Abstract
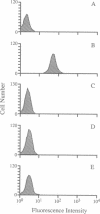
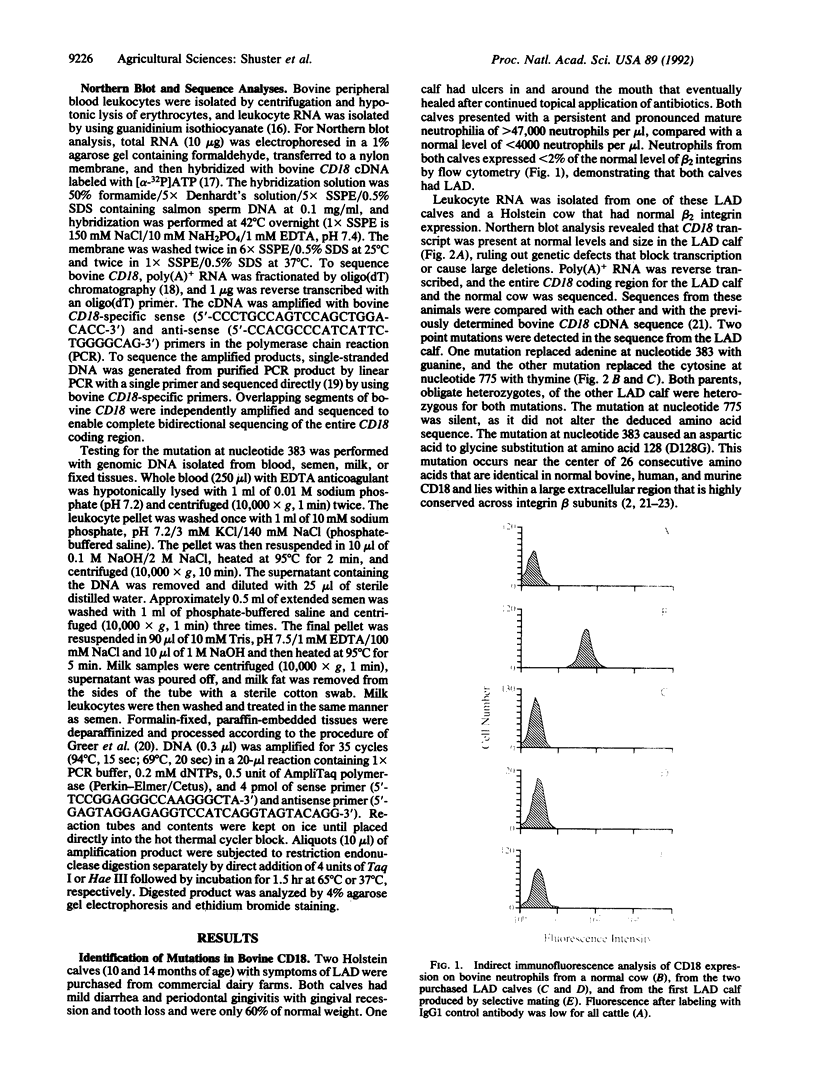
Two point mutations were identified within the gene encoding bovine CD18 in a Holstein calf afflicted with leukocyte adhesion deficiency (LAD). One mutation causes an aspartic acid to glycine substitution at amino acid 128 (D128G) in the highly conserved extracellular region of this adhesion glycoprotein, a region where several mutations have been found to cause human LAD. The other mutation is silent. Twenty calves with clinical symptoms of LAD were tested, and all were homozygous for the D128G allele. In addition, two calves homozygous for the D128G allele were identified during widespread DNA testing, and both were subsequently found to exhibit symptoms of LAD. The carrier frequency for the D128G allele among Holstein cattle in the United States is approximately 15% among bulls and 6% among cows. This mutation is also prevalent among Holstein cattle throughout the world, placing this disorder among the most common genetic diseases known in animal agriculture. All cattle with the mutant allele are related to one bull, who through the use of artificial insemination sired many calves in the 1950s and 1960s. The organization of the dairy industry and the diagnostic test described herein will enable nearly complete eradication of bovine LAD within 1 year. These results also demonstrate that bovine LAD is genetically homologous and phenotypically similar to human LAD, thus providing a useful animal model for studies of LAD and beta 2 integrin function.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allard M. W., Ellsworth D. L., Honeycutt R. L. The production of single-stranded DNA suitable for sequencing using the polymerase chain reaction. Biotechniques. 1991 Jan;10(1):24–26. [PubMed] [Google Scholar]
- Anderson D. C., Schmalsteig F. C., Finegold M. J., Hughes B. J., Rothlein R., Miller L. J., Kohl S., Tosi M. F., Jacobs R. L., Waldrop T. C. The severe and moderate phenotypes of heritable Mac-1, LFA-1 deficiency: their quantitative definition and relation to leukocyte dysfunction and clinical features. J Infect Dis. 1985 Oct;152(4):668–689. doi: 10.1093/infdis/152.4.668. [DOI] [PubMed] [Google Scholar]
- Arnaout M. A., Dana N., Gupta S. K., Tenen D. G., Fathallah D. M. Point mutations impairing cell surface expression of the common beta subunit (CD18) in a patient with leukocyte adhesion molecule (Leu-CAM) deficiency. J Clin Invest. 1990 Mar;85(3):977–981. doi: 10.1172/JCI114529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaout M. A. Leukocyte adhesion molecules deficiency: its structural basis, pathophysiology and implications for modulating the inflammatory response. Immunol Rev. 1990 Apr;114:145–180. doi: 10.1111/j.1600-065x.1990.tb00564.x. [DOI] [PubMed] [Google Scholar]
- Arnaout M. A. Structure and function of the leukocyte adhesion molecules CD11/CD18. Blood. 1990 Mar 1;75(5):1037–1050. [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back A. L., Kwok W. W., Hickstein D. D. Identification of two molecular defects in a child with leukocyte adherence deficiency. J Biol Chem. 1992 Mar 15;267(8):5482–5487. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dennis J. A., Healy P. J., Beaudet A. L., O'Brien W. E. Molecular definition of bovine argininosuccinate synthetase deficiency. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7947–7951. doi: 10.1073/pnas.86.20.7947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Greer C. E., Peterson S. L., Kiviat N. B., Manos M. M. PCR amplification from paraffin-embedded tissues. Effects of fixative and fixation time. Am J Clin Pathol. 1991 Feb;95(2):117–124. doi: 10.1093/ajcp/95.2.117. [DOI] [PubMed] [Google Scholar]
- Hagemoser W. A., Roth J. A., Lofstedt J., Fagerland J. A. Granulocytopathy in a Holstein heifer. J Am Vet Med Assoc. 1983 Nov 15;183(10):1093–1094. [PubMed] [Google Scholar]
- Hoeschele I., Meinert T. R. Association of genetic defects with yield and type traits: the weaver locus effect on yield. J Dairy Sci. 1990 Sep;73(9):2503–2515. doi: 10.3168/jds.S0022-0302(90)78936-9. [DOI] [PubMed] [Google Scholar]
- Kehrli M. E., Jr, Ackermann M. R., Shuster D. E., van der Maaten M. J., Schmalstieg F. C., Anderson D. C., Hughes B. J. Bovine leukocyte adhesion deficiency. Beta 2 integrin deficiency in young Holstein cattle. Am J Pathol. 1992 Jun;140(6):1489–1492. [PMC free article] [PubMed] [Google Scholar]
- Kehrli M. E., Jr, Schmalstieg F. C., Anderson D. C., Van der Maaten M. J., Hughes B. J., Ackermann M. R., Wilhelmsen C. L., Brown G. B., Stevens M. G., Whetstone C. A. Molecular definition of the bovine granulocytopathy syndrome: identification of deficiency of the Mac-1 (CD11b/CD18) glycoprotein. Am J Vet Res. 1990 Nov;51(11):1826–1836. [PubMed] [Google Scholar]
- Kishimoto T. K., Hollander N., Roberts T. M., Anderson D. C., Springer T. A. Heterogeneous mutations in the beta subunit common to the LFA-1, Mac-1, and p150,95 glycoproteins cause leukocyte adhesion deficiency. Cell. 1987 Jul 17;50(2):193–202. doi: 10.1016/0092-8674(87)90215-7. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. K., Larson R. S., Corbi A. L., Dustin M. L., Staunton D. E., Springer T. A. The leukocyte integrins. Adv Immunol. 1989;46:149–182. doi: 10.1016/s0065-2776(08)60653-7. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. K., O'Conner K., Springer T. A. Leukocyte adhesion deficiency. Aberrant splicing of a conserved integrin sequence causes a moderate deficiency phenotype. J Biol Chem. 1989 Feb 25;264(6):3588–3595. [PubMed] [Google Scholar]
- Kishimoto T. K., O'Connor K., Lee A., Roberts T. M., Springer T. A. Cloning of the beta subunit of the leukocyte adhesion proteins: homology to an extracellular matrix receptor defines a novel supergene family. Cell. 1987 Feb 27;48(4):681–690. doi: 10.1016/0092-8674(87)90246-7. [DOI] [PubMed] [Google Scholar]
- Nagahata H., Noda H., Takahashi K., Kurosawa T., Sonoda M. Bovine granulocytopathy syndrome: neutrophil dysfunction in Holstein Friesian calves. Zentralbl Veterinarmed A. 1987 Jun;34(6):445–451. doi: 10.1111/j.1439-0442.1987.tb00303.x. [DOI] [PubMed] [Google Scholar]
- Powell R. L., Wiggans G. R. Animal model evaluations for Mexican Holsteins. J Dairy Sci. 1991 Apr;74(4):1420–1427. doi: 10.3168/jds.S0022-0302(91)78298-2. [DOI] [PubMed] [Google Scholar]
- Robinson J. L., Dombrowski D. B., Harpestad G. W., Shanks R. D. Detection and prevalence of UMP synthase deficiency among dairy cattle. J Hered. 1984 Jul-Aug;75(4):277–280. doi: 10.1093/oxfordjournals.jhered.a109932. [DOI] [PubMed] [Google Scholar]
- Shuster D. E., Bosworth B. T., Kehrli M. E., Jr Sequence of the bovine CD18-encoding cDNA: comparison with the human and murine glycoproteins. Gene. 1992 May 15;114(2):267–271. doi: 10.1016/0378-1119(92)90586-e. [DOI] [PubMed] [Google Scholar]
- Sligh J. E., Jr, Hurwitz M. Y., Zhu C. M., Anderson D. C., Beaudet A. L. An initiation codon mutation in CD18 in association with the moderate phenotype of leukocyte adhesion deficiency. J Biol Chem. 1992 Jan 15;267(2):714–718. [PubMed] [Google Scholar]
- Stöber M., Kuczka A., Pohlenz J. Bovine Leukozyten-Adhäsions-Defizienz (BLAD = Hagemoser-Takahashi-Syndrom): Klinische, pathologisch-anatomische und -histologische Befunde. Dtsch Tierarztl Wochenschr. 1991 Dec;98(12):443–448. [PubMed] [Google Scholar]
- Takahashi K., Miyagawa K., Abe S., Kurosawa T., Sonoda M., Nakade T., Nagahata H., Noda H., Chihaya Y., Isogai E. Bovine granulocytopathy syndrome of Holstein-Friesian calves and heifers. Nihon Juigaku Zasshi. 1987 Aug;49(4):733–736. doi: 10.1292/jvms1939.49.733. [DOI] [PubMed] [Google Scholar]
- Wardlaw A. J., Hibbs M. L., Stacker S. A., Springer T. A. Distinct mutations in two patients with leukocyte adhesion deficiency and their functional correlates. J Exp Med. 1990 Jul 1;172(1):335–345. doi: 10.1084/jem.172.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. W., O'Brien W. E., Beaudet A. L. Nucleotide sequence of the cDNA from the mouse leukocyte adhesion protein CD18. Nucleic Acids Res. 1989 Jul 11;17(13):5397–5397. doi: 10.1093/nar/17.13.5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young C. W., Bonczek R. R., Johnson D. G. Inbreeding of and relationship among registered Holsteins. J Dairy Sci. 1988 Jun;71(6):1659–1666. doi: 10.3168/jds.S0022-0302(88)79730-1. [DOI] [PubMed] [Google Scholar]
- von Andrian U. H., Chambers J. D., McEvoy L. M., Bargatze R. F., Arfors K. E., Butcher E. C. Two-step model of leukocyte-endothelial cell interaction in inflammation: distinct roles for LECAM-1 and the leukocyte beta 2 integrins in vivo. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7538–7542. doi: 10.1073/pnas.88.17.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]