Abstract
Key points
We have developed a simple analytical method for quantifying the transduction of sympathetic activity into vascular tone.
This method demonstrates that as women age, the transfer of sympathetic nerve activity into vascular tone is increased, so that for a given level of sympathetic activity there is more vasoconstriction. In men, this measure decreases with age.
Test–re‐test analysis demonstrated that the new method is a reliable estimate of sympathetic transduction.
We conclude that increased sympathetic vascular coupling contributes to the age‐related increase in blood pressure that occurs in women only.
This measure is a reliable estimate of sympathetic transduction in populations with high sympathetic nerve activity. Thus, it will provide information regarding whether treatment targeting the sympathetic nervous system, which interrupts the transfer of sympathetic nerve activity into vascular tone, will be effective in reducing blood pressure in hypertensive patients. This may provide insight into which populations will respond to certain types of anti‐hypertensive medication.
Abstract
Sex and age differences in the sympathetic control of resting blood pressure (BP) may be due to differences in the transduction of sympathetic nerve activity (SNA) into vascular tone. Current methods for dynamically quantifying transduction focus on the relationship between SNA and vasoconstriction during a pressor stimulus, which increases BP and may be contra‐indicated in patients. We describe a simple analytical method for quantifying transduction under resting conditions. We performed linear regression analysis of binned muscle SNA burst areas against diastolic BP (DBP). We assessed whether the slope of this relationship reflects the transduction of SNA into DBP. To evaluate this, we investigated whether this measure captures differences in transduction in different populations. Specifically, we (1) quantified transduction in young men (YM), young women (YW), older men (OM) and postmenopausal women (PMW); and (2) measured changes in transduction during β‐blockade using propranolol in YW, YM and PMW. YM had a greater transduction vs. OM (0.10 ± 0.01 mmHg (% s)−1, n = 23 vs. 0.06 ± 0.01 mmHg (% s)−1, n = 18; P = 0.003). Transduction was lowest in YW (0.02 ± 0.01 mmHg (% s)−1, n = 23) and increased during β‐blockade (0.11 ± 0.01 mmHg (% s)−1; P < 0.001). Transduction in PMW (0.07 ± 0.01 mmHg (% s)−1, n = 23) was greater compared to YW (P = 0.001), and was not altered during β‐blockade (0.06 ± 0.01 mmHg (% s)−1; P = 0.98). Importantly, transduction increased in women with age, but decreased in men. Transduction in women intersected that in men at 55 ± 1.5 years. This measure of transduction captures age‐ and sex‐differences in the sympathetic regulation of DBP and may be valuable in quantifying transduction in disease. In particular, this measure may help target treatment strategies in specific hypertensive subpopulations.
Key points
We have developed a simple analytical method for quantifying the transduction of sympathetic activity into vascular tone.
This method demonstrates that as women age, the transfer of sympathetic nerve activity into vascular tone is increased, so that for a given level of sympathetic activity there is more vasoconstriction. In men, this measure decreases with age.
Test–re‐test analysis demonstrated that the new method is a reliable estimate of sympathetic transduction.
We conclude that increased sympathetic vascular coupling contributes to the age‐related increase in blood pressure that occurs in women only.
This measure is a reliable estimate of sympathetic transduction in populations with high sympathetic nerve activity. Thus, it will provide information regarding whether treatment targeting the sympathetic nervous system, which interrupts the transfer of sympathetic nerve activity into vascular tone, will be effective in reducing blood pressure in hypertensive patients. This may provide insight into which populations will respond to certain types of anti‐hypertensive medication.
Abbreviations
- BP
blood pressure
- DBP
diastolic blood pressure
- MAP
mean arterial blood pressure
- MSNA
muscle sympathetic nerve activity
- OM
older men
- PMW
postmenopausal women
- SBP
systolic blood pressure
- SNA
sympathetic nerve activity
- TPR
total peripheral resistance
- YM
younger men
- YW
younger women
Introduction
Action potentials in vasomotor sympathetic nerves arriving at the sympathetic neurovascular junction cause release of noradrenaline, which usually results in vasoconstriction and increased vascular resistance. This is a fundamental physiological mechanism underlying the central role of the sympathetic nervous system in regulation of arterial pressure in humans (Blessing, 1997; Guyenet, 2006; Joyner et al. 2008; Fink, 2009).
The ability of the vasculature to respond to bursts of sympathetic nerve activity (SNA) depends on the transmission process from the sympathetic postganglionic nerve terminals to the contractile machinery of the arteriole, the vascular smooth muscle cells. Sympathetic nerves release noradrenaline along with co‐transmitters, which can modulate the vascular response (Huidobro‐Toro & Donoso, 2004; Burnstock, 2008, 2009). These transmitters act presynaptically (modulating subsequent transmitter release; Ellis & Burnstock, 1990) and directly on the smooth muscle cell (Wier et al. 2009). Additionally, the relative local density and sensitivity of α‐ versus β‐adrenergic receptors in a given vascular bed may modulate the vascular response to noradrenaline itself, resulting in vasoconstriction or opposing vasodilatation (Kneale et al. 2000). Other influences, such as local concentrations of vasoactive factors (e.g. nitric oxide, endothelin) and mediators of functional hyperaemia, may also affect the relationship between sympathetic nerve activity and vasoconstriction. The transduction of sympathetic activity into vascular tone is, therefore, a major contributor to the ability of the sympathetic nervous system to control blood pressure but is highly prone to modulation.
Over the past several decades, various approaches have been developed to quantify the transduction of sympathetic nerve activity into vascular tone (Halliwill et al. 1996; Minson et al. 2000; Hogarth et al. 2007; Hart et al. 2009 b; Fairfax et al. 2013; Tan et al. 2013). Quantifying differences in the relationship between SNA and peripheral vasoconstriction (and subsequently arterial pressure) has helped progress our understanding of how the female sex hormones and menopause impact upon the control of resting blood pressure (Hart et al. 2011). This approach may also provide insight relevant to the treatment of hypertension, whilst providing a knowledge base regarding which medications might be more efficacious for different sexes and ages. Most existing reports on vascular transduction have focused on correlations between muscle SNA (MSNA) and vascular tone in groups of people at rest (Hart et al. 2009 a,b, 2011); however, using this approach makes it difficult to statistically quantify differences in transduction between individuals and/or populations of people. Other measures rely on comparing changes in vascular tone to changes in MSNA evoked by a stressful stimulus (e.g. isometric handgrip; Minson et al. 2000), which increases blood pressure and may be contra‐indicated in some patients. Additionally, these measures do not reflect sympathetic vascular transduction at rest or take into account the dynamic response of the vasculature to smaller beat‐to‐beat changes in MSNA. Others have attempted to quantify this dynamic component of sympathetic vascular transduction (Fairfax et al. 2013); however, their method cannot be reliably used in people with high MSNA burst incidence (quantified as bursts per 100 heart beats), due to the underlying analytical technique.
Therefore, our goal in this study was to develop a new, simple, automated approach to quantify sympathetic transduction in individual participants at rest, by evaluating relationships between MSNA and its effect on diastolic blood pressure (DBP). In young men, SNA is related to total peripheral resistance – a relationship that is not present in older men (Hart et al. 2009 b). Furthermore, the contractile response of male human arteries to noradrenaline is reduced with age (Dinenno et al. 2002; Smith et al. 2007). Taken together, these data support a diminishment of sympathetic transduction with age, at least in men. During mental stress, MSNA responses are similar between men and women, but the resulting vascular effects differ, with women tending to have more vasodilatation and/or smaller pressor effects compared with men (Carter & Ray, 2009; Yang et al. 2013). This suggests that men and women have different efficacies of sympathetic neurovascular transduction. Indeed, young women exhibit vasodilatory effects of noradrenaline binding to the β‐adrenergic receptors (Kneale et al. 2000; Hart et al. 2011), where the dominant receptor mediating the response to noradrenaline is a β2 adrenergic subtype. We used these insights to test our measure of transduction in a cohort of young women, young men, older men and postmenopausal women. We also verified our measure by quantifying transduction during propranolol (a non‐selective β‐adrenergic antagonist) infusion. We discuss the validity of this measure and its clinical significance.
Methods
Participants
Part of the data used in this study was retrospectively analysed from a previous study (Hart et al. 2011). This investigation had ethical approval from the Institutional Review Board of the Mayo Clinic. Other data were taken from ongoing research in our Bristol laboratory, which has ethical approval from an NHS Research Ethics Committee (11/SW/0254). Our study conforms to the standards set by the latest revision of the Declaration of Helsinki.
In total, 83 normotensive participants gave their written informed consent (40 men, 43 women). The men were classed into two groups: younger men (YM; age = 27 ± 1 years (min = 18, max = 38), n = 22) and older men (OM; age = 55 ± 2 years (43,68), n = 18). The females were also classed into two groups: younger women (YW; age = 31 ± 1 years (18,39), n = 23) and postmenopausal women (PMW; 58 ± 2 years (53,71), n = 20). The participants were non‐smokers with no history of cardiovascular or other chronic diseases. All participants had refrained from exercise in the last 24 h.
In the YW, 17 out of 23 participants were taking oral contraceptives. YW using an intra‐uterine device were excluded. PMW were excluded if they were taking hormone replacement therapy. Postmenopause was defined as at least 1 year since last menstruation (Gracia et al. 2005). All postmenopausal women had gone through naturally occurring menopause (i.e. none had received oophorectomy). To minimize the effects of reproductive hormones on autonomic control or cardiovascular function, all women were studied in the early follicular phase of the menstrual cycle or in the low hormone phase of oral contraceptive use (Minson et al. 2000). All women of child‐bearing age were asked to complete a pregnancy test at least 48 h before the study day. Participants were asked not to consume anything within 2 h before the experiment, and not to consume caffeine or alcohol for 24 h before the experiment.
Procedures
On arrival at the laboratory, participants rested in the supine position during instrumentation. A 3‐lead electrocardiogram (ECG) was used for continuous recordings of heart rate. Continuous blood pressure (BP) recordings were measured either using (A) a Finometer (Finapres Medical Systems, Amsterdam, The Netherlands), calibrated to the BP measured in the same arm using an automated cuff, or (B) by placing a 20‐gauge arterial catheter in the brachial artery of the non‐dominant arm (using aseptic technique). Forty‐two participants (11 YM, 12 YW, 13 OM, 6 PMW) had data collected from method (A) and 41 participants (11 YM, 11 YW, 5 OM, 14 PMW) from method (B). There was no significant difference in the Finometer and catheter systolic blood pressure (SBP), DBP and mean arterial blood pressure (MAP) for any of the groups (Table 2, P > 0.05). Data from methods (A) and (B) were pooled to measure differences in neurovascular transduction at rest among YM, YW, OM and PMW. Method (B) was used in the retrospective study (Hart et al. 2011); in this later method, the catheter was connected to a pressure transducer, which was interfaced with a personal computer to measure beat‐to‐beat blood pressure. Data recorded using this method were used to test the transduction measurement. These data were used to examine whether the transduction method could assess changes in neurovascular coupling following β‐blockade.
Table 2.
Difference in BP measurement according to technique in sample populations
| BP (mmHg) | Finometer (n) | Catheter (n) | P value |
|---|---|---|---|
| SBP YM | 140 ± 7 (11) | 133 ± 5 (11) | 0.15 |
| DBP YM | 70 ± 3 (11) | 73 ± 3 (11) | 0.73 |
| MAP YM | 93 ± 4 (11) | 93 ± 2 (11) | 0.36 |
| SBP YW | 131 ± 8 (12) | 140 ± 5 (11) | 0.46 |
| DBP YW | 71 ± 2 (12) | 75 ± 3 (11) | 0.50 |
| MAP YW | 91 ± 3 (12) | 97 ± 3 (11) | 0.38 |
| SBP OM | 127 ± 6 (13) | 141 ± 10 (5) | 0.21 |
| DBP OM | 73 ± 4 (13) | 64 ± 4 (5) | 0.11 |
| MAP OM | 83 ± 3 (13) | 90 ± 4 (5) | 0.12 |
| SBP PMW | 138 ± 7 (6) | 142 ± 6 (14) | 0.34 |
| DBP PMW | 71 ± 4 (6) | 73 ± 2 (14) | 0.63 |
| MAP PMW | 93 ± 4 (6) | 96 ± 3 (14) | 0.34 |
Monitoring beat‐by‐beat BP by Finometer (method A; see Methods) and arterial catheter (method B) does not produce different measurements of MAP, SBP and DBP. Data represented as means ± SEM. Unpaired t test P value. MAP, mean arterial pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; YM, younger men; YW, younger women; OM, older men; PMW, postmenopausal women.
Multiunit MSNA was measured from the peroneal nerve at the fibular head using insulated tungsten microelectrodes. A muscle sympathetic fascicle was identified when taps on the muscle belly or passive muscle stretch evoked mechanoreceptive impulses, and no afferent neural response was evoked by skin stimuli (Sundlof & Wallin, 1977). The recorded signal was amplified 80,000‐fold, band pass filtered (700–5000 Hz), rectified, and integrated (resistance–capacitance integrator circuit time constant 0.1 s) by a nerve traffic analyser. Once a recording site for MSNA was found, 5 min of baseline data were recorded with the participant resting. The peak, beginning and end of every MSNA burst were marked in a data acquisition software (therefore, artefacts were not included; Spike2, CED, Cambridge, UK). The area of the burst was then calculated as the integral of MSNA between the beginning and end of the burst. Each MSNA recording was normalized by the largest burst height in that recording, and represented as a percentage. MSNA burst area therefore had units of percentage ×·seconds. To avoid the skewing of area measurements by signal drift, the area measurement was calculated in the region bounded below MSNA by a constant line intersecting the beginning/end of the MSNA burst. Cardiac cycles including signal artefacts were excluded from analysis.
Systemic β‐blockade
Systemic β‐blockade data were taken from a retrospective study (Hart et al. 2011). In brief, after a baseline period of recording, β‐blockade was achieved via infusion of propranolol in a subpopulation of participants (12 YM, 11 YW and 13 PMW). (The retrospective study did not conduct propranolol infusion in OM.) Intravenous infusion of propranolol was used to achieve a non‐selective β‐blockade. A 0.25 mg kg−1 bolus of propranolol was followed by a continuous infusion of propranolol (4 μg kg−1 min−1) to maintain β‐blockade for 5 min. This dose of propranolol has been previously demonstrated to cause total β‐blockade in adult humans (Bell et al. 2001).
Quantification of sympathetic neurovascular transduction into diastolic blood pressure
To quantify transduction, we used DBP as a proxy for the vascular response because: (a) it is easily and reliably measured, (b) it is a target variable regulated by SNA, and (c) studies show that DBP reflects sympathetic vasomotor tone (Barnes et al. 2014). Along these lines, ganglionic blockade caused a decrease in DBP in YM, but not in YW (Christou et al. 2005). Additionally, ganglionic blockade demonstrates that in younger women DBP does not change, but falls in older women (Barnes et al. 2014).
Measuring the transduction slope
To measure transduction, we quantified the relationship between DBP and preceding MSNA burst areas. We also considered the relationship between DBP and the preceding MSNA burst height.
We measured transduction as follows. For each DBP, the summed MSNA burst area (or height) was measured at a fixed cardiac cycle lag (Fig. 1 A). In Fig. 1 A, this lag (the box region) is depicted as 8–6 cardiac cycles. This process yielded a scatter plot of DBP (the dependent variable) and MSNA burst area measured at the lag (Fig. 1 B). MSNA burst area (units of % s) was binned into 1% s bins, and the associated DBP (mean ± SEM) calculated. These data yield a relationship between MSNA burst area and DBP, the slope of which (we claim) quantifies the transduction of MSNA burst area into DBP for that individual. A non‐significant slope was not used as exclusion criteria for data, as this may reflect poor transduction (rather than a failure of the analytical method). These plots were conducted on the baseline recording for each participant, fitted with a weighted linear regression, and the transduction measure (slope of linear regression; units of mmHg (% s)−1) quantified.
Figure 1. Method of quantifying sympathetic neurovascular transduction into DBP in humans .
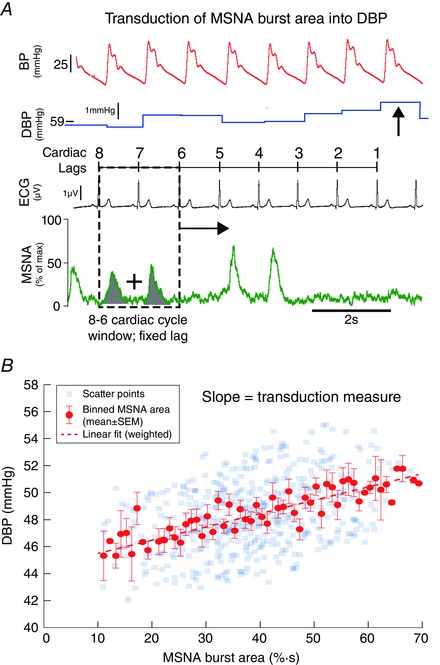
A, for each DBP (arrowed), we summed MSNA burst area (shaded) in a 2 cardiac cycle window at a fixed lag (dashed window). In this example, the fixed lag is 8–6 cardiac cycles preceding the DBP. This ‘window’ was moved across the whole baseline file, associating each DBP with an MSNA burst area. B, these data were represented as a scatter plot for a fixed lag of 8–6 cardiac cycles. MSNA burst area was then binned into 1% s bins, and the corresponding DBP (mean ± SEM) plotted. A weighted linear regression was then fitted to these data, the slope of which gave our measurement of transduction (units of mmHg (% s)−1). MSNA, muscle sympathetic nerve activity; ECG, electrocardiogram; DBP, diastolic blood pressure; BP, blood pressure.
We conducted the same analysis for a box region at 3–1, 4–2, 5–3, 6–4, 7–5, 8–6, 9–7 and 10–8 cardiac cycle lags (as is depicted in Fig. 1). The fixed lag region chosen for each group was the lag that produced the greatest transduction; in YM, this was 8–6 cardiac cycles (Fig. 2 A). This choice of lag in YM was supported by cross‐correlation analysis of beat‐to‐beat MSNA burst area and DBP (Fig. 2 B); the peak cross‐correlation occurred at 7.44 ± 0.42 cardiac cycles. Therefore, in YM, MSNA burst area is most correlated with DBP when it precedes it by 8–6 cardiac cycles. Given that the average heart rate of our YM population was 1.05 Hz (63 beats min−1; see Table 1), a fixed lag of 8–6 cardiac cycles equates to a lag of 5.7–7.6 s. These findings are therefore similar to parameter values quantifying the time to maximal effect in young men (t max = 5.6 ± 0.6; n = 10) reported previously by Tan et al. (2013), further supporting our fixed choice of lag (8–6 cardiac cycles) in YM. For each group (OM, YW and PMW), we conducted this same lag analysis to determine the optimal (i.e. that which gave peak transduction) fixed lag region over which to conduct our transduction analysis (Fig. 2 C). For all groups, this optimal lag region was 8–6 lags.
Figure 2. Determining optimal lag for analysis .
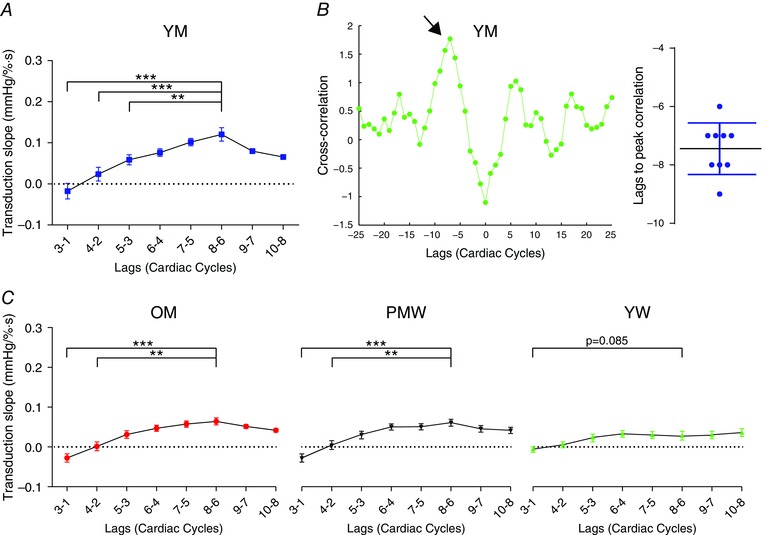
To determine the cardiac cycle lag used for our analysis (see Fig. 1), we conducted the same analysis at different cardiac cycle lags, all of which yielded a ‘window’ of 2 cardiac cycles. A, for a sample population of 10 young men, the transduction was greatest at 8–6 cardiac lags. B, cross‐correlations of beat‐to‐beat MSNA burst area with diastolic blood pressure (DBP) in YM (n = 10). Example cross‐correlation in a participant; peak correlation (arrowed) occurred at a lag of −7 cardiac cycles. Grouped data revealed that correlation peaked at a lag of −7.44 ± 0.42 cardiac cycles. This supports our choice of lag as 8–6 cardiac cycles. C, we repeated the same lag analysis in OM, PMW and YW. For OM and PMW, 8–6 cardiac cycles produced peak transduction. In YW, transduction measured at 8–6 cardiac cycles was nearly significantly greater than at 3–1 (P = 0.082). Therefore, in all subsequent analysis in all groups, we used 8–6 cardiac cycles as our fixed choice of lag. YM, younger men; OM, older men; YW, younger women; PMW, postmenopausal women; one‐way repeated measures ANOVA, ** P < 0.01, *** P < 0.001.
Table 1.
Demographics and baseline neural–haemodynamics of sample populations
| YM (n = 22) | YW (n = 23) | OM (n = 18) | PMW (n = 20) | |
|---|---|---|---|---|
| Age (years) | 27.1 ± 1.2NS | 30.8 ± 1.8** | 54.9 ± 1.9** | 57.6 ± 1.4ns |
| Height (cm) | 178 ± 1.0 | 167 ± 1.3 | 179 ± 1.7 | 166 ± 1.3 |
| Weight (kg) | 76.6 ± 2 | 66.8 ± 2.1 | 79.7 ± 3.0 | 66.9 ± 2.3 |
| BMI (kg m−2) | 24.1 ± 0.5NS | 23.7 ± 0.5ns | 24.9 ± 0.9ns | 24.4 ± 0.7NS |
| SBP (mmHg) | 126.5 ± 1.5ns | 126.1 ± 2.9** | 130.2 ± 3.0NS | 136.6 ± 4.6ns |
| DBP (mmHg) | 75.5 ± 1.8 | 73.6 ± 1.6 | 76.3 ± 2.1 | 73.3 ± 1.6 |
| MAP (mmHg) | 93.0 ± 1.6 | 91.9 ± 2.0 | 94.9 ± 2.1 | 96.1 ± 2.4 |
| HR (beats min−1) | 65.9 ± 3.1 | 62.8 ± 1.8 | 59.1 ± 1.1 | 60.7 ± 1.6 |
| MSNA (bursts (100HB)−1) | 42.4 ± 2.3** | 30.5 ± 3.0†† | 61.3 ± 4.8 | 64.1 ± 2.7** |
| MSNA (bursts min−1) | 27.6 ± 2.1** | 18.6 ± 1.6†† | 35.4 ± 2.7 | 37.6 ± 1.8ns |
Data represented as mean ± SEM. YM and YW were age‐matched (P > 0.99). OM and PMW were also age‐matched (P > 0.99). ANOVA post hoc significance vs. same sex: ** P < 0.01, NS = not significant. ANOVA post hoc significance vs. age‐matched: †† P < 0.01, ns = not significant; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; HR, heart rate; MSNA, muscle sympathetic nerve activity; 100HB, 100 heart beats; YM, younger men; YW, younger women; OM, older men; PMW, postmenopausal women.
Transduction of MSNA burst area into total peripheral resistance
We also quantified the transduction of MSNA into total peripheral resistance (TPR). Beat‐by‐beat TPR was calculated as the quotient of mean arterial pressure (MAP) and stroke volume, both quantified offline using Modelflow analysis of the pulse pressure waveform through Beatscope (TNO‐TPD; Biomedical Instrumentation, Amsterdam, The Netherlands). The transduction analysis was then repeated (see ‘Measuring the transduction slope’), but for TPR in place of DBP. Of the 83 participants, we only had reliable beat‐by‐beat stroke volume data for 26 (9 YM, 6 OM, 5 YW and 6 PMW). This MSNA to TPR transduction analysis was therefore only conducted in this subpopulation of participants (Fig. 5). These data demonstrate that this same analytical technique can produce good linear regressions (mean coefficient of determination (R 2) = 0.46 ± 0.04). However, given the availability, validity and reliability of stroke volume data, we did not attempt to quantify transduction of MSNA burst area into TPR in the entire population; all subsequent transduction analyses were conducted using DBP.
Figure 5. Method of quantifying sympathetic neurovascular transduction into TPR .
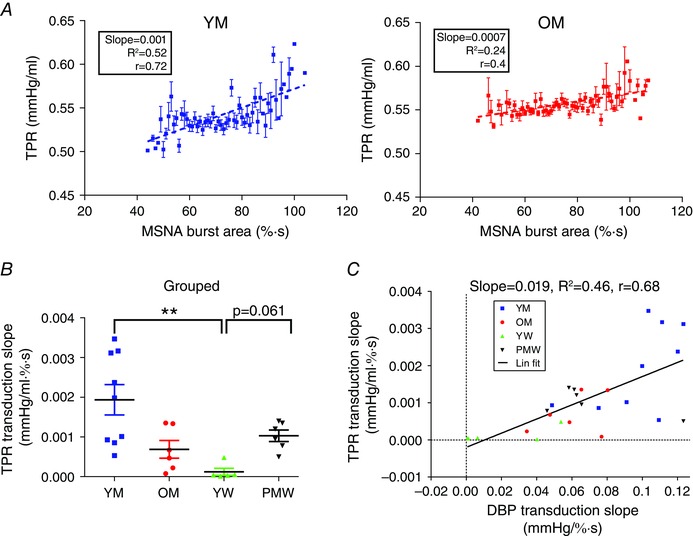
Transduction of MSNA burst area into total peripheral resistance (TPR) was also measured. Beat‐by‐beat TPR was calculated as MAP/stroke volume in a subpopulation of 9 YM, 6 OM, 5 YW and 6 PMW. For each beat‐by‐beat TPR, MSNA burst area was summed in a 2 cardiac cycle window at a lag of 8–6 cardiac cycles and associated with that TPR (similar to DBP in Fig. 1 A; see Methods). This window was moved across the whole baseline file, associating each TPR with an MSNA burst area. A, binned MSNA burst area (1% s bins) was plotted against the associated TPR (mean ± SEM). The transduction of MSNA burst area into TPR was taken as the slope of this relationship. Example for a younger male participant (left; with a steep slope/transduction) and an older male participant (right; with a gentler slope/transduction). B, grouped data for the transduction of MSNA burst area into TPR. C, transduction of MSNA burst area into TPR and DBP are linearly related. YM, younger men; OM, older men; YW, younger women; PMW, postmenopausal women; ** P < 0.01 (one‐way ANOVA; Kruskal–Wallis test with Dunn's multiple comparison).
Transduction of MSNA into DBP in the presence of β‐adrenergic blockade
In those participants that had systemic infusion of propranolol following baseline recording, the transduction analysis was repeated to quantify sympathetic neurovascular transduction during non‐selective β‐adrenergic receptor blockade.
Transduction scripts
Data were sampled at 250 Hz and stored on a personal computer for offline analysis in MATLAB (The MathWorks, Natick, MA, USA). The MATLAB scripts used to calculate transduction, together with guide documentation, are freely available from the authors on GitHub. The authors ask that users cite the current research article when using these scripts. These same analyses can be conducted in Spike2 (CED, UK) by using the ‘Active Modes’ function for locating appropriate cursor regions for MSNA burst area summation.
Statistics and data handling
MSNA was represented as a percentage of the maximal burst height recorded in the file. Data were analysed ‘blind’ to participant sex and age. Statistical tests were conducted in Prism v2.0 (GraphPad Prism, Graph pad software Inc, La Jolla, CA, USA). Results are presented as means ± SEM. Two‐sided probability values of P < 0.05 are considered to indicate statistical significance throughout the text. Statistical comparisons of groups were assessed by one‐way ANOVA (Bonferroni multiple comparison) for parametric data or Kruskal–Wallis one‐way ANOVA by ranks (Dunn's multiple comparison) for non‐parametric data. Serial within‐group comparisons were subjected to repeated measures ANOVA (Šídák multiple comparison). Relationships between variables were determined by using Pearson's correlation coefficient and linear regression analysis. For each regression, a plot of residuals was created to confirm the suitability of a weighted linear fit. Cross‐correlations between variables were conducted in MATLAB using the xcorr function. A Bland–Altman test was used to determine the level of agreement between transduction measured during a test–re‐test protocol.
Results
Baseline characteristics of groups
The YM and YW groups were matched on age (P > 0.99) and MSNA burst incidence (per 100 heart beats, (100HB)−1; P = 0.24). Similarly, the OM and PMW groups were age‐matched (P > 0.99) and MSNA burst incidence‐matched (P > 0.99). The older groups had a greater MSNA burst incidence than their younger, same‐sex counterparts (P < 0.01 in both instances). All the groups were matched on body mass index (BMI; P > 0.994). Blood pressures (systolic, diastolic and mean arterial) were similar across all groups, except for systolic, which was greater in PMW compared to YW (P = 0.005). See Table 1 for a full description of the group demographics and neuro–haemodynamic variables.
Sympathetic neurovascular transduction in sample populations
Transduction of MSNA burst area into DBP
We applied our method of quantifying transduction of MSNA burst area into DBP to the four groups (Fig. 3). Transduction slopes for an individual from each group is shown in Fig. 3 A; these reflect the grouped data (Fig. 3 B). Transduction in YM (0.10 ± 0.01 mmHg (% s)−1, n = 23; 22/23 non‐zero; Pearson r = 0.68 ± 0.05) was greater than that measured in YW (0.03 ± 0.01 mmHg (% s)−1, n = 23; 2/23 non‐zero; Pearson r = 0.13 ± 0.05; P < 0.001), OM (0.06 ± 0.01 mmHg (% s)−1, n = 18; 16/18 non‐zero; Pearson r = 0.56 ± 0.05; P = 0.003) and PMW (0.07 ± 0.01 mmHg (% s)−1, n = 20; 16/20 non‐zero; Pearson r = 0.54 ± 0.06; P = 0.017). Transduction in YW was lowest, being less than that of OM (P = 0.016) and PMW (P = 0.0013). Transduction in OM was not different to that in PMW (P = 0.92).
Figure 3. Sympathetic neurovascular transduction of MSNA burst area into DBP in the sample population .
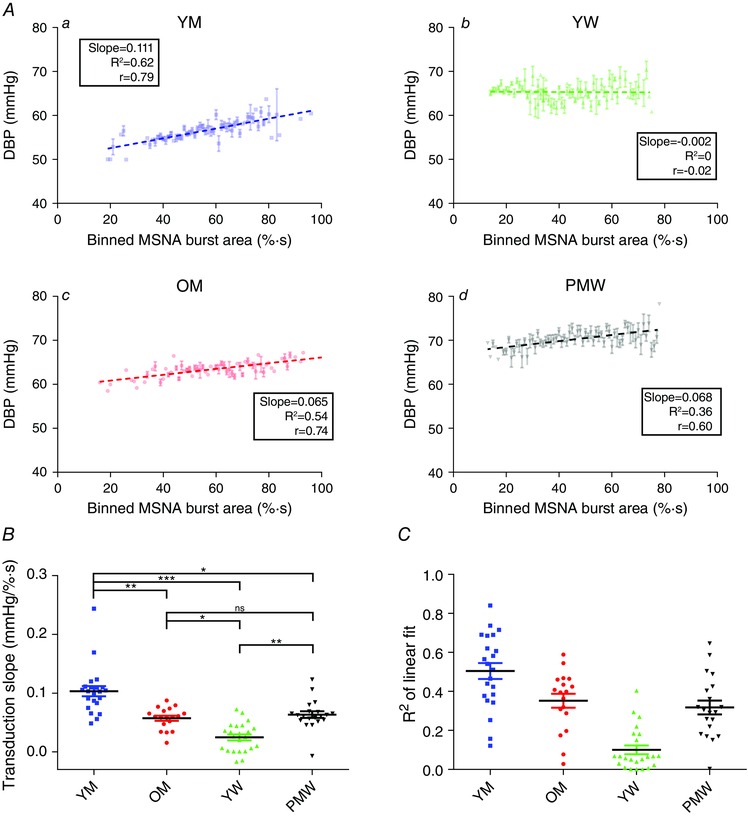
Transduction of MSNA burst area into DBP was measured in all 83 participants. A, example transductions are depicted in a younger man (YM; a), younger woman (YW; b), older man (OM; c) and postmenopausal woman (PMW; d). B, grouped data for the transduction measurement revealed that transduction was reduced in YW compared to YM (P < 0.001), OM (P < 0.001) and PMW (P < 0.01). YM had the largest transduction measurement of all groups. There was no difference in the transduction measurement between OM and PMW (P = 0.32). C, R 2 of linear fits for each transduction slope. Goodness of fit for YW was low because of a lack of relationship between MSNA and DBP. ** P < 0.01, *** P < 0.001, ns = not significant (one‐way ANOVA; Kruskal–Wallis test with Dunn's multiple comparison).
Transduction of MSNA burst height into DBP
We also investigated the transduction of MSNA burst height into DBP (Fig. 4). Transduction in YW (0.08 ± 0.02 mmHg (% s)−1, n = 23; Pearson r = 0.07 ± 0.08) was less than that in YM (0.24 ± 0.02 mmHg (% s)−1, n = 24; Pearson r = 0.58 ± 0.08, P < 0.001), PMW (0.17 ± 0.02 mmHg (% s)−1, n = 20; Pearson r = 0.45 ± 0.06; P = 0.0067) and OM (0.17 ± 0.01 mmHg (% s)−1, n = 18; Pearson r = 0.42 ± 0.1, P = 0.0074).
Figure 4. Sympathetic neurovascular transduction of MSNA burst height into DBP in the sample population .
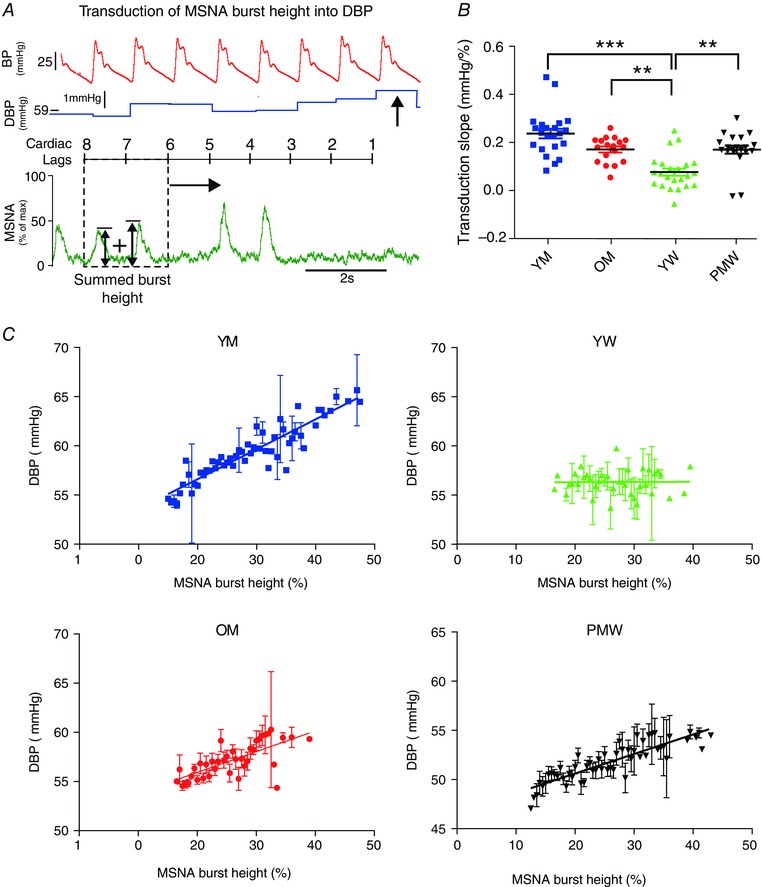
Transduction of MSNA burst height into DPB was measured in all 83 participants. A, the method of calculating transduction of MSNA burst height into DBP. Each DBP was associated with summed MSNA burst heights over a fixed cardiac lag (8–6 cardiac cycles). Subsequent regression analysis was then conducted on these paired data points (as in Fig. 1 B). B, grouped data of transduction of MSNA burst height into DBP. C, example transduction regressions in a younger man (YM), younger woman (YW), older man (OM) and postmenopausal woman (PMW). ** P < 0.01, *** P < 0.001 (one‐way ANOVA; Kruskal–Wallis test with Dunn's multiple comparison).
Since MSNA burst area and burst height are highly correlated during sinus rhythm (Eckberg et al. 1988; Welch et al. 1989) and both area‐ and height‐based measures of transduction differ similarly among the groups, we choose to focus on one measurement (burst area) for the rest of the analysis.
Transduction of MSNA burst area into TPR
In our subpopulation of participants with TPR measurements, transduction of MSNA burst area into TPR followed a similar relationship (Fig. 5 B). In particular, transduction into TPR in YW (1.2 ± 0.9 (×10−4) mmHg (ml % s)−1, n = 5) was less than in YM (19.4 ± 3.8 (×10−4) mmHg (ml % s)−1, n = 9; P = 0.002) and PMW (10.3 ± 1.4 (×10−4) mmHg (ml % s)−1, n = 6; P = 0.0611, one‐way ANOVA). In this subpopulation, transduction into TPR and into DBP were linearly related (Fig. 5 C; R 2 = 0.466, Pearson r = 0.683).
Sympathetic neurovascular transduction and β‐receptors
The same method of quantifying transduction was applied to 12 YM, 11 YW and 13 PMW before (baseline) and after β‐blockade with a systemic infusion of propranolol (Fig. 6). The grouped data (Fig. 6 D) reflect example regressions for three individuals (Fig. 6 A, B and C). In YM, propranolol did not change transduction (baseline = 0.08 ± 0.01 mmHg (% s)−1 vs. propranolol = 0.08 ± 0.01 mmHg (% s)−1; P = 0.87). Similarly in PMW, propranolol did not change transduction (baseline = 0.06 ± 0.01 mmHg (% s)−1 vs. propranolol = 0.06 ± 0.01 mmHg (% s)−1; P = 0.98). In contrast, propranolol infusion in YW greatly increased transduction (baseline = 0.03 ± 0.01 mmHg (% s)−1 vs. propranolol = 0.11 ± 0.01 mmHg (% s)−1; P < 0.001). Moreover, the transduction in YW during β‐blockade was greater than that in both YM (P < 0.001) and PMW (P < 0.01) during β‐blockade.
Figure 6. Sympathetic neurovascular transduction during β‐blockade .
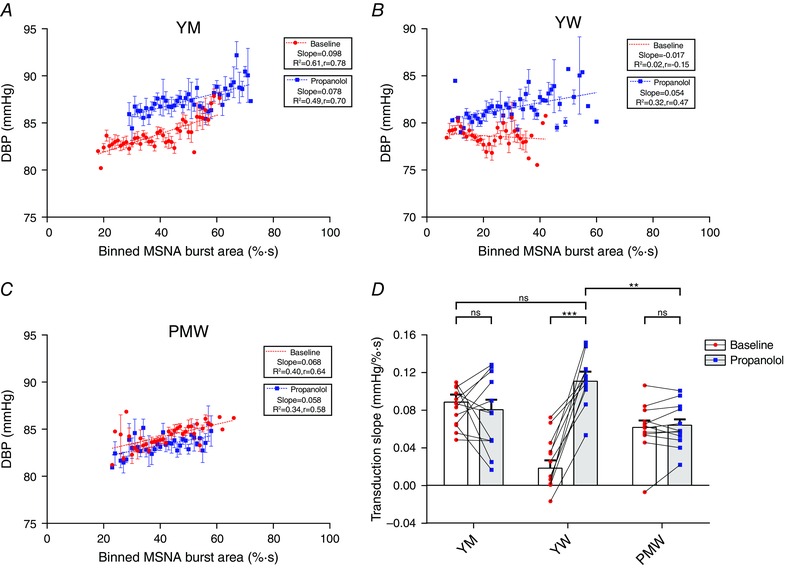
Transduction of MSNA burst area into DBP was measured in a subset of 26 participants before (baseline) and after β‐blockade with propranolol. A–C, example regressions in a young man (YM), young woman (YW) and postmenopausal woman (PMW). D, grouped data revealed that propranolol did not change transduction in YM (P = 0.87; n = 12) or PMW (P = 0.98; n = 13) but increased transduction in YW (P < 0.001; n = 11). Following β‐blockade, YW had a greater transduction than both YM (P < 0.001) and PMW (P < 0.01). Two‐way repeated‐measures ANOVA, ** P < 0.01, *** P < 0.001, ns, not significant.
Reliability of transduction measure
We next conducted a test–re‐test analysis on our transduction measure, to determine its reliability and reproducibility (Fig. 7). Eight participants had their transduction into DBP measured (test) and re‐measured during a follow‐up visit (re‐test; example for one participant in Fig. 7 A). There was no difference between the test (0.06 ± 0.02 mmHg (% s)−1) and re‐test transduction (0.07 ± 0.02 mmHg (% s)−1, P = 0.32; Fig. 7 B). A Bland–Altman test revealed that there was small negative bias (−0.006 mmHg (% s)−1) between the two measures with 95% limits of agreement of −0.031 mmHg (% s)−1 and 0.017 mmHg (% s)−1, relative to the group means (Fig. 3). This bias is small and so the test and re‐test values are in good agreement.
Figure 7. Test–re‐test analysis of transduction measurement .
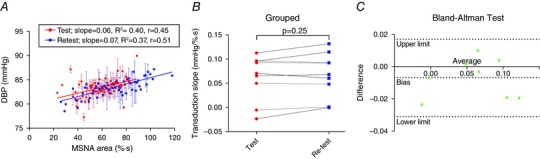
To determine the reproducibility of the measure of transduction of MSNA burst area into DBP we conducted a test–re‐test protocol on 8 participants. Transduction was measured during a baseline visit (test) and then re‐measured during a follow‐up visit on a different day (re‐test). A, example of test and re‐test transduction measurement for a participant. B, there was no difference between the test transduction and re‐test transduction (P = 0.25; paired t test). C, Bland–Altman test of agreement revealed that there was a small negative bias (which is lower than the mean differences in transduction between groups), and therefore good agreement between the variables.
Neurovascular transduction; sex and ageing
We plotted our measure of neurovascular transduction against age for all 83 participants (Fig. 8). In men, transduction had a negative linear relationship with age (slope = −0.0011 mmHg (% s years)−1, R 2 = 0.31, Pearson r = −0.56). In contrast, transduction in women had a positive relationship with age (slope = 0.0014 mmHg (% s years)−1, R 2 = 0.46, Pearson r = 0.68). The slopes of these two regressions were statistically different (P < 0.001). The age at which the linear regression lines intersected was 55 ± 1.5 years.
Figure 8. Effects of sex and ageing on sympathetic neurovascular transduction .
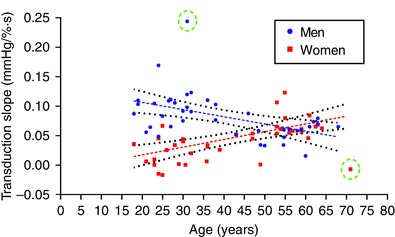
We plotted transduction of MSNA burst area into DBP against age for all 83 participants. In men, transduction increased with age and was well‐fitted by a linear regression (slope = −0.0011 mmHg (% s years)−1, R 2 = 0.31, Pearson r = −0.56; dashed lines). In contrast, transduction in women had a positive relationship with age (slope = 0.0014 mmHg (% s years)−1, R 2 = 0.46, Pearson r = 0.68). The slopes of these two regressions were statistically different (P < 0.001). Two outliers (circled) were removed from the analyses.
Discussion
In this paper we have described a new measure of sympathetic neurovascular transduction, which relates burst by burst changes in SNA to its influence on DBP in individuals at rest. Transduction was defined as the slope of this relationship. To estimate how reliable and valid this measure is in estimating sympathetic transduction, we applied our method to a cohort in which there is data on the sympathetic control of blood pressure: groups of younger and older men and women (the older female group being postmenopausal). In men, we demonstrate that our measure of transduction decreases linearly with age (Fig. 8). In contrast, we found that YW have a very low transduction that increases linearly with age. We also found that our measure of transduction was increased in YW during intravenous infusion of a non‐selective β‐adrenergic blocker (propranolol), but was unchanged in YM and PMW (Fig. 8).
A spontaneous measure of sympathetic neurovascular transduction
Currently, there are different methods available to quantify the transduction of MSNA into vascular tone. However, these measures have important limitations, which we discuss here. First, a simple technique for quantifying transduction is to divide a scalar measure of the vascular response (time‐averaged pressure, flow, resistance or conductance) by MSNA burst frequency, yielding a ratio of input (MSNA) to output (vascular response) – transduction. For a population of participants, a scatter plot of MSNA vs. the vascular response can then be made and fitted with a regression curve, the slope of which yields a transduction measure for the population. Although this technique has proved useful in quantifying transduction in specific populations under resting conditions (Minson et al. 2000; Okada et al. 2011; Jarvis et al. 2014), it uses time‐averaged values of the vascular response (e.g. average DBP over a 3 min period). The method therefore fails to account for the fact that transduction is a dynamic process. Moreover, it is only capable of quantifying transduction in a population, ignoring inter‐individual differences in transduction. Similarly, other studies have attempted to quantify sympathetic vascular conductance using this ‘ratio method’ by correlating large increases in MSNA induced by isometric handgrip exercise (Halliwill, 2000; Minson et al. 2000; Kamiya et al. 2004) or lower body negative pressure (Ray & Monahan, 2002; Notarius et al. 2012) to changes in the vascular response. Although this method has enabled researchers to quantify transduction in different populations, it does so during a pressor response and large increases in MSNA, and fails to capture the dynamic nature of transduction and inter‐individual differences in transduction. Moreover, the experimental protocols required are relatively complicated, requiring the training of participants (to avoid gasping breaths, Valsalva manoeuvres and contractions of other limbs), repeating of the exercise (to achieve maximum voluntary contraction), as well as maintenance of stable cardiovascular and microneurography measurements during these protocols. These data can be difficult to attain in patient populations. In contrast, our measurement of transduction can be conducted under resting conditions and provides information about how beat‐by‐beat changes in MSNA influence vascular tone. This dynamic component of the sympathetic vascular coupling may provide insight into the role that sympathetic vascular transduction plays in resting blood pressure control.
Fairfax et al. (2013) have quantified transduction at rest by measuring the influence of beat‐to‐beat changes in MSNA on vascular conductance. Although this well‐designed method provides information regarding how variations in MSNA influence the vasculature in young men and women, we have found the method difficult to implement in people with high MSNA (e.g. hypertensive individuals). This is because the analysis depends on locating burst ‘clusters’, i.e. single bursts, burst couplets or higher order groups of bursts, which are preceded and followed by a period of quiescence. In participants with high MSNA, there are few periods/cardiac cycles of no MSNA. However, this may be resolved by taking longer recordings of resting MSNA.
Recently, a different approach to measuring transduction has been described. Tan et al. (2013) proposed an autoregressive model to estimate sympathetic transduction during isometric handgrip exercise. The sophisticated analysis of Tan et al. describes an adapted Poiseuille relationship between blood pressure, SNA and blood flow. These analyses fully exploit the concurrent measurements of beat‐by‐beat changes in neural–haemodynamic variables to quantify transduction. This bypasses the inconsistencies that have been reported when considering transduction into vascular conductance (Dinenno et al. 1999, 2001) vs. resistance (Hart et al. 2009 b) and also the complication of whether resistance or conductance better reflects a vascular response (Lautt, 1989; O'Leary, 1991). Although Tan et al. (2013) demonstrate that such an autoregressive model explains a large percentage of the variance in the data (∼75%), the method by which they quantify transduction requires a good understanding of time‐series analysis, a careful, participant‐by‐participant fitting process of model parameters and application of spike‐sorting algorithms on microneurography data. Thus, the analysis is complex and time‐consuming. Additionally, the protocol requires measurements of blood flow in the leg during handgrip exercise, which adds extra difficulties in being able to complete this analysis in patient populations.
Given the limitations of the above methods, the goal of the present study was to provide an alternative, simple analytical technique for quantifying transduction, which may be used in participants who have high MSNA at rest (e.g. normotensive older men and women, and hypertensive individuals). Additionally, the new method allows assessment of transduction in people for whom isometric handgrip (or any intervention which causes large increases in blood pressure) might be contra‐indicated, such as those with hypertension or other cardiovascular diseases. The new method we describe here focuses on relating changes in SNA to changes in BP. This is similar to the method for analysing baroreflex sensitivity (Kienbaum et al. 2001), although there is an important difference. The baroreflex analysis looks for changes in SNA, which result from (are a reflex response to) changes in BP, whereas our transduction analysis is the converse: it looks for changes in BP which result from the effects of SNA on the circulation. Both methods take advantage of the well‐established dynamic interaction between changes in SNA and BP.
Sympathetic neurovascular transduction; sex and ageing
Using the new method proposed in the current study, we found that YM consistently exhibited a good relationship between MSNA burst area and DBP (Fig. 3), indicating a good degree of transduction. In comparison, in OM the transduction measure was decreased compared to the young men, which supports previous findings (Tan et al. 2013). Numerous studies have demonstrated that α‐adrenergic responsiveness (Dinenno et al. 2002; Smith et al. 2007; Hart et al. 2009 b) and beat‐by‐beat changes in MAP following MSNA bursts (Vianna et al. 2012) is reduced in older vs. younger men. The new estimate of transduction tested in this study is therefore in agreement with these previous data and suggests that it is a valid estimate of sympathetic transduction in OM. Additionally, the new method demonstrates that diminished vascular responsiveness to sympathetic outflow in OM manifests under resting conditions due to an impairment of transduction.
We also applied our new measure of transduction of MSNA burst area into DBP to recordings in YW and PMW. The new transduction measurement in YW was less than that of all other groups (Fig. 3). We note that a non‐significant slope was not an exclusion criterion for any data point; such a criteria would have resulted in exclusion of data from individuals with poor transduction (we interpreted slopes that did not yield a significant r value as reflecting poor transduction, particularly in young women). In PMW, the level of sympathetic vascular transduction was increased so that it was similar to that observed in OM. These results corroborate previous findings (Hart et al. 2011), suggesting that the new method of transduction is indeed a valid estimate of transduction. The lower transduction in YW compared to men and older PMW is partially due to differences in the role of the β‐adrenergic receptors in determining vasoconstrictor responses to noradrenaline. In particular, YW exhibit greater β‐adrenergic vasodilatation to noradrenaline compared with men (Kneale et al. 2000; Hart et al. 2011). This has the end effect of causing opposing vasodilatation when noradrenaline is released from the sympathetic nerve terminal, consequently blunting sympathetic vascular transduction in YW (Hart et al. 2011). This opposing vasodilator effect is diminished in women after the menopause (Hart et al. 2011). To further test the new method proposed in this study, we estimated changes in sympathetic vascular transduction in YW, YM and PMW during systemic β‐blockade with propranolol. Transduction estimated from our method was in congruence with previous results: following β‐blockade, transduction increased in the YW only (Fig. 6).
Finally, since the test may be widely used to assess sympathetic–vascular transduction, we assessed whether the measurement was reliable on different days. The Bland–Altman test showed that there was a small bias (−0.006 mmHg (% s)−1), which (for example) is considerably lower than the difference in transduction slopes in YM and YW (0.10–0.03 = 0.07 mmHg (% s)−1). This indicates that the test is repeatable with individuals and that the measurement has low day‐to‐day variability.
Together, these results demonstrate that this method (1) is a reliable measure of transduction in young and older men and women; (2) can detect changes in sympathetic vascular transduction following β‐blockade; and (3) demonstrates that the MSNA relationship to vascular resistance is age‐ and sex‐dependent.
Limitations
In older populations of men, other investigators have found that the time to peak effect of transduction in response to isometric handgrip exercise is approximately halved to 2.8 ± 0.4 s (n = 10; Tan et al. 2013). However, we found that the lag that yielded peak transduction (see Fig. 2) in older men was not different to the other groups. Thus we did not find any indication of a difference in the time course of transduction in OM vs. YM. This may, however, be because we measured the transduction of MSNA burst area into DBP (our measure does not consider concurrent changes in blood flow). The limiting factor here was the quality (/absence) of beat‐by‐beat data for stroke volume in our participants (which would yield beat‐by‐beat values of TPR). A valid measure of beat‐to‐beat stroke volume is typically hard to acquire, especially in patients with cardiovascular disease. However, DBP is known to reflect changes in autonomic tone; thus we use DBP as a surrogate of resistance or conductance. The measurement of blood flow can be difficult in a patient population, whilst collecting other data, such as MSNA. Furthermore, we found that transduction of MSNA burst area into TPR and DBP demonstrated the same differences across the groups and were linearly related (R 2 = 0.466, Pearson r = 0.683). Therefore transduction into DBP is a reliable and simple proxy for vascular transduction.
Another potential limitation is that we used burst area to quantify sympathetic transduction. In humans, typically we do not utilize the MSNA burst area due to limitations with microelectrode placement, which means that not all action potentials are measured in the nerve fibre, and that smaller bursts of MSNA may be due to the electrode simply being further away from the active fibres causing the burst. Thus, it is difficult to compare the average area of bursts between people. However, since we know that smaller bursts typically have a lower reflex latency vs. larger bursts (which inherently reflects the number of nerve fibres recruited; see Wallin et al. 1994), a negative linear regression between the height of bursts of MSNA and their latency from the R‐wave in the ECG suggests that smaller bursts with a lower reflex latency are actually smaller bursts (i.e. fewer fibres recruited) and are not smaller because of the experimental conditions. Therefore, a significant negative linear regression between these variables in a person suggests that burst area may provide useful information regarding transduction of SNA into vasoconstrictor tone in that person. Although both the area of a burst and how many bursts occur (burst incidence per 100 heart beats) are important in the sympathetic control of the peripheral vasculature, we did not use burst incidence since this causes inherent limitations in the analysis for people with high burst incidence. We aimed to quantify beat‐to‐beat changes in vasoconstrictor tone in response to bursts or no bursts. If we only used the occurrence of a burst or not, people with high burst incidence will have a flat transduction slope, ignoring any differences in the strength of these bursts. Using burst area abolishes this problem and allows quantification of beat‐to‐beat changes in vasoconstrictor tone or DBP.
Implications and conclusions
A key aim in developing a new measure of transduction was to permit application to populations of humans with high MSNA. Essential hypertension is known to be caused and maintained by overactivity of the sympathetic nervous system (Guyenet, 2006; Grassi et al. 2010, 2015; Parati & Esler, 2012). A recent report suggests that despite treatment with anti‐hypertensive medication, 47% of patients had uncontrolled blood pressure (SBP > 140 mmHg; Go et al. 2014). This may be in part due to the inappropriate targeting of pharmacotherapy; if individuals do not exhibit coupling between MSNA and the vasculature, interventions aimed at reducing sympathetic transduction would be ineffective.
Our simple analysis of transduction may provide further insights into the mechanisms driving hypertension and allow us to target research into anti‐hypertensive treatment more effectively. We show that as women age sympathetic neurovascular transduction increases, but as men age, it decreases. We found that the age at which transduction increases in women to that seen in men is 55 years. Interestingly, this corresponds to the same age at which the prevalence of hypertension in women increases to that in men (NCHS, 2010). Consequently, we suggest that the increase in sympathetic transduction contributes to the increasing prevalence of hypertension in women with age (Burt et al. 1995). In men, the role of sympathetic vascular transduction may be less important in the age‐related rise in BP. These data indicate that medications that aim to blunt sympathetic neurovascular coupling may be more effective in lowering BP in older women than in older men. Consequently, the analytical method to measure transduction outlined in this paper may help us to understand how to target anti‐hypertensive treatment more effectively, especially when considering the sex of an individual.
Additional information
Competing interests
None declared.
Author contributions
The experiments were conducted in the laboratories of E.C.H., J.F.R.P. and M.J.J. All authors designed the work. E.C.H., A.E.B., N.C., L.E.K.R. and M.J.J. acquired the data. All authors analysed and interpreted the data. All authors helped draft the manuscript and revise it critically for intellectual content. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This study was supported by the British Heart Foundation, IBSRF FS/11/1/28400 (E.C.H.). Additionally, investigations completed at the Mayo Clinic were supported by AHA 070036Z (E.C.H.) and NIH HL083947 (M.J.J., N.C.). This research was also supported by the National Institute for Health Research Biomedical Research Unit in Cardiovascular Disease at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol (A.K.N.).
Declarations
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the US Army or the US Department of Defense. Citations of commercial organizations and trade names in this report do not constitute an official US Department of the Army endorsement or approval of the products or services of these organizations. Approved for public release; distribution unlimited. This paper presents independent research funded by the National Institute for Health Research (NIHR). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Acknowledgements
We would like to thank research nurses Rissa Calsena, Jenny Wilcox and Ruth Bowles for their nursing support for the investigations completed at the Clinical Research and Imaging Centre‐Bristol and the Department of Cardiology, University Hospitals Bristol. Additionally, we would like to thank research co‐coordinators/technical managers Pamela Engrav, Nancy Meyer and Christopher Johnson, and nurses Shelly Roberts and Jean Knutson for their recruitment, nursing and technical support for the studies completed at the Mayo Clinic, USA.
Linked articles This article is highlighted by a Perspective by Carter & Cooke. To read this Perspective, visit http://dx.doi.org/10.1113/JP272569.
References
- Barnes JN, Hart EC, Curry TB, Nicholson WT, Eisenach JH, Wallin BG, Charkoudian N & Joyner MJ (2014). Aging enhances autonomic support of blood pressure in women. Hypertension 63, 303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell C, Seals DR, Monroe MB, Day DS, Shapiro LF, Johnson DG & Jones PP (2001). Tonic sympathetic support of metabolic rate is attenuated with age, sedentary lifestyle, and female sex in healthy adults. J Clin Endocrinol Metab 86, 4440–4444. [DOI] [PubMed] [Google Scholar]
- Blessing WW (1997). The Lower Brainstem and Bodily Homeostasis, pp. 165–268. Oxford University Press, New York. [Google Scholar]
- Burnstock G (2008). Non‐synaptic transmission at autonomic neuroeffector junctions. Neurochem Int 52, 14–25. [DOI] [PubMed] [Google Scholar]
- Burnstock G (2009). Purinergic cotransmission. Exp Physiol 94, 20–24. [DOI] [PubMed] [Google Scholar]
- Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ & Labarthe D (1995). Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988‐1991. Hypertension 25, 305–313. [DOI] [PubMed] [Google Scholar]
- Carter JR & Ray CA (2009). Sympathetic neural responses to mental stress: responders, nonresponders and sex differences. Am J Physiol Heart Circ Physiol 296, H847–H853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christou DD, Jones PP, Jordan J, Diedrich A, Robertson D & Seals DR (2005). Women have lower tonic autonomic support of arterial blood pressure and less effective baroreflex buffering than men. Circulation 111, 494–498. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Dietz NM & Joyner MJ (2002). Aging and forearm postjunctional α‐adrenergic vasoconstriction in healthy men. Circulation 106, 1349–1354. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Jones PP, Seals DR & Tanaka H (1999). Limb blood flow and vascular conductance are reduced with age in healthy humans – Relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation 100, 164–170. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Tanaka H, Stauffer BL & Seals DR (2001). Reductions in basal limb blood flow and vascular conductance with human ageing: role for augmented α‐adrenergic vasoconstriction. J Physiol 536, 977–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckberg DL, Rea RF, Andersson OK, Hedner T, Pernow J, Lundberg JM & Wallin BG (1988). Baroreflex modulation of sympathetic activity and sympathetic neurotransmitters in humans. Acta Physiol Scand 133, 221–231. [DOI] [PubMed] [Google Scholar]
- Ellis JL & Burnstock G (1990). Neuropeptide Y neuromodulation of sympathetic co‐transmission in the guinea‐pig vas deferens. Br J Pharmacol 100, 457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairfax ST, Credeur DP, Holwerda SW, Zuidema MY, Medley JH, Dyke PC 2nd & Wray DW (2013). The role of α‐adrenergic receptors in mediating beat‐by‐beat sympathetic vascular transduction in resting humans. FASEB J 27, 1119.2. (Meeting Abstract.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink GD (2009). Arthur C. Corcoran Memorial Lecture. Sympathetic activity, vascular capacitance, and long‐term regulation of arterial pressure. Hypertension 53, 307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go AS, Bauman MA, Coleman King SM, Fonarow GC, Lawrence W, Williams KA & Sanchez E (2014). An effective approach to high blood pressure control: a science advisory from the American Heart Association, the American College of Cardiology, and the Centers for Disease Control and Prevention. J Am Coll Cardiol 63, 1230–1238. [DOI] [PubMed] [Google Scholar]
- Gracia CR, Sammel MD, Freeman EW, Lin H, Langan E, Kapoor S & Nelson DB (2005). Defining menopause status: creation of a new definition to identify the early changes of the menopausal transition. Menopause 12, 128–135. [DOI] [PubMed] [Google Scholar]
- Grassi G, Mark A & Esler M (2015). The sympathetic nervous system alterations in human hypertension. Circ Res 116, 976–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi G, Seravalle G & Quarti‐Trevano F (2010). The ‘neuroadrenergic hypothesis’ in hypertension: current evidence. Exp Physiol 95, 581–586. [DOI] [PubMed] [Google Scholar]
- Guyenet PG (2006). The sympathetic control of blood pressure. Nat Rev Neurosci 7, 335–346. [DOI] [PubMed] [Google Scholar]
- Halliwill JR (2000). Segregated signal averaging of sympathetic baroreflex responses in humans. J Appl Physiol (1985) 88, 767–773. [DOI] [PubMed] [Google Scholar]
- Halliwill JR, Taylor JA & Eckberg DL (1996). Impaired sympathetic vascular regulation in humans after acute dynamic exercise. J Physiol 495, 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach J & Joyner MJ (2011). Sex and ageing differences in resting arterial pressure regulation: the role of the β‐adrenergic receptors. J Physiol 589, 5285–5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH & Joyner MJ (2009. a). Sex differences in sympathetic neural‐hemodynamic balance: implications for human blood pressure regulation. Hypertension 53, 571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart EC, Joyner MJ, Wallin BG, Johnson CP, Curry TB, Eisenach JH & Charkoudian N (2009. b). Age‐related differences in the sympathetic‐hemodynamic balance in men. Hypertension 54, 127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth AJ, Mackintosh AF & Mary DASG (2007). Gender‐related differences in the sympathetic vasoconstrictor drive of normal subjects. Clin Sci 112, 353–361. [DOI] [PubMed] [Google Scholar]
- Huidobro‐Toro JP & Donoso MV (2004). Sympathetic co‐transmission: the coordinated action of ATP and noradrenaline and their modulation by neuropeptide Y in human vascular neuroeffector junctions. Eur J Pharmacol 500, 27–35. [DOI] [PubMed] [Google Scholar]
- Jarvis SS, Shibata S, Okada Y, Levine BD & Fu Q (2014). Neural‐humoral responses during head‐up tilt in healthy young white and black women. Front Physiol 5, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner MJ, Charkoudian N & Wallin BG (2008). A sympathetic view of the sympathetic nervous system and human blood pressure regulation. Exp Physiol 93, 715–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya A, Michikami D, Shiozawa T, Iwase S, Hayano J, Kawada T, Sunagawa K & Mano T (2004). Bed rest attenuates sympathetic and pressor responses to isometric exercise in antigravity leg muscles in humans. Am J Physiol Regul Integr Comp Physiol 286, R844–R850. [DOI] [PubMed] [Google Scholar]
- Kienbaum P, Karlssonn T, Sverrisdottir YB, Elam M & Wallin BG (2001). Two sites for modulation of human sympathetic activity by arterial baroreceptors? J Physiol 531, 861–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneale BJ, Chowienczyk PJ, Brett SE, Coltart DJ & Ritter JM (2000). Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J Am Coll Cardiol 36, 1233–1238. [DOI] [PubMed] [Google Scholar]
- Lautt WW (1989). Resistance or conductance for expression of arterial vascular tone. Microvasc Res 37, 230–236. [DOI] [PubMed] [Google Scholar]
- Minson CT, Halliwill JR, Young TM & Joyner MJ (2000). Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation 101, 862–868. [DOI] [PubMed] [Google Scholar]
- NCHS (2010). Health, United States, 2009: with special feature on Medical Technology. Report No. 2010‐1232. National Center for Health Statistics (US), Hyattsville, MD, USA. [PubMed] [Google Scholar]
- Notarius CF, Murai H, Morris BL & Floras JS (2012). Effect of fitness on reflex sympathetic neurovascular transduction in middle‐age men. Med Sci Sports Exerc 44, 232–237. [DOI] [PubMed] [Google Scholar]
- Okada Y, Galbreath MM, Shibata S, Jarvis SS, VanGundy TB, Meier RL, Wang WD, Vongpatanasin W, Levine BD & Fu Q (2011). Relationship between arterial stiffness and sympathetic baroreflex sensitivity in elderly men and women. FASEB J 25, 842.2. (Meeting Abstract.) [Google Scholar]
- O'Leary DS (1991). Regional vascular‐resistance vs conductance – which index for baroreflex responses. Am J Physiol 260, H632–H637. [DOI] [PubMed] [Google Scholar]
- Parati G & Esler M (2012). The human sympathetic nervous system: its relevance in hypertension and heart failure. Eur Heart J 33, 1058–1066. [DOI] [PubMed] [Google Scholar]
- Ray CA & Monahan KD (2002). Sympathetic vascular transduction is augmented in young normotensive blacks. J Appl Physiol (1985) 92, 651–656. [DOI] [PubMed] [Google Scholar]
- Smith EG, Voyles WF, Kirby BS, Markwald RR & Dinenno FA (2007). Ageing and leg postjunctional α‐adrenergic vasoconstrictor responsiveness in healthy men. J Physiol 582, 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundlof G & Wallin BG (1977). The variability of muscle nerve sympathetic activity in resting recumbent man. J Physiol 272, 383–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CO, Tamisier R, Hamner JW & Taylor JA (2013). Characterizing sympathetic neurovascular transduction in humans. PLoS One 8, e53769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianna LC, Hart EC, Fairfax ST, Charkoudian N, Joyner MJ & Fadel PJ (2012). Influence of age and sex on the pressor response following a spontaneous burst of muscle sympathetic nerve activity. Am J Physiol Heart Circ Physiol 302, H2419–H2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin BG, Burke D & Gandevia S (1994). Coupling between variations in strength and baroreflex latency of sympathetic discharges in human muscle nerves. J Physiol 474, 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch WJ, Smith ML, Rea RF, Bauernfeind RA & Eckberg DL (1989). Enhancement of sympathetic nerve activity by single premature ventricular beats in humans. J Am Coll Cardiol 13, 69–75. [DOI] [PubMed] [Google Scholar]
- Wier WG, Zang WJ, Lamont C & Raina H (2009). Sympathetic neurogenic Ca2+ signalling in rat arteries: ATP, noradrenaline and neuropeptide Y. Exp Physiol 94, 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Drummer TD & Carter JR (2013). Sex differences in sympathetic neural and limb vascular reactivity to mental stress in humans. Am J Physiol Heart Circ Physiol 304, H436–H443. [DOI] [PubMed] [Google Scholar]


