Abstract
Background and Aims:
Different routes of administration of α2 adrenergic receptor agonists have been found to prolong the duration of spinal block.
Material and Methods:
One hundred and twenty patients, aged 18-60 years, of ASA physical status I or II posted for elective fixation of fractures of lower limb under spinal anesthesia were selected. Spinal anesthesia was administered with 2.5 ml of 0.5% bupivacaine mixed with 10 μg fentanyl. The patients were randomized to receive intravenous (IV) dexmedetomidine 1 μg/kg/h for 15 min followed by infusion of 0.3 μg/kg/h (Group I), IV Clonidine 2 μg/kg/h for 15 min followed by infusion of 0.5 μg kg/h (Group II) or 15 ml of normal saline for 15 min followed by infusion at 50 ml/h (Group III). Motor and sensory blockade was evaluated using bromage score and pin prick method respectively.
Results:
The median block height in all groups was T8. Time to achieve block height was fastest in Group I. Time of regression of sensory block to T12/L1 dermatome was 230.75 ± 21.25 min (Group I), 196.25 ± 20.27 min (Group II) and 163.88 ± 15.46 min (Group III) respectively. Regression of motor blocks to Bromage 0/1 was 274 ± 21.25 min, 234.25 ± 32.41 min and 130.12 ± 20.70 min in Groups I, II and III respectively. Bradycardia was seen in one patient in Group I and two patients in Group II. Hypotension was seen in five patients in Group I and seven patients in Group II. First requirement for postoperative analgesic was after 353.13 ± 39.60 min, 314.38 ± 30.64 min and 193.25 ± 17.74 min in Groups I, II and III respectively.
Conclusion:
IV α2 agonists are useful adjuvants for prolongation of the duration of spinal block. IV dexmedetomidine produces a better clinical profile compared to clonidine.
Key words: Clonidine, dexmedetomidine, orthopedic surgery, spinal block
Introduction
Spinal anesthesia is an established mode of anesthesia, especially for infraumblical area and lower extremity surgeries. Its simplicity, ease of administration and avoidance of side effects of general anesthesia are main advantages. However, a short postoperative duration of analgesia is a limitation. Alpha-2 (α2) adrenergic receptor agonists act at supraspinal and spinal level of central nervous system to modulate pain relief. Two commonly used drugs in clinical practice are clonidine and dexmedetomidine. Various routes of administration of these drugs such as oral, spinal, epidural have been found to prolong the duration of spinal block.[1,2,3] However, literature on the effects of intraoperative infusion of clonidine or dexmedetomidine on spinal block and postoperative analgesic requirement is sparse and conflicting.[4,5] This study was conducted to evaluate the effects of intravenous (IV) infusion of dexmedetomidine and clonidine on spinal block and its postoperative analgesic effect in patients undergoing elective orthopedic surgery under spinal anesthesia.
Material and Methods
The study was conducted after Institutional Ethical Committee clearance and written informed consent in 120 patients, 18-60 years of age, either sex, American Society of Anesthesiologists (ASA) I and II posted for elective fixation of long bones fractures of lower limb under spinal anesthesia. Exclusion Criteria's included patient refusal for the spinal block, BMI >30 kg/m2, history of preoperative intake of beta blockers, α2 adrenergic receptor antagonists, calcium channel blockers or angiotensin converting enzyme inhibitors. Patients with preoperative cardiac rhythm abnormalities-like bradycardia or A-V junction block were also excluded.
The patients were randomly allocated by sealed envelope method into three equal groups to receive IV dexmedetomidine1 µg/kg/h for 15 min followed by infusion of 0.3 µg/kg/h till the end of surgery (Group I), IV clonidine 2 µg/kg/h for 15 min followed by infusion of 0.5 µg/kg/h till end of surgery (Group II), or 15 ml of normal saline for 15 min followed by infusion at 50 ml/h till end of surgery (Group III).
Patients in all the groups were kept fasting for solids for 6-8 h and received oral alprazolam 0.25 mg night before and 2 h before surgery. In the operating room, the patients were preloaded with 500 ml Ringer Lactate followed by administration of spinal anesthesia with 2.5 ml of 0.5% heavy bupivacaine mixed with 10 µg fentanyl utilizing 23-25 G Quincke's needle. The patients were turned supine, and the infusion of the test drug started as per the group allotment. Time taken to achieve the sensory and motor block height of T10 or above was noted. Bromage score was used for assessing motor blockade[6] and pin prick sensation for sensory blockade.
Vital parameters such as NIBP, heart rate (HR) were recorded every 2 min for first 15 min and thereafter every 5 min till the end of surgery. Hypotension (fall in blood pressure >20% of baseline) was treated initially with fast infusion of Ringer lactate 250 ml and IV mephentermine 5 mg bolus if not responding to fluid administration. Bradycardia (HR <45 beats/min) was treated with IV Atropine 0.6 mg. None of the patients received oxygen supplementation after administration of spinal. Respiratory depression was defined as SpO2<92% or respiratory rate <10 breaths/min. Ramsay sedation score[7] was used to evaluate the perioperative sedation of the patient, after 15 min of starting the infusion and then every 30 min till end of surgery. At the end of surgery infusions were stopped. Motor and sensory block were checked and patient shifted to the recovery area. Pain assessment in postoperative period was done utilizing Visual analogue score[8](VAS) and rescue analgesic in form of IV tramadol 100 mg IV was administered when VAS score was >4.
Time for achieving sensory block to T12-L1 dermatome and bromage score of 0/1 was noted in the postoperative ward. VAS was checked at 2,4,12 and 24 h postoperatively. Cumulative consumption of tramadol over 24 h postoperatively was noted. Presence of complications such as nausea, vomiting, shivering, bradycardia, hypotension were also noted.
Sample size calculation was based on a previous study,[9] Assuming use of α2 agonist to increase the duration of analgesia by 20%, with the level of significance of 95%, power of study 80%, α error of 0.05 and β error of 0.2, 35 patients per group were needed. We took 40 patients per group for our study to compensate for any dropouts.
Results
The patients in the groups were comparable with regards to age, sex, ASA grade and weight [Table 1]. Type of surgeries according to the area involved is depicted in Table 2. Inadequate spinal block was seen in one patient, three patients and seven patients in Groups I, II and III respectively. These patients were included in the demographic profile, but excluded from the final analysis.
Table 1.
Demographic profile

Table 2.
Types of surgeries
The median height of sensory block in all the groups was T8 with the range of T6-T10. Time required to achieve the block height was 5.7 ± 2.2 min in Group I as compared to 7.4 ± 3.1 min in Group II and 7.4 ± 2.6 min in Group III. The onset of sensory block was significantly faster in Group I as compared to Group III (P = 0.000), while it was comparable between Groups I and II, and Groups II and III.
The mean duration of time for regression of sensory block to T12/L1 dermatome was 230.8 ± 21.3 min in Group I, 196.3 ± 20.3 min in Group II and 163.9 ± 15.5 min in Group III respectively. Regressionof sensory block was delayed in Group I compared to Groups II and III (P < 0.001).
The mean duration for regression of motor block to Bromage 0/1 in unaffected limb was 274 ± 21.3 min, 234.3 ± 32.4 min and 130.1 ± 20.7 min in Groups I, II and III respectively. The mean duration of motor block was significantly prolonged in Group I & II compared to Group III (P < 0.001).
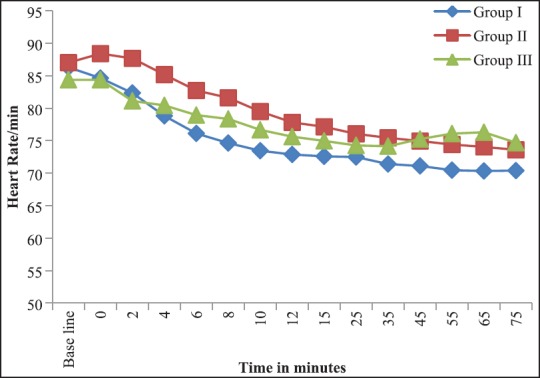
A fall in HR was seen in all the groups compared to the baseline value. The values were lowest in Group I at all time of observations [Figure 1]. One patient in Group I and two patients in Group II had bradycardia.
Figure 1.
Heart rate at different time intervals
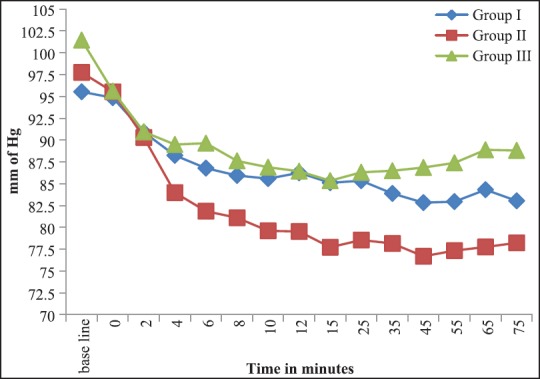
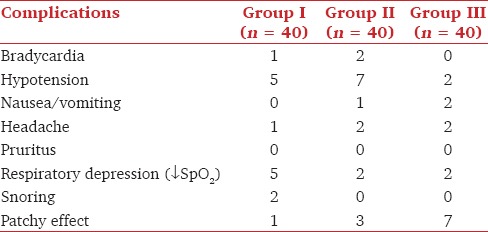
Hypotension was observed at all times of observations in all the groups. The fall was however not statistically significant [Figure 2]. Statistically Significant hypotension was seen in five patients in Group I and seven patients in Group II necessitating the use of IV mephentermine in two patients in Group I and five patients in Group II. Perioperative decrease in SpO2< 94% was observed in five patients in Group I and two patients in Groups II and III each [Table 3].
Figure 2.
Mean blood pressure at different time intervals
Table 3.
Incidence of intraoperative complications
Postoperative analgesia with IV tramadol was demanded after 353.1 ± 39.6 min, 314.4 ± 30.6 min and 193.3 ± 17.7 min in Groups I, II and III respectively of administration of spinal block. The time duration for need of rescue analgesic was significantly longer in Group I and II compared to the control group (P < 0.001). Compared to Group II, time for administration of rescue analgesic was also prolonged in Group I (P < 0.001). Total cumulative dosage of Tramadol required in first 24 h were 145.0 ± 50.4 mg, 162.5 ± 49.0 mg and 265.0 ± 48.3 mg in Groups I, II and III respectively. The cumulative doses of tramadol needed were comparable between Groups I and II (P = 0.515) while it was significant between Groups I and III (P < 0.001) and Group II and III (P < 0.001). Median VAS score was also less in Groups I and II compared to Group III.
Discussion
Use of α2 agonists is associated with prolongation of effects of local anesthetics, mechanisms postulated being peripheral,[10,11] spinal[12,13,14,15,16] and supra-spinal[17,18] in location. Supraspinal effects are a result of inhibition of locus ceruleus in brainstem resulting in disinhibition of noradrenergic nuclei and descending inhibitory effect on nociception in the spinal cord. Dexmedetomidine is 8 times more specific on α2 receptors as compared to clonidine.[17,18]
The bolus and infusion doses of dexmedetomidine and clonidine used is varied among different studies.[9,19,20] We selected the dose of 1 mcg/kg/h for dexmedetomidine and 2 mcg/kg/h for clonidine for bolus infusion over first 15 minutes instead of 1 mcg/kg for dexmedetomidine and 2 mcg/kg for clonidine. Thus the mean cumulative doses infused of dexmedetomidine and clonidine is less. The dosage for bolus infusion as well as maintenance is lower than studies of various authors.[4,9,17] The timing of starting the maintainence infusions in our studies was as soon as the patient was turned supine while in other studies[9,17] it was after 15-20 minutes of administration of spinal block. The onset of the motor and sensory block in our study demonstrated an early response to dexmedetomidine compared to clonidine and control. This was in contrast to the study of Elcicek et al.[4] and Lugo et al.[9] who did not demonstrate any difference in onset of block. A similar early onset of block was demonstrated by Harsoor et al.[5] and Reddy et al.[21]
Prolongation of motor and sensory block with the use of α2 agonist occurs as a result of differential block of Aα and C fibers. Motor blockade by α2 agonist results from the direct inhibition of impulse transmission in large, myelinated Aα fibers. The EC50 of α2 agonist is four fold less in large fibers compared to unmyelinated C fibers.[15,16] This is probably the cause of increased sensory block leading to prolonged analgesia compared to the motor block.[17,18]
α2 agonists leads to decreased myocardial contractility, systemic vascular resistance, cardiac output and systemic blood pressure. The hemodynamic response of bolus dexmedetomidine is biphasic. Rapid injection results in the initial increase in blood pressure followed by a fall in blood pressure and HR. The initial increase results from peripheral vasoconstriction due to stimulation of the peripheral α2 receptors.[22,23] Riker and Fraser[24] have postulated use of either slow IV infusion of the loading dose or complete omission of same to decrease these hemodynamic variations. The cardiovascular actions of clonidine are complex. It acts at nucleus tractus solitaries in the medulla reducing the sympathetic outflow, thereby reducing HR and arterial pressure. Peripheral activities at presynaptic α2 receptors causes vasodilatation and vasoconstriction at postsynaptic α2 and α1 receptors.[25]
The sedative effects of α2 agonist is due to its effect similar to endogenous sleep. The quality of sedation produced by dexmedetomidine is different when compared to sedatives acting through gamma-aminobutyric acid systems.[26] Patients receiving dexmedetomidine infusions are easy to wake up and follow the commands. The similarity between natural sleep and dexmedetomidine induced hypnosis results from ion conductance inhibition through L and P type calcium channels and facilitation of conductance through voltage gated potassium ion channels.
Inhibition of the locus coeruleus leads to disinhibition of the noradrenergic nuclei, exerting descending inhibitory effect on nociception at the spinal cord and contributing to prolonged postoperative analgesia.[23,26] Rescue analgesic requirements in the postoperative period were delayed in our study with the use of dexmedetomidine and clonidine. The effective doses of analgesic cannot be compared with the previous studies as most have used either patient controlled analgesia or nurse controlled analgesia pumps for rescue analgesia. Because of the lack of such facilities in our setup, we used 100 mg of tramadol IV on demand.
Hypotension, bradycardia, nausea and vomiting are the common complications of subarachnoid block. In contrast to thestudies of Kaya et al.[27] and Al Oweidi et al.[28] we observed a lower incidence of bradycardia (2.5% compared to 8% and 4% respectively) and sedation (5% vs. 8%), but a higher incidence of hypotension (12.5% vs. 8% and 4% respectively) and respiratory depression (12.5% vs. nil) with dexmedetomidine respectively. Use of Clonidine was associated with a higher incidence of hypotension (17.5% vs. 11.5% and 12% respectively) and respiratory depression (5% vs. 0% each) but a lower incidence of nausea/vomiting (2.5% vs. 8%) and bradycardia (5% vs. 15.3% and 16% respectively) compared to study of Rhee et al.[29] and Lugo et al.[9]
Conclusion
Thus co-administration of IV dexmedetomidine or clonidine improves spinal block characteristics and increases the duration of postoperative analgesia without significant motor blockade. Their use may be recommended to reduce postoperative pain, analgesic requirements and improve the patient's satisfaction in settings of limited resources. Respiratory depression is a possibility which needs vigilant monitoring
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Hamed AM, Talaat SM. Effect of intravenous versus intrathecal low-dose dexmedetomidine on spinal block in lower limb orthopedic surgery. Ain-Shams J Anaesthesiol. 2014;7:205–10. [Google Scholar]
- 2.Mahendru V, Tewari A, Katyal S, Grewal A, Singh M R, Katyal R. A comparison of intrathecal dexmedetomidine, clonidine, and fentanyl as adjuvants to hyperbaric bupivacaine for lower limb surgery: A double blind controlled study. J Anaesthesiol Clin Pharmacol. 2013;29:496–502. doi: 10.4103/0970-9185.119151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar A, Bose S, Bhattacharya A, Tandon OP, Kundra P. Oral clonidine premedication for elderly patients undergoing intraocular surgery. Acta Anaesthesiol Scand. 1992;36:159–64. doi: 10.1111/j.1399-6576.1992.tb03444.x. [DOI] [PubMed] [Google Scholar]
- 4.Elcicek K, Tekin M, Kati I. The effects of intravenous dexmedetomidine on spinal hyperbaric ropivacaine anesthesia. J Anesth. 2010;24:544–8. doi: 10.1007/s00540-010-0939-9. [DOI] [PubMed] [Google Scholar]
- 5.Harsoor S, Rani DD, Yalamuru B, Sudheesh K, Nethra S. Effect of supplementation of low dose intravenous dexmedetomidine on characteristics of spinal anaesthesia with hyperbaric bupivacaine. Indian J Anaesth. 2013;57:265–9. doi: 10.4103/0019-5049.115616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bromage PR. A comparison of the hydrochloride and carbon dioxide salt of lidocaine and prilocaine in epidural analgesia. Acta Anaesthesiologica Scandinavica. 1965;(Suppl. XVI):55–69. doi: 10.1111/j.1399-6576.1965.tb00523.x. [DOI] [PubMed] [Google Scholar]
- 7.Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. BMJ. 1974;22:656–9. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCormack HM, Horne DJ, Sheather S. Clinical applications of visual analogue scales: A critical review. Psychol Med. 1988;18:1007–19. doi: 10.1017/s0033291700009934. [DOI] [PubMed] [Google Scholar]
- 9.Lugo VW, Ramirez IG, Corral RC, Gallegos NM. Intravenous dexmedetomidinevs intravenous clonidine to prolong bupivacaine spinal anaesthesia: A double blind study. Anestesia en México. 2007;19:143–6. [Google Scholar]
- 10.Nakamura M, Ferreira SH. Peripheral analgesic action of clonidine: Mediation by release of endogenous enkephalin-like substances. EurJPharmacol. 1988;146:223–8. doi: 10.1016/0014-2999(88)90296-8. [DOI] [PubMed] [Google Scholar]
- 11.Pratap JN, Shankar RK, Goroszeniuk T. Co-injection of clonidine prolongs the anesthetic effect of lidocaine skin infiltration by a peripheral action. Anesth Analg. 2007;104:982–3. doi: 10.1213/01.ane.0000257949.46444.a8. [DOI] [PubMed] [Google Scholar]
- 12.Boico O, Bonnet F, Mazoit JX. Effects of epinephrine and clonidine on plasma concentrations of spinal bupivacaine. Acta Anaesthesiol Scand. 1992;36:684–8. doi: 10.1111/j.1399-6576.1992.tb03544.x. [DOI] [PubMed] [Google Scholar]
- 13.Eisenach JC, De Kock M, Klimscha W. alpha(2)-adrenergic agonists for regional anesthesia. A clinical review of clonidine (1984-1995) Anesthesiology. 1996;85:655–74. doi: 10.1097/00000542-199609000-00026. [DOI] [PubMed] [Google Scholar]
- 14.Lawhead RG, Blaxall HS, Bylund DB. Alpha-2A is the predominant alpha-2 adrenergic receptor subtype in human spinal cord. Anesthesiology. 1992;77:983–91. doi: 10.1097/00000542-199211000-00022. [DOI] [PubMed] [Google Scholar]
- 15.Smith MS, Schambra UB, Wilson KH, Page SO, Hulette C, Light AR, et al. alpha 2-Adrenergic receptors in human spinal cord: Specific localized expression of mRNA encoding alpha 2-adrenergic receptor subtypes at four distinct levels. Brain Res Mol Brain Res. 1995;34:109–17. doi: 10.1016/0169-328x(95)00148-l. [DOI] [PubMed] [Google Scholar]
- 16.Yaksh TL, Jage J, Takano Y. Pharmacokinetics and pharmacodynamics of medullar agents. The spinal actions of α-2 adrenergic agonists as analgesics. Clin Anaesthesiol. 1993;7:597–614. [Google Scholar]
- 17.Al-Mustafa MM, Badran IZ, Abu-Ali HM, Al-Barazangi BA, Massad IM, Al-Ghanem SM. Intravenous dexmedetomidine prolongs bupivacaine spinal analgesia. Middle East J Anesthesiol. 2009;20:225–31. [PubMed] [Google Scholar]
- 18.Fragen RJ, Fitzgerald PC. Effect of dexmedetomidine on the minimum alveolar concentration (MAC) of sevoflurane in adults age 55 to 70 years. J Clin Anesth. 1999;11:466–70. doi: 10.1016/s0952-8180(99)00081-1. [DOI] [PubMed] [Google Scholar]
- 19.Bajwa SJ, Bajwa SK, Kaur J, Singh G, Arora V, Gupta S, et al. Dexmedetomidine and clonidine in epidural anaesthesia: A comparative evaluation. Indian J Anaesth. 2011;55:116–21. doi: 10.4103/0019-5049.79883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ribeiro RN, Nascimento JP. The use of dexmedetomidine in anaesthesiology. Rev Bras Anesthesiol. 2003;53:97–113. [Google Scholar]
- 21.Reddy VS, Shaik NA, Donthu B, Reddy Sannala VK, Jangam V. Intravenous dexmedetomidine versus clonidine for prolongation of bupivacaine spinal anesthesia and analgesia: A randomized double-blind study. J Anaesthesiol Clin Pharmacol. 2013;29:342–7. doi: 10.4103/0970-9185.117101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dyck JB, Maze M, Haack C, Azarnoff DL, Vuorilehto L, Shafer SL. Computer-controlled infusion of intravenous dexmedetomidine hydrochloride in adult human volunteers. Anesthesiology. 1993;78:821–8. doi: 10.1097/00000542-199305000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Bloor BC, Ward DS, Belleville JP, Maze M. Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes. Anesthesiology. 1992;77:1134–42. doi: 10.1097/00000542-199212000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Riker RR, Fraser GL. Adverse events associated with sedatives, analgesics, and other drugs that provide patient comfort in the intensive care unit. Pharmacotherapy. 2005;25:8S–18S. doi: 10.1592/phco.2005.25.5_part_2.8s. [DOI] [PubMed] [Google Scholar]
- 25.Bousquet P, Feldman J, Bloch R, Schwartz J. The nucleus reticularis lateralis: A region highly sensitive to clonidine. EurJPharmacol. 1981;69:389–92. doi: 10.1016/0014-2999(81)90490-8. [DOI] [PubMed] [Google Scholar]
- 26.Guo TZ, Jiang JY, Buttermann AE, Maze M. Dexmedetomidine injection into the locus ceruleus produces antinociception. Anesthesiology. 1996;84:873–81. doi: 10.1097/00000542-199604000-00015. [DOI] [PubMed] [Google Scholar]
- 27.Kaya FN, Yavascaoglu B, Turker G, Yildirim A, Gurbet A, Mogol EB, et al. Intravenous dexmedetomidine, but not midazolam, prolongs bupivacaine spinal anesthesia. Can J Anaesth. 2010;57:39–45. doi: 10.1007/s12630-009-9231-6. [DOI] [PubMed] [Google Scholar]
- 28.AlOweidi AS, Al-Mustafa MM, Al-Ajlouni JM, Mas'ad DF, Hamdan MQ, Alghanem SM, Qudaisat IY. Intravenous dexmedetomidine or propofol adjuvant to spinal anaesthesia in total knee replacement surgery. Jordan Med J. 2011;45:174–83. [Google Scholar]
- 29.Rhee K, Kang K, Kim J, Jeon Y. Intravenous clonidine prolongs bupivacaine spinal anesthesia. Acta Anaesthesiol Scand. 2003;47:1001–5. doi: 10.1034/j.1399-6576.2003.00158.x. [DOI] [PubMed] [Google Scholar]


