Abstract
Key points
Recent studies have shown that high salt (HS) intake leads to endothelial dysfunction and impaired vascular reactivity in different vascular beds in both animal and human models, due to increased oxidative stress.
The objective of this study was to assess vascular response to flow‐induced dilatation (FID) and to elucidate the role of vascular oxidative stress/antioxidative capacity in middle cerebral arteries (MCAs) of HS‐fed rats in vitro.
The novelty of this study is in demonstrating impaired flow‐induced dilatation of MCAs and down‐regulation of vascular antioxidant genes with HS intake, leading to increased levels of oxidative stress in blood vessels and peripheral lymph organs, which together contribute to impaired FID.
In addition, results show increased oxidative stress in leukocytes of peripheral lymph organs, suggesting the occurrence of inflammatory processes due to HS intake.
Recirculation of leukocytes might additionally increase vascular oxidative stress in vivo.
Abstract
The aim of this study was to determine flow‐induced dilatation (FID) and the role of oxidative stress/antioxidative capacity in isolated, pressurized middle cerebral arteries (MCAs) of high salt (HS)‐fed rats. Healthy male Sprague‐Dawley rats (11 weeks old) were fed low salt (0.4% NaCl; LS group) or high salt (4% NaCl; HS group) diets for 1 week. Reactivity of MCAs in response to stepwise increases in pressure gradient (Δ10–Δ100 mmHg) was determined in the absence or presence of the superoxide dismutase (SOD) mimetic TEMPOL and/or the nitric oxide synthases (NOS) inhibitor N ω‐nitro‐l‐arginine methyl ester (l‐name). mRNA levels of antioxidative enzymes, NAPDH‐oxidase components, inducible (iNOS) and endothelial nitric oxide synthases (eNOS) were determined by quantitative real‐time PCR. Blood pressure (BP), antioxidant enzymes activity, oxidative stress in peripheral leukocytes, lipid peroxidation products and the antioxidant capacity of plasma were measured for both groups. FID was reduced in the HS group compared to the LS group. The presence of TEMPOL restored dilatation in the HS group, with no effect in the LS group. Expression of glutathione peroxidase 4 (GPx4) and iNOS in the HS group was significantly decreased; oxidative stress was significantly higher in the HS group compared to the LS group. HS intake significantly induced basal reactive oxygen species production in the leukocytes of mesenteric lymph nodes and splenocytes, and intracellular production after stimulation in peripheral lymph nodes. Antioxidant enzyme activity and BP were not affected by HS diet. Low GPx4 expression, increased superoxide production in leukocytes, and decreased iNOS expression are likely to underlie increased oxidative stress and reduced nitric oxide bioavailability, leading to impairment of FID in the HS group without changes in BP values.
Key points
Recent studies have shown that high salt (HS) intake leads to endothelial dysfunction and impaired vascular reactivity in different vascular beds in both animal and human models, due to increased oxidative stress.
The objective of this study was to assess vascular response to flow‐induced dilatation (FID) and to elucidate the role of vascular oxidative stress/antioxidative capacity in middle cerebral arteries (MCAs) of HS‐fed rats in vitro.
The novelty of this study is in demonstrating impaired flow‐induced dilatation of MCAs and down‐regulation of vascular antioxidant genes with HS intake, leading to increased levels of oxidative stress in blood vessels and peripheral lymph organs, which together contribute to impaired FID.
In addition, results show increased oxidative stress in leukocytes of peripheral lymph organs, suggesting the occurrence of inflammatory processes due to HS intake.
Recirculation of leukocytes might additionally increase vascular oxidative stress in vivo.
Abbreviations
- ACh
acetylcholine
- ANG II
angiotensin II
- BP
blood pressure
- CAT
catalase
- DCF
dichlorofluorescein
- DCF‐DA
2′,7′‐dichlorodihydrofluorescein diacetate
- EDCF
endothelium‐derived contracting factor
- EDHF
endothelium derived hyperpolarizing factor
- EDRF
endothelium‐derived relaxing factor
- eNOS
endothelial nitric oxide synthase
- ET‐1
endothelin
- Fe³+‐TPTZ
2,4,6‐Tris (2‐pyridyl)‐s‐triazine)
- FID
flow‐induced dilatation
- FRAP
ferric reducing ability of plasma assay
- FSC‐A
forward scatter area
- FSC‐H
forward scatter height
- GPx
glutathione peroxidase
- GPx1
glutathione peroxidase 1
- GPx4
glutathione peroxidase 4
- HO·
hydroxyl radical
- HPRT
hypoxanthine‐guanine phosphoribosytransferase
- HS
high salt
- iNOS
inducible nitric oxide synthase
- L
leukocyte
- l‐name
N ω‐nitro‐l‐arginine methyl ester
- LS
low salt
- MAP
mean arterial pressure
- MCA
middle cerebral artery
- MDA
malondialdehyde
- mLN
mesenteric lymph nodes
- NADPH
nicotinamide adenine dinucleotide phosphate
- NO
nitric oxide
- NOS
nitric oxide synthases
- O2−
superoxide
- ONOO−
peroxynitrite
- PGF2α
prostaglandin F2α
- PGI2
prostacyclin
- PMA
phorbol 12‐myristate 13‐acetate
- PSS
physiological salt solution
- r
vessel radius
- RBC
red blood cell
- ROS
reactive oxygen species
- rtPCR
real time polymerase chain reaction
- SD rats
Sprague‐Dawley rats
- SNP
sodium nitroprusside
- SOD
superoxide dismutase
- SSC‐A
side scatter area
- TBA
thiobarbituric acid
- TCA
trichloroacetic acid
- TBARS
thiobarbituric acid reactive substances
- TXA2
thromboxane A2
- VSMC
vascular smooth muscle cell
Introduction
As many epidemiological studies have demonstrated, stroke is the main cause of death among cerebrovascular diseases in modern Western society and high salt intake is closely linked to its occurrence (Jelakovic et al. 2009 a,b; Drenjancevic‐Peric et al. 2011). Impaired function of cerebral blood vessels, particularly resistance vessels, significantly participates in the pathophysiological mechanisms of stroke. Unfortunately, the potential impact of high salt dietary intake on cerebral resistance vessel function and its underlying mechanisms are still not clear. To date, numerous studies on experimental animals have shown that acute changes in salt intake significantly alter vascular reactivity to different physiological stimuli in different vascular beds (Drenjancevic‐Peric et al. 2003; Drenjancevic‐Peric & Lombard, 2005; Durand & Lombard, 2013), even in normotensive animals. Moreover, some of the deleterious effects of high salt (HS) diet that are independent of elevated BP also occur in normotensive individuals (Weinberger 2002; Cavka et al. 2015), and are associated with impaired endothelial function (Drenjancevic‐Peric et al. 2011).
Vasodilatation in response to flow is endothelium dependent and is an important physiological regulator of tissue perfusion. The mediator of FID is dependent on vessel and species, but typically involves the release of nitric oxide (NO), prostaglandins (PGI2) and endothelium‐derived hyperpolarizing factor (EDHF) (Matoba et al. 2002; Larsen et al. 2007; Liu et al. 2011). Although FID is one of the most important physiological regulatory mechanisms of tissue perfusion, data on the effect of HS diet on FID of resistance vessels, particularly in cerebral circulation, are still not understood.
Numerous studies in experimental animals have demonstrated that HS‐induced endothelial dysfunction is associated with increased oxidative stress (Lenda et al. 2000; Verma & Anderson, 2002; Drenjancevic‐Peric & Lombard, 2005; Durand & Lombard, 2013). HS diet increases generation of reactive oxygen species and increases vascular oxidative stress by decreasing the vascular antioxidative capacity of MCAs (Drenjancevic‐Peric & Lombard, 2005). It has been shown that the decrease in superoxide (SOD) expression in spinotrapezius muscle arterioles of HS‐fed rats is responsible for impaired acetylcholine (ACh)‐induced relaxation (Lenda et al. 2000). Durand et al. (2013) demonstrated that 3–5 days of HS intake significantly reduced protein levels of SOD isoforms (Cu/Zn SOD and Mn SOD) in cerebral arteries compared to an LS group, and that the vasodilatation of blood vessels was restored after the introduction of angiotensin II (ANG II) infusion in the same group (Durand & Lombard, 2013). Similarly, Drenjancevic‐Peric et al. (2003) reported that low levels of ANG II in consomic rats (SS.BN13) on HS diet led to impaired relaxation of MCAs in response to hypoxia and ACh, due to increased oxidative stress, and could be restored by addition of TEMPOL, a superoxide scavenger, in a tissue bath in vitro (Drenjancevic‐Peric & Lombard, 2005). SS.BN13 consomic rats are animals in which recovery of normal renin control mechanisms is achieved by substitution of chromosome 13 from the normotensive Brown Norway rat onto the Dahl S genetic background (Dahl S rats exhibit low plasma renin activity (PRA) and impaired response to vasodilator stimuli). SS.BN13 consomic rats thus have normal renin control mechanisms, which prevent the salt‐induced hypertension that occurs in the Dahl S rats (Drenjancevic‐Peric et al. 2003).
In summary, increased oxidative stress underlies the impaired endothelium‐dependent vascular relaxation responses in HS diet animal models, which mostly affect NO production or bioavailability (Verma & Anderson, 2002). However, data on the effect of HS diet on FID of resistance vessels, particularly in cerebral circulation, and the role of vascular antioxidative defense mechanisms, and the potential interaction of circulating leukocytes, are still scarce and understudied.
Thus, the aim of present study was to determine how acute, short‐term HS intake affects FID of MCAs in in vitro conditions, and if increased oxidative stress contributes to observed changes in FID in cerebral resistance vessels in otherwise healthy Sprague‐Dawley (SD) rats.
Methods
Ethical approval
All experimental procedures are conformed to the European Guidelines for the Care and Use of Laboratory Animals (directive 86/609). They were approved by the local Ethical Committee (Faculty of Medicine, University of Osijek) and Ministry of Agriculture, Croatia (HR‐POK‐005). All measurements were performed in the Laboratory for Vascular Physiology, Laboratory for Molecular and Clinical Immunology at the Department of Physiology and Immunology, Faculty of Medicine, and at the Laboratory for Biochemistry, Department of Biology, J. J. Strossmayer University of Osijek, Croatia and conform to the principles of UK regulations (Drummond, 2009).
Diet protocol
Healthy male SD rats 11 weeks old were housed in shoebox style cages, maintained on a 12:12 h light:dark cycle. Rats were divided into group of animals: an LS group fed with food containing 0.4% NaCl and an HS group fed with food containing 4% NaCl (Mucedola, Italy) with free access to food and tap water for 7 days.
Arterial blood pressure measurements
Rats were anaesthetized with a combination of ketamine (75 mg kg−1) and midazolam (2.5 mg kg−1). A PE‐50 catheter was inserted into the left femoral artery and mean arterial blood pressure was measured with a Spacelabs Medical monitoring system (Spacelabs Medical, Inc., Redmond, WA, USA). Animals were left for 10 min for blood pressure to stabilize and after that the blood pressure was recorded for 1 min and the mean value was calculated from obtained values (Kibel et al. 2015).
Preparation of isolated middle cerebral arteries
Prior to decapitation with a guillotine, rats were weighed and anesthetized with ketamine (75 mg kg−1) and midazolam (2.5 mg kg−1).
MCAs from the brain were isolated under a dissecting microscope and cannulated with a glass micropipette (diameter 100–200 μm) in a chamber filled with physiological salt solution (PSS) warmed to 37°C and oxygenated with 21% O2, 5% CO2 with the balance N2. The PSS (10−3 mol l−1) used in these experiments contained (in mmol l−1): 119 NaCl, 4.7 KCl, 1.17 MgSO4, 1.6 CaCl2, 1.18 NaH2PO4, 24 NaHCO3, 0.026 EDTA, and 5.5 glucose.
At the beginning of the experiments, arteries were pressurized at 80 mmHg to assess basal diameter. The resistances (i.e. tip sizes) of the micropipettes were matched to each other and to the diameter of the vessel to achieve equal inflow and outflow resistance (Toth et al. 2011). Inflow and outflow pressures were measured with a pressure transducer in an automated manner by the DMT pressure myograph (DMT 110P pressure myograph).
After equilibration for 60 min, vessels were subjected to flow, which was produced by simultaneous changes of inflow and outflow pressures (pressure gradients of Δ10, Δ20, Δ40, Δ60 and Δ100 mmHg) in opposite directions in an automated experimental setup. To assess the role of vascular reactive oxygen species (ROS) in vitro, FID was measured in the presence of the superoxide dismutase mimetic TEMPOL (100 μmol l−1) in the bath chamber. The role of NO in FID was determined by addition of the nitric oxide synthase (NOS) inhibitor l‐NAME (10−5 mol l−1). The NO donor sodium nitroprusside (SNP; 10−6 mol l−1) was used to test the endothelium‐independent response and acetylcholine (ACh; 10−6 mol l−1) was used to assess the endothelium‐dependent vasodilatation. The responses to ACh and SNP were assessed at 80 mmHg in pressurized MCAs (no‐flow condition). At the end of the protocol, vessels were perfused with Ca2+‐free PSS solution (maximal diameter) and vessels which did not exhibit significant levels of active tone (around 50%) were eliminated from the study.
Active tone (%) was calculated as ((D max − D bas)/D max) × 100, where D max and D bas are the maximum and baseline diameters (Δ0 mmHg, without flow) of the vessel (Table 1). Vessel diameters were recorded with infrared camera and the image was displayed on the monitor. Flow rates were measured with the DMT Flowmeter – 162FM and changes in diameter of blood vessels with the software Myograph Pressure System Model 110P MyoView Version 1.2.0 DMT (Danish Myo Technology).
Table 1.
Measurements of body mass, mean arterial pressure, basal diameter of MCAs (without flow), determination of maximal MCA diameter, active tone of vessels and indicators of oxidative stress (TBARS and FRAP)
| Diameter of MCAs at | Max. dilatation | |||||||
|---|---|---|---|---|---|---|---|---|
| Experimental | Age | Body | Mean arterial | Δ0 mmHg (without | of MCAs (Ca2+ | Active | TBARS | FRAP |
| group | (weeks) | mass (g) | pressure (mmHg) | flow) (μm) | free) (μm) | tone (%) | (μmol MDA) | (mmol Trolox) |
| LS | 11 | 349.5 ± 33.96 | 113.39 ± 5.83 | 115.20 ± 22.34 | 223.40 ± 40.06 | 48.35 ± 2.18 | 0.367 ± 0.04 | 0.202 ± 0.01 |
| HS | 11 | 369.8 ± 26.83* | 108.36 ± 9.03 | 115.30 ± 24.29 | 225.50 ± 15.37 | 48.28 ± 9.53 | 0.469 ± 0.07* | 0.213 ± 0.03 |
| P value | 0.009 | 0.324 | 0.791 | 0.545 | 0.427 | 0.013 | 0.441 | — |
Data are presented as means ± SD. * P < 0.05 LS vs. HS diet; LS, low salt; HS, high salt.
Spectrophotometric antioxidant enzyme activities assay
Preparation of tissue extracts
Fresh samples of cerebral vessels were weighed and crushed with liquid nitrogen. Buffer (100 mmol l−1 NaP + 1 mmol l−1 EDTA, pH 7.0) was then added in proportion to the quantity of weighed tissue (1 ml buffer per 100 mg tissue) and then additionally homogenized with Ultra Turrax T10 homogenizer (1300 rpm; IKA, Königswinter, Germany). Crude tissue homogenates were sonicated for 30 s, then centrifuged at 20,000 g for 15 min at 4°C and stored at −80°C until assayed.
Antioxidant enzyme activities assay
Catalase (CAT) activity was measured according to the protocol of Aebi (1984), using 0.036% hydrogen peroxide (H2O2) as a substrate in the reaction mixture with 50 mmol l−1 phosphate buffer pH (7.0). Changes in absorbance in the reaction mixture were measured at 240 nm over 2 min every 10 s after adding the sample. One unit of activity corresponds to the loss of 1 μmol of H2O2 per minute.
Glutathione peroxidase (GPx) activity (Wendel, 1981) was determined indirectly by measuring the rate of nicotinamide adenine dinucleotide phosphate (NADPH) oxidation to NADP+, accompanied by a decrease in absorbance at 340 nm over 5 min. In that assay, one unit of GPx activity catalyzes the oxidation by H2O2 of 1.0 μmol min−1 of reduced glutathione to the oxidized form at pH 7.0 and 25°C.
Total SOD activity in the supernatant was determined by inhibition of reduction of cytochrome C in the of the xanthine–xanthine oxidase system. Activity was measured according to a modification of the method described by Flohé (Flohé & Ötting, 1984).
Measured activities of all investigated enzymes were expressed as U (mg protein)−1. Enzyme activity assays were performed using a Lambda 25UV‐Vis spectrophotometer equipped with UV WinLab 6.0 software package (PerkinElmer For the Better, Waltham, MA, USA).
Determination of protein concentration
The concentration of proteins in samples (mg ml−1) was determined using Bradford reagent at 595 nm (Bradford Reagent B6916, Sigma Aldrich), following the manufacturer's protocol, using bovine serum albumin as a standard.
mRNA expression studies
In a separate group of experiments, all surface cerebral blood vessels were isolated and collected for the determination of gene expression using quantitative PCR in real time (rtPCR). Brain blood vessels were stored in RNAlater Solution (Applied Biosystems, USA), in a ratio 1:5 (weight (g) of the sample : 5× greater volume (ml) of RNAlater Solution). RNAlater Solution is a liquid, non‐toxic reagent for storing tissue that currently penetrates into the tissue and reduces the need for fast processing and freezing of samples, and inactivates the RNase. Until further processing, the samples were stored at −80°C. Homogenization of samples and total RNA was extracted using TRI reagent (Life Technologies, USA) according to the protocol of Chomczynski et al. (1987). RNA concentration and purity was assessed using Nanophotometer P300 UV/VIS, IMPLEN and RNA integrity was checked on 1% agarose gel. Samples were purified and cDNA obtained according to the manufacturer's instructions: Sigma‐Aldrich (Germany) and Applied Biosystems.
Synthetized cDNA was diluted 5× in Nuclease free water (Sigma Aldrich) and used for real‐time PCR. The expression of the following genes was determined: SOD isoforms (Cu/Zn SOD, Mn SOD, EC SOD), glutathione peroxidase (GPx) 1 and 4 (GPx1 and GPx4), CAT, iNOS, endothelial nitric oxide synthase (eNOS) and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase components (p47phox and gp91phox). Gene expression was normalized to the expression of the housekeeping gene hypoxanthine‐guanine phosphoribosytransferase (HPRT). Gene expression was performed on BioRad CFX96.
Oxidative stress in leukocytes isolated from blood and peripheral lymphoid organs measured by flow cytometry
2′,7′‐Dichlorodihydrofluorescein diacetate (DCF‐DA) is a commonly used cell‐permeable probe for detecting intracellular H2O2 and oxidative stress. It is retained in the cell after intracellular hydrolysis to the DCFH carboxylate anion. Two‐electron oxidation of DCFH results in the formation of a fluorescent product, dichlorofluorescein (DCF), which can be monitored by flow cytometry (Hempel et al. 1999; Bonini et al. 2006; Kalyanaraman et al. 2012). Several one‐electron‐oxidizing species will oxidize DCFH, including hydroxyl radicals, compounds I and II formed from peroxidase or haeme interaction with H2O2, ·NO2 formed from the myeloperoxidase–H2O2–NO2− system, hypochlorous acid, and reactive species formed from peroxynitrite decomposition (Kalyanaraman et al. 2012).
Samples of peripheral blood were collected immediately after the decapitation of animals in tubes containing 6–10% 0.5 m EDTA. Spleen, colon, mesenteric lymph nodes and inguinal, axillary and brachial peripheral lymph nodes were isolated using the forceps and dissecting microscope. Tissues were homogenized after isolation in a phosphate buffer (PBS, pH 7.2–7.4) by tearing apart the organs between the frosted ends of two microscopic slides and filtered through cotton wool. The obtained cell suspension was washed in 1× PBS (1 × 5 min centrifugation at 400g, +4°C) and resuspended in an appropriate volume of fresh PBS. Aliquots of 106 cells from the blood or peripheral lymphoid organs were incubated with 5 mmol l−1 DCF‐DA (Biomol, ThG, Germany) for 30 min at 4°C in the dark, and analysed on a flow cytometer (FACS Canto II, BD). After that, the cells were stimulated with 100 nmol l−1 phorbol 12‐myristate 13‐acetate (PMA, Calbiochem, Germany) and analysed for the second time. At each measurement a minimum of 10,000 target cells was analysed. The excitation wavelength was 488 nm supplied by a solid state, 20 mW laser output and the green fluorescence (DCFH) was evaluated using 530/30 bandpass filter. Viability of the cells was assessed by 7‐amino‐actinomycin D (Applichem, Germany). Data were analysed using Flowing software (Turku Centre for Biotechnology, Finland).
Oxidative stress measurements (TBARS and FRAP)
Arterial blood samples were collected from femoral artery, centrifugated at 3500 rpm for 10 min and serum samples were stored at −80°C until use. As a direct indicator of oxidative stress, the spectrophotometric Thiobarbituric Acid Reactive Substances (TBARS) method was used for measuring the products of lipid peroxidation with malondialdehyde (MDA) as standard (μmol l−1 MDA). The products bind to a thiobarbituric acid (TBA) at low pH. Since the method is non‐specific because the other substances bind to a TBA (including proteins), trichloroacetic acid (TCA) is first added to the sample to precipitate the proteins and after that the supernatant was used for the measurements (Oakes & Van Der Kraak, 2003). The absorbance of the sample was measured at 572 and 532 nm.
Antioxidant capacity was assessed using the ferric reducing ability of plasma assay (FRAP). Fe³+‐TPTZ (2,4,6‐tris(2‐pyridyl)‐s‐triazine) is reduced to Fe²+‐TPTZ in the presence of antioxidants and blue discoloration occurs. The absorbance of the sample was measured at 593 nm (Nanophotometer P300 UV/VIS, IMPLEN), using Trolox as a standard (mmol Trolox) (Benzie & Strain, 1996). The chemicals used to determine the oxidative stress were thiobarbituric acid (TBA; Sigma‐Aldrich, DE, USA), trichloroacetic acid (TCA; Panreac, Europe) and 1,1,3,3‐tetramethoxypropane (TMP; Sigma‐Aldrich) and for FRAP analysis Trolox (Sigma‐Aldrich), 2,4,6‐tris(2‐pyridyl)‐s‐triazine (TPTZ; Sigma‐Aldrich), iron(III) chloride hexahydrate (FeCl3.6H2O; Sigma‐Aldrich) and sodium acetate trihydrate (Kemika, Croatia).
Statistical analysis
Results are presented as means ± SD. The level of significance was determined at P < 0.05. Percentage of dilatation (%) was calculated by dividing values measured after each pressure gradient by baseline values (no flow condition, Δ0 mmHg) and delta diameter (μm) by subtracting baseline diameter (Δ0 mmHg) from the diameter at each level of flow. To test differences among groups two‐way ANOVA test was used (GraphPad Prism 5). Differences in normally distributed numerical variables between the two groups were tested with Student's t test, and in the case of deviations from the normal distribution with the Mann‐Whitney U test (SigmaPlot version 11.2, Systat Software, Inc., Chicago, USA).
Results
General information and blood pressure of studied groups
The body weight (g) of the animals fed with salt was significantly higher compared to the LS group. There was no statistically significant difference in mean arterial pressure (MAP, mmHg) between the groups (Table 1).
Endothelium‐dependent and endothelium‐independent vasodilator responses and mechanisms of FID
There were no significant differences in maximal dilatation of MCAs, baseline diameter of MCAs at Δ0 (without flow) or active tone between the groups (Table 1).
The values of the flow rates were 3.17 ± 0.38–19.77 ± 0.21 μl min−1 for LS and 2.83 ± 0.72–21.23 ± 1.68 μl min−1 for HS at pressure gradients from Δ10–Δ100 mmHg (not significantly different between groups, P > 0.05).
Isolated MCA dilated significantly in response to all pressure gradients in both experimental groups of rats, with the highest dilatation occurring at a pressure gradient of Δ40 mmHg. FID was reduced in the HS group compared to the LS group at each pressure gradient (two‐way ANOVA test), significantly at Δ40, Δ60 and Δ100 mmHg (P < 0.05) (Fig. 1 A and B).
Figure 1. Effect of increasing pressure gradient on control dilatation .
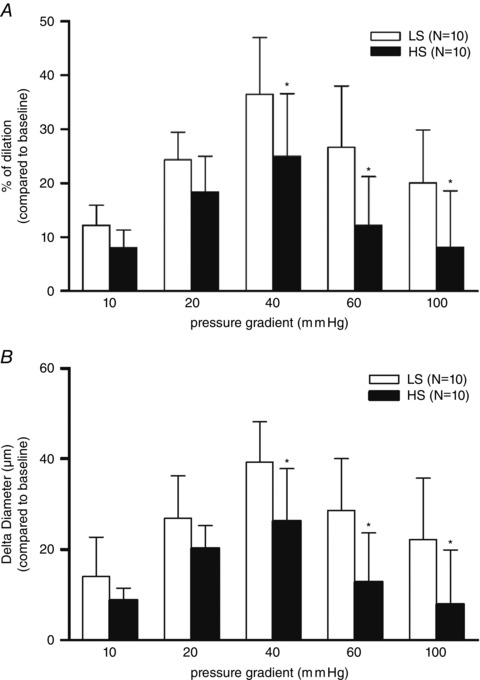
The differences in control dilatation (without inhibitors) expressed as percentage of dilatation (%; A) compared to baseline (no flow condition, Δ0 mmHg) and Delta diameter (μm; B) compared to baseline (Δ0 mmHg) of middle cerebral arteries between LS (n = 10) and HS (n = 10) groups of animals in response to stepwise increases in pressure gradient (Δ10–Δ100 mmHg). Data are presented as means ± SD. *Significant difference (P < 0.05) between LS and HS groups.
The presence of TEMPOL in vitro enhanced FID in the HS group compared to its control level (without inhibitors, measured at a different pressure gradient (Fig. 2 C and D)), while in the LS group TEMPOL had no effect on FID (Fig. 2 A and B). The addition of the NOS inhibitor l‐NAME, after TEMPOL in the HS group, returned FID again to control levels (Fig. 2 C and D).
Figure 2. Effect of TEMPOL in vitro on FID of middle cerebral arteries .
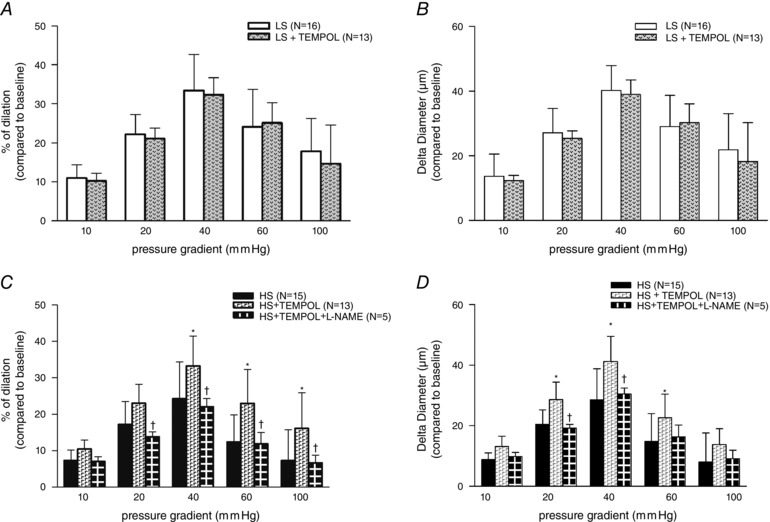
Effect of TEMPOL in vitro on FID of MCAs in LS (A and B) and HS (C and D) groups. Subsequent addition of l‐NAME with TEMPOL confirmed the increased oxidative stress in the HS group. The results are presented as percentage of dilatation (%; A and C) compared to baseline (Δ0 mmHg) and Delta Diameter (μm; B and D) compared to baseline (Δ0 mmHg). Data are presented as means ± SD. *Significant difference (P < 0.05) between HS group vs. HS group + TEMPOL (in vitro); †Significant difference (P < 0.05) between HS group + TEMPOL (in vitro) vs. HS group + TEMPOL and l‐NAME (in vitro).
Dilatation of MCA in response to endothelium‐dependent stimulus, acetylcholine (ACh; 10−6 mol l−1) was significantly reduced in the HS group compared to the LS group (LS 28.39 ± 10.31 vs. HS 16.96 ± 4.53; P = 0.008; Fig. 3 A).
Figure 3. Dilatation of MCAs in response to ACh and SNP .
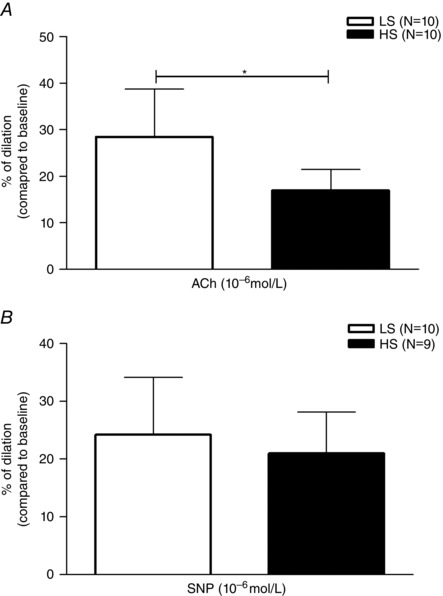
A, acetylcholine (10−6 mol l−1)‐induced vasodilatation in LS (n = 10) and HS (n = 10) groups of animals. Acetylcholine‐mediated dilatation was reduced in the HS group of animals compared to the LS‐fed rats. B, response of MCAs to an endothelium‐independent, direct NO donor, sodium nitroprusside (SNP; 10−6 mol l−1). Vasodilatation in response to SNP was preserved at both study groups, and there was no significant difference in the response between the groups. Data are presented as means ± SD. *Significant difference (P < 0.05) between LS and HS groups.
Figure 3 B presents vascular responses to the endothelium‐independent NO donor sodium‐nitroprusside (SNP, 10−6 mol l−1). In both groups vasodilatation in response to SNP was preserved and there were no differences between groups (LS 24.27 ± 9.88 vs. HS 21.07 ± 6.69; P = 0.472).
Antioxidant enzyme (SOD, GPx and CAT) activity in MCAs
The activity of antioxidant enzymes CAT, GPx and SOD did not change after 7 days of HS intake compared to the LS group (Table 2).
Table 2.
Antioxidant enzyme activities
| Experimental group | N | CAT U (mg P)−1 | GPx U (mg P)−1 | SOD U (mg P)−1 |
|---|---|---|---|---|
| LS | 5 | 17.64 ± 7.12 | 0.20 ± 0.02 | 17.25 ± 6.21 |
| HS | 4–5 | 24.68 ± 8.17 | 0.21 ± 0.04 | 19.31 ± 4.25 |
| P value | 0.231 | 0.609 | 0.592 | — |
Data are presented as means ± SD. LS, low salt; HS, high salt; P, protein.
rtPCR gene expression in brain blood vessels
One week of HS resulted in significantly decreased mRNA levels of GPx4, while expression of other antioxidant enzyme genes (SOD isoforms (Cu/Zn SOD, Mn SOD and EC SOD), CAT, PGx1, p47phox and gp91phox) were not significantly changed compared to the LS diet group (Table 3). Gene expression of iNOS was significantly decreased after 1 week of HS, while expression of eNOS tended to decrease but without reaching statistical significance.
Table 3.
Relative expression of genes for superoxide dismutase isoforms, catalase, glutathione peroxidase 1 and 4, endothelial and inducible nitric oxide synthases, and NADPH oxidase components in brain blood vessels
| Experimental | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| group | Cu/Zn SOD | Mn SOD | EC SOD | CAT | GPx1 | GPx4 | eNOS | iNOS | p47phox | gp91phox |
| LS | 1.09 ± 0.18 | 0.9 ± 0.19 | 0.60 ± 0.20 | 0.87 ± 0.11 | 1.23 ± 0.38 | 0.82 ± 0.17 | 0.589 ± 0.26 | 2.56 ± 1.49 | 0.675 ± 0.28 | 0.72 ± 0.25 |
| HS | 0.97 ± 0.14 | 0.79 ± 0.16 | 0.51 ± 0.11 | 0.74 ± 0.19 | 0.92 ± 0.22 | 0.64 ± 0.06* | 0.43 ± 0.45 | 0.47 ± 0.33* | 0.74 ± 0.33 | 0.54 ± 0.25 |
| P value | 0.226 | 0.138 | 0.295 | 0.181 | 0.096 | 0.041 | 0.516 | 0.004 | 0.657 | 0.178 |
Superoxide dismutase isoforms: Cu/Zn SOD, Mn SOD and EC SOD; catalase: CAT; glutathione peroxidase 1 and 4: GPx1, GPx4; endothelial nitric oxide synthase: eNOS; inducible nitric oxide synthase: iNOS; NADPH oxidase components: p47phox and gp91phox. Data are presented as means ± SD. * P < 0.05 LS vs. HS diet; LS, low salt; HS, high salt.
Oxidative stress in leukocytes
Reactive oxygen species (ROS) production was determined at basal and stimulated conditions in leukocytes, as presented in the Methods section.
As an example, Fig. 4 A represents an analysis of the oxidative stress level measured using flow cytometry with the dichlorofluorescein diacetate (DCFH‐DA) probe and lymphocytes isolated from mesenteric lymph nodes. After exclusion of doublets by plotting forward scatter area and height (FSC‐A vs. FSC‐H), single live cells (gate R‐1) were further analysed for DCF‐DA expression among different leukocyte subsets. Lymphocytes were defined by gate R‐2. DCF‐DA expression was analysed as the geometric mean of the positive signal in the FITC detector using the histograms. Unstained cells were used as negative control. Analyses were performed on lymphocytes (Fig. 4 B), monocytes (Fig. 4 C) and granulocytes (Fig. 4 D) isolated from peripheral blood, and lymphocytes isolated from peripheral lymphoid organs (Fig. 4 E–G). In general, leukocytes from peripheral blood presented with lower levels of ROS, at basal level and after PMA stimulation. HS diet significantly increased basal ROS production in mesenteric lymph nodes and splenocytes, and ROS production after PMA stimulation in peripheral lymph nodes (P < 0.05).
Figure 4. The effects of high salt diet on the level of oxidative stress in leukocytes isolated from blood and peripheral lymphoid organs .
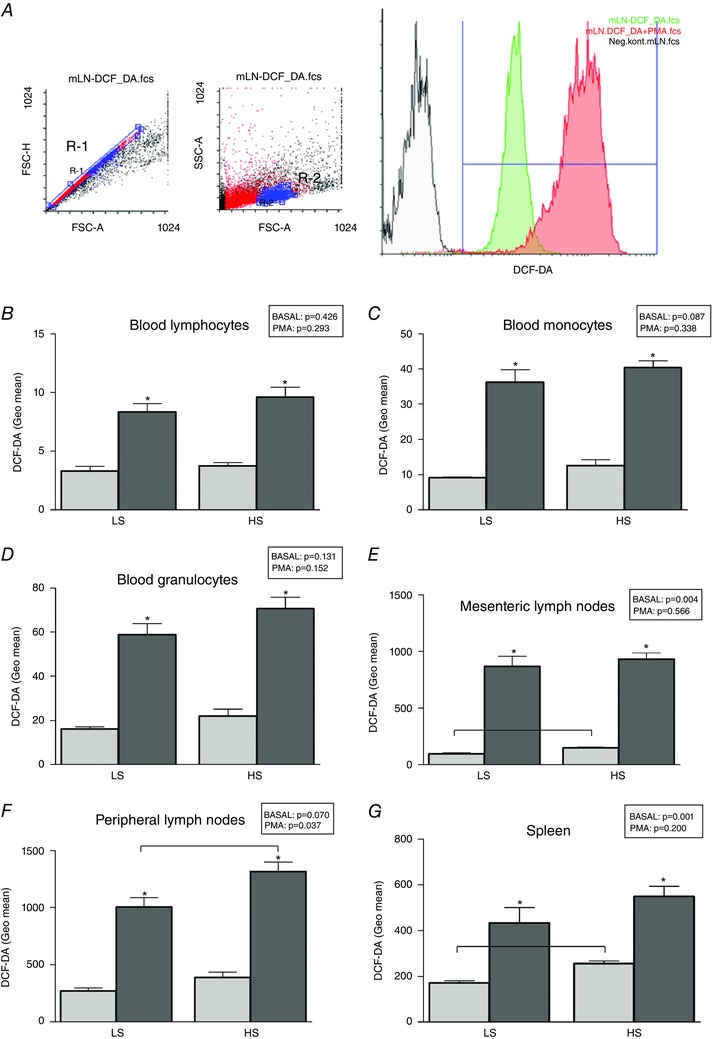
A, the gating strategy on a mesenteric lymph node sample, where positive geometric mean fluorescence was determined for gated live single cells. Unstained cells from respective tissues were used as negative controls (FSC‐A, forward scatter area; FSC‐H, forward scatter height; SSC‐A, side scatter area; mLN, mesenteric lymph nodes). Presented results are for lymphocytes (B), monocytes (C) and granulocytes (D) isolated from peripheral blood and lymphocytes isolated from peripheral lymphoid organs (E–G). Data are presented as means ± SD, P < 0.05 was considered significant. Lighter bars present level of oxidative stress before PMA stimulation (basal) and darker bars after PMA stimulation for each sample. *Significantly different compared to basal readout (within group analysis); differences between the LS and HS groups, and the P values are denoted in the panels.
Indicators of general oxidative stress in serum samples – TBARS and FRAP
Oxidative stress (TBARS) was significantly increased in the HS group compared to the LS group, while there was no sigificant difference in antioxidant capacity (FRAP values) between HS and LS groups (Table 1).
Discussion
This study is the first to demonstrate impaired FID in MCAs and to clarify the role of antioxidant defense mechanisms and the possible role of leukocytes in increased oxidative stress of rats fed HS diet for 1 week. The main findings in our study are: (a) BP levels were not affected by 7 days HS diet; (b) HS intake impaired the FID response of MCAs in vitro; (c) FID was restored by the superoxide scavenger TEMPOL, indicating that the increased level of oxidative stress leads to impaired FID; (d) the NO synthase inhibitor l‐NAME attenuated FID previously restored by the superoxide scavenger TEMPOL in the HS group, providing evidence that NO is mediating that restored response; (e) it is possible that decreased levels of NO due to decreased expression of iNOS also contribute to impaired FID response in the HS group; and (f) low expression of GPx4, in contrast to the other measured antioxidant genes, probably has the most important role of all the antioxidative enzymes examined in increasing levels of oxidative stress in MCAs, as well as increasing oxidative stress in peripheral lymphoid organs and blood lipid peroxidation products.
Earlier animal studies demonstrated that a crucial link between HS intake and the development of endothelial dysfunction is ANG II (Durand & Lombard, 2013). HS intake suppresses plasma concentration of ANG II. Low levels of ANG II are associated with an increase in oxidative stress and impairment of vascular reactivity (Drenjancevic‐Peric & Lombard, 2005). Intravenous infusion of a suppressor dose of ANG II in rats on HS diet leads to the restoration of normal vascular relaxation and a reduction in oxygen free radical concentration to normal values (McEwen et al. 2009). Numerous studies in animals and humans have already demonstrated that 3–7 days of HS intake does not increase BP (Vollmer et al. 2001; Weinberger 2002; Tzemos et al. 2008; Cavka et al. 2013, 2015), which is in agreement with present results that short‐term high salt diet increases oxidative radical production and subsequently impairs vascular reactivity independently of BP regulation (Table 1).
Vasomotor responses to increases in flow in isolated cerebral vessels have been shown to dilate (Drouin & Thorin, 2009, Drouin et al. 2011) or constrict (Madden & Christman, 1999; Bryan et al. 2001; Toth et al. 2011) the vessels, or produce a biphasic response (Garcia‐Roldan et al. 1990; Shimoda et al. 1996), depending on stimuli or conditions studied.
To our knowledge, this is the first study to examine the FID of MCAs during the acute intake of increased amounts of salt. A week of HS intake significantly reduces the FID of MCAs compared to LS intake, which once again shows how salt leads to endothelial dysfunction (Fig. 1). The smaller dilatations at the higher pressure gradient values in both groups could reflect an increasing contribution of flow‐induced constriction, as reported by Toth et al. 2011. However, our results (Fig. 1 A and B) show that at every pressure gradient MCAs dilate in response to FID, i.e. that FID is also preserved at higher pressure gradients (which is easier to see as the absolute change in diameter of the vessel at certain pressure gradients level (delta diameter, Fig. 1 B). The diameter of the vessels in response to FID at higher pressure gradients is still significantly larger than the baseline diameter (i.e. blood vessel pressurized at 80 mmHg, without flow (Δ0 mmHg). The smaller dilatation in the HS vessels is perfectly consistent with impaired vasodilatation with HS diet, probably due to reduced NO availability (Sylvester et al. 2002).
Because the difference between LS and HS vessels is similar at the higher pressure gradients, we expect that the flow‐induced constrictor response described by Toth et al. (2011) is similar in HS and LS vessels, while the vasodilator response to increased shear stress is impaired in the HS vessels, with a similar degree of flow‐induced constriction.
In in vivo conditions, parenchymal cell influences (and possibly other mechanisms) could affect the response. However, in our system, we are looking only at the response of the artery itself to increased flow velocity, which would increase shear stress on the endothelial cells. According to Koller et al. (1993), who studied isolated arterioles of the rat cremaster muscle using an experimental approach similar to the one we used in the present study, a step‐wise increase in perfusion flow elicited a gradual increase in the diameter of the vessel as a result of increased shear stress. At a constant flow rate, an increase in the viscosity of the perfusate also causes a gradual vasodilatation, which is also due to an increase in shear stress. The arteriolar dilatation maintained a calculated wall shear stress close to control values during increases in either flow or viscosity of the perfusate and both of these were endothelium dependent. In addition, those authors established that the simultaneous increases in flow and viscosity of the perfusate elicited an enhanced additive dilatation (Koller et al. 1993). Since the viscosity of the blood is 1.5× higher than the viscosity of the PSS, if we assume that the values of the flow and vessel radius (r) are constant (or equal) one may expect the shear stress would be lower by 33% with PSS, and FID would differ proportionally in magnitude in the same direction and would still be preserved. This is demonstrated in our study and the previously quoted study by Koller et al. (1993). As already mentioned, vasodilatation in response to sudden change of flow (vascular stress) is one of the main characteristics of endothelium. Endothelium also mediates vasomotor response to various stimuli, such as ACh or physical forces, by releasing various vasoactive mediators, such as endothelium‐derived relaxing factor (EDRF), NO, prostacyclin (PGI2) or endothelium‐derived contracting factors (EDCF), e.g. endothelin (ET‐1), thromboxane A2 (TXA2) and oxygen free radicals (reactive oxygen species; ROS) (Crimi et al. 2007; Sandoo et al. 2010). NO plays an important role in the maintenance of basal vascular tone (Vallance et al. 1989) and has a central role in vasodilatation (Moncada & Higgs, 2006), preventing the adhesion and migration of leukocytes into the arterial wall, and inhibits the proliferation of vascular smooth muscle cells (Ignarro, 2002). Shear force is the most important physiological factor stimulating the production of nitric oxide, by phosphorylation of endothelial NO synthase (eNOS) and tyrosine kinases, which regulate endothelial NO production through regulation of eNOS (Tran et al. 2000). Moreover, ACh, bradykinin, thrombin, adenosine diphosphate (ADP) and adenosine triphosphate (ATP) cause relaxation of vascular smooth muscle cells and also stimulate production of NO (Rubanyi et al. 1986; Ignarro, 2002; Furchgott & Zawadzki, 1980; Flammer & Lüscher, 2010). The salient finding of this study is that 1 week of HS diet impaired FID of MCAs in SD rats, and the mechanisms mediating this impairment remains to be determined in future studies. Our results confirmed that the endothelium‐dependent vasodilatation in response to ACh was reduced significantly after high intake of salt, which represents additional confirmation of endothelial dysfunction and reduced availability of NO (Fig. 3 A). Functional capacity of vascular smooth muscle cells was tested by SNP and that response was preserved in both groups (Fig. 3 B). Free oxygen radicals are produced during various physiological metabolic processes, and their overproduction in the generation of oxidative stress is well‐known. They are produced in all vascular cells (endothelial, smooth muscle) and perivascular adipocytes, as well as circulatory leukocytes (Belikov, 2015), may also produce them (Bulloch & Daly, 2014). ROS participate in vascular cell signalling in physiological and pathological conditions. Endothelial cells produce oxygen free radicals including superoxide (O2−), hydrogen peroxide (H2O2), peroxynitrite (ONOO−), hydroxyl radical (HO·) and some others, which can act directly as an EDCF, e.g. thromboxane A2 (TXA2) and prostaglandin F2α (PGF2α) (Katusic & Vanhoutte, 1989; Cavka et al. 2015), or indirectly, by reducing the bioavailability of NO (Huie & Padmaja, 1993).
Animal studies have shown that increased oxidative stress decreases NO, which is an important mediator of FID (Koller & Huang, 1994). It is well accepted that HS increases oxidative stress (Drenjancevic‐Peric & Lombard, 2005). In the present study, TEMPOL had no effets on the FID of the LS group MCAs (Fig. 2 A and B). However, in HS animals TEMPOL in vitro enhanced the FID of MCAs (Fig. 2 C and D). This enhanced response in HS group was NO mediated, because l‐NAME successfully abolished it (Fig. 2 C and D). These results confirm that high salt intake produces a significant level of oxygen free radicals and increased oxidative stress. There are several important antioxidant systems involved in reducing ROS, such as SOD, CAT and GPx (Fridovich, 1997). Since tissue antioxidant enzyme activities in brain blood vessels were not significantly different between the groups (Table 2), mRNA gene expression analysis of antioxidative enzymes iNOS and eNOS was performed. Lenda et al. (2000; 2002) presumed that a HS diet decreased the level of antioxidant enzyme expression (SOD isoforms) or activity and that, as a consequence, oxidative stress reduced dilatation of rats spinotrapezius muscle microvessels (arterioles and veins). Although the mRNA expression of SOD isoforms was not significantly different between groups in the present study (Table 3), other studies have demonstrated that protein expression of Cu/Zn SOD is significantly decreased in high salt‐fed rats (McEwen et al. 2009), supporting its role in decreased antioxidative defence mechanisms. It is well known that GPx1 and GPx4 are important enzymes for phospholipid peroxidation, particularly GPx4, which protects against lipid peroxidation (Ursini et al. 1982). To our knowledge, this is the first study to present data for GPx1 and GPx4 gene expression in brain blood vessels in animals on high salt diet. In the present study, HS intake has the greatest influence on the level of GPx4, which was significanlty decreased compared to the LS group (Table 3). Additionally, TBARS, which determines the products of lipid peroxidation (malondialdehyde), and acts as an indicator of oxidative stress, was significantly increased in the brain blood vessels after 1 week of HS diet (Table 1). Since the expression and activity of other antioxidative enzymes were not changed by HS diet, the results suggest that GPx4 is the most important isoform in cerebral vessels responsible for maintaining oxidative status.
There are also no previous data on the expression of iNOS and eNOS in the tissue of brain blood vessels after acute salt intake (Table 3). Some other studies have established that short‐term salt loading for 48 h (8% NaCl) resulted in significant reduction of iNOS in the renal cortex and aorta and of eNOS in the aorta (Ni & Vaziri, 2001). The present molecular analysis confirms functional experimental results that lack of NO, due to either low production or decreased bioavailability of NO due to high oxidative stress, is the most important reason for impaired FID in cerebral resistance arteries in HS‐fed animals.
Recent studies showed that high dietary salt intake can affect normal functioning of the immune system. In particular, it has been shown that increased local salt concentrations under physiological conditions in vivo markedly boost the induction of murine and human TH17 cells and impair Treg function, together facilitating development of autoimmunity (Kleinewietfeld et al. 2013; Hernandez et al. 2015). Furthermore, ROS have been linked to T‐cell hyporesponsiveness, apoptosis and activation (Cemerski et al. 2002; Belikov et al. 2015). The origin of intracellular ROS in lymphocytes comes from at least two sources – exogenous ROS produced by dendritic cells and phagocytes, and intracellularly produced ROS during various cellular processes, including TCR‐mediated T‐cell activation (Belikov et al. 2015). Whereas exogenous ROS can lead to oxidative stress, endogenously produced ROS primarily act as signalling molecules promoting either activation or apoptosis of T cells (Belikov et al. 2015). Under physiological conditions there is a balance between ROS and antioxidants, and the perturbation of this equilibrium can result in T‐cell hyper‐ or hyporesponsiveness, leading to the development of various pathologies (Amico et al. 2015). In this study we found that HS diet significantly increased basal intracellular ROS levels in lymphocytes isolated from mesenteric lymph nodes and spleen, as well as intracellular ROS production in peripheral lymph node lymphocytes upon PMA stimulation (Fig. 4). A limitation of this study is the lack of differentiation between different T/B‐cell subsets, as well as the inability to assess their functional status. However, our results suggest that the oxidative equilibrium of lymphocytes has been significantly altered and resulted in increased intracellular levels of ROS which might alter their normal function.
The summary of the results of the present study are depicted in Fig. 5. This study demonstrates for the first time that HS intake definitely impairs endothelium‐dependent FID in cerebral resistance vessels. The expression of antioxidant enzymes is reduced but the only significant difference is in the mRNA expression of GPx4, which may play a major role in the increased oxidative stress in MCAs. This is confirmed with significantly higher levels of lipid peroxidation with HS intake. Reduced levels of NO may occur due to decreased expression of iNOS, or reduced bioavailability due to high oxidative stress. As shown in Fig. 5, high salt dietary intake increased production of ROS in peripheral lymphoid organs and so generated additional oxidative stress, which we speculate might contribute to decreased vasodilatation of MCAs in in vivo conditions.
Figure 5.
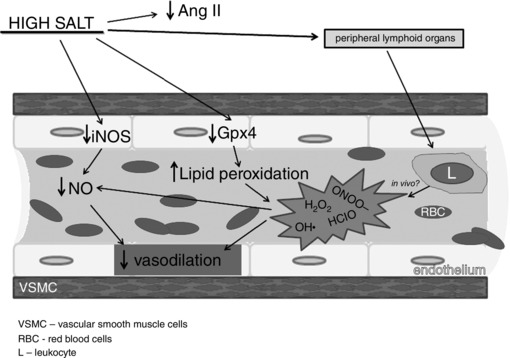
Schematic overview of the influence of high salt intake on increased production of oxidative stress and reduced vasodilation
Cerebral resistance vessels are crucial for control of the overall brain blood flow and tissue perfusion. The present study elucidates at least one segment of that complex problem and suggests lines of future research of the mechanisms of flow‐induced dilatation that may be altered by high salt diet. Since this kind of information is still rare, linking vascular functional studies with biochemical and molecular gene expression data is especially valuable for the understanding of these processes. By providing data for mechanisms in basic physiological research, the present study supports the so‐called ‘evidence‐based medicine’ approach in understanding cerebrovascular diseases, such as stroke.
Study limitations
In this study myogenic response was not assessed. However, the available literature suggests that a short‐term HS diet, either with 4% NaCl, as was used in the present study as well, or with 6% NaCl, does not significantly affect myogenic tone in any of the observed vascular beds (e.g. skeletal muscle resistance arteries in Sprague‐Dawley rats (Weber et al. 1999) or afferent arterioles in mice (Lai et al. 2010)). Moreover, Weber et al. (1999) demonstrated that even a long‐term (4—8 weeks) HS diet with 4% NaCl did not change myogenic responses of resistance arteries.
Additional information
Conflicts of interests
The authors declare no conflict of interest.
Author contributions
Anita Cosic: Conception and design; Collection and assembly of data; Data analysis and interpretation; Manuscript Writing; Final approval of manuscript Ivana Jukic: Conception and design; Collection and assembly of data; Data analysis and interpretation; Manuscript Writing; Final approval of manuscript Ana Stupin: Collection and assembly of data; Data analysis and interpretation; Manuscript Writing; Final approval of manuscript Martina Mihalj: Collection and assembly of data; Data analysis and interpretation; Manuscript Writing; Final approval of manuscript Zrinka Mihaljevic: Collection and assembly of data; Data analysis and interpretation; Manuscript Writing; Final approval of manuscript Sanja Novak: Collection and assembly of data; Data analysis and interpretation; Manuscript Writing; Final approval of manuscript Rosemary Vukovic: Collection and assembly of data; Data analysis and interpretation; Manuscript Writing; Final approval of manuscript Ines Drenjancevic: Conception and design; Collection and assembly of data; Data analysis and interpretation; Manuscript Writing; Final approval of manuscript.
Funding
This work has been supported in part by Croatian Science Foundation under the project # IP‐2014‐09‐6380 and in part with VIF‐MEFOS‐15 (Faculty of Medicine Osijek, Croatia).
Acknowledgements
The authors are grateful to Dr Aleksandar Kibel for helpful advice during the development of the study.
This is an Editor&s Choice article from the 1 September 2016 issue.
References
- Aebi H (1984). Catalase in vitro . Methods Enzymol 105, 121–126. [DOI] [PubMed] [Google Scholar]
- Amico D, Spadoni T, Rovinelli M, Serafini M, D'Amico G, Campelli N, Svegliati Baroni S & Gabrielli A (2015). Intracellular free radical production by peripheral blood T lymphocytes from patients with systemic sclerosis: role of NADPH oxidase and ERK1/2. Arthritis Res Ther 17, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belikov AV, Schraven B & Simeoni L (2015). T cells and reactive oxygen species. J Biomed Sci 22, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzie IF & Strain JJ (1996). The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: the FRAP assay. Anal Biochem 239, 70–76. [DOI] [PubMed] [Google Scholar]
- Bonini MG, Rota C, Tomasi A & Mason RP (2006). The oxidation of 2′,7′‐dichlorofluorescein to reactive oxygen species: a self‐fulfilling prophesy? Free Radic Biol Med 40, 968–975. [DOI] [PubMed] [Google Scholar]
- Bryan RM Jr, Marrelli SP, Steenberg ML, Schildmeyer LA, Johnson TD (2001). Effects of luminal shear stress on cerebral arteries and arterioles. Am J Physiol Heart Circ Physiol 280, H2011–H2022. [DOI] [PubMed] [Google Scholar]
- Bulloch JM & Daly CJ (2014). Autonomic nerves and perivascular fat: interactive mechanisms. Pharmacol Ther 143, 61–73. [DOI] [PubMed] [Google Scholar]
- Cavka A, Cosic A, Grizelj I, Koller A, Jelaković B, Lombard JH, Phillips SA & Drenjancevic I (2013). Effects of AT1 receptor blockade on plasma thromboxane A2(TXA2) level and skin microcirculation in young healthy women on low salt diet. Kidney Blood Press Res 37, 432–442. [DOI] [PubMed] [Google Scholar]
- Cavka A, Cosic A, Jukic I, Jelakovic B, Lombard JH, Phillips SA, Seric V, Mihaljevic I & Drenjancevic I (2015). The role of cyclooxygenase‐1 in high salt diet‐ induced microvascular dysfunction in humans. J Physiol 593, 5313–5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cemerski S, Cantagrel A, Van Meerwijk JP & Romagnoli P (2002). Reactive oxygen species differentially affect T cell receptor‐signaling pathways. J Biol Chem 277, 19585–19593. [DOI] [PubMed] [Google Scholar]
- Chomczynski P & Sacchi N (1987). Single‐step method of RNA isolation by acid guanidiniumthiocyanate‐phenol‐chloroform extraction. Anal Biochem 162, 156–159. [DOI] [PubMed] [Google Scholar]
- Crimi E, Ignarro LJ & Napoli C (2007). Microcirculation and oxidative stress. Free Radic Res 41, 1364–1375. [DOI] [PubMed] [Google Scholar]
- Drenjancevic‐Peric I, Frisbee JC & Lombard JH (2003). Skeletal muscle arteriolar reactivity in SS BN13 consomic rats and Dahl salt‐sensitive rats. Hypertension 41, 1012–1015. [DOI] [PubMed] [Google Scholar]
- Drenjancevic‐Peric I, Jelakovic B, Lombard JH, Kunert MP, Kibel A & Gros M (2011). High‐salt diet and hypertension: focus on the renin‐angiotensin system. Kid Blood Press Res 34, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenjancevic‐Peric I & Lombard JH (2005). Reduced angiotensin II and oxidative stress contribute to impaired vasodilation in dahl salt‐sensitive rats on low‐salt diet. Hypertension 45, 687–691. [DOI] [PubMed] [Google Scholar]
- Drouin A, Bolduc V, Thorin‐Trescases N, Belanger E, Fernandes P, Baraghis E, Lesage F, Gillis MA, Villeneuve L, Hamel E, Ferland G, Thorin E (2011). Catechin treatment improves cerebrovascular flow‐mediated dilation and learning abilities in atherosclerotic mice. Am J Physiol Heart Circ Physiol 300, H1032–H1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouin A & Thorin E (2009). Flow‐induced dilation is mediated by Akt‐dependent activation of endothelial nitric oxide synthase‐derived hydrogen peroxide in mouse cerebral arteries. Stroke 40, 1827–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond GB (2009). Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol 587, 713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand MJ, Lombard JH (2013). Low‐dose angiotensin II infusion restores vascular function in cerebral arteries of high salt‐fed rats by increasing copper/zinc superoxide dimutase expression. Am J Hypertens 26, 739–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flammer AJ & Lüscher TF (2010). Three decades of endothelium research. From the detection of nitric oxide to the everyday implementation of endothelial function measurements in cardiovascular diseases. Swiss Med Wkly 140, w13122. [DOI] [PubMed] [Google Scholar]
- Flohé L & Ötting F (1984). Superoxide dismutase assays. Method. Enzymol 105, 93–104. [DOI] [PubMed] [Google Scholar]
- Fridovich I (1997). Superoxide anion radical (O2−.), superoxide dismutases and related matters. J Biol Chem 272, 18515–18517. [DOI] [PubMed] [Google Scholar]
- Furchgott RF & Zawadzki JV (1980). The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288, 373–376. [DOI] [PubMed] [Google Scholar]
- Garcia‐Roldan JL, Bevan JA (1990). Flow‐induced constriction and dilation of cerebral resistance arteries. Circ Res 66, 1445–1448. [DOI] [PubMed] [Google Scholar]
- Hempel SL, Buettner GR, O'Malley YQ, Wessels DA & Flaherty DM (1999). Dihydrofluorescein diacetate is superior for detecting intracellular oxidants: comparison with 2′,7′‐dichlorodihydrofluorescein diacetate, 5 (and 6)‐carboxy‐2′,7′‐dichlorodihydrofluorescein diacetate, and dihydrorhodamine. Free Radic Biol Med 27, 146–159. [DOI] [PubMed] [Google Scholar]
- Hernandez AL, Kitz A, Wu C, Lowther DE, Rodriguez DM, Vudattu N, Deng S, Herold KC, Kuchroo VK, Kleinewietfeld M & Hafler DA (2015). Sodium chloride inhibits the suppressive function of FOXP3+ regulatory T cells. J Clin Invest 125, 4212–4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huie RE & Padmaja S (1993). The reaction of no with superoxide. Free Radic Res Commun 18, 195–199. [DOI] [PubMed] [Google Scholar]
- Ignarro LJ (2002). Nitric oxide as a unique signaling molecule in the vascular system: a historical overview. J Physiol Pharmacol 53, 503–514. [PubMed] [Google Scholar]
- Jelakovic B, Kaic‐Rak A, Milicic D, Premuzic V, Skupnjak B & Reiner Z (2009. a). Manje soli – više zdravlja. Hrvatska inicijativa za smanjenje nosa kuhinjske soli (CRASH). Liječnički Vjesnik 131, 87–92. [PubMed] [Google Scholar]
- Jelakovic B, Premuzic V, Skupnjak B & Reiner Z (2009. b). Kuhinjska sol – skriveni otrov u svakodnevnoj hrani. Liječnički vjesnik: glasilo Hrvatskog liječničkog zbora. Suplement 131, 146‐154. [PubMed] [Google Scholar]
- Kalyanaraman B, Darley‐Usmar V, Davies KJ, Dennery PA, Forman HJ, Grisham MB, Mann GE, Moore K, Roberts LJ 2nd & Ischiropoulos H (2012). Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic Biol Med 52, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katusic ZS & Vanhoutte PM (1989). Superoxide anion is an endothelium‐derived contracting factor. Am J Physiol Heart Circ Physiol 257, H33–H37. [DOI] [PubMed] [Google Scholar]
- Kibel A, Novak S, Cosic A, Mihaljevic Z, Falck JR & Drenjančević I (2015). Hyperbaric oxygenation modulates vascular reactivity to angiotensin‐(1‐7) in diabetic rats ‐ potential role of epoxyeicosatrienoic acids. Diab Vasc Dis Res 12, 33–45. [DOI] [PubMed] [Google Scholar]
- Kleinewietfeld M1, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, Muller DN & Hafler DA (2013). Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 496, 518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller A & Huang A (1994). Impaired nitric oxide‐mediated flow‐induced dilation in arterioles of spontaneously hypertensive rats. Circ Res 74, 416–421. [DOI] [PubMed] [Google Scholar]
- Koller A, Sun D & Kaley G (1993). Role of shear stress and endothelial prostaglandins in flow‐ and viscosity‐induced dilation of arterioles in vitro . Circ Res 72, 1276–1284. [DOI] [PubMed] [Google Scholar]
- Lai EY, Onozato ML, Solis G, Aslam S, Welch WJ & Wilcox CS (2010). Myogenic responses of mouse isolated perfused renal afferent arterioles: effects of salt intake and reduced renal mass. Hypertension 55, 983–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen BT, Campbell WB & Gutterman DD (2007). Beyond vasodilatation: non‐vasomotor roles of epoxyeicosatrienoic acids in the cardiovascular system. Trends Pharmacol Sci 28, 32–38. [DOI] [PubMed] [Google Scholar]
- Lenda DM & Boegehold MA (2002). Effect of a high salt diet on microvascular antioxidant enzymes. J Vasc Res 39, 41–50. [DOI] [PubMed] [Google Scholar]
- Lenda DM, Sauls BA & Boegehold MA (2000). Reactive oxygen species may contribute to reduced endothelium‐dependent dilation in rats fed high salt. Am J Physiol Heart Circ Physiol 279, H7–H14. [DOI] [PubMed] [Google Scholar]
- Liu Y, Bubolz AH, Mendoza S, Zhang DX & Gutterman DD (2011). H2O2 is the transferrable factor mediating flow‐induced dilation in human coronary arterioles. Circ Res 108, 566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen ST, Schmidt JR, Somber L, de la Cruz L & Lombard JH (2009). Time‐course and mechanisms of restored vascular relaxation by reduced salt intake and angiotensin II infusion in rats fed a high‐salt diet. Microcirculation 16, 220–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden JA & Christman NJ (1999). Integrin signaling, free radicals, and tyrosine kinase mediate flow constriction in isolated cerebral arteries. Am J Physiol Heart Circ Physiol 277, H2264–H2271. [DOI] [PubMed] [Google Scholar]
- Matoba T, Shimokawa H, Kubota H, Morikawa K, Fujiki T, Kunihiro I, Mukai Y, Hirakawa Y & Takeshita A (2002). Hydrogen peroxide is an endothelium‐derived hyperpolarizing factor in human mesenteric arteries. Biochem Biophys Res Commun 290, 909–913. [DOI] [PubMed] [Google Scholar]
- Moncada S & Higgs EA (2006). The discovery of nitric oxide and its role in vascular biology. Br J Pharmacol 147, S193–S201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Z & Vaziri ND (2001). Effect of salt loading on nitric oxide synthase expression in normotensive rats. Am J Hypertens 14, 155–163. [DOI] [PubMed] [Google Scholar]
- Oakes KD & Van Der Kraak GJ (2003). Utility of the TBARS assay in detecting oxidative stress in white sucker (Catostomuscommersoni) populations exposed to pulp mill effluent. Aquat Toxicol 63, 447–463. [DOI] [PubMed] [Google Scholar]
- Rubanyi GM, Romero JC & Vanhoutte PM (1986). Flow‐induced release of endothelium‐derived relaxing factor. Am J Physiol Heart Circ Physiol 250, H1145–H1149. [DOI] [PubMed] [Google Scholar]
- Sandoo A, van Zanten JJ, Metsios GS, Carroll D & Kitas GD (2010). The endothelium and its role in regulating vascular tone. Open Cardiovasc Med J 4, 302–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda LA, Norins NA, Jeutter DC & Madden JA (1996). Flow‐induced responses in piglet isolated cerebral arteries. Pediatr Res 39, 574–583. [DOI] [PubMed] [Google Scholar]
- Sylvester FA, Stepp DW, Frisbee JC & Lombard JH (2002). High‐salt diet depresses acetylcholine reactivity proximal to NOS activation in cerebral arteries. Am J Physiol Heart Circ Physiol 283, H353–H363. [DOI] [PubMed] [Google Scholar]
- Toth P, Rozsa B, Springo Z, Doczi T & Koller A (2011). Isolated human and rat cerebral arteries constrict to increases in flow: role of 20‐HETE and TP receptors. J Cereb Blood Flow Metab 31, 2096–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran QK, Ohashi K & Watanabe H (2000). Calcium signalling in endothelial cells. Cardiovasc Res 48, 13–22. [DOI] [PubMed] [Google Scholar]
- Tzemos N, Lim PO, Wong S, Struthers AD & MacDonald TM (2008). Adverse cardiovascular effects of acute salt loading in young normotensive individuals. Hypertension 51, 1525–1530. [DOI] [PubMed] [Google Scholar]
- Ursini F, Maiorino M, Valente M, Ferri L & Gregolin C (1982). Purification from pig liver of a protein which protects liposomes and biomembranes from peroxidative degradation and exhibits glutathione peroxidase activity on phosphatidylcholine hydroperoxides. Biochim Biophys Acta 710, 197–211. [DOI] [PubMed] [Google Scholar]
- Vallance P, Collier J & Moncada S (1989). Effects of endothelium‐derived nitric oxide on peripheral arteriolar tone in man. Lancet 2, 997–1000. [DOI] [PubMed] [Google Scholar]
- Verma S & Anderson TJ (2002). Fundamentals of endothelial function for the clinical cardiologist. Circulation 105, 546–549. [DOI] [PubMed] [Google Scholar]
- Vollmer WM, Sacks FM, Ard J et al (2001). DASH‐Sodium Collaborative Research Group. Effects of diet and sodium intake on blood pressure: subgroup analysis of the DASH‐Sodium trial. Ann Intern Med 135, 1019–1028. [DOI] [PubMed] [Google Scholar]
- Weber DS, Frisbee JC & Lombard JH (1999). Selective potentiation of angiotensin‐induced constriction of skeletal muscle resistance arteries by chronic elevations in dietary salt intake. Microvasc Res 57, 310–319. [DOI] [PubMed] [Google Scholar]
- Weinberger MH (2002). Salt sensitivity is associated with an increased mortality in both normal and hypertensive humans. J Clin Hypertens (Greenwich) 4, 274–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendel A (1981). Glutathione peroxidase In Enzymatic Basis of Detoxication, ed. Jakoby WB, chapter 1, pp. 333–353. Academic Press, New York. [Google Scholar]


