Abstract
Wnt signalling is involved in multiple aspects of embryonic development and adult tissue homeostasis, notably via controlling cellular proliferation and differentiation. Wnt signalling is subject to stringent positive and negative regulation to promote proper development and homeostasis yet avoid aberrant growth. Such multi‐layer regulation includes post‐translational modification and processing of Wnt proteins themselves, R‐spondin (Rspo) amplification of Wnt signalling, diverse receptor families, and intracellular and extracellular antagonists and destruction and transcription complexes. In the gastrointestinal tract, Wnt signalling is crucial for development and renewal of the intestinal epithelium. Intestinal stem cells (ISCs) undergo symmetric division and neutral drift dynamics to renew the intestinal epithelium. Sources of Wnts and Wnt amplifers such as R‐spondins are beginning to be elucidated as well as their functional contribution to intestinal homeostasis. In this review we focus on regulation of ISCs and intestinal homeostasis by the Wnt/Rspo pathway, the potential cellular sources of Wnt signalling regulators and highlight potential future areas of study.
Abbreviations
- ISC
intestinal stem cell
- LEF
lymphoid enhancer factor
- LRP5/6
low density lipoprotein receptor‐related protein 5/6
- PCP
planar cell polarity
- TCF
T‐cell factor
Wnt signalling: key players and pathways
The Wnt pathway is highly conserved across species and is broadly implicated in embryonic development, adult tissue homeostasis as well as disease pathophysiology (Clevers, 2006; van Amerongen & Nusse, 2009; Clevers & Nusse, 2012). Mammals possess genes for 19 Wnt ligands and 10 Frizzled (Fzd) receptors, which are seven‐pass transmembrane receptors that mediate downstream Wnt signalling. Secretion of Wnt proteins relies on a number of processes including palmitoylation in the endoplasmic reticulum by the palmitoyltransferase Porcupine (Porcn). Lipidated Wnts can then bind to Wntless on the Golgi membrane where they are transferred to the plasma membrane for secretion (Kikuchi et al. 2011; Willert & Nusse, 2012). Wnts travel short distances to nearby cells to initiate downstream signalling that includes both canonical and non‐canonical pathways. The canonical pathway is the best characterized and relies on β‐catenin as its main effector protein. In canonical Wnt signalling, secreted Wnt proteins act in an autocrine or paracrine fashion by binding and forming a complex with two receptors: Fzd and low density lipoprotein receptor‐related protein 5/6 (LRP5/6). This in turn activates signalling events including recruitment of proteins such as Dishevelled and conformational changes that collectively result in a block on β‐catenin degradation by a destruction complex of proteins comprising Axin, adenomatous polyposis coli (APC), casein kinase I (CKI) and glycogen synthase kinase 3β (GSK3β). Stabilized β‐catenin then translocates to the nucleus and associates with heterodimers of the lymphoid enhancer factor (LEF) and T‐cell factor (TCF) family of transcription factors to transactivate expression of Wnt target genes (Fig. 1). In the absence of Wnt signalling, the destruction complex targets cytosolic β‐catenin for proteasomal degradation (Niehrs, 2012). Non‐canonical or β‐catenin‐independent pathways have been implicated in processes such as planar cell polarity (PCP) and calcium signalling. While the Wnt pathway is involved in these processes, the role of specific pathway components in these processes is not fully elucidated. PCP describes the orientation of a group of cells in a tissue along the plane and is important in gastrulation, neural tube closure and cell/tissue polarity (extensively reviewed in Simons & Mlodzik, 2008). In vertebrates, loss of Wnt by genetic deletion or secretion of Wnt inhibitors resulted in defects in PCP in the mouse inner ear (Heisenberg et al. 2000; Dabdoub et al. 2003; Qian et al. 2007). In addition to PCP, non‐canonical Wnt signalling is implicated in calcium release mediated by Fzd (Slusarski et al. 1997; Sheldahl et al. 1999; Kuhl et al. 2000); however, it is unknown whether this effect is Wnt dependent or is mediated by interaction of Fzd with non‐Wnt ligands eliciting downstream responses.
Figure 1. Overview of the canonical Wnt pathway and points of regulation .
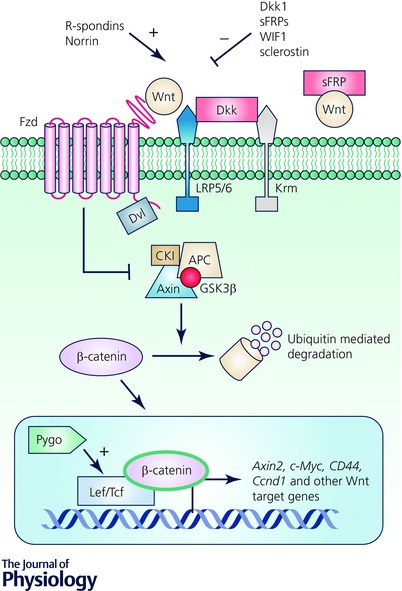
Wnts bind to their receptors Fzd/Lrp5/6, recruit Dvl and block the destruction complex from degrading cytosolic β‐catenin. Stabilized β‐catenin translocates to the nucleus where it interacts with LEF/TCF to activate Wnt target genes. Wnt antagonists such as Dkk, sFRPs, WIF1 and sclerostin block Wnt signalling by binding and blocking Wnt ligands or their receptors. Norrin and R‐spondins promote Wnt activity by binding or stabilizing membrane availability of Fzd/Lrp5/6. Pygo promotes Wnt signalling by activating transcription via interactions with the complex of proteins bound to the LEF/TCF transcription factors.
Multi‐tiered regulation of Wnt signalling
Due to its central importance and involvement in myriad physiological pathways, Wnt signalling is appropriately subject to multi‐tiered positive and negative regulation (Fig. 1). Wnt secretion itself is regulated post‐translationally through palmitoylation by Porcn and Wntless as described above. Extracellular antagonists such as Dickkopf‐1 (Dkk1), secreted Frizzled‐related proteins (sFRPs), Wif1 and sclerostin inhibit Wnt signalling by binding LRP5/6, Fzds or Wnts themselves thereby preventing pathway activation. Dkk proteins form ternary complexes with LRP and Kremen proteins, leading to LRP internalization, down‐regulation and antagonism of canonical signalling (MacDonald et al. 2009). In contrast, sFRPs antagonize Wnt signalling by directly binding and neutralizing Wnt ligands and are therefore implicated in regulating both canonical and non‐canonical Wnt pathways (Cruciat & Niehrs, 2013). At the intracellular level, Wnt signalling can be transcriptionally regulated by proteins that can bind or complex with β‐catenin/TCF/LEF such as Pygopus and transducin‐like enhancer of split proteins to activate or repress expression of Wnt target genes (Lien & Fuchs, 2014). Secreted Wnt agonists that enhance or promote Wnt signalling include Norrin and R‐spondins (Rspos). Norrin binds to Fzd and activates Wnt signalling independent of Wnts (Xu et al. 2004; Cruciat & Niehrs, 2013). Rspos potently amplify Wnt signalling by binding and forming a complex with their receptors to increase the stability of Fzds at the plasma membrane, thereby potentiating Wnt signalling ability (de Lau et al. 2014) as we discuss in greater detail below.
Renewal of the intestinal epithelium by intestinal stem cells
Stem cells are responsible for maintaining tissue function within diverse organs, such as the intestine. The intestinal epithelium is highly proliferative and is organized into invaginations (‘crypts’) and finger‐like projections that protrude out into the lumen (‘villi’) that undergo complete renewal every 3–10 days driven by intestinal stem cells (ISCs). ISCs give rise to transit‐amplifying (TA) cells that undergo differentiation as they exit the crypt and enter the villus compartment and are ultimately shed into the lumen. This rapidly proliferating and highly renewable nature renders the intestine a tissue of great interest to understanding stem cell biology. The past decade has seen an explosion of interest and advancement in our knowledge of ISC biology and regulation of ISC dynamics and behaviour. ISC maintenance is exquisitely controlled by a number of coordinated signals and pathways present in the niche in order to adapt to physiological demands as well as prevent aberrant growth.
Identification of ISC biomarkers through genetic lineage tracing has revealed two functionally distinct pools of ISCs, actively cycling and quiescent, that act in maintaining or restoring intestinal homeostasis. The two ISC pools have distinct but overlapping markers and crypt localization. Actively cycling crypt base columnar ISCs are located at the crypt base intercalated between Paneth cells. Quiescent or slowly cycling ISCs are found around the +4 region of the crypt above Paneth cells, which was identified by Potten and colleagues based on the ability of these cells to retain DNA label (label retaining cell) (Potten et al. 1974). Lgr5, encoding a seven‐pass G protein‐coupled receptor‐like protein, was the first marker of actively cycling ISCs identified using genetic approaches to irreversibly mark and lineage trace Lgr5+ cells and their multipotent progeny (Barker et al. 2007). Olfm4 and Ascl2 were also identified as actively cycling ISC markers that were enriched in Lgr5+ ISCs and Olfm4+ cells and have lineage tracing potential (van der Flier et al. 2009 a,b; Schuijers et al. 2014). Lgr5+ ISCs undergo symmetric division and niche competition with random clonal expansion and subsequent crypt monoclonality, termed neutral drift dynamics (Lopez‐Garcia et al. 2010; Snippert et al. 2010; Kozar et al. 2013). Lineage tracing of candidate quiescent ISC populations have yielded multiple biomarkers such as Bmi1, Hopx, mTert and Lrig1; however, these differ subtly in crypt position, indicating the potential co‐existence of distinct quiescent ISCs (Sangiorgi & Capecchi, 2008; Montgomery et al. 2011; Takeda et al. 2011; Powell et al. 2012). Transcriptomic and proteomic profiling and multicoloured fluorescence in situ hybridization (FISH) have revealed that quiescent ISC markers are not exclusively present in these cells and can be broadly expressed along the crypt and co‐expressed in Lgr5+ ISCs (Itzkovitz et al. 2012; Munoz et al. 2012). Furthermore, distinct ISC populations are related by lineage, as toxin‐mediated direct ablation of Lgr5+ cells revealed that Bmi1+ cells can replenish the ISC pool by giving rise to Lgr5+ cells (Tian et al. 2011). Similarly, Epstein and colleagues demonstrated analogous bi‐directional interconversion when they reported Hopx‐expressing ISCs at the +4 position generate progeny expressing Lgr5 and vice versa (Takeda et al. 2011).
Despite substantial expression overlap, the actively cycling and quiescent ISC populations are functionally distinct. Actively cycling Lgr5+ ISCs rapidly lineage trace and are responsible for physiological intestinal renewal (Barker et al. 2007). Emerging evidence supports the notion that quiescent ISCs comprise a reserve stem cell population that is injury‐inducible (Fig. 2). Both mTert+ and Bmi1+ ISCs exhibit relative baseline quiescence with rare homeostatic lineage tracing, which can be augmented upon intestinal injury (Montgomery et al. 2011; Yan et al. 2012). Van Landeghem and colleagues used the Sox9‐EGFP transgenic mouse model, where distinct levels of the Sox9‐EGFP transgene mark actively cycling Lgr5 enriched ISCs (Sox9‐EGFPLow) and quiescent ISCs (Sox9‐EGFPHigh) within the same animal. In this system, Sox9‐EGFPHigh cells, also enriched in mRNAs encoding enteroendocrine cell (EEC) hormones, re‐enter the cell cycle following radiation‐induced crypt ablation and generate actively cycling Lgr5 enriched Sox9‐EGFPLow ISCs providing additional evidence that this population containing reserve ISC are injury‐inducible (Van Landeghem et al. 2012). Additionally, Dll1+ secretory progenitors have been shown to be adopt ISC functional characteristics following radiation injury (van Es et al. 2012 b). Buczacki et al. have further revealed that label‐retaining cells (LRCs) are secretory progenitors co‐expressing Lgr5 and markers of Paneth cells and EECs under homeostatic conditions but can lineage trace following damage (Buczacki et al. 2013). Collectively, these studies support a model where the intestinal epithelium contains two categories of actively cycling versus quiescent ISC populations that coexist harmoniously and function in distinct situations to maintain intestinal homeostasis (Li & Clevers, 2010). Additionally, elegant genetic and crypt ablation studies have provided novel and exciting evidence that the concept of intestinal epithelial cell plasticity or dedifferentiation of progenitors potentially exists (van Es et al. 2012 b; Van Landeghem et al. 2012; Buczacki et al. 2013); however, conclusive evidence implicating the ability of mature differentiated cells to adopt ISC function has yet to be shown.
Figure 2. The intestinal epithelium contains two functionally distinct pools of intestinal stem cells (ISCs) .
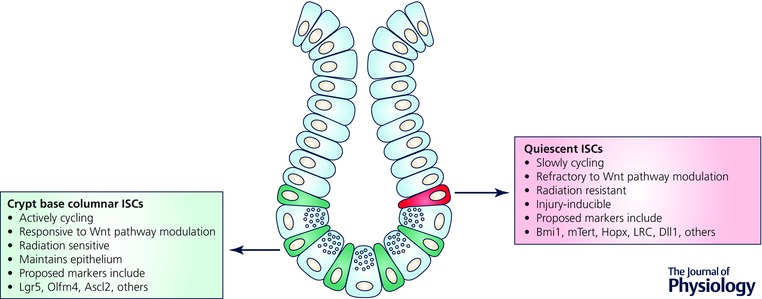
Crypt base columnar ISCs (green) and +4 quiescent ISCs (red) co‐exist in the intestinal crypt and differ in their cycling kinetics, sensitivity to extracellular Wnt pathway manipulations and radiation.
Essential Wnt pathway regulation of intestinal epithelial self‐renewal and maintenance
Wnt signalling is a critical component of the ISC niche. Clearly, Wnt pathway activation by Apc gene mutation and constitutive activation of β‐catenin, specifically in ISCs, is sufficient to induce intestinal epithelial hyperproliferation and polyposis (Barker et al. 2009; Krausova & Korinek, 2014; Powell et al. 2014). Conversely, loss‐of‐function gene ablation studies have been critical in identifying the vital role of canonical Wnt signalling in maintaining intestinal homeostasis, proliferation and ISC biology. Intestines of mice lacking the Wnt effector gene Tcf4/Tcf7l2 were devoid of intestinal crypts and consisted of solely differentiated villus cells (Korinek et al. 1998). Subsequent studies using conditional genetic deletion of Tcf4 and Wnt effector β‐catenin (Ireland et al. 2004; Fevr et al. 2007; van Es et al. 2012 a) or ectopic expression of the Wnt antagonist Dkk1 (Pinto et al. 2003; Kuhnert et al. 2004) resulted in loss of intestinal crypts and alterations in differentiated linages. Intestinal epithelial‐specific Dkk1 transgenic expression elicits non‐lethal loss of small intestine crypts, intestinal proliferation and secretory lineages (Pinto et al. 2003). An even more severe phenotype is observed upon adenovirus‐mediated circulating Dkk1 expression, which results in global Wnt inhibition, rapid crypt and villus loss in small intestine, severe colitis and fully penetrant lethality (Kuhnert et al. 2004). Genetic deletion of Wnt target gene Myc has been shown to be important for postnatal intestinal crypt formation (Bettess et al. 2005) and regulating crypt size and proliferation during adulthood (Muncan et al. 2006). The effect on crypts and differentiated lineages in these loss‐of‐function studies reveals the critical role of the Wnt pathway in ISC function, differentiation and maintenance of epithelial architecture.
The intestinal effects of impaired Wnt ligand secretion have been evaluated by genetic Porcn deletion. Epithelial‐specific Porcn loss did not affect intestinal homeostasis or intestinal regeneration following radiation injury indicating epithelial Wnt production is dispensable (Kabiri et al. 2014; San Roman et al. 2014); however, evidence for stromal Wnt contributions to intestinal homeostasis is less conclusive. Further, Porcn deletion from subepithelial myofibroblasts did not affect intestinal homeostasis (San Roman et al. 2014). On the other hand, pharmacological Porcn inhibition to mimic loss of epithelial and stromal Wnts resulted in decreased Lgr5+ ISCs, loss of the Wnt‐independent crypt base columnar ISC marker Olfm4, intestinal proliferation and impaired regenerative capacity (Kabiri et al. 2014). Taken together, these studies suggest that potential redundancy of Wnts between undefined compartments of intestinal stroma and/or epithelium may govern ISCs and intestinal homeostasis. Notably mouse knockouts of Wnt ligands have not thus far been associated with intestinal homeostatic phenotypes, although Wnt5a loss impairs colonic crypt regeneration following injury (Miyoshi et al. 2012). Recently, Foxl1+ mesenchymal cells have been shown to be a crucial cell type providing both Wnt and Rspo within the intestinal niche, as they are enriched for Wnt2b, Wnt5a and Rspo3. Toxin‐mediated loss of Foxl1+ cells resulted in shortened intestinal length along with decreased crypt depth, villus height and intestinal epithelial proliferation. More strikingly, loss of Foxl1+ cells ablated Olfm4+ signal associated with loss of Wnt2a, Wnt4 and Wnt5a mRNA (Aoki et al. 2016). This recent study implicates Foxl1+ mesenchymal cells in Wnt/Rspo synthesis and intestinal homeostasis and provides potential future possibilities to elucidate the role of other mesenchymal cells in synthesis and secretion of other Wnts.
Identifying the sources of Wnt proteins is important to understanding their function on ISCs during intestinal homeostasis, regeneration or disease, particularly since the palmitoyl modification restricts long‐range diffusion. In situ hybridization of Wnt ligands showed differential expression of various Wnts along the crypt–villus axis as well as in epithelial versus mesenchymal compartments (Gregorieff et al. 2005). Wnt3 and Wnt9b expression was observed in Paneth cells while Wnt6 was more diffusely expressed along the crypt epithelium. Expression of Wnts in the mesenchymal compartment included Wnt2b and Wnt5a (Gregorieff et al. 2005) from Foxl1+ mesenchymal cells (Aoki et al. 2016). Future studies may allow functional and phenotypical correlation of Wnts with their localization in the ISC niche and their phenotypes, if any, on ISCs.
Role of R‐spondins during development and intestinal homeostasis
R‐spondin proteins (Rspo1–4) are secreted amplifiers of Wnt signalling that are highly conserved among vertebrates. Structurally, Rspo1–4 are unified by a common domain architecture comprising a C‐terminal Thrombospondin type 1 repeat domain and two furin‐like repeats, where the latter is essential for their function in enhancing Wnt signalling (de Lau et al. 2012). In vitro TOPflash studies in HEK293 cells demonstrated the Wnt signalling ability of Rspo proteins in its potent synergism with Wnt3a (Kazanskaya et al. 2004).
Single Rspo knockout mice have not revealed any adult intestinal phenotypes despite genetic data indicating key roles and functions of Rspo1–4 during embryogenesis and development. The functions of Rspo1–4 have been explored using genetic mutation studies and are reviewed in greater detail (de Lau et al. 2012). Briefly, Rspo1 is involved in development of the sex phenotype, differentiation of the skin and predisposition to skin carcinoma (Parma et al. 2006; Tomizuka et al. 2008). Loss of Rspo2 has been shown to inhibit development of limbs, lungs, hair and ovarian follicles, associated with decreased Wnt activity, and mutants die shortly after birth due to limb defects (Nam et al. 2007; Bell et al. 2008; Cheng et al. 2013). Rspo3 is involved in angiogenesis and placental development. Targeted disruption of Rspo3 results in severe defects in placental vascularization and ultimately leads to death at E10 (Aoki et al. 2007; Kazanskaya et al. 2008). In C2C12 myoblasts, Rspo1 or ‐2 overexpression and Rspo2 or ‐3 knockdown were associated with increased and decreased myogenic differentiation, respectively, suggesting a role for Rspos in cellular differentiation (Han et al. 2011). Lastly Rspo4 is involved in fingernail development (Bergmann et al. 2006; Blaydon et al. 2006; Bruchle et al. 2008; Ishii et al. 2008). Overall, while genetic Rspo deletion phenocopies deletion of key Wnt signalling genes or that of reduced Wnt activity (Monkley et al. 1996; Vainio et al. 1999; Ishikawa et al. 2001), Rspo genetic deletion in regulation of ISCs or intestinal homeostasis has been elusive and has been potentially complicated by embryonic lethality (Aoki et al. 2007; Nam et al. 2007). Recently, Storm and colleagues treated mice with neutralizing antibodies raised against Rspo2 and Rspo3 and reported decreased Lgr5 reporter gene expression suggesting a requirement for Rspo2/3 in Lgr5+ ISC maintenance without any impairment of intestinal homeostasis or crypt architecture. However in response to radiation, anti‐Rspo2/3 treated animals displayed impaired regeneration providing evidence for the role of both Rspo2 and Rspo3 in intestinal repair (Storm et al. 2016).
Conversely, Rspo has profound effects on intestinal homeostasis and ISC in gain‐of‐function contexts. In vivo experiments using ectopic Rspo1 administration leads to expansion of many intestinal parameters including small intestinal diameter, weight, crypt density, ISC number and proliferation (Kim et al. 2005; Yan et al. 2012). Rspo1 is a critical component in the growth and expansion of in vitro organoids (‘miniguts’) in three‐dimensional Matrigel culture. In this system, a growth factor cocktail consisting of epidermal growth factor (EGF), Noggin and Rspo1 is used to mimic key signals present in the intestinal crypt to promote self‐renewal, multipotency and organoid formation (Sato et al. 2009). Although potent, these gain‐of‐function effects in vivo and in vitro could reflect ectopic and/or non‐physiological activation of ISC Wnt signalling. Thus, further genetic and/or pharmacological demonstrations of endogenous Rspo functions on ISCs and intestinal homeostasis are sorely needed.
The precise locations or sources of intestine‐relevant Rspo proteins, within the gastrointestinal tract or at remote sites, are of considerable interest. Expression of Rspo1–3 has been reported in the intestinal stromal fraction and minimally detected in the intestinal epithelium (Kabiri et al. 2014). Rspo2 expression in myofibroblasts increased in response to C. rodentium infection, leading to aberrant proliferation and impaired differentiation of colonic epithelial cells, thus providing evidence that links Rspo proteins to pathophysiological states (Papapietro et al. 2013). The overlapping expression of Wnts and Rspos within different epithelial and stromal populations of the intestine may enable significant functional redundancy, perhaps to protect from ISC dysfunction and catastrophic loss (Kabiri et al. 2014).
R‐spondins bind distinct receptors of the Lgr4–6 (seven‐pass transmembrane) and Rnf43 and Znrf3 (Rnf43/Znrf3) (transmembrane E3 ubiquitin ligase) classes (Carmon et al. 2011; de Lau et al. 2011; Glinka et al. 2011; Xie et al. 2013). In the absence of Wnt signals, Rnf43/Znrf3 act as negative regulators of Wnt signalling by ubiquitination and subsequent endocytosis and lysosomal degradation of Fzd and Lrp (Hao et al. 2012; Koo et al. 2012), which is Dishevelled‐dependent (Jiang et al. 2015). Rspo binding to Lgr4–6 and Rnf43/Znrf3, perhaps as a ternary complex, inhibits Rnf43/Znrf3‐mediated degradation of Fzd/Lrp resulting in stabilization and accumulation of Fzd/Lrp receptors on the plasma membrane to enhance Wnt signalling (Fig. 3). Intestinal epithelium‐specific Lgr5 deletion surprisingly did not show an overt intestinal phenotype while Lgr4 deletion decreased proliferation and induced crypt loss associated with decreased Olfm4 mRNA. Furthermore, Lgr4 null mice displayed impaired ex vivo organoid formation (Mustata et al. 2013). Combined Lgr4/Lgr5 deletion further exacerbated the intestinal phenotype seen with loss of Lgr4 alone (de Lau et al. 2011) and was similar to effects seen in mice ectopically expressing Dkk1 (Kuhnert et al. 2004). Conversely, conditional intestinal deletion of Rnf43/Znrf3 in mice resulted in expansion of crypt size associated with hyperproliferation and expansion of Olfm4 + cells (Koo et al. 2012). Despite evidence of alterations of ISC number by Olfm4 in situ hybridization, Rspo receptor knockout studies have not examined the subsequent fate of ISCs, as lineage‐tracing studies have not been performed. Recently, Rnf43 has been shown to be present in the nucleus and interact with and prevent TCF4‐mediated transcription, suggesting an additional mechanism by which Rnf43 negatively regulates Wnt signalling (Loregger et al. 2015) (Fig. 3).
Figure 3. R‐spondins potently amplify Wnt signalling .
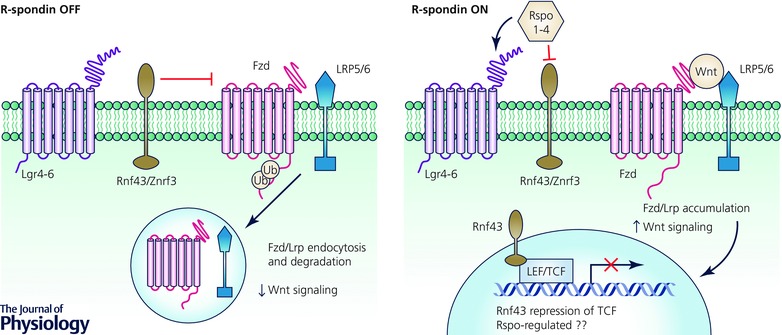
The transmembrane E3 ubiquitin ligases Rnf43/Znrf3 negatively regulate Wnt signalling by ubiquitinating the cytoplasmic tails of Fzds to promote their membrane clearance by endocytosis and degradation, ultimately downregulating Wnt signalling. When R‐spondins (Rspo1–4) are present, Rnf43/Znrf3 mediated degradation of Fzds is inhibited and Fzd/Lrp accumulation on the plasma membrane upregulates Wnt signalling. In an independent mechanism, Rnf43 present on the nuclear membrane interacts with and prevents LEF/TCF‐mediated transcription.
Differential regulation of ISCs by the Wnt/Rspo signalling pathway
The profound intestinal defects observed in animals with disrupted Wnt signalling or Rspo1 overexpression are consistent with an important role for Wnt/Rspo signalling in ISC behaviour and function. The Wnt pathway is active as a gradient in the intestinal crypt, most prominently at the base and decreasing up the crypt–villus axis (Scoville et al. 2008). This expression gradient, coupled with the differential location of actively cycling versus quiescent ISCs within the crypt suggests differential responses to Wnt levels. A direct lineage tracing comparison of Lgr5+ and Bmi1+ ISCs in Lgr5‐eGFP‐IRES‐CreERT2 and Bmi1‐CreER;Rosa26‐YFP mice, respectively, indicates that modulation of Wnt signalling differentially affects these two populations of ISCs. Rspo robustly expanded Lgr5+ or Olfm4+ cells with increased mitotic index while Bmi1+ cells remained unaffected. Conversely, the Wnt antagonist Dkk1 potently depleted Lgr5+ cells but not Bmi1+ cells. Overall, the Lgr5+ and Bmi1+ ISC pools appear functionally distinct with differential response to Wnt pathway manipulations (Yan et al. 2012) (Fig. 2). Histological analyses and single molecule FISH revealed that conditional loss of Tcf4 in intestinal epithelial cells resulted in loss of actively cycling ISC markers Lgr5 and Olfm4 with little effect on Bmi1 transcript levels (van Es et al. 2012 a). Additionally, intestinal epithelium‐specific loss of Rnf43/Znrf3 expanded the numbers of Olfm4 + cells and led to adenoma formation (Koo et al. 2012). A combination of anti‐Rspo2 and anti‐Rspo3 monoclonal antibodies reduced crypt Lgr5 expression in vivo but this was not accompanied by crypt loss or lethality (Storm et al. 2016). Thus, Lgr5+ ISCs in particular are responsive to modulation of Wnt signalling.
Future directions
There is a substantial body of evidence supporting roles of the Wnt/Rspo pathway in ISC behaviour and maintenance of the intestinal epithelium, but at the same time this raises numerous additional questions. While much of the known effects of Wnt and Rspo ligands on ISCs are inferred from gain‐of‐function or global inhibitor studies (Dkk1, Porcn antagonists), analogous loss‐of‐function data for specific individual Wnt or Rspo members have only begun to be explored (Storm et al. 2016), with open questions on regulation of ISC fate and differentiation. Indeed, genetic deletion of specific single Wnt, Fzd or Rspo family members have still not been associated with gross intestinal homeostatic phenotypes, perhaps complicated by mobilization of reserve stem cell populations, functional redundancy and/or need for conditional deletion approaches. The effects of Wnt5a deletion on colonic epithelial regeneration but not homeostasis (Miyoshi et al. 2012) suggests that additional complexity could exist whereby specific Wnt ligands might differentially regulate intestinal homeostasis versus repair or pathogenesis based on their anatomical location within the niche and their effect on ISCs. Similarly, this may also hold true for specific Rspo ligands (Storm et al. 2016). In this regard, it will be important to understand not only the spatial location of specific essential Wnt and Rspo ligands such as in Foxl1+ mesenchymal cells, but also the circuitry governing their expression during homeostasis versus regeneration. It should be interesting to overlay Wnt/Rspo functional manipulation onto the elegant framework of Lgr5+ ISC symmetric division and neutral drift dynamics, as well as interconversion between active and reserve stem cell pools. Lastly, the relative contribution of Wnt and Rspo ligands to physiological Wnt signalling and regulation of ISC fate is unknown, and could be substantially aided by development of highly specific gain‐of‐function methods for dissecting apart these two intersecting pathways. Regardless, continued exploration of Wnt or Rspo biology will illuminate how these fascinating ligand families regulate ISC fate during both intestinal homeostasis and disease.
Additional information
Competing interests
The authors declare no competing interests, financial or otherwise, contributing to this work.
Author contributions
All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by NIH/NIDDK Intestinal Stem Cell Consortium U01DK085527, U19AI116484, U01DE025188‐01, U01CA176299 and 1U01CA151920 to C.J.K.
Acknowledgements
We apologize to those whose work we are unable to cite because of space constraints. We thank members of the Kuo lab for helpful discussions.
Biographies
Amanda Mah is a postdoctoral researcher at Stanford University in Calvin Kuo's laboratory. She received her PhD in Nutrition at the University of North Carolina at Chapel Hill where she studied nutritional regulation of intestinal stem cells.

Kelley Yan received her MD and PhD in Structural Biology and Biophysics from the Mount Sinai School of Medicine of New York University. Then she moved to Stanford University to complete her clinical training in Internal Medicine and Gastroenterology as well as her postdoctoral research fellowship in Calvin Kuo's laboratory. She is currently an Instructor of Medicine at Stanford University School of Medicine where she conducts research in epithelial stem cell biology focusing on gastrointestinal tissues.
Calvin Kuo is the Maureen Lyles D'Ambrogio Professor of Medicine at Stanford University School of Medicine and Vice Chair for Basic and Translational Research within the Department of Medicine. He also co‐leads the Cancer Biology Program at the Stanford Cancer Center. His work encompasses gastrointestinal stem cells, organoid models of cancer and vascular biology.
References
- Aoki M, Mieda M, Ikeda T, Hamada Y, Nakamura H & Okamoto H (2007). R‐spondin3 is required for mouse placental development. Dev Biol 301, 218–226. [DOI] [PubMed] [Google Scholar]
- Aoki R, Shoshkes‐Carmel M, Gao N, Shin S, May CL, Golson ML, Zahm AM, Ray M, Wiser CL, Wright CV, Kaester KH (2016). Foxl1‐expressing mesenchymal cells constitute the intestinal stem cell niche. Cell Mol Gastroenterol Hepatol 2, 175–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ & Clevers H (2009). Crypt stem cells as the cells‐of‐origin of intestinal cancer. Nature 457, 608–611. [DOI] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ & Clevers H (2007). Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007. [DOI] [PubMed] [Google Scholar]
- Bell SM, Schreiner CM, Wert SE, Mucenski ML, Scott WJ & Whitsett JA (2008). R‐spondin 2 is required for normal laryngeal‐tracheal, lung and limb morphogenesis. Development 135, 1049–1058. [DOI] [PubMed] [Google Scholar]
- Bergmann C, Senderek J, Anhuf D, Thiel CT, Ekici AB, Poblete‐Gutierrez P, van Steensel M, Seelow D, Nurnberg G, Schild HH, Nurnberg P, Reis A, Frank J & Zerres K (2006). Mutations in the gene encoding the Wnt‐signaling component R‐spondin 4 (RSPO4) cause autosomal recessive anonychia. Am J Hum Genet 79, 1105–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettess MD, Dubois N, Murphy MJ, Dubey C, Roger C, Robine S & Trumpp A (2005). c‐Myc is required for the formation of intestinal crypts but dispensable for homeostasis of the adult intestinal epithelium. Mol Cell Biol 25, 7868–7878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaydon DC, Ishii Y, O'Toole EA, Unsworth HC, Teh MT, Ruschendorf F, Sinclair C, Hopsu‐Havu VK, Tidman N, Moss C, Watson R, de Berker D, Wajid M, Christiano AM & Kelsell DP (2006). The gene encoding R‐spondin 4 (RSPO4), a secreted protein implicated in Wnt signaling, is mutated in inherited anonychia. Nat Genet 38, 1245–1247. [DOI] [PubMed] [Google Scholar]
- Bruchle NO, Frank J, Frank V, Senderek J, Akar A, Koc E, Rigopoulos D, van Steensel M, Zerres K & Bergmann C (2008). RSPO4 is the major gene in autosomal‐recessive anonychia and mutations cluster in the furin‐like cysteine‐rich domains of the Wnt signaling ligand R‐spondin 4. J Invest Dermatol 128, 791–796. [DOI] [PubMed] [Google Scholar]
- Buczacki SJ, Zecchini HI, Nicholson AM, Russell R, Vermeulen L, Kemp R & Winton DJ (2013). Intestinal label‐retaining cells are secretory precursors expressing Lgr5. Nature 495, 65–69. [DOI] [PubMed] [Google Scholar]
- Carmon KS, Gong X, Lin Q, Thomas A & Liu Q (2011). R‐spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta‐catenin signaling. Proc Natl Acad Sci USA 108, 11452–11457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Kawamura K, Takae S, Deguchi M, Yang Q, Kuo C & Hsueh AJ (2013). Oocyte‐derived R‐spondin2 promotes ovarian follicle development. FASEB J 27, 2175–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H (2006). Wnt/β‐catenin signaling in development and disease. Cell 127, 469–480. [DOI] [PubMed] [Google Scholar]
- Clevers H & Nusse R (2012). Wnt/β‐catenin signaling and disease. Cell 149, 1192–1205. [DOI] [PubMed] [Google Scholar]
- Cruciat CM & Niehrs C (2013). Secreted and transmembrane Wnt inhibitors and activators. Cold Spring Harb Perspect Biol 5, a015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabdoub A, Donohue MJ, Brennan A, Wolf V, Montcouquiol M, Sassoon DA, Hseih JC, Rubin JS, Salinas PC & Kelley MW (2003). Wnt signaling mediates reorientation of outer hair cell stereociliary bundles in the mammalian cochlea. Development 130, 2375–2384. [DOI] [PubMed] [Google Scholar]
- de Lau W, Barker N, Low TY, Koo BK, Li VS, Teunissen H, Kujala P, Haegebarth A, Peters PJ, van de Wetering M, Stange DE, van Es JE, Guardavaccaro D, Schasfoort RB, Mohri Y, Nishimori K, Mohammed S, Heck AJ & Clevers H (2011). Lgr5 homologues associate with Wnt receptors and mediate R‐spondin signalling. Nature 476, 293–297. [DOI] [PubMed] [Google Scholar]
- de Lau W, Peng WC, Gros P & Clevers H (2014). The R‐spondin/Lgr5/Rnf43 module: regulator of Wnt signal strength. Genes Dev 28, 305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lau WB, Snel B & Clevers HC (2012). The R‐spondin protein family. Genome Biol 13, 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fevr T, Robine S, Louvard D & Huelsken J (2007). Wnt/β‐catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol Cell Biol 27, 7551–7559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinka A, Dolde C, Kirsch N, Huang YL, Kazanskaya O, Ingelfinger D, Boutros M, Cruciat CM & Niehrs C (2011). LGR4 and LGR5 are R‐spondin receptors mediating Wnt/β‐catenin and Wnt/PCP signalling. EMBO Rep 12, 1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorieff A, Pinto D, Begthel H, Destree O, Kielman M & Clevers H (2005). Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology 129, 626–638. [DOI] [PubMed] [Google Scholar]
- Han XH, Jin YR, Seto M & Yoon JK (2011). A WNT/β‐catenin signaling activator, R‐spondin, plays positive regulatory roles during skeletal myogenesis. J Biol Chem 286, 10649–10659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao HX, Xie Y, Zhang Y, Charlat O, Oster E, Avello M, Lei H, Mickanin C, Liu D, Ruffner H, Mao X, Ma Q, Zamponi R, Bouwmeester T, Finan PM, Kirschner MW, Porter JA, Serluca FC & Cong F (2012). ZNRF3 promotes Wnt receptor turnover in an R‐spondin‐sensitive manner. Nature 485, 195–200. [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, Geisler R, Stemple DL, Smith JC & Wilson SW (2000). Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature 405, 76–81. [DOI] [PubMed] [Google Scholar]
- Ireland H, Kemp R, Houghton C, Howard L, Clarke AR, Sansom OJ & Winton DJ (2004). Inducible Cre‐mediated control of gene expression in the murine gastrointestinal tract: effect of loss of β‐catenin. Gastroenterology 126, 1236–1246. [DOI] [PubMed] [Google Scholar]
- Ishii Y, Wajid M, Bazzi H, Fantauzzo KA, Barber AG, Blaydon DC, Nam JS, Yoon JK, Kelsell DP & Christiano AM (2008). Mutations in R‐spondin 4 (RSPO4) underlie inherited anonychia. J Invest Dermatol 128, 867–870. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Tamai Y, Zorn AM, Yoshida H, Seldin MF, Nishikawa S & Taketo MM (2001). Mouse Wnt receptor gene Fzd5 is essential for yolk sac and placental angiogenesis. Development 128, 25–33. [DOI] [PubMed] [Google Scholar]
- Itzkovitz S, Lyubimova A, Blat IC, Maynard M, van Es J, Lees J, Jacks T, Clevers H & van Oudenaarden A (2012). Single‐molecule transcript counting of stem‐cell markers in the mouse intestine. Nat Cell Biol 14, 106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Charlat O, Zamponi R, Yang Y & Cong F (2015). Dishevelled promotes Wnt receptor degradation through recruitment of ZNRF3/RNF43 E3 ubiquitin ligases. Mol Cell 58, 522–533. [DOI] [PubMed] [Google Scholar]
- Kabiri Z, Greicius G, Madan B, Biechele S, Zhong Z, Zaribafzadeh H, Edison, Aliyev J, Wu Y, Bunte R, Williams BO, Rossant J & Virshup DM (2014). Stroma provides an intestinal stem cell niche in the absence of epithelial Wnts. Development 141, 2206–2215. [DOI] [PubMed] [Google Scholar]
- Kazanskaya O, Glinka A, del Barco Barrantes I, Stannek P, Niehrs C & Wu W (2004). R‐Spondin2 is a secreted activator of Wnt/β‐catenin signaling and is required for Xenopus myogenesis. Dev Cell 7, 525–534. [DOI] [PubMed] [Google Scholar]
- Kazanskaya O, Ohkawara B, Heroult M, Wu W, Maltry N, Augustin HG & Niehrs C (2008). The Wnt signaling regulator R‐spondin 3 promotes angioblast and vascular development. Development 135, 3655–3664. [DOI] [PubMed] [Google Scholar]
- Kikuchi A, Yamamoto H, Sato A & Matsumoto S (2011). New insights into the mechanism of Wnt signaling pathway activation. Int Rev Cell Mol Biol 291, 21–71. [DOI] [PubMed] [Google Scholar]
- Kim KA, Kakitani M, Zhao J, Oshima T, Tang T, Binnerts M, Liu Y, Boyle B, Park E, Emtage P, Funk WD & Tomizuka K (2005). Mitogenic influence of human R‐spondin1 on the intestinal epithelium. Science 309, 1256–1259. [DOI] [PubMed] [Google Scholar]
- Koo BK, Spit M, Jordens I, Low TY, Stange DE, van de Wetering M, van Es JH, Mohammed S, Heck AJ, Maurice MM & Clevers H (2012). Tumour suppressor RNF43 is a stem‐cell E3 ligase that induces endocytosis of Wnt receptors. Nature 488, 665–669. [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ & Clevers H (1998). Depletion of epithelial stem‐cell compartments in the small intestine of mice lacking Tcf‐4. Nat Genet 19, 379–383. [DOI] [PubMed] [Google Scholar]
- Kozar S, Morrissey E, Nicholson AM, van der Heijden M, Zecchini HI, Kemp R, Tavare S, Vermeulen L & Winton DJ (2013). Continuous clonal labeling reveals small numbers of functional stem cells in intestinal crypts and adenomas. Cell Stem Cell 13, 626–633. [DOI] [PubMed] [Google Scholar]
- Krausova M & Korinek V (2014). Wnt signaling in adult intestinal stem cells and cancer. Cell Signal 26, 570–579. [DOI] [PubMed] [Google Scholar]
- Kuhl M, Sheldahl LC, Malbon CC & Moon RT (2000). Ca2+/calmodulin‐dependent protein kinase II is stimulated by Wnt and Frizzled homologs and promotes ventral cell fates in Xenopus . J Biol Chem 275, 12701–12711. [DOI] [PubMed] [Google Scholar]
- Kuhnert F, Davis CR, Wang HT, Chu P, Lee M, Yuan J, Nusse R & Kuo CJ (2004). Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf‐1. Proc Natl Acad Sci USA 101, 266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L & Clevers H (2010). Coexistence of quiescent and active adult stem cells in mammals. Science 327, 542–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien WH & Fuchs E (2014). Wnt some lose some: transcriptional governance of stem cells by Wnt/β‐catenin signaling. Genes Dev 28, 1517–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez‐Garcia C, Klein AM, Simons BD & Winton DJ (2010). Intestinal stem cell replacement follows a pattern of neutral drift. Science 330, 822–825. [DOI] [PubMed] [Google Scholar]
- Loregger A, Grandl M, Mejias‐Luque R, Allgauer M, Degenhart K, Haselmann V, Oikonomou C, Hatzis P, Janssen KP, Nitsche U, Gradl D, van den Broek O, Destree O, Ulm K, Neumaier M, Kalali B, Jung A, Varela I, Schmid RM, Rad R, Busch DH & Gerhard M (2015). The E3 ligase RNF43 inhibits Wnt signaling downstream of mutated β‐catenin by sequestering TCF4 to the nuclear membrane. Sci Signal 8, ra90. [DOI] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K & He X (2009). Wnt/β‐catenin signaling: components, mechanisms, and diseases. Dev Cell 17, 9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi H, Ajima R, Luo CT, Yamaguchi TP & Stappenbeck TS (2012). Wnt5a potentiates TGF‐β signaling to promote colonic crypt regeneration after tissue injury. Science 338, 108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monkley SJ, Delaney SJ, Pennisi DJ, Christiansen JH & Wainwright BJ (1996). Targeted disruption of the Wnt2 gene results in placentation defects. Development 122, 3343–3353. [DOI] [PubMed] [Google Scholar]
- Montgomery RK, Carlone DL, Richmond CA, Farilla L, Kranendonk ME, Henderson DE, Baffour‐Awuah NY, Ambruzs DM, Fogli LK, Algra S & Breault DT (2011). Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc Natl Acad Sci USA 108, 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muncan V, Sansom OJ, Tertoolen L, Phesse TJ, Begthel H, Sancho E, Cole AM, Gregorieff A, de Alboran IM, Clevers H & Clarke AR (2006). Rapid loss of intestinal crypts upon conditional deletion of the Wnt/Tcf‐4 target gene c‐Myc. Mol Cell Biol 26, 8418–8426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz J, Stange DE, Schepers AG, van de Wetering M, Koo BK, Itzkovitz S, Volckmann R, Kung KS, Koster J, Radulescu S, Myant K, Versteeg R, Sansom OJ, van Es JH, Barker N, van Oudenaarden A, Mohammed S, Heck AJ & Clevers H (2012). The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent ‘+4’ cell markers. EMBO J 31, 3079–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustata RC, Vasile G, Fernandez‐Vallone V, Strollo S, Lefort A, Libert F, Monteyne D, Perez‐Morga D, Vassart G & Garcia MI (2013). Identification of Lgr5‐independent spheroid‐generating progenitors of the mouse fetal intestinal epithelium. Cell Rep 5, 421–432. [DOI] [PubMed] [Google Scholar]
- Nam JS, Park E, Turcotte TJ, Palencia S, Zhan X, Lee J, Yun K, Funk WD & Yoon JK (2007). Mouse R‐spondin2 is required for apical ectodermal ridge maintenance in the hindlimb. Dev Biol 311, 124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehrs C (2012). The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol 13, 767–779. [DOI] [PubMed] [Google Scholar]
- Papapietro O, Teatero S, Thanabalasuriar A, Yuki KE, Diez E, Zhu L, Kang E, Dhillon S, Muise AM, Durocher Y, Marcinkiewicz MM, Malo D & Gruenheid S (2013). R‐spondin 2 signalling mediates susceptibility to fatal infectious diarrhoea. Nat Commun 4, 1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parma P, Radi O, Vidal V, Chaboissier MC, Dellambra E, Valentini S, Guerra L, Schedl A & Camerino G (2006). R‐spondin1 is essential in sex determination, skin differentiation and malignancy. Nat Genet 38, 1304–1309. [DOI] [PubMed] [Google Scholar]
- Pinto D, Gregorieff A, Begthel H & Clevers H (2003). Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev 17, 1709–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS, Kovacs L & Hamilton E (1974). Continuous labelling studies on mouse skin and intestine. Cell Tissue Kinet 7, 271–283. [DOI] [PubMed] [Google Scholar]
- Powell AE, Vlacich G, Zhao ZY, McKinley ET, Washington MK, Manning HC & Coffey RJ (2014). Inducible loss of one Apc allele in Lrig1‐expressing progenitor cells results in multiple distal colonic tumors with features of familial adenomatous polyposis. Am J Physiol Gastrointest Liver Physiol 307, G16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell AE, Wang Y, Li Y, Poulin EJ, Means AL, Washington MK, Higginbotham JN, Juchheim A, Prasad N, Levy SE, Guo Y, Shyr Y, Aronow BJ, Haigis KM, Franklin JL & Coffey RJ (2012). The pan‐ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell 149, 146–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian D, Jones C, Rzadzinska A, Mark S, Zhang X, Steel KP, Dai X & Chen P (2007). Wnt5a functions in planar cell polarity regulation in mice. Dev Biol 306, 121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Roman AK, Jayewickreme CD, Murtaugh LC & Shivdasani RA (2014). Wnt secretion from epithelial cells and subepithelial myofibroblasts is not required in the mouse intestinal stem cell niche in vivo. Stem Cell Reports 2, 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangiorgi E & Capecchi MR (2008). Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet 40, 915–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ & Clevers H (2009). Single Lgr5 stem cells build crypt‐villus structures in vitro without a mesenchymal niche. Nature 459, 262–265. [DOI] [PubMed] [Google Scholar]
- Schuijers J, van der Flier LG, van Es J & Clevers H (2014). Robust cre‐mediated recombination in small intestinal stem cells utilizing the olfm4 locus. Stem Cell Reports 3, 234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville DH, Sato T, He XC & Li L (2008). Current view: intestinal stem cells and signaling. Gastroenterology 134, 849–864. [DOI] [PubMed] [Google Scholar]
- Sheldahl LC, Park M, Malbon CC & Moon RT (1999). Protein kinase C is differentially stimulated by Wnt and Frizzled homologs in a G‐protein‐dependent manner. Curr Biol 9, 695–698. [DOI] [PubMed] [Google Scholar]
- Simons M & Mlodzik M (2008). Planar cell polarity signaling: from fly development to human disease. Annu Rev Genet 42, 517–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slusarski DC, Yang‐Snyder J, Busa WB & Moon RT (1997). Modulation of embryonic intracellular Ca2+ signaling by Wnt‐5A. Dev Biol 182, 114–120. [DOI] [PubMed] [Google Scholar]
- Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon‐Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD & Clevers H (2010). Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143, 134–144. [DOI] [PubMed] [Google Scholar]
- Storm EE, Durinck S, de Sousa EMF, Tremayne J, Kljavin N, Tan C, Ye X, Chiu C, Pham T, Hongo JA, Bainbridge T, Firestein R, Blackwood E, Metcalfe C, Stawiski EW, Yauch RL, Wu Y & de Sauvage FJ (2016). Targeting PTPRK‐RSPO3 colon tumours promotes differentiation and loss of stem‐cell function. Nature 529, 97–100. [DOI] [PubMed] [Google Scholar]
- Takeda N, Jain R, LeBoeuf MR, Wang Q, Lu MM & Epstein JA (2011). Interconversion between intestinal stem cell populations in distinct niches. Science 334, 1420–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD & de Sauvage FJ (2011). A reserve stem cell population in small intestine renders Lgr5‐positive cells dispensable. Nature 478, 255–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizuka K, Horikoshi K, Kitada R, Sugawara Y, Iba Y, Kojima A, Yoshitome A, Yamawaki K, Amagai M, Inoue A, Oshima T & Kakitani M (2008). R‐spondin1 plays an essential role in ovarian development through positively regulating Wnt‐4 signaling. Hum Mol Genet 17, 1278–1291. [DOI] [PubMed] [Google Scholar]
- Vainio S, Heikkila M, Kispert A, Chin N & McMahon AP (1999). Female development in mammals is regulated by Wnt‐4 signalling. Nature 397, 405–409. [DOI] [PubMed] [Google Scholar]
- van Amerongen R & Nusse R (2009). Towards an integrated view of Wnt signaling in development. Development 136, 3205–3214. [DOI] [PubMed] [Google Scholar]
- van der Flier LG, Haegebarth A, Stange DE, van de Wetering M & Clevers H (2009. a). OLFM4 is a robust marker for stem cells in human intestine and marks a subset of colorectal cancer cells. Gastroenterology 137, 15–17. [DOI] [PubMed] [Google Scholar]
- van der Flier LG, van Gijn ME, Hatzis P, Kujala P, Haegebarth A, Stange DE, Begthel H, van den Born M, Guryev V, Oving I, van Es JH, Barker N, Peters PJ, van de Wetering M & Clevers H (2009. b). Transcription factor achaete scute‐like 2 controls intestinal stem cell fate. Cell 136, 903–912. [DOI] [PubMed] [Google Scholar]
- van Es JH, Haegebarth A, Kujala P, Itzkovitz S, Koo BK, Boj SF, Korving J, van den Born M, van Oudenaarden A, Robine S & Clevers H (2012. a). A critical role for the Wnt effector Tcf4 in adult intestinal homeostatic self‐renewal. Mol Cell Biol 32, 1918–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Es JH, Sato T, van de Wetering M, Lyubimova A, Nee AN, Gregorieff A, Sasaki N, Zeinstra L, van den Born M, Korving J, Martens AC, Barker N, van Oudenaarden A & Clevers H (2012. b). Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat Cell Biol 14, 1099–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Landeghem L, Santoro MA, Krebs AE, Mah AT, Dehmer JJ, Gracz AD, Scull BP, McNaughton K, Magness ST & Lund PK (2012). Activation of two distinct Sox9‐EGFP‐expressing intestinal stem cell populations during crypt regeneration after irradiation. Am J Physiol Gastrointest Liver Physiol 302, G1111–G1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert K & Nusse R (2012). Wnt proteins. Cold Spring Harb Perspect Biol 4, a007864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Zamponi R, Charlat O, Ramones M, Swalley S, Jiang X, Rivera D, Tschantz W, Lu B, Quinn L, Dimitri C, Parker J, Jeffery D, Wilcox SK, Watrobka M, LeMotte P, Granda B, Porter JA, Myer VE, Loew A & Cong F (2013). Interaction with both ZNRF3 and LGR4 is required for the signalling activity of R‐spondin. EMBO Rep 14, 1120–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Wang Y, Dabdoub A, Smallwood PM, Williams J, Woods C, Kelley MW, Jiang L, Tasman W, Zhang K & Nathans J (2004). Vascular development in the retina and inner ear: control by Norrin and Frizzled‐4, a high‐affinity ligand‐receptor pair. Cell 116, 883–895. [DOI] [PubMed] [Google Scholar]
- Yan KS, Chia LA, Li X, Ootani A, Su J, Lee JY, Su N, Luo Y, Heilshorn SC, Amieva MR, Sangiorgi E, Capecchi MR & Kuo CJ (2012). The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci USA 109, 466–471. [DOI] [PMC free article] [PubMed] [Google Scholar]


