K, et al. Different risk factors related to adenovirusor BK virus-associated hemorrhagic cystitis following allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18:458–465. doi: 10.1016/j.bbmt.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 23.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laskin BL, Denburg M, Furth S, et al. BK Viremia Precedes Hemorrhagic Cystitis in Children Undergoing Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2013;19:1175–1182. doi: 10.1016/j.bbmt.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erard V, Kim HW, Corey L, et al. BK DNA viral load in plasma: evidence for an association with hemorrhagic cystitis in allogeneic hematopoietic cell transplant recipients. Blood. 2005;106:1130–1132. doi: 10.1182/blood-2004-12-4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laskin BL, Nehus E, Goebel J, Khoury JC, Davies SM, Jodele S. Cystatin Cestimated glomerular filtration rate in pediatric autologous hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18:1745–1752. doi: 10.1016/j.bbmt.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bia M, Adey DB, Bloom RD, Chan L, Kulkarni S, Tomlanovich S. KDOQI US commentary on the 2009 KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Kidney Dis. 2010;56:189–218. doi: 10.1053/j.ajkd.2010.04.010. [DOI] [PubMed] [Google Scholar]