Abstract
Adult tissues maintain function and architecture through robust homeostatic mechanisms mediated by self‐renewing cells capable of generating all resident cell types. However, severe injury can challenge the regeneration potential of such a stem/progenitor compartment. Indeed, upon injury adult tissues can exhibit massive cellular plasticity in order to achieve proper tissue regeneration, circumventing an impaired stem/progenitor compartment. Several examples of such plasticity have been reported in both rapidly and slowly self‐renewing organs and follow conserved mechanisms. Upon loss of the cellular compartment responsible for maintaining homeostasis, quiescent or slowly proliferating stem/progenitor cells can acquire high proliferation potential and turn into active stem cells, or, alternatively, mature cells can de‐differentiate into stem‐like cells or re‐enter the cell cycle to compensate for the tissue loss. This extensive cellular plasticity acts as a key mechanism to respond to multiple stimuli in a context‐dependent manner, enabling tissue regeneration in a robust fashion. In this review cellular plasticity in the adult liver and stomach will be examined, highlighting the diverse cell populations capable of repairing the damaged tissue.
Introduction: cellular plasticity in tissue homeostasis and regeneration
Throughout adult life, tissues maintain cellular function and constant cell number through robust homeostatic mechanisms that maintain the fragile equilibrium between proliferation and differentiation. The rate of cellular proliferation depends on the turnover requirement of the tissue (Sanchez Alvarado & Yamanaka, 2014). For example, in the mammalian system, the intestine and the skin are amongst the organs with the highest cellular turnover (Blanpain & Fuchs, 2009, 2014; Barker, 2014; Tetteh et al. 2015). Such tissues take advantage of specific adult stem cell compartments that are able to generate all the cell types of the resident tissue in order to support homeostasis (Morrison & Spradling, 2008). Adult tissues with low cellular turnover, such as the liver, have been shown to be maintained by differentiated adult cells (Malato et al. 2011; Tarlow et al. 2014; Yanger et al. 2014) or by quiescent/slowly proliferating subpopulations with stem/progenitor properties (Miyajima et al. 2014; Font‐Burgada et al. 2015; Wang et al. 2015 a). Importantly, damage experiments pushing tissues beyond the regenerative capacity of their resident stem cell compartments have recently indicated that adult tissues might possess vast cellular plasticity. Here plasticity is defined as the ability of a cell to acquire novel features or adopt alternative fates in a tissue‐specific, controlled manner, in response to distinct context‐dependent intracellular or extracellular cues. Of note, unlike trans‐differentiation, where cell fate can change between different lineages, cellular plasticity occurs within a specific tissue lineage. There are examples of cellular plasticity in both rapidly self‐renewing organs (e.g. skin, intestine and stomach) and slowly self‐renewing organs (e.g. liver, pancreas, kidney, lung) (Blanpain & Fuchs, 2009, 2014; Goulas et al. 2012; Zhu et al. 2013; Barker, 2014; Richmond et al. 2015; Tetteh et al. 2015; Wang et al. 2015 b). In a slowly proliferating tissue such as the liver, cellular plasticity may allow differentiated cell types to de‐differentiate into stem/progenitor‐like cells upon tissue damage or may serve to turn resident slowly proliferating and quiescent stem/progenitor cells into highly proliferating stem/progenitor populations. One could argue that rapidly self‐renewing organs do not require cellular plasticity in order to maintain tissue architecture and function upon injury. However, a fascinating prospect is that in rapidly self‐renewing organs, cellular plasticity could add a layer of redundancy during tissue regeneration. This becomes evident in situations where the stem cell compartment is compromised during severe damage (Blanpain & Fuchs, 2014). For instance, the highly proliferative isthmus region of the corpus epithelium in the stomach is thought to be the major stem cell zone (Karam & Leblond, 1993; Hayakawa et al. 2015). However, under conditions where the isthmus region is lost or unable to perform its function, mature chief cells gain stem cell properties and replenish the corpus epithelium (Stange et al. 2013). Altogether, cellular plasticity allows for robust tissue repair by providing redundant mechanisms that enable regeneration. This review aims to examine the role of cellular plasticity in tissue homeostasis and regeneration in the adult liver and stomach (plasticity in other tissues has been elegantly reviewed in Blanpain & Fuchs, 2014; Tetteh et al. 2015). The molecular markers of the different populations exhibiting cellular plasticity in liver and stomach and how these organs respond to injury and repair the damaged tissue will be the main focus of this review.
Plasticity of the adult liver
The liver is a critical organ for regulating homeostasis and metabolism. It has a highly organized architecture and contains several cell types, including hepatocytes, cholangiocytes (also named ductal cells), endothelial cells, Kupffer cells and stellate cells. Most of the metabolic functions are carried out by hepatocytes, which account for the greater part of the organ size. Ductal cells are the epithelial cells forming the biliary ducts, which export bile (secreted by the hepatocytes) to the duodenum (Miyajima et al. 2014; Gordillo et al. 2015).
In the adult, the liver exhibits low physiological turnover. Several reports had indicated that tissue self‐renewal under physiological conditions is maintained by mature cells (Malato et al. 2011; Tarlow et al. 2014; Yanger et al. 2014). However, lineage‐labelling studies have also recently supported a role for stem/progenitor compartments in liver homeostasis (Espanol‐Suner et al. 2012; Font‐Burgada et al. 2015; Lu et al. 2015; Wang et al. 2015 a). A recent study has identified a diploid population of hepatocytes surrounding the central vein in the liver lobule expressing Axin2 (indicating that it is responsive to Wnt signalling) and the early hepatoblast marker Tbx3 (Wang et al. 2015 a). Axin2+/Tbx3+ hepatocytes exhibit proliferation potential and are capable of generating hepatocytes during homeostasis, thus suggesting that they function as progenitor cells sustaining tissue homeostasis. However, as these are unipotent progenitors (only generating hepatocytes, albeit of different sub‐types), it could be argued as to whether they strictly fulfil the definition of a stem cell, since they lack multipotency. Similarly, Sox9+ cells, marking both periportal hepatocytes (Font‐Burgada et al. 2015) as well as ductal cells (Furuyama et al. 2011) might also act as progenitors contributing to the homeostasis of the hepatocyte and ductal compartment of the adult liver. The stemness potential of the ductal compartment during homeostasis has been elegantly reviewed elsewhere (Turner et al. 2011; Cardinale et al. 2012; Miyajima et al. 2014; Dolle et al. 2015; Verhulst et al. 2015).
Importantly, the liver exerts remarkable regenerative capacity following damage (Zaret & Grompe, 2008). In those scenarios where the hepatocyte compartment is not severely compromised, adult hepatocytes re‐enter the cell cycle in order to replenish the lost tissue (Malato et al. 2011; Schaub et al. 2014; Yanger et al. 2014; Jors et al. 2015). Whether diploid cells are responsible for regeneration or whether the tissue can exploit multiple sources to recover the lost liver volume remains to be addressed. Notably, transplantation studies have shown the ability of both diploid and polyploid hepatocytes to engraft into damaged livers (Duncan et al. 2010). Upon chronic hepatocyte‐depleting injuries, multiple potential scenarios have been described whereby cells interchange states and acquire non‐default abilities in order to cope with the regenerative demand. A subset of periportal hepatocytes (expressing low amounts of Sox9 and other ductal markers) has been shown to contribute to regeneration (Font‐Burgada et al. 2015). Whether Sox9+ periportal hepatocytes represent a subpopulation of mature hepatocytes or a unipotent progenitor compartment (capable of generating hepatocytes) remains to be clarified. In addition, the constant injury to the hepatocyte compartment might result in hepatocytes acquiring the features of proliferating bipotent progenitors (capable of generating both hepatocytes and ductal cells) with ductal features (Tarlow et al. 2014). One intriguing hypothesis is that, upon damage, the liver microenvironment may generate signals required to convert hepatocytes into ductal cells. Consistent with this hypothesis, it has been shown that ectopic Notch signalling is able to convert hepatocytes into ductal cells (Chen et al. 2012; Fan et al. 2012; Yanger et al. 2013). Significant evidence indicates that the ductal compartment also plays an important role during regeneration upon significant hepatocyte loss. Indeed, upon severe liver damage, ductal cells have been shown to significantly increase their proliferative capacity acting as bipotent stem/progenitor cells capable of generating both hepatocytes and ductal cells (Schmelzer et al. 2007; Dorrell et al. 2011; Shin et al. 2011; Espanol‐Suner et al. 2012; Huch et al. 2013 a; Choi et al. 2014 b; Lu et al. 2015). Whether all the cells of the ductal compartment or a specific subpopulation of putative stem/progenitor cells adopt these changes in proliferation and gene expression remains to be addressed. The extent of ductal cell contribution to regeneration seems to be both injury‐ and specie‐dependent. Although in most of the studies involving mouse models the contribution of ductal stem/progenitor cells to regeneration seems low, a recent study providing extensive damage has shown a major contribution of ductal cells to regeneration of the mouse liver (Lu et al. 2015), as observed in other species such as zebrafish (Choi et al. 2014 b) and rat (Michalopoulos, 2007). Importantly, ductal cells isolated from the healthy liver rapidly acquire proliferation and differentiation potential in culture and upon transplantation (Huch et al. 2013 b). The different approaches used to isolate these cells from the healthy liver have been elegantly discussed elsewhere (Tanaka & Miyajima, 2012; Miyajima et al. 2014). Of note, two of these most recent approaches include the isolation of a CD45−/CD31−/CD11b−/CD26−/MIC1‐1C3+/CD133+ population (Dorrell et al. 2014) and a CD45−/CD31−/Ter119−/EpCAM+/CD133+/CD24+ population (Lu et al. 2015). Interestingly, both populations exhibit a robust ability to self‐renew in culture either as 3D‐proliferating organoids (Dorrell et al. 2011; Huch et al. 2013 b) or as a 2D‐monolayer cultures (Lu et al. 2015). Also, both populations have the ability to differentiate into hepatocytes and ductal cells in vitro, and engraft in vivo in either the FAH mouse model (Huch et al. 2013 b; Dorrell et al. 2014) or in a novel model of hepatocyte senescence based on Mdm2 knock‐out (Lu et al. 2015). Further characterization will be required to establish whether these two populations represent multiple progenitor populations or whether they are different sides of the same coin. A side‐by‐side comparison of the respective markers, transcriptomes, self‐renewal ability in the same culture system and engraftment ability in the same liver damage model would answer this question. Bipotent ductal stem/progenitor cells retain the expression of ductal markers including EpCAM (Yovchev et al. 2008), MIC1‐1C3 (Dorrell et al. 2008), SOX9 (Dorrell et al. 2011; Furuyama et al. 2011) and osteopontin (Espanol‐Suner et al. 2012) but also acquire a specific molecular signature. Genetic lineage‐tracing experiments have elucidated the transcription factor FoxL1 as a marker of actively proliferating ductal stem/progenitor cells (Sackett et al. 2009; Shin et al. 2011) and ablation of this specific population results in impaired tissue regeneration (Shin et al. 2015). Similarly, the transmembrane glycoprotein marker TROP2 (which is not expressed in healthy liver) has been shown to be induced in liver stem/progenitor cells following damage (Okabe et al. 2009). Interestingly, Trop2 can undergo regulated proteolysis (Stoyanova et al. 2012) in a similar fashion to the related protein EpCAM (Maetzel et al. 2009). Of note, the Trop2 intracellular domain has been reported to promote Wnt signalling (Stoyanova et al. 2012), thus suggesting that Wnt signalling might play a role in liver regeneration. Supporting this, several components of the Wnt signalling pathway (including Wnt6, R‐spondin family members and Lgr5) are significantly up‐regulated upon liver injury (Hu et al. 2007; Huch et al. 2013 b). Taken together, these observations suggest an interesting concept whereby Wnt signalling levels would rise during regeneration to rapidly activate or reprogramme a highly proliferative state either in mature cells, quiescent or slowly proliferating progenitors that could help to achieve faster recovery of the lost tissue. Furthermore, cells expressing Lgr5 were shown by lineage tracing studies to repopulate the hepatocyte and ductal compartments upon tissue damage (Huch et al. 2013 a). Lgr5 is the receptor for the R‐spondin family members (de Lau et al. 2014) and an enhancer of Wnt signalling through the inhibition of Rnf43 and Znrf3‐dependent Wnt‐receptor degradation (Koo et al. 2012). Interestingly, Lgr5‐positive cells isolated from injured livers show self‐renewal and bipotentiality in vitro. This injury‐derived Lgr5‐positive population can be clonally expanded as proliferating 3D organoid cultures that resemble proliferative ductal progenitors, while still retaining the ability to differentiate into functional hepatocytes both in vitro and in vivo upon liver transplantation (Huch et al. 2013 b). Whether this injury‐induced Lgr5‐positive population is also a bipotential population in vivo or whether independent Lgr5‐expressing populations regenerate the ductal and hepatocyte lineages separately is still to be investigated. Of note, biliary ducts derived from healthy mouse and human liver, when cultured in a medium containing regenerative niche signals such as Wnt ligands, FGFs and HGF, also establish long‐term expanding, 3D organoid cultures that, similar to the ones generated from Lgr5‐positive cells derived from damaged liver, not only self‐renew but also preserve the ability to differentiate into hepatocytes and ductal cells in vitro (Huch et al. 2013 b, 2015). Therefore, organoid cultures represent an excellent tool for studying the activation of liver ductal cells and the potential regulatory mechanisms behind their plasticity. The use of 3D organoid cultures as a model of human and mouse development, adult homeostasis and regeneration has been reviewed elsewhere (Huch & Koo, 2015).
Taken together, the studies mentioned here highlight the high plasticity of the resident hepatocyte and ductal populations in the adult liver. Diploid hepatocytes (Font‐Burgada et al. 2015; Wang et al. 2015 a), fully differentiated parenchymal cells (Malato et al. 2011; Schaub et al. 2014; Tarlow et al. 2014; Yanger et al. 2014) and putative stem/progenitor cell populations (Dorrell et al. 2008; Yovchev et al. 2008; Okabe et al. 2009; Espanol‐Suner et al. 2012; Tanaka & Miyajima, 2012; Dorrell et al. 2014; Font‐Burgada et al. 2015; Wang et al. 2015 a), have all been reported to contribute to tissue homeostasis. In line with this, upon damage, both mature cells and putative stem/progenitor cells belonging to both hepatocyte and ductal compartments have been shown to contribute significantly to tissue regeneration (Okabe et al. 2009; Dorrell et al. 2011; Shin et al. 2011; Espanol‐Suner et al. 2012; Huch et al. 2013 b; Choi et al. 2014 b; Lu et al. 2015). Therefore, we can speculate that liver cellular plasticity is behind all these observations and that according to the place where the injury occurs and/or the type of toxic insult, resident ductal cells, hepatocytes and/or subpopulations of cells with stem cell‐like properties contribute to tissue repair (Fig. 1).
Figure 1. Plasticity of the adult liver .
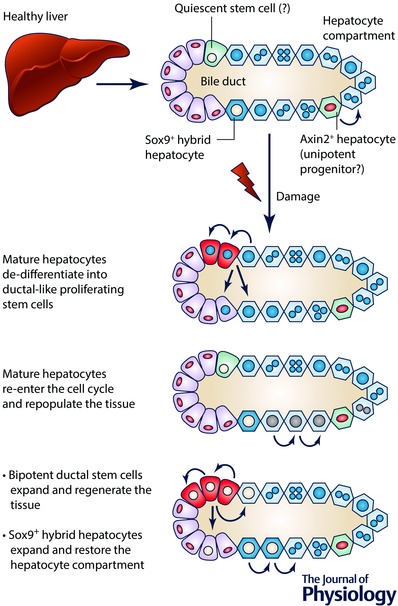
A simplified scheme of a liver lobule composed of a biliary duct and surrounding hepatocytes is shown. Epithelial ductal cells (also named cholangiocytes) constitute the bile ducts that collect the bile secreted by hepatocytes. A facultative quiescent stem/progenitor population in the ductal compartment (depicted in green) has been suggested by several reports. Axin2‐positive and Sox9‐positive hepatocytes have been recently described as drivers of liver homeostasis and potential facultative stem/progenitor populations. Different mechanisms involving mature cells and stem/progenitor cells have been reported during tissue regeneration. Mature hepatocytes might de‐differentiate into proliferating ductal‐like stem/progenitor cells (in red) that, in turn, expand and differentiate. Alternatively, they might re‐enter the cell cycle, re‐acquiring proliferative potential and restoring the damaged parenchyma. Regarding stem/progenitor cell response, two main mechanisms have been reported: (i) the ductal compartment can activate bipotential stem/progenitor cells (in red), which rapidly expand and differentiate into hepatocyte and ductal cells; (ii) Sox9‐positive hybrid hepatocytes can undergo extensive proliferation and generate mature hepatocytes.
Plasticity of the adult stomach
The stomach is an endoderm‐derived organ. In the embryo, the specification of the gastric epithelium is driven by the transcription factor Barx1, which mediates inhibition of Wnt signalling (Kim et al. 2005; Mills & Shivdasani, 2011). Importantly, 3D organoid models of the stomach have recently been established from pluripotent stem cells, thus allowing the study of stomach development in a dish (McCracken et al. 2014; Noguchi et al. 2015). The adult stomach is a single‐cell layer epithelium that can be divided into three anatomically distinct regions: the forestomach, the corpus and the antro‐pylorus. The epithelium is invaginated into tubular structures called gastric units. Each gastric unit can be further stratified by distinct cellular composition (Vries et al. 2010). The uppermost section (named the pit) contains mainly surface mucous cells. Deeper in the gland there is the isthmus region, which contains rapidly dividing cells (Karam & Leblond, 1993). At the base of the gastric unit there are the chief cells, which produce digestive enzymes, while enteroendocrine cells and parietal cells are distributed across the whole unit (Barker et al. 2010 a; Goldenring et al. 2011; Mills & Shivdasani, 2011; Choi et al. 2014 a).
In the adult, the stomach is constantly undergoing self‐renewal. Several reports have elucidated that each gastric unit is monoclonal (derived from a single stem/progenitor cell) (Bjerknes & Cheng, 2002; Giannakis et al. 2006; McDonald et al. 2008; Leushacke et al. 2013; Choi et al. 2014 a) and gastric units contain multiple stem/progenitor populations (Bjerknes & Cheng, 2002; Giannakis et al. 2006; McDonald et al. 2008; Barker et al. 2010 b; Choi et al. 2014 a). Utilizing lineage tracing experiments, a self‐renewing population marked by the expression of Lgr5, residing at the base of the antro‐pyloric gastric unit, was shown to be a bona fide stem cell population in the stomach (Barker et al. 2010 b). Hence, Lgr5‐positive cells act as multipotent stem/progenitor cells capable of self‐renewing and giving rise to all cell types of the adult antro‐pyloric gland. Interestingly, while Lgr5‐positive cells do not contribute to the adult maintenance of the corpus region, they do contribute to postnatal development of both antro‐pylorus and corpus (Barker et al. 2010 b). The vast majority of Lgr5‐positive cells in the antro‐pyloric region described above divide symmetrically. However, a small fraction of the Lgr5‐positive compartment has been shown to adopt asymmetric cell division, thus suggesting that they can change fate in response to environmental changes (Leushacke et al. 2013).
Further supporting the rich plasticity of the antro‐pyloric cells, the study of stomach epithelial regeneration in this region has identified several cell types that can change fate upon certain types of damage. Thus, lineage tracing experiments have revealed the presence of rare villin‐positive cells below the isthmus region in the adult stomach (Qiao et al. 2007). These villin‐positive cells act as a reservoir of multipotent stem/progenitor cells, exhibiting rapid proliferation and stem cell properties upon inflammation‐mediated damage (Qiao et al. 2007). It is also worth mentioning that a Sox2‐positive population of multipotent stem/progenitor cells has also been identified both in the corpus and in the antro‐pylorus (Arnold et al. 2011). Sox2‐positive cells are able to self‐renew and generate all the different cell types of the gland under physiological conditions. Interestingly, the Sox2‐positive stem/progenitor population does not overlap with Lgr5‐positive cells in the pylorus, indicating that Lgr5 and Sox2 mark two different populations. According to the number of cells identified per gland and their localization, the Sox2‐positive population might overlap with villin‐positive stem/progenitor cells. However, villin‐positive cells have been shown to proliferate only in response to inflammation, whereas Sox2‐positive cells proliferate under homeostatic conditions (Arnold et al. 2011). This suggests that Sox2 and villin also mark two different populations that can acquire stem cell capacity (stem cell potential) under different conditions. Altogether, these reports suggest that, in the adult antro‐pylorus, at least four different populations with stem cell potential exist: the isthmic stem/progenitor compartment, Sox2‐positive cells, self‐renewing Lgr5‐positive cells at the base of the glands and the quiescent villin‐positive cells.
Similar to the pylorus, several cell types that exhibit high cellular plasticity have also been identified in the corpus epithelium. Several reports indicate that differentiated chief cells are plastic by nature, as they can be activated upon parietal cell loss (Mills & Shivdasani, 2011). Interestingly, upon massive parietal cell loss post‐mitotic chief cells generate a metaplastic cell lineage known as SPEM (spasmolytic polypeptide‐expressing metaplasia). SPEM cells exhibit markers of both mucous neck cells and chief cells, as shown by genetic lineage tracing of the transcription factor Mist1 in several models of stomach injury (Nam et al. 2010). Importantly, it was recently reported that Mist1 marks a quiescent stem cell population in the corpus (Hayakawa et al. 2015). Further supporting the high plasticity of mature chief cells, lineage‐tracing experiments have demonstrated that, in the corpus, mature chief cells expressing Tnfsrf19 (also known as Troy) act as multipotent stem/progenitor cells upon damage to the proliferative isthmus compartment (Stange et al. 2013). Under these conditions, non‐proliferative, Troy‐positive chief cells re‐enter the cell cycle, expand and contribute to repopulate the entire corpus gastric unit. Of note, Troy‐positive cells can be expanded as proliferating 3D organoids and eventually differentiate into mucous neck and pit cells according to culture conditions (Stange et al. 2013). Thus, Troy‐positive chief cells act as a quiescent reservoir of stem/progenitor population in the adult corpus, primed to restore tissue integrity upon damage. Interestingly, Troy is a Wnt target gene and Troy‐positive cells present a ‘high Wnt’ signature (Stange et al. 2013). Furthermore, Troy has been reported to be involved in inhibiting Wnt signalling (Fafilek et al. 2013). These observations, together with the reports discussed above in the liver section, lead us to speculate that the Wnt signalling pathway might play a crucial role in tissue regeneration and the acquisition of cellular plasticity, at least in the two organs discussed in this review.
Taken together, these reports demonstrate the high diversity and plasticity present in the gastric epithelium and highlight the presence of several cells capable of responding to specific injury stimuli in a context‐dependent manner and activating a stem cell programme to reinstate homeostasis (Fig. 2).
Figure 2. Plasticity of the adult stomach .
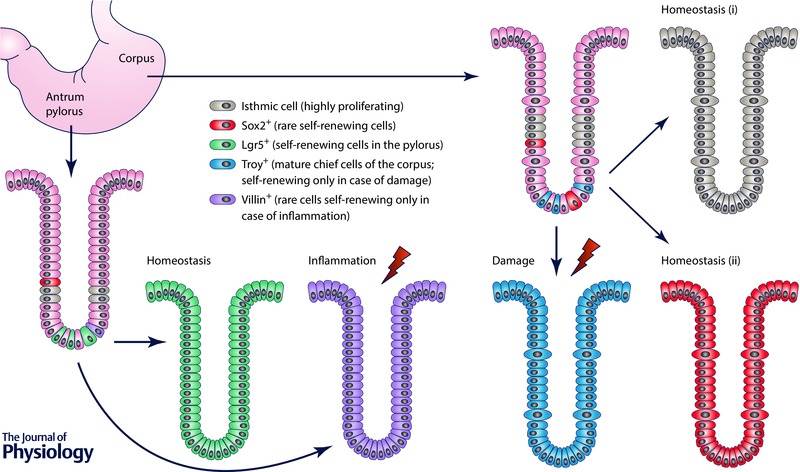
A simplified scheme of a gastric unit of the corpus and pylorus of the adult stomach is shown. In both the corpus and pylorus, highly proliferative isthmic cells (depicted in brown) and rare, self‐renewing Sox2‐positive multipotent stem/progenitor cells (depicted in red) have been observed. In the corpus, both isthmic cells and Sox2‐positive cells are able to generate the entire gastric unit during homeostasis. However, mature, Troy‐positive chief cells (in light blue) act as a quiescent stem/progenitor population, re‐entering the cell cycle and repopulating the gland upon damage. In the pylorus, Lgr5‐positive cells (in green), located at the base of the gland, act as self‐renewing, multipotent stem/progenitor cells capable of repopulating the entire gastric unit under physiological conditions. Of note, Sox2‐positive cells are also capable of generating the entire gland under physiological conditions (not shown). Upon inflammation, rare, quiescent villin‐positive stem/progenitor cells (in purple) become highly proliferating cells, which regenerate the damaged epithelium in the pylorus.
Conclusions
Adult tissues have to be prepared to react to a huge variety of different insults, stimuli and intra‐ and extracellular cues. To achieve this, both rapidly and slowly self‐renewing adult organs possess multiple populations which are able to change fate, thus allowing the maintenance of tissue function in response to changes in environmental cues (Li & Clevers, 2010). Importantly, upon tissue damage, resident quiescent stem/progenitor cells (such as villin‐positive cells in the pylorus and the ductal compartment in the liver) exhibit high plasticity by becoming actively proliferating stem/progenitor cells capable of repopulating the tissue. As an additional mechanism, mature cells (such as Troy‐positive chief cells of the stomach or ductal and hepatocyte cells in the liver) can act as ‘reserve’ stem/progenitor cells, which upon injury will de‐differentiate, allowing them to proliferate, and subsequently re‐differentiate to regenerate the tissue. Therefore, increasing evidence supports a role for cellular plasticity in injury response. Further studies will be needed in order to elucidate the diverse cell types involved and the molecular mechanisms responsible for cellular plasticity during tissue regeneration.
Of note, different adult tissues of the gastro‐intestinal tract undergoing regeneration acquire a common molecular signature, including the expression of Sox9 (Furuyama et al. 2011), Bmi1 (Sangiorgi & Capecchi, 2008; Zhu et al. 2013) and the Wnt‐target gene Lgr5 (Tetteh et al. 2015), suggesting that regenerative mechanisms are conserved between tissues. Supporting this, the expression of several components of the Wnt cascade is increased in actively proliferating cells of the stomach, liver and intestine (Giannakis et al. 2006; Huch et al. 2013 b; 2015; Stange et al. 2013; Clevers et al. 2014). The identification of stem cell markers has to be carefully confirmed by using independent experimental approaches ranging from lineage tracing to transplantation assays. It is important to note that lineage tracing experiments based on inducible Cre activity, so far representing the gold standard approach for identifying stem cell markers, have generated controversy and have highlighted important limitations due to the different efficiencies of recombinase induction and possible non‐specific expression of the transgenes (Lemaigre, 2015). So far, little is known about the downstream targets of adult stem cell markers. For example, the epigenetic regulation of adult stem/progenitor cells of the gastro‐intestinal tract is poorly characterised. Of note, it has recently been reported that the Polycomb repressive complex, PRC1, sustains Wnt signalling in intestinal stem cells (Chiacchiera et al. 2016). In agreement with this, the Polycomb‐member Bmi1 has been reported as a marker of adult stem/progenitor cells (Sangiorgi & Capecchi, 2008; Tian et al. 2011; Zhu et al. 2013; López‐Arribillaga et al. 2015; Rinaldi & Benitah, 2015; Tetteh et al. 2015). Therefore, it is feasible to speculate that the Polycomb proteins play a role in the epigenetic regulation of adult stem cells, as it has been described for the embryo (Aloia et al. 2013).
Increasing evidence indicates that differentiated cells provide Wnt ligands and signalling enhancers to the stem/progenitor compartment, thus generating a niche and promoting the expansion of stem/progenitor cells (Clevers & Bevins, 2013; Huch et al. 2013 b; Clevers et al. 2014; Wang et al. 2015 a). Therefore, the niche might play a crucial role in stimulating cell plasticity and tissue regeneration. An intriguing hypothesis is that depending on the place and type of injury, specific ‘niche’ cells might stimulate specific regenerative processes required to repair the tissue. However, so far, little is known about the function and the identity of such niche cells in vivo. Identifying these niche factors and/or cells will provide novel insights into the regenerative processes and acquisition of plasticity.
An interesting hypothesis is that molecular mechanisms responsible for the plasticity of adult tissues in response to injury might be similar to the ones involved in the reprogramming of somatic cells into induced pluripotent stem cells (Yamanaka & Blau, 2010), albeit within the constraints of germ layer or tissue specificity. Therefore, a detailed comparative analysis of the molecular signature of stem/progenitor cells and cells involved in tissue regeneration versus somatic cells reprogrammed into induced pluripotent stem cells might provide novel insights into plasticity.
It is possible that cellular plasticity in adult tissues might be a double‐edged sword. There are many theories that cells with the ability to acquire stem cell fate could be the source of tumour‐initiating cells (Goding et al. 2014; Laugesen & Helin, 2014; Zeuner et al. 2014; Jeter et al. 2015). Accordingly, it was recently shown that tumour‐initiating cells emerging during chronic liver disease exhibit the same molecular features of Lgr5‐positive liver stem/progenitor populations (Nikolaou et al. 2015). Such reports suggest that alterations in plasticity processes turning quiescent stem/progenitor cells into actively proliferating cells may ultimately result in carcinogenesis (Rountree et al. 2012). Therefore, understanding how cellular plasticity works might provide novel insights to the molecular mechanisms involved in carcinogenesis and disease.
Additional information
Competing interests
None declared.
Funding
M.H. is a Wellcome Trust Sir Henry Dale Fellow and is jointly funded by the Wellcome Trust and The Royal Society (104151/Z/14/Z). M.A.M. is an Medical Research Council (MRC) PhD fellow (PMAG/440).
Acknowledgements
We are grateful to Dr Christopher J. Hindley for critical reading of the manuscript.
Biography
Meritxell Huch is a Group Leader at the Gurdon Institute and affiliated group leader at the MRC/WT Cambridge Stem Cell Institute and an academic member in the Physiology, Development and Neuroscience department of the University of Cambridge. She obtained her PhD degree in 2007 at the Centre for Genomic Regulation in Barcelona, Spain. In 2008, she moved to the Netherlands to study adult stem cell biology. Between 2008 and 2014 she has been working on the adult stem cells of several gastrointestinal organs, including the liver, pancreas and stomach. In 2013 she published a seminal paper describing the identification of adult liver progenitors and their contribution to tissue regeneration. At the same time, she developed a culture system that allows the unlimited expansion of liver and pancreas progenitors from an adult tissue. In February 2014, she joined the Gurdon Institute as a junior Group Leader, where she continues her research on stem cells and tissue regeneration. In 2014 the liver organoid technology she developed was awarded the International NC3Rs Prize and the Beit Prize. Luigi Aloia has been a postdoctoral research associate in the laboratory of Dr Huch at the Gurdon Institute since November 2014. He received his PhD in 2010 at University Federico II in Naples (Italy) studying novel players involved in pluripotency and differentiation of embryonic stem cells. In September 2010 he joined the laboratory of Luciano Di Croce at the Centre for Genomic Regulation in Barcelona (Spain), where he worked on the epigenetic regulation driving specification of embryonic neural progenitors. Mikel McKie is a PhD student in the laboratory of Dr Huch at the Gurdon Institute after joining the group in 2015. He studied Natural Sciences at the University of Cambridge and received a Master of Natural Sciences degree from the Department of Biochemistry in 2014.

L. Aloia and M. A. McKie contributed equally to this work.
References
- Aloia L, Di Stefano B & Di Croce L (2013). Polycomb complexes in stem cells and embryonic development. Development 140, 2525–2534. [DOI] [PubMed] [Google Scholar]
- Arnold K, Sarkar A, Yram MA, Polo JM, Bronson R, Sengupta S, Seandel M, Geijsen N & Hochedlinger K (2011). Sox2+ adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell 9, 317–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N (2014). Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol 15, 19–33. [DOI] [PubMed] [Google Scholar]
- Barker N, Bartfeld S & Clevers H (2010. a). Tissue‐resident adult stem cell populations of rapidly self‐renewing organs. Cell Stem Cell 7, 656–670. [DOI] [PubMed] [Google Scholar]
- Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, Danenberg E, van den Brink S, Korving J, Abo A, Peters PJ, Wright N, Poulsom R & Clevers H (2010. b). Lgr5+ve stem cells drive self‐renewal in the stomach and build long‐lived gastric units in vitro. Cell Stem Cell 6, 25–36. [DOI] [PubMed] [Google Scholar]
- Bjerknes M & Cheng H (2002). Multipotential stem cells in adult mouse gastric epithelium. Am J Physiol Gastrointest Liver Physiol 283, G767–G777. [DOI] [PubMed] [Google Scholar]
- Blanpain C & Fuchs E (2009). Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol 10, 207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C & Fuchs E (2014). Stem cell plasticity. Plasticity of epithelial stem cells in tissue regeneration. Science 344, 1242281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinale V, Wang Y, Carpino G, Mendel G, Alpini G, Gaudio E, Reid LM & Alvaro D (2012). The biliary tree – a reservoir of multipotent stem cells. Nat Rev Gastroenterol Hepatol 9, 231–240. [DOI] [PubMed] [Google Scholar]
- Chen Y, Wong PP, Sjeklocha L, Steer CJ & Sahin MB (2012). Mature hepatocytes exhibit unexpected plasticity by direct dedifferentiation into liver progenitor cells in culture. Hepatology 55, 563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiacchiera F, Rossi A, Jammula S, Piunti A, Scelfo A, Ordonez‐Moran P, Huelsken J, Koseki H & Pasini D (2016). Polycomb complex PRC1 preserves intestinal stem cell identity by sustaining Wnt/β‐catenin transcriptional activity. Cell Stem Cell 18, 91–103. [DOI] [PubMed] [Google Scholar]
- Choi E, Roland JT, Barlow BJ, O'Neal R, Rich AE, Nam KT, Shi C & Goldenring JR (2014. a). Cell lineage distribution atlas of the human stomach reveals heterogeneous gland populations in the gastric antrum. Gut 63, 1711–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi TY, Ninov N, Stainier DY & Shin D (2014. b). Extensive conversion of hepatic biliary epithelial cells to hepatocytes after near total loss of hepatocytes in zebrafish. Gastroenterology 146, 776–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H, Loh KM & Nusse R (2014). Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 346, 1248012. [DOI] [PubMed] [Google Scholar]
- Clevers HC & Bevins CL (2013). Paneth cells: maestros of the small intestinal crypts. Annu Rev Physiol 75, 289–311. [DOI] [PubMed] [Google Scholar]
- de Lau W, Peng WC, Gros P & Clevers H (2014). The R‐spondin/Lgr5/Rnf43 module: regulator of Wnt signal strength. Genes Dev 28, 305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolle L, Theise ND, Schmelzer E, Boulter L, Gires O & van Grunsven LA (2015). EpCAM and the biology of hepatic stem/progenitor cells. Am J Physiol Gastrointest Liver Physiol 308, G233–G250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrell C, Erker L, Lanxon‐Cookson KM, Abraham SL, Victoroff T, Ro S, Canaday PS, Streeter PR & Grompe M (2008). Surface markers for the murine oval cell response. Hepatology 48, 1282–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrell C, Erker L, Schug J, Kopp JL, Canaday PS, Fox AJ, Smirnova O, Duncan AW, Finegold MJ, Sander M, Kaestner KH & Grompe M (2011). Prospective isolation of a bipotential clonogenic liver progenitor cell in adult mice. Genes Dev 25, 1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrell C, Tarlow B, Wang Y, Canaday PS, Haft A, Schug J, Streeter PR, Finegold MJ, Shenje LT, Kaestner KH & Grompe M (2014). The organoid‐initiating cells in mouse pancreas and liver are phenotypically and functionally similar. Stem Cell Res 13, 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan AW, Taylor MH, Hickey RD, Hanlon Newell AE, Lenzi ML, Olson SB, Finegold MJ & Grompe M (2010). The ploidy conveyor of mature hepatocytes as a source of genetic variation. Nature 467, 707–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espanol‐Suner R, Carpentier R, Van Hul N, Legry V, Achouri Y, Cordi S, Jacquemin P, Lemaigre F & Leclercq IA (2012). Liver progenitor cells yield functional hepatocytes in response to chronic liver injury in mice. Gastroenterology 143, 1564–1575.e7. [DOI] [PubMed] [Google Scholar]
- Fafilek B, Krausova M, Vojtechova M, Pospichalova V, Tumova L, Sloncova E, Huranova M, Stancikova J, Hlavata A, Svec J, Sedlacek R, Luksan O, Oliverius M, Voska L, Jirsa M, Paces J, Kolar M, Krivjanska M, Klimesova K, Tlaskalova‐Hogenova H & Korinek V (2013). Troy, a tumor necrosis factor receptor family member, interacts with Lgr5 to inhibit Wnt signaling in intestinal stem cells. Gastroenterology 144, 381–391. [DOI] [PubMed] [Google Scholar]
- Fan B, Malato Y, Calvisi DF, Naqvi S, Razumilava N, Ribback S, Gores GJ, Dombrowski F, Evert M, Chen X & Willenbring H (2012). Cholangiocarcinomas can originate from hepatocytes in mice. J Clin Invest 122, 2911–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Font‐Burgada J, Shalapour S, Ramaswamy S, Hsueh B, Rossell D, Umemura A, Taniguchi K, Nakagawa H, Valasek MA, Ye L, Kopp JL, Sander M, Carter H, Deisseroth K, Verma IM & Karin M (2015). Hybrid periportal hepatocytes regenerate the injured liver without giving rise to cancer. Cell 162, 766–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T, Hosokawa S, Elbahrawy A, Soeda T, Koizumi M, Masui T, Kawaguchi M, Takaori K, Doi R, Nishi E, Kakinoki R, Deng JM, Behringer RR, Nakamura T & Uemoto S (2011). Continuous cell supply from a Sox9‐expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet 43, 34–41. [DOI] [PubMed] [Google Scholar]
- Giannakis M, Stappenbeck TS, Mills JC, Leip DG, Lovett M, Clifton SW, Ippolito JE, Glasscock JI, Arumugam M, Brent MR & Gordon JI (2006). Molecular properties of adult mouse gastric and intestinal epithelial progenitors in their niches. J Biol Chem 281, 11292–11300. [DOI] [PubMed] [Google Scholar]
- Goding CR, Pei D & Lu X (2014). Cancer: pathological nuclear reprogramming? Nat Rev Cancer 14, 568–573. [DOI] [PubMed] [Google Scholar]
- Goldenring JR, Nam KT & Mills JC (2011). The origin of pre‐neoplastic metaplasia in the stomach: Chief cells emerge from the Mist. Exp Cell Res 317, 2759–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordillo M, Evans T & Gouon‐Evans V (2015). Orchestrating liver development. Development 142, 2094–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulas S, Conder R & Knoblich JA (2012). The Par complex and integrins direct asymmetric cell division in adult intestinal stem cells. Cell Stem Cell 11, 529–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa Y, Ariyama H, Stancikova J, Sakitani K, Asfaha S, Renz BW, Dubeykovskaya ZA, Shibata W, Wang H, Westphalen CB, Chen X, Takemoto Y, Kim W, Khurana SS, Tailor Y, Nagar K, Tomita H, Hara A, Sepulveda AR, Setlik W, Gershon MD, Saha S, Ding L, Shen Z, Fox JG, Friedman RA, Konieczny SF, Worthley DL, Korinek V & Wang TC (2015). Mist1 expressing gastric stem cells maintain the normal and neoplastic gastric epithelium and are supported by a perivascular stem cell niche. Cancer Cell 28, 800–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Kurobe M, Jeong YJ, Fuerer C, Ghole S, Nusse R & Sylvester KG (2007). Wnt/β‐catenin signaling in murine hepatic transit amplifying progenitor cells. Gastroenterology 133, 1579–1591. [DOI] [PubMed] [Google Scholar]
- Huch M, Boj SF & Clevers H (2013. a). Lgr5+ liver stem cells, hepatic organoids and regenerative medicine. Regen Med 8, 385–387. [DOI] [PubMed] [Google Scholar]
- Huch M, Dorrell C, Boj SF, van Es JH, Li VS, van de Wetering M, Sato T, Hamer K, Sasaki N, Finegold MJ, Haft A, Vries RG, Grompe M & Clevers H (2013. b). In vitro expansion of single Lgr5+ liver stem cells induced by Wnt‐driven regeneration. Nature 494, 247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huch M, Gehart H, van Boxtel R, Hamer K, Blokzijl F, Verstegen MM, Ellis E, van Wenum M, Fuchs SA, de Ligt J, van de Wetering M, Sasaki N, Boers SJ, Kemperman H, de Jonge J, Ijzermans JN, Nieuwenhuis EE, Hoekstra R, Strom S, Vries RR, van der Laan LJ, Cuppen E & Clevers H (2015). Long‐term culture of genome‐stable bipotent stem cells from adult human liver. Cell 160, 299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huch M & Koo BK (2015). Modeling mouse and human development using organoid cultures. Development 142, 3113–3125. [DOI] [PubMed] [Google Scholar]
- Jeter CR, Yang T, Wang J, Chao HP & Tang DG (2015). Concise review: NANOG in cancer stem cells and tumor development: an update and outstanding questions. Stem Cells 33, 2381–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jors S, Jeliazkova P, Ringelhan M, Thalhammer J, Durl S, Ferrer J, Sander M, Heikenwalder M, Schmid RM, Siveke JT & Geisler F (2015). Lineage fate of ductular reactions in liver injury and carcinogenesis. J Clin Invest 125, 2445–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam SM & Leblond CP (1993). Dynamics of epithelial cells in the corpus of the mouse stomach. I. Identification of proliferative cell types and pinpointing of the stem cell. Anat Rec 236, 259–279. [DOI] [PubMed] [Google Scholar]
- Kim BM, Buchner G, Miletich I, Sharpe PT & Shivdasani RA (2005). The stomach mesenchymal transcription factor Barx1 specifies gastric epithelial identity through inhibition of transient Wnt signaling. Dev Cell 8, 611–622. [DOI] [PubMed] [Google Scholar]
- Koo BK, Spit M, Jordens I, Low TY, Stange DE, van de Wetering M, van Es JH, Mohammed S, Heck AJ, Maurice MM & Clevers H (2012). Tumour suppressor RNF43 is a stem‐cell E3 ligase that induces endocytosis of Wnt receptors. Nature 488, 665–669. [DOI] [PubMed] [Google Scholar]
- Laugesen A & Helin K (2014). Chromatin repressive complexes in stem cells, development, and cancer. Cell Stem Cell 14, 735–751. [DOI] [PubMed] [Google Scholar]
- Lemaigre FP (2015). Determining the fate of hepatic cells by lineage tracing: facts and pitfalls. Hepatology 61, 2100–2103. [DOI] [PubMed] [Google Scholar]
- Leushacke M, Ng A, Galle J, Loeffler M & Barker N (2013). Lgr5+ gastric stem cells divide symmetrically to effect epithelial homeostasis in the pylorus. Cell Rep 5, 349–356. [DOI] [PubMed] [Google Scholar]
- Li L & Clevers H (2010). Coexistence of quiescent and active adult stem cells in mammals. Science 327, 542–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López‐Arribillaga E, Rodilla V, Pellegrinet L, Guiu J, Iglesias M, Roman AC, Gutarra S, González S, Munoz‐Cánoves P, Fernandez‐Salguero P, Radtke F, Bigas A & Espinosa L (2015). Bmi1 regulates murine intestinal stem cell proliferation and self‐renewal downstream of Notch. Development 142, 41–50. [DOI] [PubMed] [Google Scholar]
- Lu WY, Bird TG, Boulter L, Tsuchiya A, Cole AM, Hay T, Guest RV, Wojtacha D, Man TY, Mackinnon A, Ridgway RA, Kendall T, Williams MJ, Jamieson T, Raven A, Hay DC, Iredale JP, Clarke AR, Sansom OJ & Forbes SJ (2015). Hepatic progenitor cells of biliary origin with liver repopulation capacity. Nat Cell Biol 17, 971–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken KW, Cata EM, Crawford CM, Sinagoga KL, Schumacher M, Rockich BE, Tsai YH, Mayhew CN, Spence JR, Zavros Y & Wells JM (2014). Modelling human development and disease in pluripotent stem‐cell‐derived gastric organoids. Nature 516, 400–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald SA, Greaves LC, Gutierrez‐Gonzalez L, Rodriguez‐Justo M, Deheragoda M, Leedham SJ, Taylor RW, Lee CY, Preston SL, Lovell M, Hunt T, Elia G, Oukrif D, Harrison R, Novelli MR, Mitchell I, Stoker DL, Turnbull DM, Jankowski JA & Wright NA (2008). Mechanisms of field cancerization in the human stomach: the expansion and spread of mutated gastric stem cells. Gastroenterology 134, 500–510. [DOI] [PubMed] [Google Scholar]
- Maetzel D, Denzel S, Mack B, Canis M, Went P, Benk M, Kieu C, Papior P, Baeuerle PA, Munz M & Gires O (2009). Nuclear signalling by tumour‐associated antigen EpCAM. Nat Cell Biol 11, 162–171. [DOI] [PubMed] [Google Scholar]
- Malato Y, Naqvi S, Schurmann N, Ng R, Wang B, Zape J, Kay MA, Grimm D & Willenbring H (2011). Fate tracing of mature hepatocytes in mouse liver homeostasis and regeneration. J Clin Invest 121, 4850–4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos GK (2007). Liver regeneration. J Cell Physiol 213, 286–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills JC & Shivdasani RA (2011). Gastric epithelial stem cells. Gastroenterology 140, 412–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyajima A, Tanaka M & Itoh T (2014). Stem/progenitor cells in liver development, homeostasis, regeneration, and reprogramming. Cell Stem Cell 14, 561–574. [DOI] [PubMed] [Google Scholar]
- Morrison SJ & Spradling AC (2008). Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 132, 598–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam KT, Lee HJ, Sousa JF, Weis VG, O'Neal RL, Finke PE, Romero‐Gallo J, Shi G, Mills JC, Peek RM Jr, Konieczny SF & Goldenring JR (2010). Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology 139, 2028–2037 e2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaou KC, Moulos P, Chalepakis G, Hatzis P, Oda H, Reinberg D & Talianidis I (2015). Spontaneous development of hepatocellular carcinoma with cancer stem cell properties in PR‐SET7‐deficient livers. EMBO J 34, 430–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi TA, Ninomiya N, Sekine M, Komazaki S, Wang PC, Asashima M & Kurisaki A (2015). Generation of stomach tissue from mouse embryonic stem cells. Nat Cell Biol 17, 984–993. [DOI] [PubMed] [Google Scholar]
- Okabe M, Tsukahara Y, Tanaka M, Suzuki K, Saito S, Kamiya Y, Tsujimura T, Nakamura K & Miyajima A (2009). Potential hepatic stem cells reside in EpCAM+ cells of normal and injured mouse liver. Development 136, 1951–1960. [DOI] [PubMed] [Google Scholar]
- Qiao XT, Ziel JW, McKimpson W, Madison BB, Todisco A, Merchant JL, Samuelson LC & Gumucio DL (2007). Prospective identification of a multilineage progenitor in murine stomach epithelium. Gastroenterology 133, 1989–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond CA, Shah MS, Deary LT, Trotier DC, Thomas H, Ambruzs DM, Jiang L, Whiles BB, Rickner HD, Montgomery RK, Tovaglieri A, Carlone DL & Breault DT (2015). Dormant intestinal stem cells are regulated by PTEN and nutritional status. Cell Rep 13, 2403–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi L & Benitah SA (2015). Epigenetic regulation of adult stem cell function. FEBS J 282, 1589–1604. [DOI] [PubMed] [Google Scholar]
- Rountree CB, Mishra L & Willenbring H (2012). Stem cells in liver diseases and cancer: recent advances on the path to new therapies. Hepatology 55, 298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackett SD, Li Z, Hurtt R, Gao Y, Wells RG, Brondell K, Kaestner KH & Greenbaum LE (2009). Foxl1 is a marker of bipotential hepatic progenitor cells in mice. Hepatology 49, 920–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Alvarado A & Yamanaka S (2014). Rethinking differentiation: stem cells, regeneration, and plasticity. Cell 157, 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangiorgi E & Capecchi MR (2008). Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet 40, 915–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub JR, Malato Y, Gormond C & Willenbring H (2014). Evidence against a stem cell origin of new hepatocytes in a common mouse model of chronic liver injury. Cell Rep 8, 933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelzer E, Zhang L, Bruce A, Wauthier E, Ludlow J, Yao HL, Moss N, Melhem A, McClelland R, Turner W, Kulik M, Sherwood S, Tallheden T, Cheng N, Furth ME & Reid LM (2007). Human hepatic stem cells from fetal and postnatal donors. J Exp Med 204, 1973–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S, Upadhyay N, Greenbaum LE & Kaestner KH (2015). Ablation of Foxl1‐Cre‐labeled hepatic progenitor cells and their descendants impairs recovery of mice from liver injury. Gastroenterology 148, 192–202.e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S, Walton G, Aoki R, Brondell K, Schug J, Fox A, Smirnova O, Dorrell C, Erker L, Chu AS, Wells RG, Grompe M, Greenbaum LE & Kaestner KH (2011). Foxl1‐Cre‐marked adult hepatic progenitors have clonogenic and bilineage differentiation potential. Genes Dev 25, 1185–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange DE, Koo BK, Huch M, Sibbel G, Basak O, Lyubimova A, Kujala P, Bartfeld S, Koster J, Geahlen JH, Peters PJ, van Es JH, van de Wetering M, Mills JC & Clevers H (2013). Differentiated Troy + chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell 155, 357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoyanova T, Goldstein AS, Cai H, Drake JM, Huang J & Witte ON (2012). Regulated proteolysis of Trop2 drives epithelial hyperplasia and stem cell self‐renewal via β‐catenin signaling. Genes Dev 26, 2271–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M & Miyajima A (2012). Identification and isolation of adult liver stem/progenitor cells. Methods Mol Biol 826, 25–32. [DOI] [PubMed] [Google Scholar]
- Tarlow BD, Pelz C, Naugler WE, Wakefield L, Wilson EM, Finegold MJ & Grompe M (2014). Bipotential adult liver progenitors are derived from chronically injured mature hepatocytes. Cell Stem Cell 15, 605–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetteh PW, Farin HF & Clevers H (2015). Plasticity within stem cell hierarchies in mammalian epithelia. Trends Cell Biol 25, 100–108. [DOI] [PubMed] [Google Scholar]
- Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD & de Sauvage FJ (2011). A reserve stem cell population in small intestine renders Lgr5‐positive cells dispensable. Nature 478, 255–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner R, Lozoya O, Wang Y, Cardinale V, Gaudio E, Alpini G, Mendel G, Wauthier E, Barbier C, Alvaro D & Reid LM (2011). Human hepatic stem cell and maturational liver lineage biology. Hepatology 53, 1035–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst S, Best J, van Grunsven LA & Dollé L (2015). Advances in hepatic stem/progenitor cell biology. EXCLI J 14, 33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vries RG, Huch M & Clevers H (2010). Stem cells and cancer of the stomach and intestine. Mol Oncol 4, 373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Zhao L, Fish M, Logan CY & Nusse R (2015. a). Self‐renewing diploid Axin2+ cells fuel homeostatic renewal of the liver. Nature 524, 180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Yamamoto Y, Wilson LH, Zhang T, Howitt BE, Farrow MA, Kern F, Ning G, Hong Y, Khor CC, Chevalier B, Bertrand D, Wu L, Nagarajan N, Sylvester FA, Hyams JS, Devers T, Bronson R, Lacy DB, Ho KY, Crum CP, McKeon F & Xian W (2015. b). Cloning and variation of ground state intestinal stem cells. Nature 522, 173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka S & Blau HM (2010). Nuclear reprogramming to a pluripotent state by three approaches. Nature 465, 704–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanger K, Knigin D, Zong Y, Maggs L, Gu G, Akiyama H, Pikarsky E & Stanger BZ (2014). Adult hepatocytes are generated by self‐duplication rather than stem cell differentiation. Cell Stem Cell 15, 340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanger K, Zong Y, Maggs LR, Shapira SN, Maddipati R, Aiello NM, Thung SN, Wells RG, Greenbaum LE & Stanger BZ (2013). Robust cellular reprogramming occurs spontaneously during liver regeneration. Genes Dev 27, 719–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yovchev MI, Grozdanov PN, Zhou H, Racherla H, Guha C & Dabeva MD (2008). Identification of adult hepatic progenitor cells capable of repopulating injured rat liver. Hepatology 47, 636–647. [DOI] [PubMed] [Google Scholar]
- Zaret KS & Grompe M (2008). Generation and regeneration of cells of the liver and pancreas. Science 322, 1490–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuner A, Todaro M, Stassi G & De Maria R (2014). Colorectal cancer stem cells: from the crypt to the clinic. Cell Stem Cell 15, 692–705. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Huang YF, Kek C & Bulavin DV (2013). Apoptosis differently affects lineage tracing of Lgr5 and Bmi1 intestinal stem cell populations. Cell Stem Cell 12, 298–303. [DOI] [PubMed] [Google Scholar]


