Abstract
Key points
Activation of central chemoreceptors by CO2 increases sympathetic nerve activity (SNA), arterial blood pressure (ABP) and breathing. These effects are exaggerated in spontaneously hypertensive rats (SHRs), resulting in an augmented CO2 chemoreflex that affects both breathing and ABP.
The augmented CO2 chemoreflex and the high ABP are measureable in young SHRs (postnatal day 30–58) and become greater in adult SHRs.
Blockade of orexin receptors can normalize the augmented CO2 chemoreflex and the high ABP in young SHRs and normalize the augmented CO2 chemoreflex and significantly lower the high ABP in adult SHRs.
In the hypothalamus, SHRs have more orexin neurons, and a greater proportion of them increase their activity with CO2.
The orexin system is overactive in SHRs and contributes to the augmented CO2 chemoreflex and hypertension. Modulation of the orexin system may be beneficial in the treatment of neurogenic hypertension.
Abstract
Activation of central chemoreceptors by CO2 increases arterial blood pressure (ABP), sympathetic nerve activity and breathing. In spontaneously hypertensive rats (SHRs), high ABP is associated with enhanced sympathetic nerve activity and peripheral chemoreflexes. We hypothesized that an augmented CO2 chemoreflex and overactive orexin system are linked with high ABP in both young (postnatal day 30–58) and adult SHRs (4–6 months). Our main findings are as follows. (i) An augmented CO2 chemoreflex and higher ABP in SHRs are measureable at a young age and increase in adulthood. In wakefulness, the ventilatory response to normoxic hypercapnia is higher in young SHRs (mean ± SEM: 179 ± 11% increase) than in age‐matched normotensive Wistar–Kyoto rats (114 ± 9% increase), but lower than in adult SHRs (226 ± 10% increase; P < 0.05). The resting ABP is higher in young SHRs (122 ± 5 mmHg) than in age‐matched Wistar–Kyoto rats (99 ± 5 mmHg), but lower than in adult SHRs (152 ± 4 mmHg; P < 0.05). (ii) Spontaneously hypertensive rats have more orexin neurons and more CO2‐activated orexin neurons in the hypothalamus. (iii) Antagonism of orexin receptors with a dual orexin receptor antagonist, almorexant, normalizes the augmented CO2 chemoreflex in young and adult SHRs and the high ABP in young SHRs and significantly lowers ABP in adult SHRs. (iv) Attenuation of peripheral chemoreflexes by hyperoxia does not abolish the augmented CO2 chemoreflex (breathing and ABP) in SHRs, which indicates an important role for the central chemoreflex. We suggest that an overactive orexin system may play an important role in the augmented central CO2 chemoreflex and in the development of hypertension in SHRs.
Key points
Activation of central chemoreceptors by CO2 increases sympathetic nerve activity (SNA), arterial blood pressure (ABP) and breathing. These effects are exaggerated in spontaneously hypertensive rats (SHRs), resulting in an augmented CO2 chemoreflex that affects both breathing and ABP.
The augmented CO2 chemoreflex and the high ABP are measureable in young SHRs (postnatal day 30–58) and become greater in adult SHRs.
Blockade of orexin receptors can normalize the augmented CO2 chemoreflex and the high ABP in young SHRs and normalize the augmented CO2 chemoreflex and significantly lower the high ABP in adult SHRs.
In the hypothalamus, SHRs have more orexin neurons, and a greater proportion of them increase their activity with CO2.
The orexin system is overactive in SHRs and contributes to the augmented CO2 chemoreflex and hypertension. Modulation of the orexin system may be beneficial in the treatment of neurogenic hypertension.
Abbreviations
- ABP
arterial blood pressure
- Almxt
almorexant
- DMH
dorsomedial hypothalamus
- fR
respiratory frequency
- Hyp
the hypothalamus
- LHA
lateral hypothalamic area
- NREM
non‐rapid eye movement
- OX
orexin
- OXA
orexin A
- OXR
orexin receptor
- OX1R
orexin receptor‐1
- OX2R
orexin receptor‐2
- PBST
0.1% Triton X‐100 phosphate‐buffered saline
- PeF
perifornical hypothalamus
- REM
rapid eye movement
- SHR
spontaneously hypertensive rat
- SNA
sympathetic nerve activity
ventilation
- WKY rat
Wistar–Kyoto rat
Introduction
It is well established that activation of central chemoreceptors by CO2/H+ increases breathing, sympathetic nerve activity (SNA) and blood pressure in humans and in anaesthetized and conscious animals (Hanna et al. 1981; Lioy & Trzebski, 1984; Somers et al. 1989; Nattie et al. 1992, 1993; Oikawa et al. 2005; Guyenet et al. 2010; Li & Nattie, 2014). Many putative central chemoreceptors sites are involved in the regulation of breathing, SNA and blood pressure. For example, the CO2/pH chemosensitive orexin neurons in the hypothalamus send projections to many brainstem putative central chemoreceptor sites, e.g. retrotrapezoid nucleus, medullary raphe and nucleus of the solitary tract, as well as to many blood pressure regulatory sites, e.g. hypothalamic paraventricular nucleus and brainstem rostral ventrolateral medulla (Peyron et al. 1998; Date et al. 1999; Marcus et al. 2001; Young et al. 2005; Nixon & Smale, 2007; Lazarenko et al. 2011), that participate in the regulation of respiration, SNA and blood pressure (Shahid et al. 2011, 2012; Li & Nattie, 2014). The neurons in the retrotrapezoid nucleus, a key putative central chemoreceptor site, are innervated by orexin neurons (Lazarenko et al. 2011) and in turn innervate much of the ventrolateral medulla, including the ventral respiratory column, the rostral ventrolateral medulla and the caudal ventrolateral medulla (Rosin et al. 2006). In conscious rats, hypercapnia induces significant increases in arterial blood pressure (ABP), renal SNA and breathing, even after denervation of carotid and aortic bodies (Oikawa et al. 2005).
We previously reported that blocking orexin receptor‐1 (OX1R; Dias et al. 2009, 2010) or both orexin receptors (OX1R and OX2R; Dias et al. 2009, 2010; Li & Nattie, 2010) can significantly decrease the CO2 chemoreflex in normal conscious rats. We also found that in adult spontaneously hypertensive rats (SHRs) blocking both OX1R and OX2R can significantly decrease SNA and ABP (Li et al. 2013 a). In the present study, using SHRs at young (postnatal day 30–58) and adult ages (4–6 months), we investigated the following factors: (i) whether an enhanced CO2 chemoreflex is progressively associated with the increase in ABP from young to adult age; (ii) whether the enhanced CO2 chemoreflex is predominantly attributable to overactive central chemoreceptors; (iii) whether hypercapnia activates more orexin neurons in the hypothalamus; and (iv) whether blocking OXRs can normalize and/or lower (a) the augmented CO2 chemoreflex in both young and adult SHRs and (b) the ABP in young SHRs. We hypothesized that the augmented central CO2 chemoreflex is progressively associated with the increase in ABP from young to adult SHRs and that the overactive orexin system plays an important role in development of the augmented CO2 chemoreflex and neurogenic hypertension in SHRs.
Methods
Ethical approval
All animal experiments and surgical protocols were within the guidelines of the National Institutes of Health for animal use and care and the American Physiological Society's Guiding Principles in the Care and Use of Animals and were approved by the Institutional Animal Care and Use Committee at the Geisel School of Medicine at Dartmouth. The authors have read and complied with guidelines for research in rodents outlined for The Journal of Physiology and Experimental Physiology (Grundy, 2015).
General methods
Spontaneously hypertensive rats and their background normotensive Wistar–Kyoto (WKY) control rats were used for the experiments in this study. All the rats were housed in a temperature‐ and light‐controlled environment set on a 12 h–12 h light–dark cycle (lights on at 00.00on h; lights off at 12.00 h). Food and water were provided ad libitum. The general methods are those in common use in our laboratory (Li et al. 2008, 2013 a). At the conclusion of the experiments, the rats were killed with an overdose of sodium pentobarbital (>75 mg kg−1, i.p.; Euthasol; Virbac Inc., Fort Worth, TX, USA). Two different sets of animals were used for the physiological and anatomical studies. For physiological experiments, SHR and normotensive WKY rats at two ages were used. The young SHRs (male, n = 10) and WKY rats (male, n = 6) were between postnatal days 30 and 58, while adult SHRs (male, n = 11) and WKY rats (male, n = 6) were between 4 and 6 months of age. For a CO2‐activated c‐fos and orexin immunostaining study, four separate groups of young and adult SHRs and WKY rats (n = 6 in each) were used, and the ages and sexes of these rats were similar to those used in the physiological experiments.
Surgery
Animals were anaesthetized with a ketamine (Putney, Inc., Portland, ME, USA) and xylazine (Lloyd Labs, Walnut, CA, USA) cocktail (90/15 mg kg−1, i.m.). All the rats were implanted with a blood pressure telemeter, which allows us to record ABP chronically in the rats continuously and reliably, as described in our previous publications (Li et al. 2008, 2013 a). In brief, the tip of the blood pressure catheter was placed into the descending aorta through the femoral artery. The adult rats were implanted with a PhysioTel C50‐PXT telemetric transmitter (DSI, St Paul, MN, USA), which allowed us to measure ABP, ECG and temperature simultaneously. The positive ECG lead was secured to the xyphoid process, while the negative lead was sutured to the muscle tissue in the area of the right shoulder. The body of the transmitter was placed inside the abdominal cavity and secured to the abdominal wall. The young rats were implanted with a PA‐C10 blood pressure telemetric transmitter (DSI), and the body of the transmitter was placed in the right flank region subcutaneously. All the rats were implanted with EEG and neck EMG electrodes for sleep–wake study as previously described (Li et al. 2008, 2013 a; Li & Nattie, 2010). In brief, three EEG electrodes were screwed onto the skull and two EMG electrodes were sutured onto the dorsal neck muscles. All the electrodes were connected to a six‐prong plastic pedestal. The rats were then allowed at least 7 days of recovery before conducting any experiment. All the experiments were conducted in a Plexiglas chamber with the lights controlled as appropriate for the phase of the rat's diurnal cycle under study.
Ventilatory and ABP measurements and data collection
The methods used to measure the ventilation (), ABP, ECG, body temperature, EEG and EMG were those in common use in our laboratory (Nattie & Li, 2000; Taylor et al. 2005; Li et al. 2008, 2013 a; Li & Nattie, 2010). In brief, whole‐body plethysmography was used to measure breathing, with the analog output signal from the pressure transducer sampled at 150 Hz and converted into a digital signal by a computer using LabChart (ADInstruments, Colorado Springs, CO, USA). To calibrate the plethysmograph before each experiment, we obtained pressure data from five 0.3 ml air injections made with a 1 ml syringe. The rates of inflow and outflow to the plethysmograph were balanced with a flowmeter so that the plethysmograph remained at atmospheric pressure. The CO2 and O2 fractions were sampled from the outflow line at ∼100 ml min−1 by a CO2 and O2 gas analyser (Applied Electrochemistry, Pittsburgh, PA, USA). Oxygen consumption was calculated by application of the Fick principle, using the difference in O2 content between inflow gas and outflow gas and the flow rate, as described previously (Nattie & Li, 2000; Taylor et al. 2005; Li & Nattie, 2010). Core body temperature was measured continuously via the signal from the telemetric temperature probe within the abdomen, and the temperature of the experimental chamber was measured using a thermometer within the plethysmograph.
To measure ABP, the signals of blood pressure or the blood pressure–ECG–body temperature combination telemeter were converted into an analog signal using a calibrated pressure output adapter and recorded continuously using LabChart (ADinstruments). For adult animals with a PhysioTel C50‐PXT telemeter, ABP and ECG (heart rate) were sampled at 1000 Hz, whereas body temperature was sampled at 150 Hz. For the young animals with PA‐C10 blood pressure only telemeters, ABP was sampled at 1000 Hz, heart rate was derived from pulse pressure of the ABP using LabChart, and the rectal body temperature was measured before and after the experiment. Raw EEG and EMG outputs from the skull and neck skeletal muscle electrodes were filtered at 0.3–70 and 0.1–100 Hz, respectively, using a Grass Physiodata Amplifier System (Natus Neurology Inc., Grass Products, Middleton, WI, USA) and sampled at 150 Hz via LabChart.
Inspired gases and drugs
To test the hypercapnic chemoreflex, the following three inspired gases were used: (i) room air (21% O2 balanced with nitrogen) to measure the resting control conditions; (ii) 7% CO2–21% O2 balanced with nitrogen to measure the responses to normoxic hypercapnia; and (iii) 7% CO2 –93% O2 to measure the responses to the hyperoxic hypercapnia when the peripheral chemoreceptors were attenuated.
Almorexant (Almxt; (2R)‐2‐{(1S)‐6,7‐dimethoxy‐1‐[2‐(4‐trifluoromethylphenyl)‐ethyl]‐3,4‐dihydro‐1H‐isoquinolin‐2‐yl}‐N‐methyl‐2‐phenyl‐acetamide) is a selective potent dual orexin receptor antagonist that can be administrated orally, rapidly crosses the blood–brain barrier, and blocks both OX1R and OX2R in the central nervous system (Brisbare‐Roch et al. 2007). Adult SHRs received Almxt gavage (200 mg kg−1) made of Almxt dissolved in 0.25% methylcellulose solution as previously described (Brisbare‐Roch et al. 2007; Li & Nattie, 2010; Li et al. 2013 a). Young rats received injection of Almxt (32 mg kg−1, s.c.). The doses used for gavage and subcutaneous injection are comparable based on previous studies (Brisbare‐Roch et al. 2007; Dugovic et al. 2009), in which the authos examined the selectivity and pharmacodynamics of the drug and the brain and systemic concentrations after treatment. Almorexant was generously supplied by Actelion Pharm, Ltd.
Experimental design and protocol
The rats were divided into three testing groups. In group 1, SHRs, young (n = 5) and adult (n = 9), were tested with normoxic hypercapnia (7% CO2–21% O2) before and after Almxt treatment. In group 2, SHRs, young (n = 5) and adult (n = 6), were tested with normoxic hypercapnia and hyperoxic hypercapnia (7% CO2–93% O2). In group 3, normotensive WKY control rats, young (n = 6) and adult (n = 6), were tested with normoxic hypercapnia.
All the animals were studied in the dark period of the diurnal cycle, when the orexin neurons are more active. On the day of the experiment, the rat was placed into the experimental chamber and allowed to acclimate for at least 1 h until the rat started to have normal wake–sleep cycles. Then 1.5–2 h of air‐breathing baseline data were collected before switching the inspired gas to 7% CO2–21% O2 or 7% CO2–93% O2 for 1–1.5 h to ensure sufficient data in wakefulness, non‐rapid eye movement (NREM) and rapid eye movement (REM) sleep. For Almxt treatment, the rats received Almxt after baseline data collection and were then returned to the recording chamber; the treatment data were collected from 30 min post‐treatment.
Carbon dioxide‐activated c‐fos expression experiments
To test whether CO2 activated more orexin neurons in the hypothalamus, we evaluated CO2‐induced c‐fos in orexin neurons in SHRs and WKY rats at young and adult ages (n = 6 in each group). After exposure to either 7% CO2–21% O2 or air for 1.5 h during the dark period, the rats were overdosed with the ketamine and xylazine cocktail, then transcardially perfused with saline followed by 4% paraformaldehyde. The brains were collected and postfixed overnight in 4% paraformaldehyde at 4°C, after which they were cryoprotected in 30% sucrose for 48 h. The general methods were similar to those described previously (Li & Nattie, 2006). In short, the brains were cut into 40‐μm‐thick coronal sections, with every other section processed for double immunohistochemical staining of c‐fos and orexin A, using the diaminobenzidine reaction for visualization. The free‐floating sections were washed using 0.1% Triton X‐100 phosphate‐buffered saline (PBST), incubated in a rabbit polyclonal anti‐c‐fos antibody (1:10,000 dilution; Ab‐5, catalogue no. PC38; Calbiochem, Billerica, MA, USA) for 48 h, followed by a biotinylated goat anti‐rabbit IgG secondary antibody (1:1000 dilution; Vector Lab, Burlingame, CA, USA) overnight at 4°C or for 2 h at room temperature. The peroxidase and diaminobenzidine with nickel were used to visualize the c‐fos expression (black). After finishing c‐fos staining, all the sections were washed with PBST–azide and PBST, then incubated in a goat polyclonal anti‐orexin A (OXA) antibody (1:10,000 dilution; SC‐8070; Santa Cruz, Dallas, TX, USA) for 48 h, a biotinylated horse anti‐goat IgG secondary antibody (1:100 dilution; Vector Lab) overnight at 4°C, and then the peroxidase and diaminobenzidine with no nickel to visualize the OXA expression (brown). All sections were mounted and dehydrated with graded alcohol (from 25 to 100% EtOH), cleared with xylene and coverslipped. The OXA‐immunoreactive (OXA‐ir) and c‐fos‐expressing orexin (fos–OXA‐ir) neurons were counted using the Stereo Investigator System (MBF Bioscience, Williston, VT, USA).
For the cell counts, we divided the hypothalamus into three zones, the dorsomedial hypothalamus (DMH), the perifornical hypothalamus (PeF) and the lateral hypothalamic area (LHA), by drawing four vertical lines in the hypothalamus (see Fig. 5), modified from Clifford et al. (2015). The five vertical placement lines were positioned as follows (see Fig. 5 A): line 1 at the centre of the fornix; line 2 at the edge of the third ventricle; line 3 two‐thirds of the distance from the second line (edge of the third ventricle); line 4 at the same distance between lines 1 and 3 on the lateral side of the fornix; and line 5 at the edge of the LHA. The DMH zone was between the second and third vertical lines, the PeF zone between the third and fourth vertical lines, and the LHA zone between the fourth and and fifth lines (Fig. 5 A). We identified fos–OXA‐ir cells as brown with black nuclei double stain (Fig. 5 B, red arrows), non‐fos OXA‐ir cells as brown cells without black nuclei (Fig. 5 B, blue arrows), and non‐OXA fos‐ir as black nucleic stain only (Fig. 5 B, green arrow). All OX‐ir and fos–OXA‐ir positive cells within these three zones of both sides of the sections were counted and averaged based on their location, and grouped as young WKY rat air control and 7% CO2, young SHR air and 7% CO2, adult WKY rat air and 7% CO2, and adult SHR air and 7% CO2.
Figure 5. Distribution of orexin A‐immunoreactive (OXA‐ir) and c‐fos‐expressing orexin (fos–OXA‐ir) neurons in the hypothalamus .
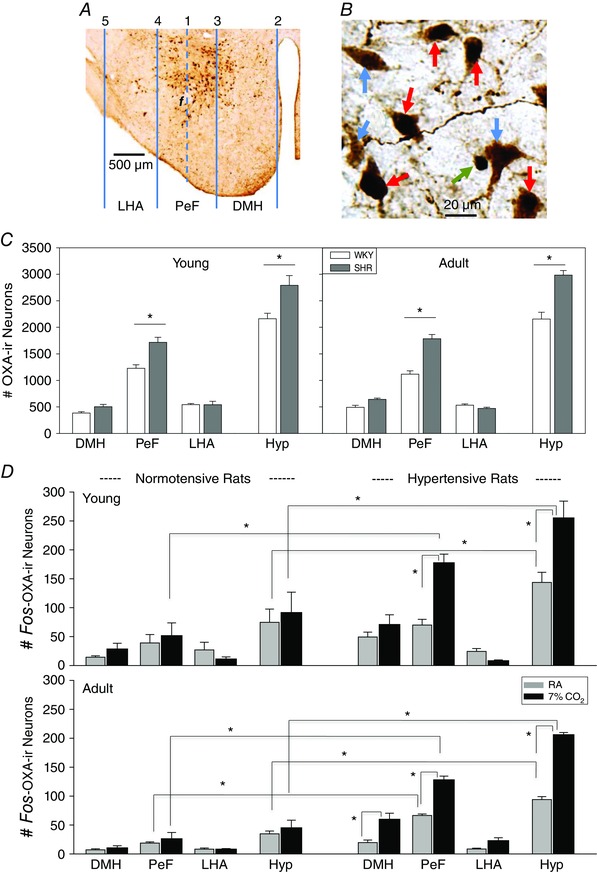
Representative sections show the distribution of OX‐ir and fos–OXA‐ir neurons and the three subdivided zones in the hypothalamus (A) and an example of OXA‐ir (blue arrows; brown cells with no staining of nuclei), fos–OXA‐ir (red arrows; brown cells with black nuclei) and non‐OXA fos‐ir neurons (green arrow; black nucleus only; B). C shows the distributions of OXA‐ir in the three zones and Hyp in young and adult SHRs and WKY rats. D shows the distributions of fos–OXA‐ir neurons in room air and 7% CO2 in the three zones and Hyp (all three zones) in young and adult SHRs and WKY rats. Values are shown as means + SEM. *Values are significantly different with Student–Newman–Keuls post hoc tests (P ≤ 0.03). Abbreviations: DMH, dorsomedial hypothalamus; f, fornix; Hyp, hypothalamus (all three zones); LHA, lateral hypothalamic area; PeF, perifornical hypothalamus; and RA, room air.
Data and statistical analysis
The methods for sleep analysis were as described previously (Nattie & Li, 2001; Li & Nattie, 2010). In brief, a fast Fourier transform was performed on the EEG signal at 4.0‐s‐long epochs using SleepSign software (Kissei Comtec Co. Ltd, Matsumoto, Nagano, Japan). The vigilance states were categorized as wakefulness, NREM and REM sleep. The and mean ABP were averaged based on the vigilance state in different inspired gases in each rat, and the data are presented as means ± SEM (Figs 1, 2, 3, 4).
Figure 1. Comparisons of ventilation ( ) between spontaneously hypertensive rats (SHRs) and age‐matched Wistar–Kyoto (WKY) rats at young and adult ages in wakefulness, non‐rapid eye movement (NREM) and rapid eye movement (REM) sleep .
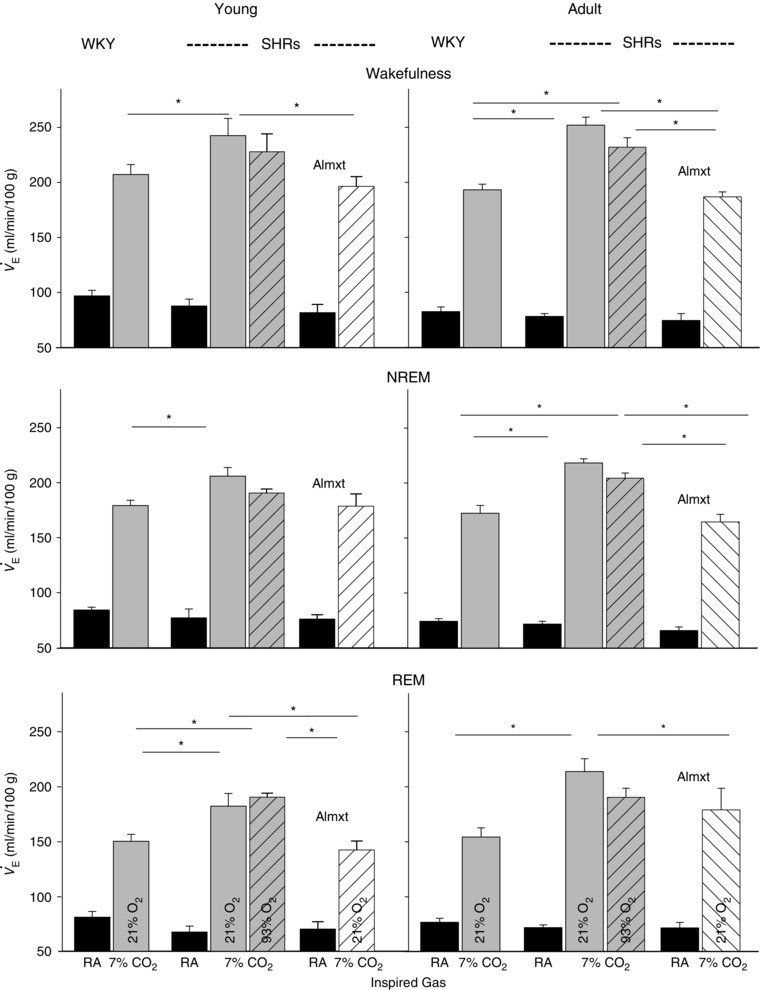
Data are shown as absolute values (means + SEM). Abbreviations: Almxt, almorexant; and RA, room air. *Values are significantly different in hypercapnia with Holm–Sidak or Student–Newman–Keuls post hoc test (P < 0.05).
Figure 2. Ventilatory response ( ; expressed as the percentage change) to hypercapnia .
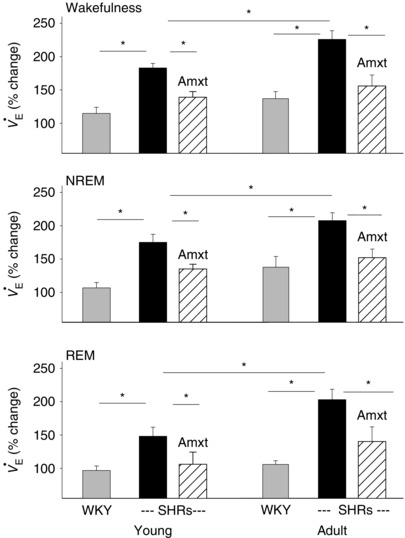
The percentage change of in normoxic hypercapnia with almorexant (Amxt) treatment (hatched bars) and without (black bars) in young and adult SHRs vs. that in normotensive young and adult WKY rats (grey bars). Value are shown as means + SEM. *Values are significantly different with Holm–Sidak or Student–Newman–Keuls post hoc test (P < 0.05–0.001).
Figure 3. Resting mean arterial blood pressure (MABP) with almorexant (Amxt) treatment (hatched bars) and without (black bars) in young and adult SHRs vs. that in age‐matched WKY rats (grey bars) .
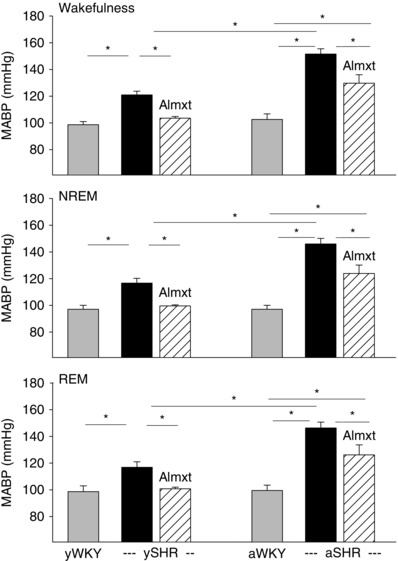
Data are shown as absolute values (means + SEM). *Values are significantly different with Student–Newman–Keuls post hoc tests (P < 0.001).
Figure 4. The change of mean arterial blood pressure (ΔMABP) in response to normoxic hypercapnia with almorexant (Amxt) treatment (hatched bars) and without (black bars) in young and adult SHRs vs. that in age‐matched WKY rats (grey bars) .
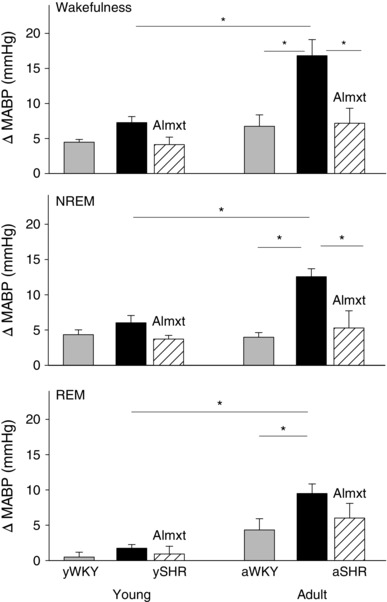
Data are shown as absolute values (means + SEM). *Values are significantly different with Student–Newman–Keuls post hoc tests (P < 0.001).
Breath‐by‐breath analysis was performed using the pressure deflections and the respiratory cycle time for each breath as described previously (Nattie & Li, 2000; Taylor et al. 2005; Li et al. 2008; Li & Nattie, 2010). Any breaths occurring during activity (sniffing, grooming and moving) were excluded. Ventilation was the product of tidal volume and breathing frequency (f R). Tidal volume and were calculated for each breath, with rat body temperature, plethysmograph chamber temperature and the barometric pressure of the day the experiment was performed, and normalized with body weight (Bartlett & Tenney, 1970). Ventilatory parameters are expressed as mean values ± SEM for quiet wakefulness, NREM and REM sleep during room air or 7% CO2 conditions. To evaluate the central chemoreflex, and mean ABP responses to 7% CO2–21% O2 with and without Almxt treatment and to 7% CO2–93% O2, we first categorized the vigilance states into wakefulness, NREM and REM sleep in each group, and then compared the changes in 7% CO2–21% O2 with and without Almxt treatment and 7% CO2–93% O2 using a one‐way ANOVA separately for each vigilance state, applying the Holm–Sidak or Student–Newman–Keuls post hoc test when appropriate in each age group.
To compare the changes of CO2 chemoreflex ( and ABP; the value in hypercapnia minus the value in resting air) in SHRs and WKY rats at young and adult ages, a two‐way ANOVA was used separately for each vigilance state. The two factors were age (young and adult) and genotype (SHRs and WKY rats), applying the Holm–Sidak post hoc test when appropriate.
To evaluate whether SHRs have more orexin neurons and whether hypercapnia activated more orexin neurons in SHRs, the differences in the total number of OXA‐ir and fos–OXA‐ir neurons in each of the three zones and in the hypothalamus (all three zones combined) between SHRs and WKY rats at young and adult age were compared using a one‐way ANOVA and applying the Holm–Sidak or Student–Newman–Keuls post hoc test when appropriate. To evaluate whether there was an age difference in the total number of OXA‐ir or fos–OXA‐ir neurons between young and adult SHRs or WKY rats, a one‐way ANOVA was used with post hoc analysis using the Holm–Sidak method when appropriate.
Results
Ventilatory CO2 chemoreflex in young and adult SHRs
Room‐air breathing
There was no significant difference in air breathing between young and adult SHRs and between age‐matched SHRs and WKY rats in wakefulness, NREM and REM sleep (P ≥ 0.05, one‐way ANOVA; Fig. 1).
Ventilatory response to normoxic and hyperoxic hypercapnia
At each age, young and adult, the SHRs had a significantly higher ventilatory () response to normoxic hypercapnia and to hyperoxic hypercapnia than age‐matched normotensive WKY rats in wakefulness and NREM and REM sleep (P < 0.05, one‐way ANOVA; Figs 1 and 2). In SHRs during wakefulness, the response to normoxic hypercapnia and to hyperoxic hypercapnia in young (179 ± 11 and 162 ± 18% increase, respectively) and adult animals (226 ± 10 and 218 ± 9% increase, respectively) was higher than that of age‐matched normotensive young (114 ± 9 and 113 ± 6% increase, respectively) and adult WKY rats (137 ± 10 and 126 ± 28% increase, respectively; P < 0.05, one‐way ANOVA; Figs 1 and 2). In SHRs, there was no significant difference between the response to normoxic hypercapnia and to hyperoxic hypercapnia, when peripheral chemoreceptors are attenuated, at either young or adult age in wakefulness or NREM or REM sleep (P ≥ 0.05, one‐way ANOVA; Fig. 1).
When comparing age and genotype, a two‐way ANOVA with Holm–Sidak post hoc test showed that there was a significant difference in the response to CO2 (expressed as a percentage change) between the genotypes (SHR and WKY rat, P < 0.001) and ages (young and adult, P = 0.009), with significant interaction (P ≤ 0.001). The Holm–Sidak post hoc test showed that SHRs had a significantly higher CO2 chemoreflex than age‐matched normotensive WKY rats at both young and adult ages (P = 0.002 and P < 0.001, respectively), and adult SHRs had significantly higher CO2 chemoreflex than young SHRs (P = 0.01; Fig. 2). In WKY rats, there was no significant difference in the response to CO2 between young and adult age (P = 0.2; Figs 1 and 2).
There was no significant difference between the percentage change of the response to normoxic and hyperoxic hypercapnia in SHRs (P > 0.05, one‐way ANOVA); therefore, the data are not shown here.
In both young and adult SHRs, Almxt treatment significantly lowered the augmented respiratory CO2 chemoreflex to a level that was no longer significantly different from that of age‐matched normotensive WKY rats in wakefulness, NREM and REM sleep (P > 0.05, one‐way ANOVA; Figs 1 and 2). In adult WKY rats (n = 5), Almxt treatment decreased the respiratory response to hypercapnia by ∼20% within the range we previously reported in Sprague–Dawley rats (Li & Nattie, 2010; data not shown).
Arterial blood pressure in young and adult SHRs
Resting ABP
There was a significant difference in resting mean ABP between the genotypes (SHR and WKY rat, P < 0.001) and ages (young and adult, P < 0.001), whereby SHRs had significantly higher mean ABP than that of age‐matched normotensive WKY rats at both young and adult ages (P < 0.001) and an age‐dependent increase in mean ABP (P < 0.001; two‐way ANOVA with Holm–Sidak post hoc test; Fig. 3). The resting mean ABP in SHRs at postnatal day 30–58 was already higher than that of age‐matched normotensive WKY rats in wakefulness, NREM and REM sleep (e.g. in wakefulness 122 ± 5 and 99 ± 5 mmHg, respectively, P < 0.05), but lower than that of adult SHRs (152 ± 4 mmHg in wakefulness, P < 0.05, one‐way ANOVA; Fig. 3). A complete study on the effect of Almxt treatment on ABP in adult SHRs has been reported previously (Li et al. 2013 a,b), and here we provide further comparison of the effect of Almxt on ABP in both young and adult SHRs in resting, air‐breathing conditions. In young SHRs, Almxt significantly lowered ABP from 121 to 103 mmHg, a level similar to that of age‐matched WKY rats (99 mmHg), while in adult SHRs, Almxt significantly lowered ABP from 152 to 130 mmHg, a level that was still significantly higher than that of age‐matched normotensive WKY rats (103 mmHg; Fig. 3).
Arterial blood pressure response to hypercapnia
Hypercapnic stimulation induced an increase in ABP in both hypertensive and normotensive rats (Fig. 4). In SHRs, however, the increase in ABP was greater than in age‐matched normotensive WKY rats, a difference that increased with age (Fig. 4). In wakefulness in young SHRs and WKY rats, hypercapnia increased ABP by 6.6 and 4.5 mmHg, respectively, and the difference between young SHRs and WKY rats was not significant (P ≥ 0.05, one‐way ANOVA; Fig. 4). In adult SHRs and WKY rats, however, hypercapnia increased ABP by 16.8 and 6.8 mmHg, respectively, and the difference between adult SHRs and WKY rats was highly significant (P < 0.001, one‐way ANOVA; Fig. 4). In adult SHRs, Almxt treatment significantly lowered the CO2‐augmented ABP response to a level similar to that of normotensive WKY rats in wakefulness and sleep (Fig. 4). It was also noted that in SHRs the augmented ABP response to hypercapnia was progressively increased with age, and the difference between young (6.6 mmHg) and adult SHRs (16.8 mmHg) was significant (P < 0.001, one‐way ANOVA; Fig. 4).
Other physiological parameters
The changes of body temperature and metabolic rate before and after Almxt were not statistically significant in wakefulness, NREM and REM sleep in SHRs and WKY rats, or between SHRs and WKY rats, possibly because of the lower dose of Almxt used in this set of experiments relative to our previous experiment (Li & Nattie, 2010; data not shown). The effects of Almxt have been reported in detail previously (Brisbare‐Roch et al. 2007), and in the present experiment we focused on the cardiorespiratory changes in different vigilance states, namely wakefulness, NREM and REM sleep.
The number of orexin neurons and CO2‐activated c‐fos‐expressing orexin neurons in SHRs
The numbers of OXA‐ir neurons in the hypothalamus (Hyp; three zones combined) and the three individual zones (DMH, PeF and LHA) are summarized and shown in Fig. 5 C. The total number of OXA‐ir neurons in the hypothalamus was significantly higher in both young (2791 ± 184) and adult SHRs (2984 ± 86) than that in age‐matched normotensive WKY rats (2160 ± 104 and 2155 ± 128, respectively, P = 0.01 and P = 0.02, one‐way ANOVA applied to SHRs and WKY rats; Fig. 5 C). The significance was solely attributable to the difference in the PeF zone, [young SHRs (1718 ± 93) vs. young WKY rats (1228 ± 67), P < 0.001; adult SHRs (1783 ± 86) vs. adult WKY rats (1118 ± 64), P < 0.001; one‐way ANOVA with Holm–Sidak post hoc test]; there was no significant difference in the DMH and LHA zones between SHRs and WKY rats at either age (P > 0.05, one‐way ANOVA; Fig. 5 C).
The numbers of fos–OXA‐ir double stained neurons in the hypothalamus (Hyp; three zones combined) and the three individual zones (DMH, PeF and LHA) are summarized and shown in Fig. 5 D. In room‐air control conditions, fewer fos–OXA‐ir positive neurons were found in both SHRs and WKY rats; however, both young and adult SHRs (143 ± 17 and 87 ± 8, respectively) had more fos–OXA‐ir neurons than age‐matched WKY rats (75 ± 20 and 35 ± 5, respectively) in Hyp (P ≤ 0.011, one‐way ANOVA; Fig. 5 D, grey bars). The difference was mainly contributed by DMH and PeF regions, and the only individual area that showed a significant difference was the PeF region between adult SHRs and WKY rats (P ≤ 0.001, one‐way ANOVA; Fig. 5). Hypercapnia (7% CO2) activated more orexin neurons (fos–OXA‐ir) in the hypothalamus in both young (255 ± 29) and adult SHRs (206 ± 4) than in age‐matched WKY rats (92 ± 35 and 44 ± 13, respectively, P = 0.002, one‐way ANOVA with Student–Newman–Keuls post hoc test; Fig. 5 D). In this situation, both the PeF and DMH zones contributed to the effect. In the Pef zone, both young and adult SHRs had significantly more fos–OXA‐ir neurons than age‐matched WKY control rats (P < 0.001, one‐way ANOVA with Student–Newman–Keuls post hoc test; Fig. 5 D). In the DMH zone, both young and adult SHRs had more fos–OXA‐ir neurons than age‐matched WKY rats, but only the difference between the adult SHRs and WKY rats reached significance (P = 0.007, one‐way ANOVA with Student–Newman–Keuls post hoc test). In the LHA, very few orexin neurons were activated by the hypercapnia (7% CO2), and there was no significant difference between SHRs and WKY rats at either age.
When comparing the difference in numbers of fos–OXA‐ir neurons between room air and 7% CO2 in the three individual zones and combined Hyp between SHRs and WKY rats at young or adult ages, we found that the significant differences were only in the PeF zone and Hyp in young SHRs (P ≤ 0.001, one‐way ANOVA) and in the PeF and DMH zones and Hyp in adult SHRs (P ≤ 0.002, one‐way ANOVA; Fig. 5 D).
When comparing young and adult animals within each strain, we found no significant difference in the total number of OXA‐ir or fos–OXA‐ir neurons in the hypothalamus or in any of the three zones between young and adult SHRs or WKY rats (P > 0.05, one‐way ANOVA).
Discussion
The main findings of this study are as follows: (i) SHRs have an augmented CO2 chemoreflex compared with age‐matched normotensive rats; (ii) both the augmented CO2 chemoreflex and the higher ABP are measureable in young rats (postnatal day 30–58) and become greater in adult SHRs; (iii) attenuation of the peripheral chemoreflexes by hyperoxia does not diminish the augmented CO2 chemoreflex in SHRs; (iv) SHRs have more orexin neurons in the hypothalamic PeF zone, and hypercapnia activates significantly more orexin neurons (PeF zone in young rats; PeF and DMH zones in adult rats); and (v) blocking orexin receptors with a dual orexin receptor antagonist, Almxt, in SHRs normalizes the augmented CO2 chemoreflex in both young and adult animals, normalizes the higher ABP in the young rats, and significantly lowers the higher ABP in the adults.
Animal models
Spontaneously hypertensive rats are the most commonly used animal model to study neurogenic hypertension. The SHR strain was obtained from selective breading of the Wistar rats at Kyoto University with high blood pressure during 1960s by Dr Okamoto and his colleagues (Okamoto & Aoki, 1963), and the inbred WKY rats were used as the background normotensive control animals for the SHR (Okamoto & Aoki, 1963). There is a mild elevation in ABP in normotensive rats between the fourth and 10th week of age, but it is more severe in SHRs, in which ABP increases by up to 30% above that of WKY rats (Zicha & Kunes, 1999). Spontaneously hypertensive rats develop an established hypertension between 8 and 10 weeks of age without physiological, pharmacological, environmental or surgical intervention (Dickhout & Lee, 1998; Zicha & Kunes, 1999; Dornas & Silva, 2011). In the present study, we focused on two ages, postnatal day 30–58, when hypertension is actively developing, and 4–6 months, when the hypertension is completely established. Our data show that a higher CO2 chemoreflex and ABP are already measureable at the age of postnatal day 30–58 in SHRs and progressively increase with age relative to the age‐matched normotensive WKY control rats, at least at the ages we studied.
More OXA‐ir and CO2‐activated fos–OXA‐ir neurons in hypothalamus in SHRs
Overall, SHRs have more orexin neurons in the hypothalamus than normotensive WKY control rats at both young and adult ages, and our data are consistent with two recent publications (Clifford et al. 2015; Lee et al. 2015) and a recent report on the genetically hypertensive BPH/2J mice (Jackson et al. 2016). Moreover, hypercapnia (7% CO2) activated more orexin neurons in the hypothalamus in both young and adult SHRs than in age‐matched normotensive rats. Both the DMH and PeF zones contribute to the significant changes in SHRs. It is known that orexin neurons in the hypothalamus, particularly in the PeF and DMH areas, are involved in cardiorespiratory function (Young et al. 2005; Furlong & Carrive, 2007; Dampney et al. 2008) and responses to stress (Johnson et al. 2010; Nollet et al. 2011; Iigaya et al. 2012).
It is worth noting that in room‐air control conditions, fewer fos–OXA‐ir neurons were found in the hypothalamus in both SHRs and WKY rats, but the difference in total numbers between SHRs and age‐matched WKY rats was significant. When comparing the change in numbers of fos–OXA‐ir neurons in the hypothalamus between room‐air control conditions and hypercapnia in SHRs and WKY rats, we found that this change is much more profound in SHRs than in age‐matched WKY rats. For example, in Hyp (all three hypothalamic zones combined) relative to room‐air control conditions, hypercapnia (7% CO2) increased the number of fos–OXA‐ir neurons by 77 and 122% in young and adult SHRs, respectively, and by only 24 and 33%, respectively, in age‐matched WKY rats (Fig. 5 D). From these data we suggest that orexin neurons in SHRs are hyperactive in room‐air conditions and hyper‐responsive to hypercapnia relative to normotensive WKY control rats. We speculate that orexin signalling is higher in SHRs from a very young age and contributes significantly to the development of an augmented CO2 chemoreflex and hypertension. More studies may be needed to draw a definitive conclusion on this issue.
It has been demonstrated that CO2 can activate orexin neurons in vitro and in vivo in normal animals (Williams et al. 2007; Sunanaga et al. 2009). In the present study, three indices [(i) numbers of CO2‐activated fos–OXA‐ir neurons; (ii) magnitude of changes in ventilation; and (iii) magnitude of changes in ABP] were used to evaluate the CO2 chemoreflex, and we showed that all three indices were significantly higher in SHRs than in age‐matched normotensive WKY control rats (Figs 1, 2, 3, 4, 5). It is very interesting to note that Almxt significantly decreased the response to hypercapnia in both SHRs and WKY rats; however, the magnitude of change of response to CO2 with Almxt treatment was much larger in SHRs than in WKY rats. For example, the response to hypercapnia with Almxt was ∼20 and ∼71% lower than no‐treatment control in adult WKY rats and SHRs respectively, and in SHRs the Almxt treatment decreased the augmented CO2 chemoreflex to a level that was no longer significantly higher than that of the normotensive control rats. The results suggest that Almxt may be more potent when the orexin system is highly activated. A similar phenomenon has been observed previously, e.g. an orexin receptor antagonist has little effect on resting cardiorespiratory parameters, but it can block the cardiorespiratory response to exogenous orexin, fear and anxiety panic (Shirasaka et al. 2002; Johnson et al. 2010; Iigaya et al. 2012).
Carbon dioxide chemoreception in SNA, ABP and hypertension
The sympathetic nervous system plays a crucial role in the regulation of ABP, and increased central sympathetic drive is one of the hallmarks of essential hypertension in humans and in SHRs. Many factors, including chemoreflexes, are known to affect sympathetic tone, and both hypoxia and hypercapnia are capable of inducing increased ventilation, sympathoexcitation and increased ABP (Hanna et al. 1981; Trzebski & Kubin, 1981; Lioy & Trzebski, 1984; Somers et al. 1989; Nattie et al. 1992, 1993; Oikawa et al. 2005; Guyenet et al. 2010; Li & Nattie, 2014). The role of peripheral chemoreceptors in neurogenic hypertension and in heart failure has generated attention recently (Paton et al. 2013 a; Schultz et al. 2015). Studies have shown that an enhanced peripheral chemoreflex may contribute to the development of hypertension by altering respiratory–sympathetic coupling and increasing the sympathetic vascular tone (Trzebski & Kubin, 1981; Trzebski et al. 1982; Somers et al. 1988; Zoccal et al. 2009; Sinski et al. 2012) and that denervation of the carotid body can significantly lower ABP in patients with persistent hypertension and in SHRs (Abdala et al. 2012; Paton et al. 2013 b). However, few studies have focused on the role of central chemoreceptors or the CO2 chemoreflex in pathological conditions, such as neurogenic hypertension, even though it is known that in conscious normal rats hypercapnia induces significant increases in renal SNA, mean ABP and respiratory rate in rats with and without peripheral carotid and/or aortic chemoreceptors (Oikawa et al. 2005).
How central and peripheral chemoreceptors interact in regulating the CO2 chemoreflex remains a controversial topic (Duffin & Mateika, 2013; Dempsey et al. 2014). Studies in humans and dogs suggest that central and peripheral chemoreceptors are not functionally separate but rather that they are interdependent in responding to the change in arterial CO2/pH (Duffin & Mateika, 2013; Dempsey et al. 2014). It is unknown what the relationship between central and peripheral chemoreceptors is in pathological conditions, i.e. neurogenic hypertension. In the present study, we showed that SHRs have an augmented CO2 chemoreflex that is progressively associated with the increase in ABP from postnatal day 30–58 to adulthood, and the enhanced CO2 chemoreflex may be predominantly attributable to overactive central chemoreceptors, because it persisted while the activity of the peripheral chemoreceptors was attenuated by hyperoxia. We speculate that both central and peripheral chemoreceptors are overactive in SHRs and contribute to the enhanced SNA and respiratory–sympathetic coupling in SHRs.
Orexin in enhanced CO2 chemoreflex and high ABP in SHRs
The finding that hypothalamic orexin participates in the regulation of the CO2 chemoreflex (Williams et al. 2007; Dias et al. 2009, 2010; Sunanaga et al. 2009; Kuwaki et al. 2010; Li & Nattie, 2010; Lazarenko et al. 2011), SNA and blood pressure (Chen et al. 2000; Matsumura et al. 2001; Shirasaka et al. 2002; Shahid et al. 2012) has been well documented. In normal rats, antagonism of orexin receptors can significantly decrease the CO2 chemoreflex (Dias et al. 2009, 2010; Li & Nattie, 2010), and central injection of orexin‐A can significantly increase breathing, SNA and ABP (Chen et al. 2000; Matsumura et al. 2001; Shirasaka et al. 2002; Shahid et al. 2012). In adult SHRs, we have shown that blocking both orexin receptors via an oral administration of Almxt can significantly lower SNA and ABP (by up to 30 mmHg) in both wakefulness and sleep, an effect accompanied by decreased levels of noradrenaline in cerebrospinal fluid and blood (Li et al. 2013 a). In the present study, we provide further evidence supporting our hypothesis that SHRs have an overactive orexin system, as follows. (i) Blocking orexin receptors with Almxt can not only significantly lower ABP but also normalize the augmented CO2 chemoreflex in SHRs. (ii) Spontaneously hypertensive rats have more orexin neurons, and a greater proportion of orexin neurons are activated by hypercapnia (7% CO2) than in age‐matched normotensive WKY rats. This suggests that SHRs not only have more orexin neurons but they also have more hyperactive or hypersensitive orexin neurons in the hypothalamus, at least in response to CO2. It is likely that these hyperactive orexin neurons are responsible for the augmented CO2 chemoreflex and possibly the overactive SNA and the development of hypertension in SHRs.
In SHRs, the increased ABP and CO2 chemoreflex are measureable from a young age (postnatal day 30–58) and become progressively greater with age. Almorexant treatment in young SHRs significantly lowered ABP to a level that was no longer significantly different from that of age‐matched normotensive WKY rats, whereas in adult SHRs the ABP post‐treatment was still significantly higher than that of age‐matched WKY rats. We speculate that in SHRs the upregulated orexin system produces a long‐term excitatory drive to many cardiorespiratory‐related nuclei in the brainstem and spinal cord projection sites and results in a long‐term increase in the CO2 chemoreflex, SNA and blood pressure. The sustained increase in SNA may also induce partly irreversible changes in vascular smooth muscle of resistance vessels. Downregulation of the orexin system, e.g. by blocking orexin receptors, can, at least in part, remove the elevated orexin excitatory input on these cardiovascular nuclei and vascular smooth muscle cells, resulting in a depressor effect, that lowers SNA, ABP and the CO2 chemoreflex. The overactive orexin system could also overexcite hypothalamic–pituitary–adrenal axis functions, which could also lead to altered cardiorespiratory changes in SHRs.
Perspectives
It is not clear at present why there is an increased number of orexin neurons in SHRs and whether the increased number of orexin neurons is the cause of hypertension in SHRs. Mounting evidence in the past decade has shown that the orexin system is linked with hyperarousal, anxiety/stress and autonomic functions (Kayaba et al. 2003; Furlong et al. 2009; Huang et al. 2010; Johnson et al. 2010; Li & Nattie, 2010; de Lecea, 2012; Shahid et al. 2012; Li et al. 2013 a; Bonnavion et al. 2015; Clifford et al. 2015; de Lecea, 2015), and it has been proposed that the orexin system may be the link between autonomic function and stress (Kuwaki et al. 2008, 2010). Some reports suggest that stress can lead to an upregulated orexin system. For example, exposing normal rats to an acute episode of footshock led to an increase in prepro‐orexin mRNA for 6 and 14 days (Chen et al. 2014), whereas daily footshock for 2 weeks induced hypertension and doubled the number of orexin neurons (Xiao et al. 2013). The number of orexin neurons increased by 20% when rats were REM sleep deprived using the technique of multiple small platforms over water for a week (Allard et al. 2007). These data suggest that stress or extreme conditions can alter the orexin system in the healthy normotensive individual. In SHRs, in contrast, a genetic trait may play an important role in development of hypertension, because SHRs will develop hypertension without any physiological, pharmacological, environmental or surgical intervention. More studies are needed to gain a better understanding of why SHRs have an augmented CO2 chemoreflex and upregulated orexin system, and their role in the development of neurogenic hypertension.
Summary and conclusions
The augmented CO2 chemoreflex and overactive orexin system are associated with high ABP in neurogenic hypertension in SHRs. This link between the overactive orexin system and the augmented CO2 chemoreflex and high ABP is already present at an age of postnatal day 30–58 in SHRs, and downregulation of the overactive orexin system has significant antihypertensive effects. We hypothesize that this overactive orexin system may overstimulate many key central chemoreceptor and cardiovascular sites as well as vascular smooth muscle of resistance vessels, causing or facilitating the increase of SNA, CO2 chemoreflex and blood pressure in the neurogenic hypertension of SHRs. Modulation of the orexin system and central CO2 chemoreflex may be beneficial in treatment of neurogenic hypertension.
Additional information
Competing interests
None declared.
Author contributions
All experiments were conducted at Geisel School of Medicine at Dartmouth. A.L.: designed the experiments, performed physiology experiments and data analysis, and wrote the manuscript. S.H.R.: performed cell counts and staining. A.L. & E.E.N.: contributed equally to conceive the hypothesis and conceptualize the findings, revised and finalized the manuscript. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
The study was supported by the National Heart, Lung and Blood Institute (NHLBI), HL 28066.
References
- Abdala AP, McBryde FD, Marina N, Hendy EB, Engelman ZJ, Fudim M, Sobotka PA, Gourine AV & Paton JFR (2012). Hypertension is critically dependent on the carotid body input in the spontaneously hypertensive rat. J Physiol 590, 4269–4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard JS, Tizabi Y, Shaffery JP & Manaye K (2007). Effects of rapid eye movement sleep deprivation on hypocretin neurons in the hypothalamus of a rat model of depression. Neuropeptides 41, 329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett D Jr & Tenney SM (1970). Control of breathing in experimental anemia. Respir Physiol 10, 384–395. [DOI] [PubMed] [Google Scholar]
- Bonnavion P, Jackson AC, Carter ME & de Lecea L (2015). Antagonistic interplay between hypocretin and leptin in the lateral hypothalamus regulates stress responses. Nat Commun 6, 6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisbare‐Roch C, Dingemanse J, Koberstein R, Hoever P, Aissaoui H, Flores S, Mueller C, Nayler O, van Gerven J, de Haas SL, Hess P, Qiu C, Buchmann S, Scherz M, Weller T, Fischli W, Clozel M & Jenck F (2007). Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat Med 13, 150–155. [DOI] [PubMed] [Google Scholar]
- Chen CT, Hwang LL, Chang JK & Dun NJ (2000). Pressor effects of orexins injected intracisternally and to rostral ventrolateral medulla of anesthetized rats. Am J Physiol Regul Integr Comp Physiol 278, R692–R697. [DOI] [PubMed] [Google Scholar]
- Chen X, Wang H, Lin Z, Li S, Li Y, Bergen HT, Vrontakis ME & Kirouac GJ (2014). Orexins (hypocretins) contribute to fear and avoidance in rats exposed to a single episode of footshocks. Brain Struct Funct 219, 2103–2118. [DOI] [PubMed] [Google Scholar]
- Clifford L, Dampney BW & Carrive P (2015). Spontaneously hypertensive rats have more orexin neurons in their medial hypothalamus than normotensive rats. Exp Physiol 100, 388–398. [DOI] [PubMed] [Google Scholar]
- Dampney RA, Horiuchi J & McDowall LM (2008). Hypothalamic mechanisms coordinating cardiorespiratory function during exercise and defensive behaviour. Auton Neurosci 142, 3–10. [DOI] [PubMed] [Google Scholar]
- Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, Sakurai T, Yanagisawa M & Nakazato M (1999). Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci USA 96, 748–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L (2012). Hypocretins and the neurobiology of sleep‐wake mechanisms. Prog Brain Res 198, 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L (2015). Optogenetic control of hypocretin (orexin) neurons and arousal circuits. Curr Top Behav Neurosci 25, 367–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey JA, Powell FL, Bisgard GE, Blain GM, Poulin MJ & Smith CA (2014). Role of chemoreception in cardiorespiratory acclimatization to, and deacclimatization from, hypoxia. J Appl Physiol 116, 858–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias MB, Li A & Nattie EE (2009). Antagonism of orexin receptor‐1 in the retrotrapezoid nucleus inhibits the ventilatory response to hypercapnia predominantly in wakefulness. J Physiol 587, 2059–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias MB, Li A & Nattie E (2010). The orexin receptor 1 (OX1R) in the rostral medullary raphe contributes to the hypercapnic chemoreflex in wakefulness, during the active period of the diurnal cycle. Respir Physiol Neurobiol 170, 96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickhout JG & Lee RM (1998). Blood pressure and heart rate development in young spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 274, H794–H800. [DOI] [PubMed] [Google Scholar]
- Dornas WC & Silva ME (2011). Animal models for the study of arterial hypertension. J Biosci 36, 731–737. [DOI] [PubMed] [Google Scholar]
- Duffin J & Mateika JH (2013). Cross‐Talk opposing view: peripheral and central chemoreflexes have additive effects on ventilation in humans. J Physiol 591, 4351–4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugovic C, Shelton JE, Aluisio LE, Fraser IC, Jiang X, Sutton SW, Bonaventure P, Yun S, Li X, Lord B, Dvorak CA, Carruthers NI & Lovenberg TW (2009). Blockade of orexin‐1 receptors attenuates orexin‐2 receptor antagonism‐induced sleep promotion in the rat. J Pharmacol Exp Ther 330, 142–151. [DOI] [PubMed] [Google Scholar]
- Furlong T & Carrive P (2007). Neurotoxic lesions centered on the perifornical hypothalamus abolish the cardiovascular and behavioral responses of conditioned fear to context but not of restraint. Brain Res 1128, 107–119. [DOI] [PubMed] [Google Scholar]
- Furlong TM, Vianna DM, Liu L & Carrive P (2009). Hypocretin/orexin contributes to the expression of some but not all forms of stress and arousal. Eur J Neurosci 30, 1603–1614. [DOI] [PubMed] [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology . J Physiol 593, 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Abbott SB, Depuy SD, Fortuna MG & Kanbar R (2010). Central CO2 chemoreception and integrated neural mechanisms of cardiovascular and respiratory control. J Appl Physiol 108, 995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna BD, Lioy F & Polosa C (1981). Role of carotid and central chemoreceptors in the CO2 response of sympathetic preganglionic neurons. J Auton Nerv Syst 3, 421–435. [DOI] [PubMed] [Google Scholar]
- Huang SC, Dai YW, Lee YH, Chiou LC & Hwang LL (2010). Orexins depolarize rostral ventrolateral medulla neurons and increase arterial pressure and heart rate in rats mainly via orexin 2 receptors. J Pharmacol Exp Ther 334, 522–529. [DOI] [PubMed] [Google Scholar]
- Iigaya K, Horiuchi J, McDowall LM, Lam ACB, Sediqi Y, Polson JW, Carrive P & Dampney RAL (2012). Blockade of orexin receptors with Almorexant reduces cardiorespiratory responses evoked from the hypothalamus but not baro‐ or chemoreceptor reflex responses. Am J Physiol Regul Integr Comp Physiol 303, R1011–R1022. [DOI] [PubMed] [Google Scholar]
- Jackson KL, Dampney BW, Moretti JL, Stevenson ER, Davern PJ, Carrive P & Head GA (2016). Contribution of orexin to the neurogenic hypertension in BPH/2J mice. Hypertension 67, 959–969. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Truitt W, Fitz SD, Minick PE, Dietrich A, Sanghani S, Traskman‐Bendz L, Goddard AW, Brundin L & Shekhar A (2010). A key role for orexin in panic anxiety. Nat Med 16, 111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayaba Y, Nakamura A, Kasuya Y, Ohuchi T, Yanagisawa M, Komuro I, Fukuda Y & Kuwaki T (2003). Attenuated defense response and low basal blood pressure in orexin knockout mice. Am J Physiol Regul Integr Comp Physiol 285, R581–R593. [DOI] [PubMed] [Google Scholar]
- Kuwaki T, Li A & Nattie E (2010). State‐dependent central chemoreception: a role of orexin. Respir Physiol Neurobiol 173, 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwaki T, Zhang W, Nakamura A & Deng BS (2008). Emotional and state‐dependent modification of cardiorespiratory function: role of orexinergic neurons. Auton Neurosci 142, 11–16. [DOI] [PubMed] [Google Scholar]
- Lazarenko RM, Stornetta RL, Bayliss DA & Guyenet PG (2011). Orexin A activates retrotrapezoid neurons in mice. Respir Physiol Neurobiol 175, 283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Tsai MC, Li TL, Dai YW, Huang SC & Hwang LL (2015). Spontaneously hypertensive rats have more orexin neurons in the hypothalamus and enhanced orexinergic input and orexin 2 receptor‐associated nitric oxide signalling in the rostral ventrolateral medulla. Exp Physiol 100, 993–1007. [DOI] [PubMed] [Google Scholar]
- Li A, Emond L & Nattie E (2008). Brainstem catecholaminergic neurons modulate both respiratory and cardiovascular function. Adv Exp Med Biol 605, 371–376. [DOI] [PubMed] [Google Scholar]
- Li A, Hindmarch CCT, Nattie EE & Paton JFR (2013. a). Antagonism of orexin receptors significantly lowers blood pressure in spontaneously hypertensive rats. J Physiol 591, 4237–4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Hindmarch CC, Nattie EE & Paton JF (2013. b). Reply from Aihua Li, Charles C. T. Hindmarch, Eugene E. Nattie and Julian F. R. Paton. J Physiol 591, 6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A & Nattie E (2006). Catecholamine neurones in rats modulate sleep, breathing, central chemoreception and breathing variability. J Physiol 570, 385–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A & Nattie E (2010). Antagonism of rat orexin receptors by almorexant attenuates central chemoreception in wakefulness in the active period of the diurnal cycle. J Physiol 588, 2935–2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A & Nattie E (2014). Orexin, cardio‐respiratory function, and hypertension. Front Neurosci 8, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lioy F & Trzebski A (1984). Pressor effect of CO2 in the rat: different thresholds of the central cardiovascular and respiratory responses to CO2 . J Auton Nerv Syst 10, 43–54. [DOI] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M & Elmquist JK (2001). Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol 435, 6–25. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Tsuchihashi T & Abe I (2001). Central orexin‐A augments sympathoadrenal outflow in conscious rabbits. Hypertension 37, 1382–1387. [DOI] [PubMed] [Google Scholar]
- Nattie E & Li A (2000). Muscimol dialysis in the retrotrapezoid nucleus region inhibits breathing in the awake rat. J Appl Physiol 89, 153–162. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Blanchford C & Li A (1992). Retrofacial lesions: effects on CO2‐sensitive phrenic and sympathetic nerve activity. J Appl Physiol 73, 1317–1325. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Gdovin M & Li A (1993). Retrotrapezoid nucleus glutamate receptors: control of CO2‐sensitive phrenic and sympathetic output. J Appl Physiol 74, 2958–2968. [DOI] [PubMed] [Google Scholar]
- Nattie EE & Li A (2001). CO2 dialysis in the medullary raphe of the rat increases ventilation in sleep. J Appl Physiol 90, 1247–1257. [DOI] [PubMed] [Google Scholar]
- Nixon JP & Smale L (2007). A comparative analysis of the distribution of immunoreactive orexin A and B in the brains of nocturnal and diurnal rodents. Behav Brain Funct 3, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nollet M, Gaillard P, Minier F, Tanti A, Belzung C & Leman S (2011). Activation of orexin neurons in dorsomedial/perifornical hypothalamus and antidepressant reversal in a rodent model of depression. Neuropharmacology 61, 336–346. [DOI] [PubMed] [Google Scholar]
- Oikawa S, Hirakawa H, Kusakabe T, Nakashima Y & Hayashida Y (2005). Autonomic cardiovascular responses to hypercapnia in conscious rats: the roles of the chemo‐ and baroreceptors. Auton Neurosci 117, 105–114. [DOI] [PubMed] [Google Scholar]
- Okamoto K & Aoki K (1963). Development of a strain of spontaneously hypertensive rats. Jpn Circ J 27, 282–293. [DOI] [PubMed] [Google Scholar]
- Paton JFR, Ratcliffe L, Hering D, Wolf J, Sobotka PA & Narkiewicz K (2013. a). Revelations about carotid body function through its pathological role in resistant hypertension. Curr Hypertens Rep 15, 273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JFR, Sobotka PA, Fudim M, Engleman ZJ, Hart ECJ, McBryde FD, Abdala AP, Marina N, Gourine AV, Lobo M, Patel N, Burchell A, Ratcliffe L & Nightingale A (2013. b). The carotid body as a therapeutic target for the treatment of sympathetically mediated diseases. Hypertension 61, 5–13. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG & Kilduff TS (1998). Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 18, 9996–10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin DL, Chang DA & Guyenet PG (2006). Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol 499, 64–89. [DOI] [PubMed] [Google Scholar]
- Schultz HD, Marcus NJ & Del Rio R (2015). Mechanisms of carotid body chemoreflex dysfunction during heart failure. Exp Physiol 100, 124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahid IZ, Rahman AA & Pilowsky PM (2011). Intrathecal orexin A increases sympathetic outflow and respiratory drive, enhances baroreflex sensitivity and blocks the somato‐sympathetic reflex. Br J Pharmacol 162, 961–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahid IZ, Rahman AA & Pilowsky PM (2012). Orexin A in rat rostral ventrolateral medulla is pressor, sympatho‐excitatory, increases barosensitivity and attenuates the somato‐sympathetic reflex. Br J Pharmacol 165, 2292–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasaka T, Kunitake T, Takasaki M & Kannan H (2002). Neuronal effects of orexins: relevant to sympathetic and cardiovascular functions. Regul Pept 104, 91–95. [DOI] [PubMed] [Google Scholar]
- Sinski M, Lewandowski J, Przybylski J, Bidiuk J, Abramczyk P, Ciarka A & Gaciong Z (2012). Tonic activity of carotid body chemoreceptors contributes to the increased sympathetic drive in essential hypertension. Hypertens Res 35, 487–491. [DOI] [PubMed] [Google Scholar]
- Somers VK, Mark AL & Abboud FM (1988). Potentiation of sympathetic nerve responses to hypoxia in borderline hypertensive subjects. Hypertension 11, 608–612. [DOI] [PubMed] [Google Scholar]
- Somers VK, Mark AL, Zavala DC & Abboud FM (1989). Contrasting effects of hypoxia and hypercapnia on ventilation and sympathetic activity in humans. J Appl Physiol 67, 2101–2106. [DOI] [PubMed] [Google Scholar]
- Sunanaga J, Deng BS, Zhang W, Kanmura Y & Kuwaki T (2009). CO2 activates orexin‐containing neurons in mice. Respir Physiol Neurobiol 166, 184–186. [DOI] [PubMed] [Google Scholar]
- Taylor NC, Li A & Nattie EE (2005). Medullary serotonergic neurones modulate the ventilatory response to hypercapnia, but not hypoxia in conscious rats. J Physiol 566, 543–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzebski A & Kubin L (1981). Is the central inspiratory activity responsible for pCO2‐dependent drive of the sympathetic discharge? J Auton Nerv Syst 3, 401–420. [DOI] [PubMed] [Google Scholar]
- Trzebski A, Tafil M, Zoltowski M & Przybylski J (1982). Increased sensitivity of the arterial chemoreceptor drive in young men with mild hypertension. Cardiovasc Res 16, 163–172. [DOI] [PubMed] [Google Scholar]
- Williams RH, Jensen LT, Verkhratsky A, Fugger L & Burdakov D (2007). Control of hypothalamic orexin neurons by acid and CO2 . Proc Natl Acad Sci USA 104, 10685–10690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F, Jiang M, Du D, Xia C, Wang J, Cao Y, Shen L & Zhu D (2013). Orexin A regulates cardiovascular responses in stress‐induced hypertensive rats. Neuropharmacology 67, 16–24. [DOI] [PubMed] [Google Scholar]
- Young JK, Wu M, Manaye KF, Kc P, Allard JS, Mack SO & Haxhiu MA (2005). Orexin stimulates breathing via medullary and spinal pathways. J Appl Physiol 98, 1387–1395. [DOI] [PubMed] [Google Scholar]
- Zicha J & Kunes J (1999). Ontogenetic aspects of hypertension development: analysis in the rat. Physiol Rev 79, 1227–1282. [DOI] [PubMed] [Google Scholar]
- Zoccal DB, Bonagamba LGH, Paton JFR & Machado BH (2009). Sympathetic‐mediated hypertension of awake juvenile rats submitted to chronic intermittent hypoxia is not linked to baroreflex dysfunction. Exp Physiol 94, 972–983. [DOI] [PubMed] [Google Scholar]


