Abstract
The niche constitutes a unique category of cells that support the microenvironment for the maintenance and self‐renewal of stem cells. Intestinal stem cells reside at the base of the crypt, which contains adjacent epithelial cells, stromal cells and smooth muscle cells, and soluble and cell‐associated growth and differentiation factors. We summarize here recent advances in our understanding of the crucial role of the niche in regulating stem cells. The stem cell niche maintains a balance among quiescence, proliferation and regeneration of intestinal stem cells after injury. Mesenchymal cells, Paneth cells, immune cells, endothelial cells and neural cells are important regulatory components that secrete niche ligands, growth factors and cytokines. Intestinal homeostasis is regulated by niche signalling pathways, specifically Wnt, bone morphogenetic protein, Notch and epidermal growth factor. These insights into the regulatory stem cell niche during homeostasis and post‐injury regeneration offer the potential to accelerate development of therapies for intestine‐related disorders.

Keywords: endothelial cell, immune response, Intestinal stem cells, neural cells, Paneth cells, Signaling pathways, Stem cell niche, Stromal cells
Abbreviations
- BMP
bone morphogenetic protein
- CBC
crypt base columnar
- EGF
epidermal growth factor
- ISC
intestinal stem cell
- Lgr5
leucine‐rich repeat‐containing G‐protein coupled receptor 5
- LRC
label retaining cell
Introduction
The intestinal epithelium, with its rapid turnover, is an excellent system for the study of adult stem cells. It is composed of crypt and villus, and intestinal stem cells (ISCs) are responsible for ongoing epithelial regeneration throughout life. The ISC niche is a complex cellular structure that plays a key role in stem cell maintenance, proliferation and differentiation. The term ‘niche’, coined by Schofield (1978), defines a group of cells playing a crucial role in controlling stem cell fate and maintaining stem cell number by regulating the balance between self‐renewal and differentiation, thus supporting tissue regeneration (Li & Clevers, 2010). Stem cell homeostasis is maintained by mesenchyme and crypt‐based epithelial cells. ISCs reside at the base of crypts, adjacent to Paneth cells, and are surrounded by stromal cells. The niche consists of different cellular components, namely myofibroblasts, endothelial cells, neural cells, lymphocytes, macrophages and smooth muscle cells (Fig. 1). In this review, our focus is mainly on stromal cells, Paneth cells, immune cells, endothelial cells and neural cells. The ISC niche is likely to comprise several different cell types, each of which contributes cell‐associated ligands and chemokines, soluble cytokines and growth factors that regulate stem cell behaviour (Tan & Barker, 2014). To unravel the mysteries of intestinal disease mechanisms and stem cell regeneration, insights into niche components are of high importance. It is known that Wnt, bone morphogenetic protein (BMP), Notch and epidermal growth factor (EGF) signalling pathways are the regulators of stem cell activity, and further studies on these associations with the niche would improve understanding of ISC homeostasis.
Figure 1. Diagram of the regulatory niche of intestinal stem cells .

The stem cell compartment resides at the base of the crypt consisting of CBC and +4 stem cells. Rapidly dividing transit‐amplifying cells arise from these stem cells and differentiate into absorptive lineages (enterocytes) or secretory lineages (enteroendocrine cells, goblet cells, tuft cells and Paneth cells). The niche consists of multiple components and cell types, including extracellular matrix, fibroblasts, myofibroblasts, smooth muscle cells, neural cells, endothelial cells, lymphocytes and macrophages along with secreted factors (Wnt3, EGF), and BMP inhibitors (Noggin, Gremlin and chordin) that support the regulation of stem cell activity. Wnt, BMP, Notch, Hh and EGF signalling pathways are the regulators of stem cell activity (left).
Intestinal stem cells in the niche
Stem cells reside at the base of the crypt and are supported by the microenvironment. Active stem cells are considered to be crypt base columnar (CBC) cells and quiescent stem cells mark the +4 position (Li & Clevers, 2010). CBC cells respond quickly to regenerative niche signals, while +4 label retaining cells (LRCs) remain quiescent during normal homeostasis, but retain the ability to produce other cells after injury (Scoville et al. 2008). Lgr5 (leucine‐rich repeat‐containing G‐protein coupled receptor 5) emerged as a specific and robust marker for CBC cells (Barker et al. 2007). Ascl2, Olfm4, Rnf43, Znrf3, Smoc2, Troy, Prom1, Sox9, and Msi1 were also identified as markers of stem cell populations (Snippert et al. 2009; van der Flier et al. 2009; Hao et al. 2012; Munoz et al. 2012; Fafilek et al. 2013; Schuijers et al. 2014, 2015; Roche et al. 2015). Recently Klf5 (Krüppel‐like factor 5), an additional stem cell marker, was reported in the maintenance of stem cells in the intestinal crypt (Bell & Shroyer, 2015).
The +4 LRCs were discovered by Potten through tritium or Brdu LRC assays (Potten et al. 2002). One of the initial studies showed that phosphatase and tensin homologue (PTEN), especially p‐PTEN, marks +4 stem cells (He et al. 2004; He et al. 2007). The functional validation of +4 stem cells was done by in vivo lineage tracing using Bmi1‐CreER induced mice (Sangiorgi & Capecchi, 2008). Consistent with this observation, Bmi1+ cells were characterized as radioresistant and quiescent in contrast to the Lgr5 stem cell population (Yan et al. 2012). mTert expresses at the +4 position identical to LRCs (Breault et al. 2008). Following injury, mTert+ cells give rise to all intestinal cell lineages, which was demonstrated by lineage tracing in vivo (Montgomery et al. 2011). A recent study showed that the mTert+ dormant stem cell population is regulated by PTEN phosphorylation and nutritional status (Richmond et al. 2015; Sailaja et al. 2015). Takeda et al. demonstrated that Hopx is a +4 stem cell marker and that these Hopx‐expressing cells generate all intestinal epithelial lineages (Takeda et al. 2011 a). Lrig1 is a regulator of the ErbB signalling pathway and is profoundly expressed by ISCs. ISC self‐renewal and proliferation are stimulated by ErbB signalling and controlled by BMP inhibition and Wnt activation (Sato et al. 2009; Wong et al. 2012). In a recent study, Dclk1‐expressing small intestinal epithelial tuft cells, a rare population, were reported to show hallmarks of quiescence with self‐renewal ability (Chandrakesan et al. 2015).
Bmi1, mTert, Hopx and Lrig1 were later reported to be expressed not only in +4 cells, but also in CBC and other progenitor cells (Itzkovitz et al. 2012; Munoz et al. 2012). Some studies reported that +4 cells also express the endocrine marker and that enteroendocrine cells maintain the quiescent stem cell niche (Radford & Lobachevsky, 2006). So, there is considerable debate in the field regarding +4 markers. Recently, similar to the haematopoietic approach, combinatorial surface markers were shown to be able to sort ISCs, and the sorted ISCs could be functionally characterized using an efficient in vitro organoid culture assay (Wang et al. 2013). Characterization and identification of candidate markers must be further validated by gene expression profiling and lineage tracing methods. Broad understanding of intrinsic mechanisms, microenvironmental interactions, and communication with surrounding cells will yield much information about regulatory niche signals during homeostasis.
Differential responses of stem cell subsets in response to injury implicate roles of the niche
The identity of the stem cell is maintained by intrinsic cell and environmental (niche) factors. While substantial efforts have led to progress in uncovering the mechanisms regarding homeostatic ISC maintenance, the regenerative process after ISC injury is still controversial. The deletion of Lgr5 stem cells upon radiation‐caused or diphtheria toxin‐induced ablation does not perturb epithelial homeostasis, indicating the existence of a reserve ISC population (Montgomery et al. 2011; Tian et al. 2011; Takeda et al. 2011 b; Yan et al. 2012). However, following crypt injury, Dll1+ secretory progenitor cells are able to reacquire stem cell properties and generate all four secretory cell types, arguing for cellular plasticity as another mechanism (van Es et al. 2012 b). However, the remaining question is what mechanism supports a robust regeneration in response to injury? Recently, keratin‐19 (Krt19) was shown to be expressed at the +4 position, continuing up to the isthmus. These are radiation‐resistant cells that robustly give rise to all intestinal epithelial lineages, including Lgr5 CBCs, in the colon and intestine. Krt19 also marks radioresistant cancer‐initiating cells and regenerates after injury to Lgr5 stem cells (Asfaha et al. 2015).
These studies propose that different subpopulations of stem cells reside in different niches, which provide the required signals to maintain stem cells in different cycling and metabolic states. Hence, the niche regulates stem cells not only in homeostatic conditions, but also in stressed conditions following injury (Scoville et al. 2008). Differential niche regulation may also have a role in gastrointestinal tumours, allowing a cancer stem cell population to be drug‐resistant. Therefore, understanding the different components of the ISC niche following injury and the signals emanating from it is essential in the future.
Stromal microenvironment
The connective tissue cells that support the functioning of parenchymal cells in an organ are called stromal cells. These are also often referred to as non‐haematopoietic, non‐epithelial and non‐endothelial in origin (Owens, 2015). Stromal cells and subepithelial myofibroblasts exist in the lamina propria, located beneath the intestinal crypts, which supports ISCs during intestinal morphogenesis, differentiation and proliferation (McLin et al. 2009; Mifflin et al. 2011). Intestinal subepithelial myofibroblasts exhibit qualities of smooth muscle cells and fibroblasts (Pinchuk et al. 2010; Powell et al. 2011). Myofibroblasts maintain and support stem cells and lead to expansion of intestinal epithelium. The linkage among crypt stem cells, Paneth cells and myofibroblasts requires investigation to further delineate their molecular interactions (Powell et al. 2011). Farin et al. revealed that stromal cells support the formation of intestinal epithelium by the Wnt signalling pathway (Farin et al. 2012). A recent study showed that epithelial Wnt is non‐essential, and stromal cells endogenously express Rspo3, a secreted Wnt agonist (an R‐spondin family member), which supports regeneration of intestinal epithelium (Kabiri et al. 2014). Hedgehog (Hh) signalling, an important component of epithelial–mesenchymal cross‐talk, also contributes to establishing epithelial stem cells in the niche by inducing stromal BMP synthesis (Vries et al. 2010). Hh signalling within the stroma results in decreased epithelial proliferation and expansion of smooth muscle cells and myofibroblasts. A similar phenotype was observed in mice overexpressing Noggin (a BMP inhibitor) indicating the importance of BMPs as downstream mediators of Hh signalling (Vries et al. 2010; Zacharias et al. 2010). Snai1, which also regulates the epithelial‐to‐mesenchymal transition, is reported to be necessary for maintenance of CBC stem cells (Horvay et al. 2011).
Stromal cells are the mesenchymal elements that have also been implicated as key players in regenerating injured tissue following trauma. These mesenchymal cells possess similar marker molecules, origins and coordinated biological functions and provide a microenvironment for ISC maintenance (He et al. 2004; Pinchuk et al. 2010). The isolation and characterization of mesenchymal niche cells are critical in determining their functional regulation of the ISC niche. Furthermore, the mechanisms that regulate these interactions between mesenchymal and crypt base epithelial cells remain unclear. A better knowledge of stromal cells in the niche is indispensable for understanding the mechanisms of homeostasis and disease. During inflammation, tissue stromal cells experience immunological changes (Pinchuk et al. 2010). Since the effects of these stromal cells are poorly understood and advances in the field are minimal, further studies on stromal cell functions and the ISC niche may lead to next‐generation cell‐based therapies for intestinal bowel disorders.
Immune, endothelial and neural cells in the niche
In the small intestine, endothelial cells and neural cells are present along with connective tissue fibroblasts. The study by Bjerknes and Cheng introduced enteric neurons and blood vessels as niche cells surrounding the intestinal crypt (Bjerknes & Cheng, 2001; Mills & Gordon, 2001). Neural cells play a key role in regulating epithelial growth. Glucagon‐like peptide 2 (GLP‐2) produced by eneteroendocrine cells signals to underlying enteric neurons that express the GLP‐2 receptor, which stimulates the proliferation of enterocytes (Bjerknes & Cheng, 2001). Endothelial cells were identified as key players of the niche as there was no epithelial cell loss when endothelial apoptosis was blocked by using basic fibroblast growth factor (bFGF) after radiation damage (Bjerknes & Cheng, 2001; Paris et al. 2001). Immune cells contribute to the protection of epithelial surfaces by releasing factors and promoting tissue repair. Both epithelial cells and dendritic cells interact to maintain immune balance in small intestine (Rimoldi et al. 2005). Regulatory T cells, which have the capacity to modulate the immune system, along with other mesenchymal cells, macrophages and endothelial cells, also play an important role in maintaining the regulatory niche (Paris et al. 2001; Akcora et al. 2013). Regulatory T cells, a subset of CD4+ T cells, are central players for maintaining intestinal homeostasis (Korn et al. 2014). Colony stimulating factor (CSF1) receptor, which is expressed on macrophages, fashions the ISC niche (Akcora et al. 2013). Growth factors, cytokines and ligands are secreted by intestinal immune cells and stromal cells that regulate ISCs (Pinchuk et al. 2010). Stromal cells respond to the immune cell‐derived cytokines interleukin (IL)‐1α and IL‐1β, with relevance to intestinal inflammatory diseases (Okuno et al. 2002). The crypt is also influenced by external signals, such as IL‐22 produced by innate lymphoid cells, that maintain epithelial integrity and protect stem cells against damage (Hanash et al. 2012). Hence, it is critically important to investigate the role of endothelial, neural and immune cells as niche cells in the intestine, as their functional properties are least known.
Paneth cells as a regulatory niche
Paneth cells are the cells identified by Joseph Paneth as columnar epithelial cells of the secretory lineage with cytoplasm filled with large granules and located at the base of the crypt (Clevers, 2013). CBC cells are interdigitated between Paneth cells, and the function of Paneth cells involves production of growth factors such as Wnt, Notch and EGF as niche signals to CBC stem cells. Yilmaz et al. proposed that Paneth cells, a vital member of the ISC niche, augment stem cell function in response to fasting. Refeeding starved mice regulates mTORC1 in Paneth cells. These results establish that mTORC1 regulates self‐renewal of the ISC niche and emphasize its significance in supporting the functionality of stem cells (Yilmaz et al. 2012).
In intestinal crypts, Paneth cells constitute the niche for stem cells. The communication between CBC cells and Paneth cells is seen in in vitro intestinal organoid culture. Recent research revealed that co‐culturing ISCs with Paneth cells or exogenous Wnt3a improves growth efficiency of Lgr5 stem cells (Sato et al. 2011). As colon is devoid of Paneth cells, CD24+ cells and C‐Kit+ colonic goblet cells located adjacent to the Lgr5+ stem cells at the base of crypt have been proposed to be niche components (Rothenberg et al. 2012). There is controversy about the role of Paneth cells as a niche since ablation of Paneth cells did not affect number and function of Lgr5+ ISCs (Durand et al. 2012; Kim et al. 2012). Interestingly, another report showed that deletion of Wnt3 had no effect on stem cell function in adult mice, although Wnt is necessary for organoid cultures (Farin et al. 2012). However, Paneth cells play a role in facilitating ISC recovery post‐intestinal injury (Parry et al. 2013). Taken together, Paneth cells provide several niche factors in vivo, and there is also redundancy when compared with stromal cells.
Regulatory signalling pathways in the niche
Several regulatory signalling pathways are known for their importance in the niche. The Wnt pathway is considered to be the crucial pathway for maintaining self‐renewal and proliferation of ISCs. Wnt signals are exhibited more along the base of the crypt and less towards the villus (Vries et al. 2010). Knock‐out of Tcf, Dkk1 (a secreted Wnt antagonist), Ctnnb1 and c‐Myc (a Wnt target gene) greatly affects the intestine's proliferative compartments, indicating the importance of Wnt signals in forming stem cell compartments (Kuhnert et al. 2004; Muncan et al. 2006; Fevr et al. 2007; van Es et al. 2012 a) . The WNT agonist roof plate‐specific spondin 1 (R‐spondin) and knock‐out of the APC gene readily drive hyperplasia in intestine and colon (Kim et al. 2005). Simultaneous deletion of Rnf43 (ring finger protein 43) and Znrf3 (zinc and ring finger 3), which are Wnt target genes, is key to modulation of the Wnt signal and drives hyperplasia in intestine (Hao et al. 2012). Wnt ligands are secreted by pericryptal stromal cells and epithelial cells, including Paneth cells that produce Wnt3 (Sato et al. 2011). Even without Wnt3, Wnt2b can support the growth of enteroids (Farin et al. 2012), and Lgr receptors mediate R‐spondin signals in enhancing the Wnt signalling pathway (de Lau et al. 2011). Although numerous studies show that Wnt is critical for stem cell function, other studies question the need for secreted Wnt and its source in vivo (San Roman et al. 2014). Very recent findings reveal Fzd7 is highly expressed in Lgr5 stem cells and mediates Wnt signalling (Flanagan et al. 2015).
Mesenchymal derived BMPs belong to the transforming growth factor β (TGFβ) family. In contrast to Wnt signalling, BMP signals are exhibited high at the villus and less towards the base of the crypt, inhibiting stem cell renewal and supporting epithelial differentiation (He et al. 2004). Mesenchymal cells express BMP4, including cells that are adjacent to ISCs. In the submucosal region, the BMP inhibitor Noggin was expressed predominantly adjacent to the crypt bottom and was occasionally detected only in ISCs, thus supporting CBC cell proliferation via inhibiting BMP (He et al. 2004). In addition, gremlin 1, gremlin 2 and chordin are also highly expressed in the submucosa to suppress BMP signalling (He et al. 2004; Pinchuk et al. 2010).
Notch is another regulator of the niche that is required for ISC maintenance, and furthermore, it requires cell–cell interactions, suggesting the stem cell is regulated by adjacent epithelial cells and not stromal cells to deliver the Notch signal (Pellegrinet et al. 2011; VanDussen et al. 2012; Tian et al. 2015). Knock‐out of RBPjκ, simultaneous inactivation of Dll1 and Dll4 (membrane bound notch ligands), or double deletion of Notch1 and ‐2 impairs secretory lineage and cellular proliferation (van Es et al. 2005; Riccio et al. 2008; Pellegrinet et al. 2011). Further understanding of how the various signalling pathways are integrated to regulate ISC function is a major challenge in the field. A recent study presented data suggesting Notch and Wnt pathway interactions regulate ISCs (Tian et al. 2015). EGF‐like growth factors also regulate stem cell activities through different signalling pathways such as phosphoinositide 3‐kinase (PI3K), protein kinase B (Akt), Ras, Raf, mitogen‐activated protein kinase kinase (MEK), mitogen‐activated protein kinase (MAPK) and protein kinase C (PKC) (Normanno et al. 2006). Hence, there is a need to further understand how the various niche factors are integrated to maintain homeostasis as well as to respond to challenges that modulate the ISC compartment.
Understanding signals emanating from the niche provided researchers with the critical insight to add different growth factors such as EGF, R‐spondin‐1 and Noggin to in vitro organoid culture (Fig. 2) (Sato et al. 2009). Interestingly, enteroids derived from mouse crypts display spontaneous oscillations of gene expression (Moore et al. 2014), suggesting the presence of a clock mechanism inherent to the intestinal epithelium. Of practical consequence is the observation that these rhythms appear to influence the responses to growth factors. The organoids grown in culture with niche factors self‐renew and produce all types of epithelial cells, resembling the intestinal epithelium and mimicking the stem cell niche in vivo, and hence can be used instead of cell lines (van de Wetering et al. 2015). Organoid culture may also represent a cheap and robust alternative to xenograft‐based drug studies. Hence, these organoids are useful in cancer genetics to allow design of personalized therapy.
Figure 2. Ex vivo organoid culture .
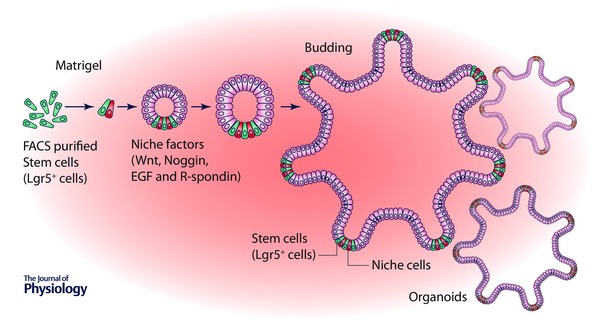
Stem cells (Lgr5+) plated into laminin‐rich matrigel supplemented with a cocktail of niche factors including Noggin, R‐spondin 1 and EGF generate self‐renewing epithelial organoids resembling the stem cell niche in vivo.
Summary and perspectives
The signals emanating from the niche in epithelial cells and stromal cells regulate intestinal homeostasis. Wnt signals are indispensable for ISC self‐renewal and the differentiation of Paneth cells, BMP signals for balancing ISC maintenance and activation, and Notch signals for specifying the absorptive and secretory lineage crucial to establishing the niche during homeostatic and injury conditions. The ISC niche maintains stem cell number. Although there has been much progress over the last few years in identifying which important cellular components regulate the ISC niche, many unresolved issues remain. The identity of ‘+4 stem cells’ and how they respond to multiple niche signals during homeostasis, and particularly in response to injury, remain largely unknown. The origin of stromal cells and their functional identity are largely unclear. Identification of proper markers to define immune cells, endothelial cells and neural cells in regulating ISCs will help us understand niche interactions in homeostatic and stressed or injury conditions. There are certain enduring questions in understanding the signalling mechanisms which regulate the niche. Uncovering the role of Wnt, Hh, BMP, Notch and EGF signalling in stromal cells will help illuminate these questions. Further research on the function of stromal cells in response to the immune system with a variety of cytokines will enlighten the field and may lead to new therapies in treating gastrointestinal disorders. In the future, artificial ex vivo niches may provide intriguing opportunities for regeneration‐based treatment.
Additional information
Competing interests
None of the authors has any conflicts of interests.
Author contributions
All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was funded by NIDDK (U01DK085507).
Acknowledgements
Thanks to Karen Tannen for editing and Mark Miller for scientific illustration.
Biographies
Badi Sri Sailaja is a postdoctoral fellow at Stowers Institute for Medical Research, working on characterization of intestinal stem cells and their niche. She received her PhD from Hebrew University of Jerusalem, Israel.
Xi He is a senior research specialist at Stowers, investigating intestinal and haematopoietic stem cells. She has participated in numerous studies and published over 30 papers.
Linheng Li is an investigator at Stowers, best known for combining genomics and genetics to study haematopoietic and intestinal stem cells and their associated niches. He was among the first to identify the endosteal niche and among the first to propose a model of co‐existing quiescent and active adult stem cells in the same tissue in mammals. He received his PhD from New York University Medical Center and has contributed to the field by characterizing and demonstrating the roles of several developmental signalling pathways in regulation of stem cells.

References
- Akcora D, Huynh D, Lightowler S, Germann M, Robine S, de May JR, Pollard JW, Stanley ER, Malaterre J & Ramsay RG (2013). The CSF‐1 receptor fashions the intestinal stem cell niche. Stem Cell Res 10, 203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asfaha S, Hayakawa Y, Muley A, Stokes S, Graham TA, Ericksen RE, Westphalen CB, von Burstin J, Mastracci TL, Worthley DL, Guha C, Quante M, Rustgi AK & Wang TC (2015). Krt19+/Lgr5 − cells are radioresistant cancer‐initiating stem cells in the colon and intestine. Cell Stem Cell 16, 627–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ & Clevers H (2007). Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007. [DOI] [PubMed] [Google Scholar]
- Bell KN & Shroyer NF (2015). Krupple‐like factor 5 is required for proper maintenance of adult intestinal crypt cellular proliferation. Dig Dis Sci 60, 86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerknes M & Cheng H (2001). Modulation of specific intestinal epithelial progenitors by enteric neurons. Proc Natl Acad Sci USA 98, 12497–12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breault DT, Min IM, Carlone DL, Farilla LG, Ambruzs DM, Henderson DE, Algra S, Montgomery RK, Wagers AJ & Hole N (2008). Generation of mTert‐GFP mice as a model to identify and study tissue progenitor cells. Proc Natl Acad Sci USA 105, 10420–10425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrakesan P, May R, Qu D, Weygant N, Taylor VE, Li JD, Ali N, Sureban SM, Qante M, Wang TC, Bronze MS & Houchen CW (2015). Dclk1+ small intestinal epithelial tuft cells display the hallmarks of quiescence and self‐renewal. Oncotarget 6, 30876–30886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H (2013). The intestinal crypt, a prototype stem cell compartment. Cell 154, 274–284. [DOI] [PubMed] [Google Scholar]
- de Lau W, Barker N, Low TY, Koo BK, Li VS, Teunissen H, Kujala P, Haegebarth A, Peters PJ, van de Wetering M, Stange DE, van Es JE, Guardavaccaro D, Schasfoort RB, Mohri Y, Nishimori K, Mohammed S, Heck AJ & Clevers H (2011). Lgr5 homologues associate with Wnt receptors and mediate R‐spondin signalling. Nature 476, 293–297. [DOI] [PubMed] [Google Scholar]
- Durand A, Donahue B, Peignon G, Letourneur F, Cagnard N, Slomianny C, Perret C, Shroyer NF & Romagnolo B (2012). Functional intestinal stem cells after Paneth cell ablation induced by the loss of transcription factor Math1 (Atoh1). Proc Natl Acad Sci USA 109, 8965–8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fafilek B, Krausova M, Vojtechova M, Pospichalova V, Tumova L, Sloncova E, Huranova M, Stancikova J, Hlavata A, Svec J, Sedlacek R, Luksan O, Oliverius M, Voska L, Jirsa M, Paces J, Kolar M, Krivjanska M, Klimesova K, Tlaskalova‐Hogenova H & Korinek V (2013). Troy, a tumor necrosis factor receptor family member, interacts with lgr5 to inhibit wnt signaling in intestinal stem cells. Gastroenterology 144, 381–391. [DOI] [PubMed] [Google Scholar]
- Farin HF, Van Es JH & Clevers H (2012). Redundant sources of Wnt regulate intestinal stem cells and promote formation of Paneth cells. Gastroenterology 143, 1518–1529.e7. [DOI] [PubMed] [Google Scholar]
- Fevr T, Robine S, Louvard D & Huelsken J (2007). Wnt/β‐catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol Cell Biol 27, 7551–7559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan DJ, Phesse TJ, Barker N, Schwab RH, Amin N, Malaterre J, Stange DE, Nowell CJ, Currie SA, Saw JT, Beuchert E, Ramsay RG, Sansom OJ, Ernst M, Clevers H & Vincan E (2015). Frizzled7 functions as a Wnt receptor in intestinal epithelial Lgr5+ stem cells. Stem Cell Reports 4, 759–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanash AM, Dudakov JA, Hua G, O'Connor MH, Young LF, Singer NV, West ML, Jenq RR, Holland AM, Kappel LW, Ghosh A, Tsai JJ, Rao UK, Yim NL, Smith OM, Velardi E, Hawryluk EB, Murphy GF, Liu C, Fouser LA, Kolesnick R, Blazar BR & van den Brink MR (2012). Interleukin‐22 protects intestinal stem cells from immune‐mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity 37, 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao HX, Xie Y, Zhang Y, Charlat O, Oster E, Avello M, Lei H, Mickanin C, Liu D, Ruffner H, Mao X, Ma Q, Zamponi R, Bouwmeester T, Finan PM, Kirschner MW, Porter JA, Serluca FC & Cong F (2012). ZNRF3 promotes Wnt receptor turnover in an R‐spondin‐sensitive manner. Nature 485, 195–200. [DOI] [PubMed] [Google Scholar]
- He XC, Yin T, Grindley JC, Tian Q, Sato T, Tao WA, Dirisina R, Porter‐Westpfahl KS, Hembree M, Johnson T, Wiedemann LM, Barrett TA, Hood L, Wu H & Li L (2007). PTEN‐deficient intestinal stem cells initiate intestinal polyposis. Nat Genet 39, 189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, Mishina Y & Li L (2004). BMP signaling inhibits intestinal stem cell self‐renewal through suppression of Wnt‐β‐catenin signaling. Nat Genet 36, 1117–1121. [DOI] [PubMed] [Google Scholar]
- Horvay K, Casagranda F, Gany A, Hime GR & Abud HE (2011). Wnt signaling regulates Snai1 expression and cellular localization in the mouse intestinal epithelial stem cell niche. Stem Cells Dev 20, 737–745. [DOI] [PubMed] [Google Scholar]
- Itzkovitz S, Lyubimova A, Blat IC, Maynard M, van Es J, Lees J, Jacks T, Clevers H & van Oudenaarden A (2012). Single‐molecule transcript counting of stem‐cell markers in the mouse intestine. Nat Cell Biol 14, 106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabiri Z, Greicius G, Madan B, Biechele S, Zhong Z, Zaribafzadeh H, Edison, Aliyev J , Wu Y, Bunte R, Williams BO, Rossant J & Virshup DM (2014). Stroma provides an intestinal stem cell niche in the absence of epithelial Wnts. Development 141, 2206–2215. [DOI] [PubMed] [Google Scholar]
- Kim KA, Kakitani M, Zhao J, Oshima T, Tang T, Binnerts M, Liu Y, Boyle B, Park E, Emtage P, Funk WD & Tomizuka K (2005). Mitogenic influence of human R‐spondin1 on the intestinal epithelium. Science 309, 1256–1259. [DOI] [PubMed] [Google Scholar]
- Kim TH, Escudero S & Shivdasani RA (2012). Intact function of Lgr5 receptor‐expressing intestinal stem cells in the absence of Paneth cells. Proc Natl Acad Sci USA 109, 3932–3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn LL, Hubbeling HG, Porrett PM, Yang Q, Barnett LG & Laufer TM (2014). Regulatory T cells occupy an isolated niche in the intestine that is antigen independent. Cell Rep 9, 1567–1573. [DOI] [PubMed] [Google Scholar]
- Kuhnert F, Davis CR, Wang HT, Chu P, Lee M, Yuan J, Nusse R & Kuo CJ (2004). Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf‐1. Proc Natl Acad Sci USA 101, 266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L & Clevers H (2010). Coexistence of quiescent and active adult stem cells in mammals. Science 327, 542–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLin VA, Henning SJ & Jamrich M (2009). The role of the visceral mesoderm in the development of the gastrointestinal tract. Gastroenterology 136, 2074–2091. [DOI] [PubMed] [Google Scholar]
- Mifflin RC, Pinchuk IV, Saada JI & Powell DW (2011). Intestinal myofibroblasts: targets for stem cell therapy. Am J Physiol Gastrointest Liver Physiol 300, G684–G696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills JC & Gordon JI (2001). The intestinal stem cell niche: there grows the neighborhood. Proc Natl Acad Sci USA 98, 12334–12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery RK, Carlone DL, Richmond CA, Farilla L, Kranendonk ME, Henderson DE, Baffour‐Awuah NY, Ambruzs DM, Fogli LK, Algra S & Breault DT (2011). Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc Natl Acad Sci USA 108, 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SR, Pruszka J, Vallance J, Aihara E, Matsuura T, Montrose MH, Shroyer NF & Hong CI (2014). Robust circadian rhythms in organoid cultures from PERIOD2::LUCIFERASE mouse small intestine. Dis Model Mech 7, 1123–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muncan V, Sansom OJ, Tertoolen L, Phesse TJ, Begthel H, Sancho E, Cole AM, Gregorieff A, de Alboran IM, Clevers H & Clarke AR (2006). Rapid loss of intestinal crypts upon conditional deletion of the Wnt/Tcf‐4 target gene c‐Myc. Mol Cell Biol 26, 8418–8426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz J, Stange DE, Schepers AG, van de Wetering M, Koo BK, Itzkovitz S, Volckmann R, Kung KS, Koster J, Radulescu S, Myant K, Versteeg R, Sansom OJ, van Es JH, Barker N, van Oudenaarden A, Mohammed S, Heck AJ & Clevers H (2012). The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent ‘+4’ cell markers. EMBO J 31, 3079–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F & Salomon DS (2006). Epidermal growth factor receptor (EGFR) signaling in cancer. Gene 366, 2–16. [DOI] [PubMed] [Google Scholar]
- Okuno T, Andoh A, Bamba S, Araki Y, Fujiyama Y, Fujiyama M & Bamba T (2002). Interleukin‐1β and tumor necrosis factor‐alpha induce chemokine and matrix metalloproteinase gene expression in human colonic subepithelial myofibroblasts. Scand J Gastroenterol 37, 317–324. [DOI] [PubMed] [Google Scholar]
- Owens BM (2015). Inflammation, innate immunity, and the intestinal stromal cell niche: opportunities and challenges. Front Immunol 6, 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D, Haimovitz‐Friedman A, Cordon‐Cardo C & Kolesnick R (2001). Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science 293, 293–297. [DOI] [PubMed] [Google Scholar]
- Parry L, Young M, El Marjou F & Clarke AR (2013). Evidence for a crucial role of Paneth cells in mediating the intestinal response to injury. Stem Cells 31, 776–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrinet L, Rodilla V, Liu Z, Chen S, Koch U, Espinosa L, Kaestner KH, Kopan R, Lewis J & Radtke F (2011). Dll1‐ and Dll4‐mediated notch signaling are required for homeostasis of intestinal stem cells. Gastroenterology 140, 1230–1240.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinchuk IV, Mifflin RC, Saada JI & Powell DW (2010). Intestinal mesenchymal cells. Curr Gastroenterol Rep 12, 310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS, Owen G & Booth D (2002). Intestinal stem cells protect their genome by selective segregation of template DNA strands. J Cell Sci 115, 2381–2388. [DOI] [PubMed] [Google Scholar]
- Powell DW, Pinchuk IV, Saada JI, Chen X & Mifflin RC (2011). Mesenchymal cells of the intestinal lamina propria. Annu Rev Physiol 73, 213–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford IR & Lobachevsky PN (2006). An enteroendocrine cell‐based model for a quiescent intestinal stem cell niche. Cell Prolif 39, 403–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio O, van Gijn ME, Bezdek AC, Pellegrinet L, van Es JH, Zimber‐Strobl U, Strobl LJ, Honjo T, Clevers H & Radtke F (2008). Loss of intestinal crypt progenitor cells owing to inactivation of both Notch1 and Notch2 is accompanied by derepression of CDK inhibitors p27Kip1 and p57Kip2. EMBO Rep 9, 377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond CA, Shah MS, Deary LT, Trotier DC, Thomas H, Ambruzs DM, Jiang L, Whiles BB, Rickner HD, Montgomery RK, Tovaglieri A, Carlone DL & Breault DT (2015). Dormant intestinal stem cells are regulated by PTEN and nutritional status. Cell Rep 13, 2403–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoldi M, Chieppa M, Larghi P, Vulcano M, Allavena P & Rescigno M (2005). Monocyte‐derived dendritic cells activated by bacteria or by bacteria‐stimulated epithelial cells are functionally different. Blood 106, 2818–2826. [DOI] [PubMed] [Google Scholar]
- Roche KC, Gracz AD, Liu XF, Newton V, Akiyama H & Magness ST (2015). SOX9 maintains reserve stem cells and preserves radioresistance in mouse small intestine. Gastroenterology 149, 1553–1563.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg ME, Nusse Y, Kalisky T, Lee JJ, Dalerba P, Scheeren F, Lobo N, Kulkarni S, Sim S, Qian D, Beachy PA, Pasricha PJ, Quake SR & Clarke MF (2012). Identification of a cKit+ colonic crypt base secretory cell that supports Lgr5+ stem cells in mice. Gastroenterology 142, 1195–1205.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailaja BS, He XC & Li L (2015). Stem cells matter in response to fasting. Cell Rep 13, 2325–2326. [DOI] [PubMed] [Google Scholar]
- San Roman AK, Jayewickreme CD, Murtaugh LC & Shivdasani RA (2014). Wnt secretion from epithelial cells and subepithelial myofibroblasts is not required in the mouse intestinal stem cell niche in vivo. Stem Cell Rep 2, 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangiorgi E & Capecchi MR (2008). Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet 40, 915–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M & Clevers H (2011). Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469, 415–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ & Clevers H (2009). Single Lgr5 stem cells build crypt‐villus structures in vitro without a mesenchymal niche. Nature 459, 262–265. [DOI] [PubMed] [Google Scholar]
- Schofield R (1978). The relationship between the spleen colony‐forming cell and the haemopoietic stem cell. Blood Cells 4, 7–25. [PubMed] [Google Scholar]
- Schuijers J, Junker JP, Mokry M, Hatzis P, Koo BK, Sasselli V, van der Flier LG, Cuppen E, van Oudenaarden A & Clevers H (2015). Ascl2 acts as an R‐spondin/Wnt‐responsive switch to control stemness in intestinal crypts. Cell Stem Cell 16, 158–170. [DOI] [PubMed] [Google Scholar]
- Schuijers J, van der Flier LG, van Es J & Clevers H (2014). Robust cre‐mediated recombination in small intestinal stem cells utilizing the olfm4 locus. Stem Cell Rep 3, 234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville DH, Sato T, He XC & Li L (2008). Current view: intestinal stem cells and signaling. Gastroenterology 134, 849–864. [DOI] [PubMed] [Google Scholar]
- Snippert HJ, van Es JH, van den Born M, Begthel H, Stange DE, Barker N & Clevers H (2009). Prominin‐1/CD133 marks stem cells and early progenitors in mouse small intestine. Gastroenterology 136, 2187–2194.e1. [DOI] [PubMed] [Google Scholar]
- Takeda K, Kinoshita I, Shimizu Y, Matsuno Y, Shichinohe T & Dosaka‐Akita H (2011. a). Expression of LGR5, an intestinal stem cell marker, during each stage of colorectal tumorigenesis. Anticancer Res 31, 263–270. [PubMed] [Google Scholar]
- Takeda N, Jain R, LeBoeuf MR, Wang Q, Lu MM & Epstein JA (2011. b). Interconversion between intestinal stem cell populations in distinct niches. Science 334, 1420–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan DW & Barker N (2014). Intestinal stem cells and their defining niche. Curr Top Dev Biol 107, 77–107. [DOI] [PubMed] [Google Scholar]
- Tian H, Biehs B, Chiu C, Siebel CW, Wu Y, Costa M, de Sauvage FJ & Klein OD (2015). Opposing activities of Notch and Wnt signaling regulate intestinal stem cells and gut homeostasis. Cell Rep 11, 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD & de Sauvage FJ (2011). A reserve stem cell population in small intestine renders Lgr5‐positive cells dispensable. Nature 478, 255–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Flier LG, van Gijn ME, Hatzis P, Kujala P, Haegebarth A, Stange DE, Begthel H, van den Born M, Guryev V, Oving I, van Es JH, Barker N, Peters PJ, van de Wetering M & Clevers H (2009). Transcription factor achaete scute‐like 2 controls intestinal stem cell fate. Cell 136, 903–912. [DOI] [PubMed] [Google Scholar]
- van de Wetering M, Francies HE, Francis JM, Bounova G, Iorio F, Pronk A, van Houdt W, van Gorp J, Taylor‐Weiner A, Kester L, McLaren‐Douglas A, Blokker J, Jaksani S, Bartfeld S, Volckman R, van Sluis P, Li VS, Seepo S, Sekhar Pedamallu C, Cibulskis K, Carter SL, McKenna A, Lawrence MS, Lichtenstein L, Stewart C, Koster J, Versteeg R, van Oudenaarden A, Saez‐Rodriguez J, Vries RG, Getz G, Wessels L, Stratton MR, McDermott U, Meyerson M, Garnett MJ & Clevers H (2015). Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 161, 933–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDussen KL, Carulli AJ, Keeley TM, Patel SR, Puthoff BJ, Magness ST, Tran IT, Maillard I, Siebel C, Kolterud A, Grosse AS, Gumucio DL, Ernst SA, Tsai YH, Dempsey PJ & Samuelson LC (2012). Notch signaling modulates proliferation and differentiation of intestinal crypt base columnar stem cells. Development 139, 488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Es JH, Haegebarth A, Kujala P, Itzkovitz S, Koo BK, Boj SF, Korving J, van den Born M, van Oudenaarden A, Robine S & Clevers H (2012. a). A critical role for the Wnt effector Tcf4 in adult intestinal homeostatic self‐renewal. Mol Cell Biol 32, 1918–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Es JH, Sato T, van de Wetering M, Lyubimova A, Nee AN, Gregorieff A, Sasaki N, Zeinstra L, van den Born M, Korving J, Martens AC, Barker N, van Oudenaarden A & Clevers H (2012. b). Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat Cell Biol 14, 1099–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F & Clevers H (2005). Notch/γ‐secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 435, 959–963. [DOI] [PubMed] [Google Scholar]
- Vries RG, Huch M & Clevers H (2010). Stem cells and cancer of the stomach and intestine. Mol Oncol 4, 373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Scoville D, He XC, Mahe MM, Box A, Perry JM, Smith NR, Lei NY, Davies PS, Fuller MK, Haug JS, McClain M, Gracz AD, Ding S, Stelzner M, Dunn JC, Magness ST, Wong MH, Martin MG, Helmrath M & Li L (2013). Isolation and characterization of intestinal stem cells based on surface marker combinations and colony‐formation assay. Gastroenterology 145, 383–395.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong VW, Stange DE, Page ME, Buczacki S, Wabik A, Itami S, van de Wetering M, Poulsom R, Wright NA, Trotter MW, Watt FM, Winton DJ, Clevers H & Jensen KB (2012). Lrig1 controls intestinal stem‐cell homeostasis by negative regulation of ErbB signalling. Nat Cell Biol 14, 401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan KS, Chia LA, Li X, Ootani A, Su J, Lee JY, Su N, Luo Y, Heilshorn SC, Amieva MR, Sangiorgi E, Capecchi MR & Kuo CJ (2012). The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci USA 109, 466–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz OH, Katajisto P, Lamming DW, Gultekin Y, Bauer‐Rowe KE, Sengupta S, Birsoy K, Dursun A, Yilmaz VO, Selig M, Nielsen GP, Mino‐Kenudson M, Zukerberg LR, Bhan AK, Deshpande V & Sabatini DM (2012). mTORC1 in the Paneth cell niche couples intestinal stem‐cell function to calorie intake. Nature 486, 490–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharias WJ, Li X, Madison BB, Kretovich K, Kao JY, Merchant JL & Gumucio DL (2010). Hedgehog is an anti‐inflammatory epithelial signal for the intestinal lamina propria. Gastroenterology 138, 2368–2377.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]


